Abstract
Background:
Suicide is a leading cause of death among youth. Prior research using transcranial magnetic stimulation (TMS) has implicated deficits in GABAergic cortical inhibition in adolescent suicidal behavior, yet no studies have assessed whether cortical inhibition varies over time in conjunction with changes in suicidal ideation (SI). This study examined dynamic changes in long-interval intracortical inhibition (LICI), a TMS measure of GABAB-mediated inhibition, and their relationship with changes in SI in a small sample of adolescents undergoing pharmacologic treatment for depression.
Methods:
Ten depressed adolescents (age 13-17) underwent clinical assessment and TMS testing at baseline and again at follow-up. All were treated with antidepressant medication in the interim. SI was measured with the Columbia Suicide Severity Rating Scale (C-SSRS) Intensity of Ideation subscale. LICI was measured at interstimulus intervals of 100 and 150 ms.
Results:
There was a significant partial correlation, controlling for change in depression severity, between ΔLICI-100 and change in SI as measured by ΔC-SSRS (ρ=.746, p=.021), which remained after also controlling for time to follow-up assessment (ρ=.752, p=.032). No significant correlation was observed between ΔLICI-150 and change in SI.
Limitations:
Sample size; variable follow-up interval; inability to control for age, sex, and potential treatment effects.
Conclusions:
These data offer preliminary signal of an association between increases in GABAB-mediated cortical inhibition and reduction in SI over time in adolescents treated for depression. Further studies are warranted to explore the role of cortical inhibition in adolescent suicidal ideation and behavior.
Keywords: adolescent, cortical inhibition, depression, suicidal ideation, transcranial magnetic stimulation
1. Introduction
Suicide is the second-leading cause of mortality in adolescents (World Health Organization, 2014), and youth suicide rates have been increasing over the past decade (Centers for Disease Control and Prevention, 2017). Additionally, suicidal ideation (SI) and suicidal behavior (SB) during the adolescent years are associated with suicide-related outcomes later in life (Copeland et al., 2017). However, despite considerable research efforts aimed at understanding the psychological and biological underpinnings of suicidality, suicidal events are notoriously challenging to predict (Chang et al., 2016; Franklin et al., 2017), and there is a pressing need for a better understanding of the physiologic brain states associated with suicide risk.
Inhibitory regulation of neural circuits via the γ-aminobutyric acid (GABA) neurotransmitter system has gained increasing attention for its potential impact on suicidal ideation and behavior. Postmortem gene association research (Sequeira et al., 2007; Klempan et al., 2009) has indicated altered regulation of the metabotropic GABAB receptor and associated binding proteins in various brain regions of suicide victims. In vivo evidence from noninvasive techniques such as transcranial magnetic stimulation (TMS) also suggests that inhibitory cortical physiology mediated by the GABAB system may play a role in SI and SB. Prior work indicates that higher levels of TMS-measured cortical inhibition predict greater improvement of SI in adults undergoing a novel brain stimulation treatment for depression (Sun et al., 2016). Another recent cross-sectional study utilizing single- and paired-pulse TMS (sp/ppTMS) found significant impairment in long-interval intracortical inhibition (LICI), a marker of GABAB receptor-mediated functioning, at interstimulus intervals of 100 ms and 150 ms in depressed youth with histories of SB compared to depressed adolescents without suicidal histories and healthy controls (Lewis et al., 2018). These measures of cortical inhibition also significantly distinguished depressed youth with and without histories of SB, with LICI-100 demonstrating greater specificity and LICI-150 demonstrating greater sensitivity. However, it remains unknown whether current SI, rather than historical SB, is associated with similar deficits LICI, and whether changes in SI over time are associated with concurrent changes in LICI.
This study sought to investigate dynamic changes in LICI and their potential association with changes in SI in a small sample of depressed youth who underwent TMS testing at two time points. We hypothesized that increases in GABAB receptor-mediated cortical inhibition, indexed by LICI-100 and LICI-150, would correlate significantly with decreases in suicidal ideation.
