Abstract
Introduction:
Environmental exposure to toxic metals and metalloids is pervasive and occurs from multiple sources. The Health Effects of Arsenic Longitudinal Study (HEALS) is an ongoing prospective study predominantly focused on understanding health effects associated with arsenic exposure from drinking water. The goal of this project was to measure a suite of elements in urine to better understand potential exposure patterns and to identify common environmental sources of exposure among this semi-rural Bangladeshi population.
Methods:
In a random sample of 199 adult HEALS participants (50% female), the concentrations of 15 urinary elements (As, Ba, Cd, Co, Cs, Cu, Mn, Mo, Ni, Pb, Se, Sr, Tl, W, Zn) were assessed by Inductively-Coupled Plasma Mass Spectrometry (ICP-MS) to assess commonalities with sociodemographic characteristics and potential sources of exposure. We used principal component analysis (PCA) with varimax normalized rotations, and hierarchical cluster analysis (CA), using Ward’s method with Euclidean distances, to evaluate these relationships.
Results:
PCA and CA showed similar patterns, suggesting 6 principal components (PC) and 5 clusters: 1)(PC: Sr-Ni-Cs/ CA: Sr-Ni-Co; 2) Pb-Tl/Pb-Tl-Se-Cs; 3) As-Mo-W/As-Mo-W; 4) Ba-Mn/Ba-Mn; 5) Cu-Zn/Cu-Zn-Cd; and 6) Cd. There was a strong significant association between the As-Mo-W PC/cluster and water arsenic levels (p<0.001) and between the Cd PC and betel nut use (p=0.003). The Sr-Ni-Cs PC was not related to any of the socio-demographic characteristics investigated, including smoking status and occupation. The first PC, Sr-Ni-Cs, explained 21% of the variability; the third PC, As-Mo-W, explained 12.5% of the variability; and the sixth PC, Cd, explained 10% of the variability. Day laborers appeared to have the highest exposure.
Conclusions:
Groundwater and betel nut use are likely important sources of metal and metalloid exposure in this population. These findings will guide future exposure assessment research in Bangladesh and future epidemiologic research investigating the degree to which metal mixtures play a role in disease development.
Keywords: Metals, metalloids, metal mixtures, biomonitoring, groundwater, betel nut use, air pollution
1. Introduction
Chronic exposure to certain metals and metalloids (herein referred to as “metals” for simplicity) can lead to the development of cardiovascular disease (Cosselman et al., 2015; Nigra et al., 2016; Solenkova et al., 2014), adverse neurocognitive outcomes (Karri et al., 2016; Wright and Baccarelli, 2007) and/or some cancers (Cogliano et al., 2011). Relatively little is known, however, about health effects of exposures to mixtures of metals. Because metals are ubiquitous in the environment, metal exposure is rarely limited to one source or one metal. Identifying metal clusters and relevant sources for metal mixtures (air, water, diet, occupation) can guide research needs and public health interventions. Urine is a typical biospecimen used to assess metal exposure and internal dose, as many metals are excreted via the kidneys. Metal concentrations in the urine can be inter-related due to common exposure sources and/or similarities in metabolism. Dimensionality reduction methods, including principal component analysis (PCA) (Hotelling, 1933) and cluster analysis (CA) (Everitt et al., 2011), are often used in environmental research to identify underlying patterns of metal exposures (Bhowmick et al., 2015; Hoover et al., 2018; Pang et al., 2016; Yongming et al., 2006). By reducing the dimensionality of the original data, these methods can facilitate the identification of common sources and metabolic pathways of metals.
In Bangladesh, a large body of evidence has investigated the major sources (Kile et al., 2007; Nickson et al., 1998; Smith et al., 2000; Yunus et al., 2016) and health effects of arsenic exposure, including cancers of the lung, skin, bladder kidney and liver; cardiovascular disease; and neurological effects (Argos et al., 2010; Huyck et al., 2007; Kile et al., 2015; Naujokas et al., 2013; Smith et al., 2000; Vahter et al., 2006). Less is known, however, about sources of exposure and related health effects for other toxic metals. A few studies have investigated two or three metals in addition to arsenic, such as lead, manganese and cadmium (Berglund et al., 2011; Forsyth et al., 2018; Gleason et al., 2014; Hawkesworth et al., 2013; Khan et al., 2010). One study conducted among children living in Dhaka, has investigated the environmental sources of multiple metals and persistent organic pollutants (POPs) (Linderholm et al., 2011). The study found that while POPs were higher among children working and living near waste disposal plants, blood metals were not clearly associated with distance to waste disposal plants; however, blood metals were elevated compared to non-Bangladeshi populations, signifying that these exposures might affect the population as a whole, perhaps through air pollution via combustion by- products (Begum et al., 2005). Exposure patterns amongst people living in urban centers may greatly differ from exposures amongst a rural population. In rural Bangladesh, the majority of the population relies on biomass fuels to meet their energy needs (Khan et al., 2017). Biomass burning is a potential source of several metals, including lead and cadmium (Hasan et al., 2009).
Our study population was drawn from the Health Effects of Arsenic Longitudinal Study (HEALS) cohort, located in the semi-rural Upazila of Araihazar, Bangladesh. HEALS is an ongoing cohort study which has predominantly focused on understanding health effects associated with arsenic exposure from drinking water (Ahsan et al., 2006). Araihazar is located about 25 km southeast of Dhaka. It was chosen as the study site for HEALS in part because the area contains a population with relatively homogenous sociocultural characteristics and, while similar to other parts of Bangladesh in that it is affected by arsenic in drinking water, the area also has a heterogenous water arsenic exposure distribution. Some of the main sources of income in the area include agricultural work and commerce, with roughly 50% of Araihazar residents owning agricultural land (Islam, 2015).
