Abstract
Background and aims
Colectomy with ileoanal pouch is the standard of care for most patients with ulcerative colitis (UC) who require surgery. However, 5%–38% of patients with ileoanal pouch develop pouch strictures that can severely impact the functional results. We retrospectively evaluated the efficacy and safety of endoscopic balloon dilation of ileoanal pouch strictures in patients with inflammatory bowel disease (IBD).
Methods
All consecutive patients with IBD that underwent endoscopic balloon dilatation of a pouch stricture at our institution between January 1, 2011, and April 31, 2016, were included. Clinical, endoscopic, and surgical variables were collected retrospectively. Stricture-related pouch failure was defined by the need for surgical management of pouch stricture including pouch excision, diversion ileostomy, or stricturoplasty. Secondary outcomes included technical success, clinical success, and safety.
Results
Eighty-eight endoscopic balloon dilatations were identified in 20 patients. Sixty percent of patients were female, with a median age at ileoanal pouch of 28.6 years (interquartile range [IQR], 25.5–37.2). Ileoanal pouch was performed for UC in 95% of cases; 95% of patients underwent J pouch; and 65% had a stapled anastomosis, whereas 35% had a handsewn anastomosis. Strictures were diagnosed at a median of 4.6 years (0.2–10.6) after surgery, and half of patients were symptomatic. The most frequent location of stricture was the anal-pouch anastomosis (87%). Half of patients were found to have associated pouchitis, 4 (20%) had at least 1 fistula, and 5 (25%) had ulcerations of the pouch. At the end of follow-up, 6 patients (30%) underwent a change in diagnosis from UC to Crohn’s disease (CD) of the pouch, and in 1 patient (5%) a diagnosis of ischemic stricture was made. A median of 3.5 dilatations per patient (IQR, 2.0–7.0) were performed. Technical efficacy was observed in 87 procedures (98%). Twenty-two procedures were preceded by obstructive symptoms, and a clinical improvement after endoscopic balloon dilatation was observed in 95% of cases. After a median follow-up of 3.0 years (2.1–3.5), only 1 patient had stricture-related pouch failure. After the first dilatation, 4 patients were hospitalized for obstructive symptoms. Conservative management with another endoscopic balloon dilation was clinically effective in all cases. No major complications related to dilation were observed.
Conclusion
Endoscopic balloon dilatation of ileoanal pouch strictures is largely effective and safe and can be recommended as the first line strategy to treat ileoanal pouch strictures in patients with IBD.
INTRODUCTION
It is estimated that 15% of patients with ulcerative colitis (UC) require colectomy within 10 years of diagnosis.1 Ileal pouch anal anastomosis (IPAA) is recognized as the best surgical procedure that allows bowel continuity in these patients.2 Although many studies have shown excellent quality of life in patients who have undergone IPAA, complications are common after this operation including pouchitis, dysplasia, Crohn’s disease of the pouch, and strictures.3–5 Five to 38% of patients with IPAA develop pouch strictures6–9 that could severely impact functional results.10 The most common locations for strictures to develop are at the pouch-anal anastomosis (pouch outlet) and the junction of neoterminal ileum and pouch body (pouch inlet).11 Limited data is available regarding the management of pouch strictures, and treatment is still challenging in clinical practice. Because of the fibrotic nature of pouch strictures, medical therapies are usually ineffective.11 Endoscopic therapy has emerged as an effective and safe alternative to surgery for the management of intestinal strictures.12–14 Although endoscopic dilation is commonly used for Crohn’s diseases (CD) strictures, little data is available in patients with pouch strictures. To date, there is only 1 published cohort from the Cleveland Clinic that reported long-term efficacy in 150 patients. After a median follow-up of 10 years, 87% of patients were able to retain their pouches. In this cohort, the number of strictures and underlying CD of the pouch were the only factors associated with pouch failure.
We aimed to retrospectively review the efficacy and safety of endoscopic balloon dilation of ileoanal pouch strictures in patients with IBD, at a single center.
MATERIALS AND METHODS
Study Population
All consecutive patients that underwent endoscopic dilatation of a pouch stricture between January 1, 2011, and April 31, 2016, at the UCSD IBD center were retrospectively identified. Inclusion criteria were the following: (a) IBD patients 18 years or older with IPAA; (b) presence of stricture of the ileoanal anastomosis, pouch body, inlet, or afferent limb during endoscopy; and (c) treatment of stricture with endoscopic dilatation performed by a gastroenterologist at the UCSD IBD center. Stricture was defined as a fixed, localized, luminal narrowing identified on endoscopy or imaging. The study was approved by the University of California, San Diego institutional review board (IRB # 160991).