2. Methods
Participants were 10 treatment-seeking adolescents (aged 13-17) with depressive symptoms recruited from a pediatric psychopharmacology clinic. These participants were a subset of the larger sample from our previous cross-sectional study (Lewis et al., 2018) whose baseline data were included in that previous analysis. All participants in the present longitudinal study underwent clinical assessment and sp/ppTMS at baseline and again approximately 8 weeks later. All were treated with an antidepressant medication between baseline and follow-up assessments. Informed consent was obtained from adolescent participants’ parents/guardians; adolescent participants provided informed assent. All study procedures were approved by the Mayo Clinic institutional review board.
SI severity was assessed on the “Intensity of Ideation” subscale of the Columbia Suicide Severity Rating Scale (C-SSRS; Posner et al., 2011). The subscale consists of the sum of five items rating intensity of SI in various dimensions (frequency, duration, controllability, deterrents, reasons for ideation); each item is scored 0 to 5, with subscale scores ranging from 0 (no SI) to 25 (most intense SI). Change in SI score (ΔC-SSRS) was calculated as the baseline score subtracted from the follow-up score, divided by the baseline score (to control for the impact of the baseline SI value and the regression toward the mean observed in SI scores between baseline and follow-up; see supplementary materials for plots of change in raw scores). Note that in order to avoid division by zero, all baseline and follow-up C-SSRS Intensity of Ideation scores were transformed by increasing scores by a value of 1 (giving a possible range of 1 to 26). Thus, negative ΔC-SSRS values indicated improvement in suicidal ideation from baseline to follow-up.
Depression severity was assessed using the Children’s Depression Rating Scale, Revised (CDRS-R; Poznanski et al., 1984), a 17-item, clinician-rated instrument of depressive symptomatology. Potential CDRS-R total scores range from 17 to 113. Change in depression severity (ΔCDRS-R total score) was calculated as the baseline score subtracted from the followup score, divided by the baseline score (to control for the impact of baseline depression severity and regression toward the mean observed in depression severity scores between baseline and follow-up). All clinical assessments were conducted by a board-certified child and adolescent psychiatrist (PEC).
TMS testing was conducted according to methods published previously (Daskalakis et al., 2002), using paired Magstim 200 stimulators with a BiStim module (Magstim Co. Ltd., Whitland, Wales, UK) and a 70-mm figure-of-eight coil to stimulate the left primary motor cortex (M1). In brief, the optimal scalp location for eliciting motor evoked potentials (MEPs) in the right abductor pollicis brevis (APB) was determined with simultaneous electromyographic (EMG) recording, followed by measurement of the resting motor threshold (RMT). For the LICI paradigm, two suprathreshold stimuli (i.e., above the RMT) calibrated to result in a 1-mV peak-to-peak amplitude were applied to the left M1. The first magnetic pulse (conditioning stimulus, CS) was followed by a test stimulus (TS) after an interstimulus interval (ISI) of 100 or 150 ms. Ten trials at each ISI were conducted in a randomized, counterbalanced order. LICI was measured as the ratio of the amplitude of the conditioned MEP (i.e., the MEP following the TS) to the amplitude of the unconditioned MEP. The suppression of the conditioned MEP in the LICI paradigm is posited to result from the inhibitory activity of cortical GABAB receptors (Valls-Solé et al., 1992; Nakamura et al., 1997; Chen et al., 1999). Thus, lower amplitude ratios indicate greater inhibition. Change in LICI amplitude ratios (ΔLICI-100 and ΔLICI-150) were calculated as the baseline ratio subtracted from the follow-up ratio, with negative values indicating an increase in cortical inhibition between baseline and follow-up assessments.
In order to examine the dynamic relationships between cortical inhibition and SI, we examined the partial correlations (two-tailed) between changes in LICI (ΔLICI-100 and ΔLICI-150) and change in SI (ΔC-SSRS), controlling for the change in depression severity (ΔCDRS-R total score). Statistical analyses were performed with IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA).
3. Results
The sample consisted of ten adolescents (6 female, 4 male) with a mean age of 15.50 ± 1.18 years. Four participants had prior SB (suicidal planning, aborted/interrupted attempt, or suicide attempt), and one had attempted suicide previously. Mean depression severity (CDRS-R total score) was 52.10±7.88 at baseline and 30.20±7.19 at follow-up. Mean ΔCDRS-R (dividing by the baseline CDRS-R score) was −0.41±0.15. Nine participants were unmedicated at the time of baseline clinical and TMS assessments, while one was taking fluoxetine; all were taking an antidepressant at follow-up (8 taking fluoxetine; 1 escitalopram; 1 bupropion), which occurred a median of 8 weeks (range 2-20 weeks) after baseline assessment. On the C-SSRS Intensity of Ideation subscale, mean (transformed) baseline SI score was 7.00±6.88 (range 1 to 18), while mean score at follow-up was 3.10±4.43 (range 1 to 12). Mean ΔC-SSRS (dividing by the baseline C-SSRS score) was −0.33±0.41.