In this study, we leveraged the extensive resources of the HEALS cohort to measure a panel of metals in urine, in addition to the metalloid As and the non-metal Se, and characterize patterns of mixtures with the goal to identify common sources of exposure and/or metabolic pathways among a semi-rural population in Bangladesh. We hypothesized that urinary metals highly correlated with arsenic, in urine and/or in water (as both have been measured in HEALS), could reflect water as a common source. For metals not correlated with urinary and water arsenic, these preliminary data on metal mixtures may generate hypotheses to guide future research on the sources of exposure and potential health effects of metal mixtures in rural and semi-rural populations of Bangladesh.
2. Materials and methods
2.1. Study Population
Between 2000 and 2002, 11,746 married men and women age 18 and older were recruited into the HEALS cohort. HEALS participants had to be married, a resident of the study area for at least 5 years and primarily drinking water from one of the 5,966 study wells for at least 3 years to be eligible. Since then, HEALS has expanded to over 30,000 participants. For this study, we randomly selected 200 HEALS participants (50% female) with a urine sample available at the third HEALS follow-up (2007–2009) to conduct a metal panel assay (Supplementary Table 1). One participant, however, was missing urinary specific gravity and was excluded. The samples size was based on available funding. HEALS study protocols were approved by the Columbia University Institutional Review Board and the Ethical Committee of the Bangladesh Medical Research Council. All participants provided oral informed consent.
2.2. Urinary Metals
Spot urine samples were collected from all HEALS participants at the time of the clinical examination at the third HEALS follow-up (2007–2009). Urine samples were initially stored at −20°C in Bangladesh, shipped in dry ice to the United States and stored at −20°C at Columbia University. All laboratory analyses were performed blinded to participants’ characteristics. Total urinary arsenic (As) was measured shortly after arrival by graphite furnace atomic absorption spectrometry (GFAAS) at the Trace Metals Laboratory at Columbia University following a previously used protocol (Nixon et al., 1991). For the multi-elemental urinary metal panel specifically conducted for this study, we used inductively coupled plasma mass spectrometry (ICP-MS) to measure antimony (Sb), barium (Ba), beryllium (Be), cadmium (Cd), cobalt (Co), cesium (Cs), copper (Cu), lead (Pb), manganese (Mn), molybdenum (Mo), nickel (Ni), platinum (Pt), selenium (Se), strontium (Sr), tin (Sn), thallium (Tl), tungsten (W), uranium (U) and zinc (Zn) concentrations using a Perkin-Elmer NexION 350S ICP-MS-DRC equipped with an Autosampler SC-4 Dx, manufactured by Elemental Scientific, as highly automated and set up as a FAST System for NexION measurements. The ICP-MS-DRC method used for metals in urine was developed in our laboratory according to published procedures (Pruszkowski et al., 1998; Stroh, 1993) the Center for Disease Control (CDC) method (Centers for Disease Control and Prevention (CDC), 2014) and with modifications suggested by the Perkin Elmer application laboratory. Urine samples were diluted 50x with 2% HNO3 + 0.02% Triton-X-100 + 500 µg/L gold (Au). One multi-element standard solution, made of stocks of single elements, was used for instrument calibration. The same diluent used for urine samples was used for calibration standards. The metal concentrations of calibration solution were chosen to cover the expected ranges of analyte concentrations in urine samples: for Sb, Ba, Cd, Cs, Co, Mn, Pt, Tl, W and U they were 0.01, 0.02, 0.05, 0.1, 0.2 µg/L; for Be and Ni they were 0.05, 0.1, 0.25, 0.5, 1.0 µg/L; for Mo, Pb, Se, Sn, Cu and Sr they were 0.25, 0.50, 1.25, 2.5 and 5.0 µg/L; and for Zn they were 0.5, 1.0, 2.5, 5.0, 10.0 µ/L. Special attention was given to correct for matrix-induced interferences. Matrix suppression is compensated very well by the selection of suitable internal standards (IS), which were matched to masses and, if possible, to ionization properties of the analytes. For low masses (Co, Ni, Zn, Cu and Mn) we used gallium (Ga) and for Be we used scandium (Sc), for medium masses (Sr, Mo, Sn, Sb, Cs, Ba, Se and Cd) we used rhodium (Rh) and for high masses (W, Pt, Tl, Pb and U) we used iridium (Ir). A stock IS spiking solution was prepared which ultimately delivered to each tube 25 ng of each IS. Polyatomic interferences are suppressed with the instrument’s Dynamic Reaction Cell (DRC) technology feature, oxygen was used as a second gas for Se and Cd and ammonia was used as a second gas for Mn. The other metals (Be, Co, Ni, Zn, Cu, Sr, Mo, Sn, Sb, Cs, Ba, W, Pt, Tl, Pb and U) were run in standard mode without a second gas. Samples were analyzed in batches of 20 with a set of calibration blanks, sample preparation blanks, and commercially available reference urine samples from NIST and Institut de Santé Publique du Québec with a broad range of metals concentration. Also, about 10% of samples were measured in duplicate to determine intraprecision, and another 10% were measured on a different day to determine interprecision. These 20 elements were selected because of their relevance to a prior study of urinary metal mixtures in the United States (Pang et al., 2016). The limits of detection (LOD) were 2.00 µg/L for As, 0.23 µg/L for Ba, 0.27 µg/L for Be, 0.07 µg/L for Cd, 0.05 µg/L for Co, 0.03 µg/L for Cs, 0.64 µg/L for Cu, 0.18 µg/L for Mn, 0.09 µg/L for Mo, 0.21 µg/L for Ni, 0.30 µg/L for Pb, 0.05 µg/L for Pt, 0.14 µg/L for Sb, 1.10 µg/L for Se, 1.34 µg/L for Sn, 0.34 µg/L for Sr, 0.02 µg/L for Tl, 0.02 µg/L for U, 0.03 µg/L for W and 5.49 µg/L for Zn. Five metals (Be, Pt, Sb, Sn and U) were excluded from further analysis because >80% had values below the LOD (Supplementary Table 2). For the 15 metals retained in the analysis, the percent of samples with values below the LOD ranged from 0% for As, Cs, Cu, Mo, Ni, Se, Sr and Zn to 27% for Mn. Values below the LOD were replaced by the LOD divided by the square root of two. We accounted for urinary dilution by standardizing metal concentrations to urinary specific gravity (SG) using urinary metal concentration*(mean SG −1)/(SG −1).