Clinical Variables
Clinical, endoscopic, and surgical data was extracted from patients’ hospital medical records retrospectively by using a standardized questionnaire specifically developed for this study. The following clinical data was collected: age; sex; date of IBD diagnosis; previous exposure to IBD-related medications; chronic use of NSAIDs; date and type of pouch; and indications of IPAA. The following information regarding pouch strictures was collected: presence of acute or chronic pouchitis, ulceration, fistula, or cuffitis; date of stricture diagnosis; stricture location; length and diameter of stricture and ability to traverse with endoscope; presence of symptoms including abdominal pain, bloating, or obstructive symptoms. We also collected data regarding technical aspects of stricture dilation including the number of endoscopies with dilation performed, smallest and largest balloon size used, number of balloon sizes used for dilation, and associated finger dilatation. We evaluated the efficacy and complications for each procedure, as well as the need for pouch surgery or hospitalizations related to pouch stricture during the follow-up period. Crohn’s disease of the pouch was diagnosed based on evidence of penetrating disease originating from the pouch or pre-pouch ileum away from surgical anastomosis, non-anastomotic strictures, or severe inflammation of prepouch inflammation extending >10 cm proximal to pouch inlet.
Endoscopic Procedure
Four different operators performed pouchoscopies on patients using no sedation, moderate sedation (midazolam and either fentanyl or meperidine), or monitored anesthesia care with propofol. A flexible, single-channel, video upper endoscope, and through-the-scope hydraulic balloon (Boston Scientific CRE) was used. Segmental endoscopic evaluation with biopsies of the prepouch ileum, pouch body, and anal transitional zone was systematically performed. Location, length, and diameter of the stricture were evaluated. A pouch-anal anastomosis stricture was suspected by digital examination, and digital dilatation was performed if necessary. In our clinical practice, once a pouch stricture was detected, endoscopic dilation therapy was performed regardless of patient symptoms. Sequential dilations were performed based on the location, degree, and length of stricture, at the discretion of the endoscopist. Passage through the stricture was attempted immediately after the dilation. Patients were observed in the recovery area for a minimum of 30 minutes after the procedure to monitor for any complications related to the dilation or sedation medications. Procedures were systematically repeated after a delay of 1 to 6 months until there was improvement or resolution of the stricture according to physician judgment.
Outcome
The primary outcome of this study was the rate of stricture-related pouch failure defined by the need for surgical management of pouch stricture including pouch excision, diversion ileostomy, or stricturoplasty. Secondary outcomes included technical success (ie, increase of stricture diameter with ability to pass endoscope without resistance after therapy), clinical success (ie, resolution of obstructive symptoms if present) and safety (ie, major complications related to the procedure which required hospitalization, transfusion, urgent interventional endoscopy, or surgical intervention).
Statistical Analysis
Continuous variables were calculated as medians with interquartile ranges (IQR, Q1–Q3). Categorical variables were presented as frequencies and percentages. Kaplan-Meier curves for stricture-related pouch failure and hospitalizations were performed, and data was analyzed using GraphPad software V.9.3 (San Diego, CA, USA).
RESULTS
Patients
Eighty-eight dilatations were performed in 20 patients. Sixty percent of patients were female, with a median age at IPAA of 28.6 years (IQR, 25.5–37.2). IPAA was performed for UC in 95% of cases, primarily for medically refractory disease (60%). Ninety-five percent of patients underwent J pouch, and 65% had a stapled pouch, while the remaining 35% had a hand-sewn pouch (Table 1). Strictures were diagnosed at a median of 4.6 years (0.2–10.6) after IPAA surgery, and half of patients were symptomatic. Five (25%) patients had diverting ileostomy at the time of stricture diagnosis, created at the time of the original pouch and never taken down.