Mean conditioned/unconditioned MEP amplitude ratios at baseline were 0.395±0.471 in the LICI-100 paradigm and 0.512±.0701 in the LICI-150 paradigm. At follow-up assessment, the mean conditioned/unconditioned MEP amplitude ratio was 0.310±0.286 for LICI-100 and 0.443±0.417 for LICI-150. Participants with prior SB had higher follow-up conditioned/unconditioned MEP amplitude ratios than those with no prior SB in the 100-ms LICI paradigm (p=.038), but not in the 150-ms paradigm (p=.352). Mean change in conditioned/unconditioned MEP amplitude ratios (ΔLICI-100 and ΔLICI-150) between baseline and follow-up were −0.086±0.208 and −0.069±0.431, respectively.
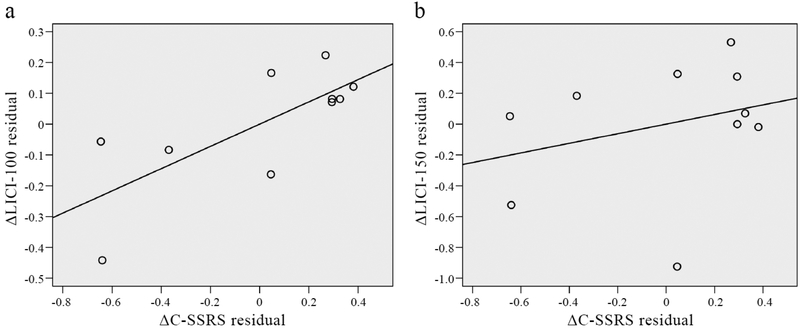
The partial correlation analysis, controlling for ΔCDRS-R total score, revealed a significant correlation between ΔLICI-100 and change in SI as measured by ΔC-SSRS (ρ=.746, p=.021). The relationship between ΔLICI-150 and ΔC-SSRS, controlling for ΔCDRS-R total score, was not significant (ρ=.293, p=.444). Partial residual plots depicting these relationships are shown in Figure 1. The ΔLICI-100 – ΔC-SSRS partial correlation remained significant when the one patient taking fluoxetine at baseline was excluded (ρ=.864, p=.006).
Figure 1. Relationships between change in LICI and change in SI, controlling for change in depression severity.
Partial residual plots depicting relationships between a) ΔLICI-100 and ΔC-SSRS; and b) ΔLICI-150 and ΔC-SSRS.
In consideration of the substantial variability in the time between baseline and follow-up assessments in this naturalistic sample, we conducted a second partial correlation analysis that also included time-to-follow-up as a control variable alongside ΔCDRS-R total score (despite a further restriction of the degrees of freedom). The correlation between ΔLICI-100 and ΔC-SSRS remained significant (ρ=.752, p=.032), while the correlation between ΔLICI-150 and ΔC-SSRS was not significant (ρ=.331, p=.424).
4. Discussion
These preliminary data represent, to our knowledge, the first indication that dynamic increases in cortical inhibition, as measured by LICI-100, are associated with reductions in SI. Notably, this finding was observed while controlling for the overall change in depression severity, suggesting that the LICI-SI relationship may be distinct from cortical inhibitory changes that occur with changes in the severity of affective illness during antidepressant treatment. This builds upon prior findings demonstrating that cortical inhibition may distinguish the presence or absence of past SB in depressed adolescents (Lewis et al., 2018) and may predict resolution of SI with neuromodulatory treatment (Sun et al., 2016). However, unlike prior work on TMS-measured cortical inhibition and suicidality, our initial results from the present study suggest that cortical inhibitory deficits may be associated with a state of increased SI. To date, the majority of previously identified correlates and risk factors for SI, SB, and suicide have been either static trait factors or have not been evaluated at multiple time points to assess temporal association with SB and suicidal events (Chang et al., 2016; Franklin et al., 2017; Glenn et al., 2017), thus limiting their clinical applicability. For a quantitative neurobiological index of risk to have meaningful clinical utility, its changes must be commensurate with changes in SI, SB, or other aspects of suicide risk. Larger, more definitive studies are necessary to determine whether measures of cortical inhibition are associated with states of elevated suicide risk, long-term suicidal traits, or a combination of the two.