2.3. Other variables
Interviews, physical examinations and biospecimen collection were conducted by trained field staff in Araihazar, Bangladesh, using standardized HEALS procedures (Ahsan et al, 2006). Information on age, sex, occupation, TV ownership (as a marker of socioeconomic status), and fertilizer/pesticide/fabric dye use was abstracted from a standardized questionnaire that was administered at baseline (2000–2002). Information on current cigarette and betel nut use was abstracted from the questionnaire administered at follow-up 3 (2007–2009), thus concurrent with the time of urine panel metals assessment. These variables were selected because they were available in the questionnaire and we believed they could be relevant to identify different sources of metal exposure (e.g. occupation) or metabolic factors that influence internal dose (e.g. sex, age). Water As and Mn concentrations collected from the participants’ well at baseline were previously analyzed at Lamont-Doherty Earth Observatory of Columbia University by GFAAS. Samples with As concentrations below 5 µg/L were subsequently re-analyzed by HR ICPMS. Details have been published elsewhere (Cheng et al., 2004; van Geen et al., 2003).
2.4. Statistical analysis
The distributions of urinary metal concentrations were right skewed and thus log-transformed for all analyses to achieve normality (Supplementary figure 1). Spearman’s correlation coefficients were calculated to examine the correlation between each metal pair (Supplementary figure 1). Basic descriptive statistics were calculated for each metal overall and by participant characteristics.
Principal Component Analysis (PCA) is commonly used to reduce data dimensionality when there are many interrelated variables in a dataset while minimizing the loss of original information. This reduction is achieved by extracting the principal components (PCs) from the observed variables. The resulting PCs are uncorrelated and ordered by decreasing explanation of the variation present in all of the original variables (Hotelling, 1933; Jolliffe, 2002). The coefficients defining these linear combinations (factor loadings) are the correlation coefficients of each variable with that component. We used PCA with varimax normalized rotation to maximize the variances of the factor loadings across variables for each factor (Kaiser, 1958).
Hierarchical cluster analysis (CA) is used to identify relatively homogeneous groups of variables by combining them into agglomerative clusters until only one cluster is left (Everitt et al., 2011). We used Ward’s method with Euclidean distances as the criterion for forming clusters. Dendrograms were constructed to assess the cohesiveness of the clusters formed. Because urinary metal concentrations varied by differing orders of magnitude, we normalized each variable to unit variance and zero mean before conducting PCA and CA. We ran PCA and CA then compared the findings of PCA and CA to assess common patterns between the two approaches.
We used one-way ANOVA to evaluate differences in geometric mean urinary metal levels and in arithmetic mean PC values by participant characteristics. Statistical analyses and graphical displays were performed using STATA (StataCorp, Version 14.2) and R software (R Studio Version 1.0.143, R Version 3.4.0).
3. Results
3.1. Metal levels in urine
The mean age of the study participants was 38 years. By design, 50% were male. Men had lower urinary Co and Mn and higher Se and Zn compared to women (Table 1 and Figure 1). Thirty percent of participants were smokers and smokers were predominantly male (95%). Smokers had higher urinary Ni and Zn levels compared to non-smokers. Thirty-five percent of participants (36% of men and 33% of women) reported chewing betel nut. Betel nut users had higher Cd, Co, Cs and Ni levels compared to non-betel nut users. Male betel nut users had higher Co, Cs and Tl levels compared to male non-users (Supplementary Table 3). Female betel nut users had higher Cd levels compared to female non-users. Television owners, used as a marker of socioeconomic status, had significantly lower As, Mo and W levels and higher Pb levels, although associations with urinary As, Mo and W were attenuated after adjustment for water As (Supplemental Table 4). Forty percent of participants self-reported using fertilizers and/or pesticides. Those who worked with pesticides had higher As, Mo, Ni, W and Zn levels. Levels of urinary As and Mo were higher in farmers compared to men with other occupations, whereas day laborers had higher mean Cs, Cu, Mo, Pb, Se, Sr, Tl and Zn levels compared to men with other occupations. Women who worked outside the home (4%) had higher Ba and Mn levels and lower Zn levels compared to homemakers. Water arsenic was positively associated with urinary As, Mo and W levels. Water manganese was moderately correlated with water arsenic (r=0.35, p<0.0001, n=135). Water manganese was also positively associated with urinary As, Mn, and Mo, but not urinary W (results not shown).
Table 1.