Table 1:
Characteristics of the Population
| Male gender (n, %) | 8, 40% |
| Median age at IBD diagnosis (y, IQR) | 22.6 (17.0–31.5) |
| Median age at IPAA (y, IQR) | 28.6 (25.5–37.2) |
| IBD diagnosis at pouch surgery (n,%) | |
| Ulcerative colitis | 19 (95%) |
| Crohn’s disease (diagnosis before IPAA) | 1 (5%) |
| Treatment before IPAA | |
| 5ASA | 18 (90%) |
| Thiopurines | 12 (60%) |
| Infliximab | 12 (60%) |
| Adalimumab | 4 (20%) |
| Type of Pouch (n,%) | |
| J pouch | 19 (95%) |
| W pouch | 1 (5%) |
| Stapled | 13 (65%) |
| Hand-sewn | 7 (35%) |
| Pouch indication (n,%) | |
| Refractory disease | 12 (60%) |
| Severe acute colitis | 7 (35%) |
| Dysplasia/Cancer | 1 (5%) |
Stricture Characteristics
Characteristics of the strictures are detailed in Table 2. Fifteen percent of patients had more than one stricture, and the median diameter was 10 mm (7.5–11.7). The most frequent location of stricture was the pouch-anal anastomosis (87%). Half the patients were found to have endoscopic or histologic evidence of pouchitis (or both) during pouchoscopy; 4 (20%) had at least one fistula and 5 (25%) had ulcerations of the pouch. At the end of the follow-up period, 6 patients (30%) were diagnosed with CD of the pouch, and 1 (5%) of ischemic stricture.
Table 2:
Disease and Strictures Characteristics
| Median age at stricture diagnosis (y, IQR) | 39.0 (30.6–48.9) |
| Median duration after IPAA (y, IQR) | 4.6 (0.2–10.6) |
| Asymptomatic (before dilatation) (n,%) | 9 (45%) |
| Including diversion ileostomy | 5 |
| Symptomatic (before dilatation) (n,%) | 11 (55%) |
| Including obstructive symptoms | 6 |
| Hospitalization for obstructive symptoms | 5 |
| Radiological diagnosis of pouch stricture before endoscopic dilatation (n,%) | 5 (25%) |
| No. of strictures (n) | |
| 1 | 17 (85%) |
| 2 | 3 (15%) |
| Median diameter (mm) | 10 (7.5–11.7) |
| Ulcerated stricture (n,%) | 2 (10%) |
| Stricture location (n,%) | |
| Anastomosis | 20 (87%) |
| Inlet | 2 (8.6%) |
| Afferent limb | 1 (4.3%) |
| Associated lesion of the pouch | |
| Acute pouchitis | 4 (20%) |
| Chronic pouchitis | 5 (25%) |
| Pouch fistula | 4 (20%) |
| Pouch ulceration | 5 (25%) |
| Final stricture diagnosis | |
| Surgery related | 13 (65%) |
| Ischemic | 1 (5%) |
| Crohn’s disease | 6 (30%) |
| IBD related treatment at 1st dilatation | |
| Antibiotics | 6 (30%) |
| Ustekinumab | 1 (5%) |
| Ustekinumab and azathioprine | 1 (5%) |
Endoscopic Balloon Dilation
A median of 3.5 dilatations per patient (2.0–7.0) was performed. The median sizes of the smallest and largest balloons were respectively 12 mm (12–15) and 18 mm (15–20). At the time of the first dilatation, 8 (40%) patients were receiving IBD-related treatments including antibiotics (n = 6), ustekinumab (n = 1), and ustekinumab with azathioprine (n = 1). There was a modification of medical therapy at time of dilation in 11 (55%) patients, as detailed in Table 3.