The present study was limited by its small number of participants. Although we assessed the ΔLICI-ΔSI relationship while controlling for change in depression severity, the small sample did not permit controlling for additional covariates such as age and sex. LICI has been found to vary with age in children and adolescents (Croarkin et al., 2014), while other TMS measures of GABAergic cortical inhibition have been found to vary with menstrual phase in adult women (Smith et al., 1999; Smith et al., 2003). Additionally, prior work in adults indicates that LICI has good reliability over time (Farzan et al., 2010), but studies in children and adolescents are lacking. Future investigations will require larger samples and greater power to assess dynamic LICI-SI relationships in the context of neural development and other effects.
Another significant limitation is that all participants were treated with antidepressant medication during the interval between baseline and follow-up assessments. The primary aim of this study was to examine the dynamic LICI-SI relationship as both changed over time, rather than to evaluate any causal relationship or treatment effect. However, antidepressant medications have been found to impact cortical inhibition (Robol et al., 2004; Minelli et al., 2010). Considering the substantial variability in time between baseline and follow-up assessments in our sample, it is conceivable that participants’ variable cumulative exposure to medications could have impacted the degree of change in cortical inhibition. Further investigations of LICI in adolescents undergoing pharmacologic and nonpharmacologic treatments, as well as test-retest studies of healthy control adolescents, may help to elucidate the impact of medications on the LICI-SI relationship.
In summary, this small study in a sample of adolescents undergoing pharmacologic treatment for depression demonstrated preliminary evidence that improvement in cortical inhibition is associated with improvement in SI, controlling for simultaneous improvement in depression severity. However, additional longitudinal studies of cortical inhibition in larger samples of youth with diverse presentations of suicidality are necessary to understand the role of cortical inhibition in SI and SB. Further investigations of inhibitory cortical physiology may inform future developments in the assessment and stratification of suicide risk, and may reveal neurobiological targets for novel treatment approaches in this population.
Supplementary Material
Highlights.
Suicide is the second-leading cause of death in adolescents
Prior work has implicated GABAergic cortical inhibition in suicidality
Cortical inhibition was measured by TMS before and after antidepressant treatment
Increases in cortical inhibition correlated with improvement in suicidal ideation
Acknowledgements
This study was supported by a National Institute of Mental Health (NIMH) grant, R01MH113700 “Glutamatergic and GABAergic Biomarkers in TMS for Adolescent Depression” (Dr. Croarkin).
Abbreviations
- APB
abductor pollicis brevis
- CDRS-R
Children’s Depression Rating Scale, Revised
- C-SSRS
Columbia Suicide Severity Rating Scale
- CS
conditioning stimulus
- EMG
electromyography
- GABA
γ-aminobutyric acid
- ISI
interstimulus interval
- LICI
long-interval intracortical inhibition
- M1
primary motor cortex
- MEP
motor evoked potential
- RMT
resting motor threshold
- SB
suicidal behavior
- SI
suicidal ideation
- TMS
transcranial magnetic stimulation
- TS
test stimulus
Footnotes
Disclaimer statement
The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Plots of baseline and follow-up scores on the C-SSRS Intensity of Ideation score, CDRS-R total score, and LICI-100 and LICI-150 cortical inhibition measures.