Geometric means of urine metals (µg/L) by participant characteristics. All metals standardized by specific gravity.
| N | As | Ba | Cd | Co | Cs | Cu | Mn | Mo | Ni | Pb | Se | Sr | Tl | W | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||||||||||
| Men | 100 | 79.84 | 1.07 | 0.41 | 0.35 | 9.30 | 9.58 | 0.36 | 64.72 | 3.39 | 2.41 | 10.59 | 79.04 | 0.16 | 0.25 | 208.51 |
| Women | 99 | 68.72 | 1.11 | 0.45 | 0.41 | 9.68 | 8.67 | 0.70 | 58.56 | 2.92 | 2.29 | 9.03 | 75.19 | 0.17 | 0.19 | 112.17 |
| p-value | 0.2 | 0.8 | 0.4 | 0.03 | 0.6 | 0.2 | 0.001 | 0.4 | 0.1 | 0.6 | 0.03 | 0.6 | 0.5 | 0.1 | <0.001 | |
| Current smoker | ||||||||||||||||
| Yes | 60 | 81.45 | 1.02 | 0.48 | 0.38 | 9.03 | 9.68 | 0.39 | 70.11 | 3.67 | 2.53 | 10.8 | 88.23 | 0.16 | 0.26 | 210.61 |
| No | 139 | 71.52 | 1.12 | 0.41 | 0.38 | 9.68 | 8.94 | 0.56 | 57.97 | 2.94 | 2.27 | 9.39 | 72.97 | 0.17 | 0.20 | 132.95 |
| p-value | 0.3 | 0.6 | 0.2 | 0.8 | 0.4 | 0.4 | 0.1 | 0.1 | 0.03 | 0.3 | 0.07 | 0.08 | 0.6 | 0.1 | <0.001 | |
| Betel nut user | ||||||||||||||||
| Yes | 69 | 82.27 | 1.28 | 0.53 | 0.44 | 10.7 | 9.39 | 0.57 | 57.97 | 3.60 | 2.25 | 10.28 | 80.64 | 0.17 | 0.20 | 154.47 |
| No | 130 | 70.81 | 1.00 | 0.39 | 0.35 | 8.94 | 8.94 | 0.47 | 63.43 | 2.94 | 2.39 | 9.49 | 75.19 | 0.16 | 0.23 | 152.93 |
| p-value | 0.3 | 0.1 | 0.01 | 0.009 | 0.02 | 0.5 | 0.4 | 0.5 | 0.04 | 0.6 | 0.3 | 0.5 | 0.5 | 0.4 | >0.9 | |
| Owns TV | ||||||||||||||||
| Yes | 58 | 54.60 | 1.28 | 0.42 | 0.38 | 9.12 | 9.12 | 0.58 | 50.40 | 3.22 | 2.77 | 9.97 | 75.94 | 0.18 | 0.17 | 169.02 |
| No | 141 | 84.77 | 1.02 | 0.44 | 0.38 | 9.68 | 9.12 | 0.47 | 66.69 | 3.13 | 2.18 | 9.68 | 77.48 | 0.16 | 0.24 | 146.94 |
| p-value | 0.001 | 0.2 | 0.8 | 0.8 | 0.5 | >0.9 | 0.4 | 0.02 | 0.8 | 0.02 | 0.7 | 0.8 | 0.2 | 0.02 | 0.3 | |
| Fabric dye use | ||||||||||||||||
| Yes | 15 | 75.94 | 0.82 | 0.45 | 0.33 | 8.85 | 8.00 | 0.33 | 60.34 | 2.97 | 2.34 | 8.76 | 57.97 | 0.17 | 0.17 | 167.34 |
| No | 184 | 74.44 | 1.12 | 0.43 | 0.38 | 9.58 | 9.21 | 0.52 | 61.56 | 3.16 | 2.34 | 9.87 | 79.04 | 0.17 | 0.22 | 152.93 |
| p-value | 0.9 | 0.3 | 0.8 | 0.3 | 0.6 | 0.3 | 0.2 | 0.9 | 0.7 | >0.9 | 0.4 | 0.1 | >0.9 | 0.3 | 0.7 | |
| Fertilizer/pesticide use | ||||||||||||||||
| Yes | 81 | 86.49 | 1.20 | 0.47 | 0.38 | 9.49 | 9.39 | 0.48 | 72.24 | 3.53 | 2.25 | 10.07 | 79.84 | 0.16 | 0.26 | 194.42 |
| No | 118 | 67.36 | 1.02 | 0.41 | 0.38 | 9.58 | 8.94 | 0.52 | 55.15 | 2.92 | 2.41 | 9.58 | 75.19 | 0.17 | 0.20 | 130.32 |