Table 3:
Characteristics of Procedures and Final Outcomes
| Median no. of dilatation (n, IQR) | 3.5 (2.0–7.0) |
| Median size of smallest balloon (mm, IQR) | 12 (12–15) |
| Median size of largest balloon (mm, IQR) | 18 (15–20) |
| Associated finger dilatation (n, %) | 12 (13.6%) |
| Modifications of IBD related treatment at dilatation | 18 (20.5%) |
| Antibiotics initiation | 4 (4.5%) |
| Budesonide initiation | 4 (4.5%) |
| Antibiotics and budesonide initiation | 1 (1.1%) |
| Anti-TNF optimization | 2 (2.3%) |
| Anti-TNF with immunosuppressants initiation | 3 (3.4%) |
| Anti-TNF with immunosuppressants and budesonide initiation | 1 (1.1%) |
| Anti-TNF with immunosuppressants and oral steroids initiation | 1 (1.1%) |
| Ustekinumab with immunosuppressants, oral steroids and antibiotics initiation | 1 (1.1%) |
| Hydrocortisone suppository | 1 (1.1%) |
| Technical efficacy (n, %) | 87 (98%) |
| Clinical improvement of obstructive symptoms (n, %) | 21 (95%) |
| Pouch surgery related to stricture (n,%) | 1 (5%) |
| Hospitalizations after 1 st dilatation related to stricture (n,%) | 5 (25%) |
| Complications (n,%) | 0 |
| Final outcomes | |
| Complete disappearance of the pouch stricture | 9 (45%) |
| Persistent passable strictures without related symptoms | 7 (35%) |
| Diverting stoma because of resistant Crohn of the pouch | 3 (15%) |
| Death (not related to IBD) | 1 (5%) |
Efficacy
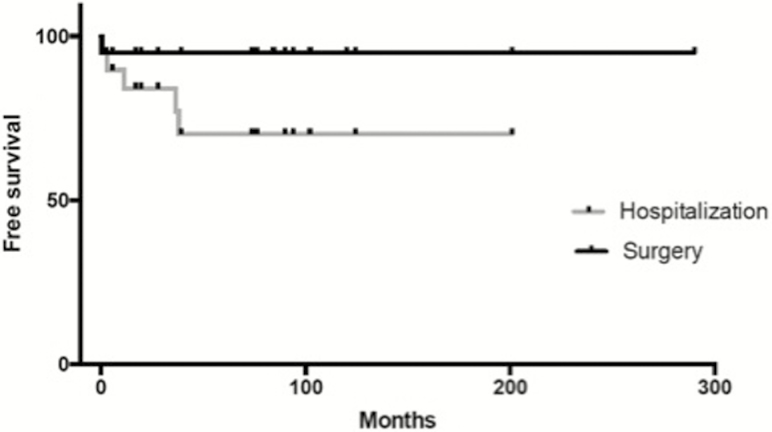
Technical efficacy was observed in 87 procedures (98%). Twenty-two procedures were preceded by obstructive symptoms, and a clinical improvement after endoscopic dilatation was observed in 95% of these patients. After a median follow-up of 3.0 years (2.1–3.5), only 1 patient had stricture-related pouch failure (Fig. 1). This patient had 2 strictures: an ileal pouch-anal anastomosis stricture effectively treated by endoscopic dilatation and a pre-pouch ileal stricture, which could not be clearly visualized and was not accessible to dilatation. A pouch reconstruction with excision of inflammatory stricture of the prepouch ileum, re-anastomosis to the pouch inlet, and diverting loop ileostomy was performed without complications. After the first dilatation, 5 hospitalizations for persistent obstructive symptoms were observed in 4 patients. Conservative management with new endoscopic dilation was clinically effective in all these cases. At the date of the last news, 9 patients presented complete disappearance of the pouch stricture, 7 patients presented persistent passable strictures without related symptoms, 3 patients had diverting stoma because of refractory CD of the pouch, and 1 patient died for non-IBD related reason.
FIGURE 1.
Kaplan-Meir curve on risk of stricture-related surgery and hospitalizations.
Safety
During the follow-up period, no major complications related to dilation were observed. Specifically, there were no hospitalizations, pouch/intestinal perforations, gastrointestinal bleeding requiring transfusion, urgent interventional endoscopy, or surgical intervention within the follow-up period.
DISCUSSION
In this series of 88 pouch strictures dilatation, we observed that endoscopic dilatation could be safely performed and that the pouch could be preserved in 95% of patients with endoscopic management. To our knowledge, only 1 study previously reported the efficacy and safety of pouch strictures dilatation. Shen et al reported similar results with 87% of patients that were able to retain their pouches after a median follow-up of 9.6 years. The 5-, 10-, and 25-year pouch retention rates were 97%, 91%, and 86%, respectively.11
Most of the data on endoscopic dilatations in IBD come from ileocolonic anastomotic strictures. Endoscopic dilatation is now considered a first-line option for the management of luminal strictures in IBD.14 A systematic review by Hassan et al of 13 studies reported a technical success rate of 86% and a long-term efficacy of 58%, with a major complication rate of 2% overall. In this study, the most consistent factor associated with dilatation failure was the stricture length. Based on this, dilatation is now typically reserved for short strictures (<50 mm).15 Endoscopic dilatation has been shown to be effective after long-term follow-up. A recent study observed that endoscopic dilatation is effective in about 80% after 6 years of follow-up.12
Despite our increasing knowledge of luminal strictures in IBD, pouch strictures are a different entity. It remains unclear if their natural history is comparable to luminal enteral or colonic strictures. Pouch strictures occur at different locations, including the anastomosis, the body, and the outlet, and they can present with or without inflammation, pouchitis, or Crohn’s disease. As previously reported, the majority of strictures observed in our cohort were located on pouch-anal anastomosis.9, 16 These strictures are observed in up to 38% of patients undergoing IPAA.17 Risk factors include hand-sewn technique, small diameter of the staple gun, use of a quadruplicated reservoir, use of a defunctioning ileostomy, anastomotic dehiscence, pelvic sepsis, “W”-shaped pouch, excessive operative blood loss, and overweight male gender—and perhaps associated cuffitis.9, 17, 18 Prudhomme et al categorized anal anastomosis strictures as non-fibrotic or fibrotic based on the presence of a palpable fibrotic segment at the anal canal anastomosis.9 Dilatation was successful in 95% of non-fibrotic strictures as compared with 45% of fibrotic strictures.9 Overall, our results and those of Shen et al support the argument that pouch dilatation in the appropriate clinical setting is safe and effective in the management of strictures at different locations of the pouch.11