Disclosures
CPL receives research support from the Mayo Clinic Foundation Departmental Small Grant Program and is a site investigator for multicenter studies funded by Neuronetics, Inc. and NeoSync, Inc. DDC receives research support from the Mayo Clinic Foundation Departmental Small Grant Program. ZJD has received research and equipment in-kind support for investigator-initiated studies from Brainsway Ltd. and MagVenture, Inc.; he also has served on the advisory board for F. Hoffmann-La Roche Ltd. and Merck & Co., Inc. and has received speaker support from Eli Lilly and Co. PEC has received research grant support from Pfizer, Inc., NIMH, the Brain and Behavior Research Foundation, and the Mayo Clinic Foundation. He has served as a site subprincipal or principal investigator (without additional compensation) for Eli Lilly and Co., Forest Laboratories, Inc., Merck & Co., Inc., and Pfizer, Inc.; has received equipment support from Neuronetics, Inc.; and receives supplies and genotyping services from Assurex Health, Inc. for an investigator-initiated study. He is the primary investigator for a multicenter study funded by Neuronetics, Inc. and primary site investigator for a multicenter study funded by NeoSync, Inc. AIS, ALN, and MAG have no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2017. Web-based Injury Statistics Query and Reporting System (WISQARS) [online]. www.cdc.gov/injury/wisqars (accessed 22 Mar 2018).
- Chang BP, Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Nock MK, 2016. Biological risk factors for suicidal behaviors: a meta-analysis. Transl Psychiatry 6, e887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P, 1999. Mechanism of the silent period following transcranial magnetic stimulation: evidence from epidural recordings. Exp Brain Res 128, 539–542. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Goldston DB, Costello EJ, 2017. Adult associations of childhood suicidal thoughts and behaviors: a prospective, longitudinal analysis. J Am Acad Child Adolesc Psychiatry 56, 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croarkin PE, Nakonezny PA, Lewis CP, Zaccariello MJ, Huxsahl JE, Husain MM, Kennard BD, Emslie GJ, Daskalakis ZJ, 2014. Developmental aspects of cortical excitability and inhibition in depressed and healthy youth: an exploratory study. Front Hum Neurosci 8, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S, 2002. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry 59, 347–354. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, Daskalakis ZJ, 2010. Reliability of long-interval cortical inhibition in healthy human subjects: a TMS-EEG study. J Neurophysiol 104, 1339–1346. [DOI] [PubMed] [Google Scholar]
- Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, Huang X, Musacchio KM, Jaroszewski AC, Chang BP, Nock MK, 2017. Risk factors for suicidal thoughts and behaviors: a meta-analysis of 50 years of research. Psychol Bull 143, 187–232. [DOI] [PubMed] [Google Scholar]
- Glenn CR, Cha CB, Kleiman EM, Nock MK, 2017. Understanding suicide risk within the Research Domain Criteria (RDoC) framework: insights, challenges, and future research considerations. Clin Psychol Sci 5, 568–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G, 2009. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry 14, 175–189. [DOI] [PubMed] [Google Scholar]
- Lewis CP, Nakonezny PA, Blacker CJ, Vande Voort JL, Port JD, Worrell GA, Jo HJ, Daskalakis ZJ, Croarkin PE, 2018. Cortical inhibitory markers of lifetime suicidal behavior in depressed adolescents. Neuropsychopharmacology 43, 1822–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Bortolomasi M, Scassellati C, Salvoro B, Avesani M, Manganotti P, 2010. Effects of intravenous antidepressant drugs on the excitability of human motor cortex: a study with paired magnetic stimulation on depressed patients. Brain Stimul 3, 15–21. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H, 1997. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol 498, 817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ, 2011. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R, 1984. Preliminary studies of the reliability and validity of the Children's Depression Rating Scale. J Am Acad Child Psychiatry 23, 191–197. [DOI] [PubMed] [Google Scholar]
- Robol E, Fiaschi A, Manganotti P, 2004. Effects of citalopram on the excitability of the human motor cortex: a paired magnetic stimulation study. J Neurol Sci 221, 41–46. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Klempan T, Canetti L, ffrench-Mullen J, Benkelfat C, Rouleau GA, Turecki G, 2007. Patterns of gene expression in the limbic system of suicides with and without major depression. Mol Psychiatry 12, 640–655. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM, 2003. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry 54, 757–762. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM, 1999. Menstrual cycle effects on cortical excitability. Neurology 53, 2069–2072. [DOI] [PubMed] [Google Scholar]
- Sun Y, Farzan F, Mulsant BH, Rajji TK, Fitzgerald PB, Barr MS, Downar J, Wong W, Blumberger DM, Daskalakis ZJ, 2016. Indicators for remission of suicidal ideation following magnetic seizure therapy in patients with treatment-resistant depression. JAMA Psychiatry 73, 337–345. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M, 1992. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85, 355–364. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2014. Preventing suicide: a global imperative. Geneva: WHO Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.