| p-value | 0.05 | 0.3 | 0.3 | 0.8 | 0.9 | 0.6 | 0.6 | 0.02 | 0.04 | 0.5 | 0.6 | 0.6 | 0.3 | 0.05 | <0.001 | |
| Occupation, male | ||||||||||||||||
| Day labor | 8 | 86.49 | 1.07 | 0.68 | 0.45 | 10.07 | 12.68 | 0.31 | 75.94 | 5.47 | 2.72 | 13.2 | 97.51 | 0.19 | 0.38 | 278.66 |
| Farmer | 18 | 130.32 | 0.95 | 0.51 | 0.36 | 9.87 | 8.17 | 0.38 | 87.36 | 3.35 | 1.90 | 9.12 | 61.56 | 0.14 | 0.36 | 186.79 |
| Factory | 24 | 99.48 | 0.85 | 0.36 | 0.34 | 9.78 | 9.12 | 0.27 | 77.48 | 3.19 | 2.39 | 10.07 | 84.77 | 0.15 | 0.27 | 165.67 |
| Other | 50 | 59.74 | 1.25 | 0.37 | 0.34 | 8.85 | 9.97 | 0.42 | 51.94 | 3.25 | 2.56 | 10.91 | 81.45 | 0.17 | 0.19 | 230.44 |
| p-value | 0.01 | 0.4 | 0.1 | 0.5 | 0.8 | 0.3 | 0.3 | 0.05 | 0.2 | 0.4 | 0.3 | 0.4 | 0.5 | 0.06 | 0.2 | |
| Occupation, female | ||||||||||||||||
| Homemaker | 95 | 68.72 | 1.05 | 0.46 | 0.41 | 9.78 | 8.76 | 0.62 | 59.74 | 2.94 | 2.32 | 9.21 | 75.19 | 0.17 | 0.19 | 115.58 |
| Other | 4 | 75.94 | 3.35 | 0.35 | 0.38 | 8.76 | 7.32 | 11.70 | 36.23 | 2.39 | 1.45 | 6.42 | 66.02 | 0.15 | 0.21 | 50.91 |
| p-value | 0.8 | 0.05 | 0.6 | 0.7 | 0.7 | 0.5 | <0.001 | 0.2 | 0.5 | 0.2 | 0.2 | 0.7 | 0.6 | 0.8 | 0.02 | |
| Water arsenic quartiles (µg/L) | ||||||||||||||||
| <15 | 50 | 38.09 | 1.20 | 0.49 | 0.43 | 9.49 | 9.68 | 0.36 | 61.56 | 3.29 | 2.23 | 10.38 | 85.63 | 0.17 | 0.14 | 154.47 |
| 15-<67 | 50 | 72.24 | 1.15 | 0.39 | 0.34 | 8.94 | 8.58 | 0.66 | 50.40 | 2.83 | 2.08 | 9.78 | 67.36 | 0.17 | 0.21 | 133.95 |
| 67-<167 | 48 | 94.69 | 1.14 | 0.39 | 0.37 | 10.07 | 9.21 | 0.56 | 58.56 | 3.06 | 2.51 | 9.49 | 81.45 | 0.17 | 0.23 | 172.43 |
| ≥167 | 51 | 119.10 | 0.90 | 0.46 | 0.39 | 9.58 | 9.12 | 0.48 | 79.04 | 3.49 | 2.64 | 9.49 | 75.19 | 0.16 | 0.34 | 156.02 |
| p-value | <0.001 | 0.5 | 0.4 | 0.2 | 0.7 | 0.7 | 0.2 | 0.04 | 0.4 | 0.3 | 0.8 | 0.4 | 0.8 | <0.001 | 0.4 | |
Figure 1.
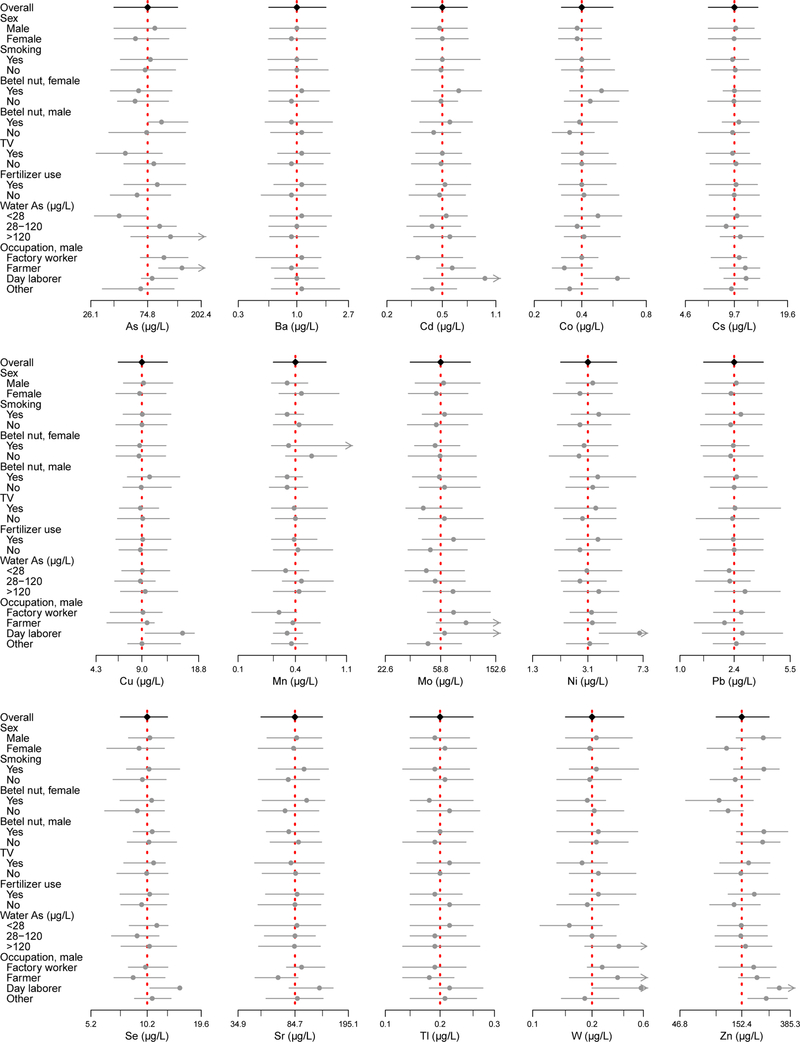
Forest plot of medians and interquartile range of 15 urinary metals (µg/L) by participant characteristics. X-axis is on a natural-log scale. Minor tick marks represent overall interquartile range.
3.2. Principal component and cluster analysis
Six PCs explained 80.1% of the total variance (Table 2). The six PCs (% variance explained) were Sr-Ni-Cs (21.1%), Pb- Tl (15.6%), As-Mo-W (12.5%), Ba-Mn (10.7%), Cu-Zn (10.3%) and Cd (10.0%). Cluster analysis showed similar although not identical patterns with PCA (Figure 2). The five clusters were Sr-Co-Ni, Pb-Se-Cs-Tl, Cd-Cu-Zn, Ba-Mn and As-Mo-W.
Table 2.