The impact of IBD-related treatment on pouch stricture natural history is unknown. Because of their fibrotic nature, medical therapies are usually ineffective. We can hypothesize that the mechanism of the stricture is different depending on the location of stricture and presence of underlined pouchitis or CD of the pouch. Anal anastomosis strictures have also been observed after IPAA for familial adenomatous polyposis, and it could be hypothesized that the influence of inflammation in this stricture is low, but is strongly associated with the technique. However, CD of the pouch is usually associated with strictures, and underlying pouchitis was observed in half of our patients. Half of the patients included in our cohort had optimization of medical therapy associated with pouch dilatation, including antibiotics, local or oral corticosteroids, and biologics. Even for ileocolonic anastomotic strictures, limited data is available on whether escalation of medical therapy following dilatation of strictures may prevent the need for a repeat dilatation or surgery. Disease activity was associated with future surgical resection after anastomotic ileocolonic dilatations, and recently, the St. Marks Hospital of London observed that the only factor associated with the decreased need for repeated dilatation combination therapy was escalation of medical therapy with combination of anti-TNF and azathioprine.12 Another small study of 25 patients with intestinal strictures due to CD showed that patients who had begun immunomodulator therapy prior to initial dilatation had fewer repeat dilatations than those who commenced postdilatation (1.6 versus 4.8, P = 0.04).19 It is unclear if strictures due to CD of the pouch may also be less likely to recur when dilation is combined with immunomodulator treatment.
The major concern for endoscopic treatment of digestive strictures is the risk for perforation and bleeding. In our series of 88 dilatations, we did not observe any major complications related to the procedure which required hospitalization, transfusion, urgent interventional endoscopy, or surgical intervention. Shen et al observed very few complications, including 2 perforations (0.5%) and 4 transfusion-required bleeding (0.1%) among 406 dilatations.11
Despite the results above, our study has some weaknesses. Due to the very low rate of pouch failures in our group, we were not able to assess risk factor of failure of endoscopic dilatation. Shen et al identified that an underlying diagnosis of a CD-associated pouch stricture was associated with an increased risk for pouch failure.11 In our study, the only patient with pouch failure related to strictures had 2 strictures, 1 of the anal anastomosis and 1 of the preileum. In addition, our study may not represent a typical cohort of IBD patients as this study was performed in a referral center caring for many patients with more severe diseases or diseases refractory to medical therapy. This study also has some strengths. We were able to consecutively include all the dilatations performed in our center during a 5-year period. The median follow-up of 3 years allows us to observe long-term effectiveness of endoscopic dilatation. Because this study was performed at a single center, peri-procedure monitoring was standardized, and the equipment used, including the balloon, was consistent.
In conclusion, endoscopic dilatation of pouch stricture is effective and safe. It should be the first-line strategy to treat pouch stricture in IBD patients.
Author Contribution: Study concept and design: MF, NP, BB, WJS. Acquisition of data: MF, NP. Analysis and interpretation of data: MF, NP, BB, SS, PSD, WJS. Drafting of the manuscript: MF, NP. Critical revision of the manuscript for important intellectual content: MF, NP, BB, SS, PSD, WJS. Approval of the final manuscript: MF, NP, BB, SS, PSD, WJS. Guarantor of the article: WJS.
Conflicts of Interest: Dr. Fumery is supported by the French Society of Gastroenterology (SNFGE, bourse Robert Tournut). Dr. Dulai is supported by the NIDDK training grant 5T32DK007202. Dr. Singh is supported by the NIH/NLM training grant T15LM011271 and the American College of Gastroenterology Junior Faculty Development Award and Crohn’s and Colitis Foundation of American Career Development Award. None of the other authors have any relevant financial disclosures.