Standardized rotated factor loading and communalities for specific gravity standardized metals (n=199).
| Component | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| As | −0.16 | 0.12 | 0.63 | 0.09 | −0.16 | 0.22 |
| Ba | 0.16 | 0.08 | −0.08 | 0.61 | 0.10 | −0.20 |
| Cd | 0.02 | −0.05 | −0.01 | 0.03 | 0.15 | 0.64 |
| Co | 0.20 | 0.26 | 0.06 | −0.02 | −0.23 | 0.36 |
| Cs | 0.52 | −0.03 | −0.05 | −0.01 | −0.13 | 0.21 |
| Cu | 0.10 | −0.04 | 0.01 | 0.09 | 0.46 | 0.28 |
| Mn | −0.08 | −0.06 | 0.07 | 0.75 | −0.06 | 0.11 |
| Mo | 0.27 | −0.06 | 0.44 | −0.15 | 0.05 | −0.08 |
| Ni | 0.55 | −0.10 | −0.01 | −0.05 | 0.03 | 0.06 |
| Pb | −0.03 | 0.60 | 0.06 | −0.03 | 0.15 | −0.24 |
| Se | 0.09 | 0.38 | −0.02 | −0.04 | 0.18 | 0.06 |
| Sr | 0.48 | 0.12 | −0.03 | 0.10 | −0.01 | −0.31 |
| Tl | −0.04 | 0.59 | −0.05 | 0.01 | −0.12 | 0.15 |
| W | 0.05 | −0.09 | 0.62 | 0.01 | 0.14 | −0.19 |
| Zn | −0.05 | 0.04 | −0.01 | −0.03 | 0.76 | 0.02 |
| | ||||||
| Eigenvalue | 3.17 | 2.33 | 1.88 | 1.61 | 1.50 | 1.49 |
| Total variance (%) | 21.11% | 15.55% | 12.51% | 10.72% | 10.27% | 9.98% |
| Cumulative (%) | 21.11% | 36.65% | 49.17% | 59.89% | 70.16% | 80.14% |
Factor loadings are bolded if >0.40.
Figure 2.
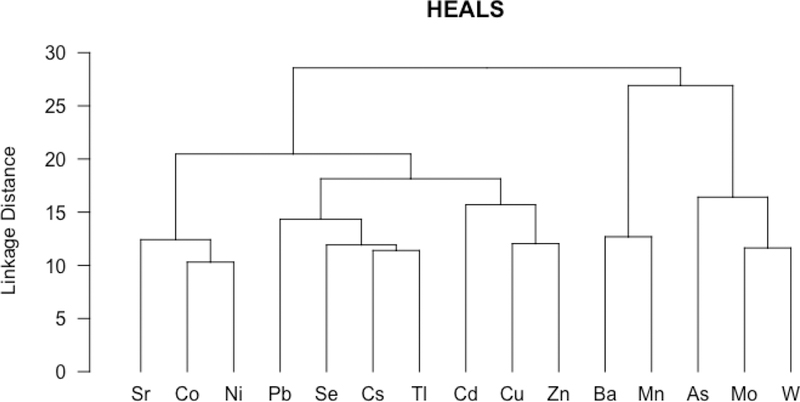
Dendrogram of metals in urine in 199 HEALS participants. All metals standardized by specific gravity, log- transformed and centered before analysis.
3.3. PCA commonalities with sociodemographic characteristics and exposure sources
The first two components, Sr-Ni-Cs and Pb-Tl, were not related to any of the sociodemographic characteristics investigated, including smoking status and occupation (Table 3). For the third component, As-Mo-W, there was a strong significant association between the component and water arsenic levels (p<0.001), TV ownership (a marker of socioeconomic status) (p<0.001), fertilizer/pesticide use (0.01) and occupation among males (0.003). For the fourth component, Ba-Mn was associated with sex (p=0.03) and occupation among women (p=0.001). The fifth component, Cu-Zn, was associated with sex (p<0.001), smoking status (p<0.001), fertilizer/pesticide use (p=0.004) and occupation among females (p=0.03). The sixth component, Cd, was associated with betel nut use (p=0.003).
Table 3.
Mean Principal Component (PC) value by participant characteristics
| N | PC 1 Sr-Ni-Cs |
PC 2 Pb-Tl |
PC 3 As-Mo-W |
PC 4 Ba-Mn |
PC 5 Cu-Zn |
PC 6 Cd |
|
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Men | 100 | 0.03 | 0.06 | 0.14 | −0.20 | 0.42 | −0.09 |
| Women | 99 | −0.03 | −0.06 | −0.15 | 0.20 | −0.43 | 0.10 |
| p-value | 0.8 | 0.6 | 0.1 | 0.03 | <0.001 | 0.3 | |
| Current smoker | |||||||
| Yes | 60 | 0.24 | 0.08 | 0.23 | −0.19 | 0.49 | −0.02 |
| No | 139 | −0.11 | −0.04 | −0.10 | 0.08 | −0.21 | 0.01 |
| p-value | 0.2 | 0.6 | 0.1 | 0.2 | <0.001 | 0.9 | |
| Betel nut user | |||||||
| Yes | 69 | 0.31 | 0.10 | −0.03 | 0.18 | −0.03 | 0.35 |
| No | 130 | −0.17 | −0.05 | 0.02 | −0.10 | 0.02 | −0.19 |
| p-value | 0.07 | 0.5 | 0.8 | 0.1 | 0.8 | 0.003 | |
| Owns TV | |||||||
| Yes | 58 | −0.05 | 0.24 | −0.50 | 0.15 | 0.15 | −0.10 |
| No | 141 | 0.02 | −0.10 | 0.20 | −0.06 | −0.06 | 0.04 |
| p-value | 0.8 | 0.2 | 0.001 | 0.3 | 0.3 | 0.5 | |
| Dye use | |||||||
| Yes | 15 | −0.48 | −0.12 | −0.14 | −0.42 | −0.04 | 0.06 |
| No | 184 | 0.04 | 0.01 | 0.01 | 0.03 | 0.00 | 0.00 |
| p-value | 0.3 | 0.8 | 0.7 | 0.2 | 0.9 | 0.9 | |
| Fertilizer/pesticide use | |||||||
| Yes | 81 | 0.16 | −0.08 | 0.29 | 0.00 | 0.31 | 0.04 |
| No | 118 | −0.11 | 0.06 | −0.20 | 0.00 | −0.21 | −0.03 |
| p-value | 0.3 | 0.5 | 0.01 | 0.9 | 0.004 | 0.7 | |
| Occupation, male | |||||||
| Day labor | 8 | 0.99 | 0.42 | 0.51 | −0.28 | 1.10 | 0.49 |
| Farmer | 18 | −0.09 | −0.45 | 0.92 | −0.28 | 0.10 | 0.22 |
| Factory | 24 | 0.02 | 0.03 | 0.47 | −0.49 | 0.09 | −0.23 |
| Other | 50 | −0.08 | 0.20 | −0.35 | −0.01 | 0.59 | −0.24 |
| p-value | 0.5 | 0.4 | 0.003 | 0.2 | 0.09 | 0.2 | |
| Occupation, female | |||||||
| Homemaker | 95 | −0.01 | −0.02 | −0.14 | 0.10 | −0.38 | 0.10 |
| Other | 4 | −0.60 | −0.93 | −0.23 | 2.49 | −1.58 | −0.13 |
| p-value | 0.5 | 0.3 | 0.9 | 0.001 | 0.03 | 0.7 | |
| Water arsenic quartiles | |||||||
| <15 µg/L | 50 | 0.37 | −0.02 | −0.82 | −0.17 | 0.14 | 0.05 |
| 15-<67 µg/L | 50 | −0.38 | −0.13 | −0.15 | 0.20 | −0.21 | −0.07 |
| 67-<167 µg/L | 48 | −0.07 | 0.17 | 0.19 | 0.12 | 0.04 | −0.03 |
| ≥167 µg/L | 51 | 0.08 | −0.01 | 0.78 | −0.14 | 0.03 | 0.05 |
| p-value | 0.2 | 0.8 | <0.001 | 0.4 | 0.5 | 0.9 | |
4. Discussion
We measured 15 urinary metals and assessed commonalities with sociodemographic characteristics and potential sources of exposure in a random sample of HEALS participants. Metal levels in our study were similar to studies in Bangladesh (similar for As, Cd, Mn, Pb, Sb, Se, U and Zn) (Berglund et al., 2011) and Cambodia (similar for As, Co, Cu, Mo, and Pb) (Chanpiwat et al., 2015) but differed from levels in the US (lower As, higher Sb, Se, and Zn) (Pang et al., 2016) (Supplementary Table 5). PCA and CA showed similar patterns, resulting in six distinct components and five clusters. The As-Mo-W component/cluster likely reflects groundwater contamination, as the component (PC3) and each of the metals were significantly associated with water arsenic levels. One of the components/clusters (Sr-Ni-Cs/Sr-Ni-Co) may reflect air pollution as a potential exposure source, as the component (PC1) was not associated with any of the sociodemographic characteristics evaluated. The Cd component (PC6) appears to reflect betel nut usage but could also be related to smoking.
4.1. PCA versus CA
Unsupervised dimensionality reduction methods, including PCA and CA are useful during data exploration, particularly to generate hypotheses. Each method comes with its strengths and limitations when applied to biomonitoring. While CA offers a useful visualization and silos all elements into neat clusters, CA will always calculate clusters, even if there is not a strong signal in the data. Further, because of its hierarchical design, there is no ability to see potential cross-loading of elements across multiple clusters. Alternatively, in PCA, only the strongest PCs are presented and some of the weaker, but potentially important, signals in the filtered data may be discarded. These differences likely explain why our PCA and CA results are not identical. For example in our CA, Cd most strongly clustered with Cu and Zn, however, in our PCA, there is evidence that Cd loads on both PC6 and PC5, indicating that there are multiple potential signatures for this element. Taken together, PCA and CA are useful methods to characterize co-exposure patterns.
4.2. Arsenic-Molybdenum-Tungsten
The As-Mo-W component/cluster supports that groundwater is an important environmental source of exposure to these metals. This finding regarding urinary metal concentrations is supported by two tubewell surveys conducted in Bangladesh that found a correlation between elevated water As levels and elevated Mo (BGS/DPHE, 2001; Frisbie et al., 2002). Similar to the geochemical mechanism of As release, Mo may co-occur with As in groundwater by desorption from reduced iron oxides (BGS/DPHE, 2001). As far as we are aware, W in water has not been measured in previous studies in Bangladesh. Arsenic and W commonly co-occur in groundwater in parts of the United States, India and China (Gao et al., 2018; Mohajerin et al., 2014). For instance, a cluster between urinary As, W and U was found in the Strong Heart Study, a multi-center study in American Indian communities in the United States (Pang et al., 2016). Uranium, however, was very low in our study (87% of participants had levels below the LOD of 0.02 µg/L) and in a sensitivity analysis did not cluster with As (data not shown). Relatively little is known about the health effects of W, although this is a priority metal for risk assessment due to its increased use in many industrial goods, including lightbulb filaments, jewelry, electronics, ammunition and implanted medical devices (Bolt and Mann, 2016). Tungsten and Mo interact in biological systems and W can replace Mo and interfere with Mo-enzymes. In the Strong Heart Study, there was an antagonistic interaction between urinary Mo and W levels, with higher Mo showing protection from potential adverse effects of W on cardiovascular diseases. In a post-hoc analysis of water As, Mo and W levels measured in a subset of water samples (n=45) from HEALS participants (unpublished results), there was a relatively strong correlation between water As and W (r=0.63) and between As and Mo (r=0.71) but the correlation between water W and Mo was moderate (r=0.49). The likely co-occurrence of As-W-Mo in water in the HEALS population, and the possible joint effects of these metals highlights the need to evaluate the potential health effects of co-exposure to these metals (Cheng et al., 2004).