REFERENCES
- 1. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 2. Magro F, Gionchetti P, Eliakim R, et al. ; European Crohn’s and Colitis Organisation [ECCO] Third european evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–70. [DOI] [PubMed] [Google Scholar]
- 3. Fazio VW, Kiran RP, Remzi FH, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257:679–85. [DOI] [PubMed] [Google Scholar]
- 4. Selvaggi F, Pellino G, Canonico S, et al. Systematic review of cuff and pouch cancer in patients with ileal pelvic pouch for ulcerative colitis. Inflamm Bowel Dis. 2014;20:1296–308. [DOI] [PubMed] [Google Scholar]
- 5. Barton JG, Paden MA, Lane M, et al. Comparison of postoperative outcomes in ulcerative colitis and familial polyposis patients after ileoanal pouch operations. Am J Surg. 2001;182:616–20. [DOI] [PubMed] [Google Scholar]
- 6. Marcello PW, Roberts PL, Schoetz DJ Jr, et al. Obstruction after ileal pouch-anal anastomosis: a preventable complication?Dis Colon Rectum. 1993;36:1105–11. [DOI] [PubMed] [Google Scholar]
- 7. Fazio VW, Ziv Y, Church JM, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewis WG, Kuzu A, Sagar PM, et al. Stricture at the pouch-anal anastomosis after restorative proctocolectomy. Dis Colon Rectum. 1994;37:120–5. [DOI] [PubMed] [Google Scholar]
- 9. Prudhomme M, Dozois RR, Godlewski G, et al. Anal canal strictures after ileal pouch-anal anastomosis. Dis Colon Rectum. 2003;46:20–23. [DOI] [PubMed] [Google Scholar]
- 10. Sagar PM, Pemberton JH. Intraoperative, postoperative and reoperative problems with ileoanal pouches. Br J Surg. 2012;99:454–68. [DOI] [PubMed] [Google Scholar]
- 11. Shen B, Lian L, Kiran RP, et al. Efficacy and safety of endoscopic treatment of ileal pouch strictures. Inflamm Bowel Dis. 2011;17:2527–35. [DOI] [PubMed] [Google Scholar]
- 12. Ding NS, Yip WM, Choi CH, et al. Endoscopic dilatation of crohn’s anastomotic strictures is effective in the long term, and escalation of medical therapy improves outcomes in the biologic era. J Crohns Colitis. 2016;10:1172–8. [DOI] [PubMed] [Google Scholar]
- 13. Greener T, Shapiro R, Klang E, et al. Clinical outcomes of surgery versus endoscopic balloon dilation for stricturing Crohn’s disease. Dis Colon Rectum. 2015;58:1151–7. [DOI] [PubMed] [Google Scholar]
- 14. Morar PS, Faiz O, Warusavitarne J, et al. ; Crohn’s Stricture Study (CroSS) Group Systematic review with meta-analysis: endoscopic balloon dilatation for Crohn’s disease strictures. Aliment Pharmacol Ther. 2015;42:1137–48. [DOI] [PubMed] [Google Scholar]
- 15. Hassan C, Zullo A, De Francesco V, et al. Systematic review: endoscopic dilatation in Crohn’s disease. Aliment Pharmacol Ther. 2007;26:1457–64. [DOI] [PubMed] [Google Scholar]
- 16. Meagher AP, Farouk R, Dozois RR, et al. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. Br J Surg. 1998;85:800–3. [DOI] [PubMed] [Google Scholar]
- 17. Lewis WG, Kuzu A, Sagar PM, et al. Stricture at the pouch-anal anastomosis after restorative proctocolectomy. Dis Colon Rectum. 1994;37:120–5. [DOI] [PubMed] [Google Scholar]
- 18. Fleshman JW, Cohen Z, McLeod RS, et al. The ileal reservoir and ileoanal anastomosis procedure. Factors affecting technical and functional outcome. Dis Colon Rectum. 1988;31:10–6. [DOI] [PubMed] [Google Scholar]
- 19. Honzawa Y, Nakase H, Matsuura M, et al. Prior use of immunomodulatory drugs improves the clinical outcome of endoscopic balloon dilation for intestinal stricture in patients with Crohn’s disease. Dig Endosc. 2013;25:535–43. [DOI] [PubMed] [Google Scholar]