4.3. Cadmium
In PC6, Cd was the only element with a factor loading >0.40, suggesting that the main source of Cd is distinct and specific compared to other metals in the population; however, this PC also had smaller positive loads for other metals including As, Co, Cs and Cu and in cluster analysis, Cd clustered with Cu and Zn. Both PC6 and urinary Cd levels were significantly associated with betel nut use. Betel nut (areca nut) is a mild stimulant that is often mixed with tobacco leaves and chewed. Betel nut may be a significant source of some metals, including Cd (Al-Rmalli et al., 2011). However, research investigating the relationship between metal levels in betel nut, or betel nut use, with metal biomarker levels is sparse. A case-control study in Matlab, Bangladesh found that betel nut use was a risk factor for developing As-related skin lesions in women (Lindberg et al., 2010). Women who chewed betel nut had considerably higher odds of having skin lesions [OR=3.8 (95% CI=1.4–10)] compared to women who did not use betel nut, after controlling for differences in As metabolites (%monomethylarsonic acid) (Lindberg et al., 2010). A follow-up study in the same population found that female betel nut users had higher urinary Cd levels (median=0.96 µg/L) than women who did not use betel nut (median=0.84 µg/L) (Berglund et al., 2011). Our findings also support that betel nut use may contribute to increased Cd exposure, particularly among women (median urinary Cd among female betel nut users=0.59 µg/L vs 0.44 µg/L among female non- users). Cadmium retention is known to be higher in women than men at equal levels of exposure (Vahter et al., 2007). This sex-difference is related to lower ferritin values in women caused by lower body iron stores (Flanagan et al., 1978). Iron-deficient individuals have a higher Cd level because of increased expression of the divalent metal transporter SLC11A2, formerly DCT-1 (Gunshin et al., 1997; Vesey, 2010). This component points to a new opportunity to further study the health consequences associated with Cd exposure via betel nut use, particularly among women and iron-deficient populations. Alternatively, this Cd component could also reflect dietary exposure. An exploratory study in Matlab, Bangladesh, found that As and Cd are detected in both raw and cooked rice and vegetables (Khan et al., 2010). Cadmium is believed to enter the food chain in Bangladesh through the heavy use of Cd-containing fertilizers and pesticides. In the Strong Heart Study, a cohort study among American Indians in the US, PCA identified a Cd-Zn component that was attributed to common metabolic pathways or common dietary sources (Pang et al., 2016). The metal transporter SLC11A2 has a broad substrate range, including Cd, Zn, Co, and Cu (Gunshin et al., 1997). Our cluster analysis found Cu, Zn and Cd to cluster together, and in PCA, Cd, Cu and Zn also loaded on PC5, even though Zn levels in our study were much lower (geometric mean=153 µg/L) than that of the US population studied in the Strong Heart Study (geometric mean=907 µg/L). Surprisingly, the Cd component (PC6) was not significantly associated with smoking status; however, smokers had higher urinary Cd levels after stratifying by sex. The lack of association between smoking and the PC for Cd may be due to our small sample size or the particular type of cigarette smoked (the questionnaire administered did not differentiate between regular cigarettes and bidis, which are locally produced cigarettes).
4.4. Strontium-Nickel-Cesium, Lead-Thallium
We were unable to identify a possible exposure source for the first two principal components from the sociodemographic variables available in the HEALS database. The lack of any association suggests that these exposures might be affecting the population as a whole, maybe through air pollution or through a major staple food. Based on prior research, we speculate that potential sources of metal exposure for these elements is likely from air pollution sources, including biomass and/or oil combustion (Begum et al., 2005) and potentially brick kilns (Sikder et al., 2016). Air pollution sources have unique metal signatures. For example, oil combustion- derived metals in Bangladesh have been found to include a signature of sulfur, aluminum, and other crustal metals (Begum et al., 2013) whereas brick kilns, one of the fastest growing industrial sectors in Bangladesh (Guttikunda et al., 2013), are known to emit several other metals, including Pb, Zn, Cu and Ni (Sikder et al., 2016).
4.5. Strengths and limitations
The major study limitation is the small sample size, which was due to budgetary restraints. Due to the sample size, we were not able to look deeper into potential sources of metal exposure, for example 17 of 18 farmers reported using pesticides. There were also some discrepancies in the characteristics of our study population subset and the larger HEALS cohort, particularly by TV ownership and smoking status. The sample, however, was obtained at random and we were able to leverage the high quality and extensive HEALS data to examine differences by relevant participant characteristics in the metal groupings. For some metals, such as Mn, Pb, Se and Zn, urine is considered a less reliable biomarker to assess exposure and internal dose (Abadin et al., 2007; ATSDR, 2012, 2003). For all metals except arsenic we used samples that had been stored for several years; however, metals were unlikely to be affected by the storage/handling conditions (Scheer et al., 2012). These data are likely relevant to the surrounding HEALS area and other rural and semi-rural populations in Bangladesh and in other countries in the region; however, results might not be representative for populations in urban settings or for other rural communities with different socioeconomic, geological and climatological conditions.
5. Conclusions
We identified several distinct metal groupings likely related to multiple sources of exposure. None of the PCs were more predominant over the others, with the variability explained ranging from 21% for PC1 (Sr-Ni-Cs) to 10% for PC6 (Cd). Groundwater, betel nut use and air pollution are likely important sources of metal exposure in the HEALS study population. Given the well-established health effects of As in HEALS and the possibility that As interacts with other metals, these findings can contribute to guiding future exposure assessment research in rural Bangladesh and future epidemiologic research investigating the degree to which metal mixtures play a role in disease development. Future research and public health initiatives should focus on reducing metal and metalloid exposure from groundwater and betel nut and on identifying potential exposure to airborne metals.
Supplementary Material
Highlights:
Examined 15 urinary metals and their sociodemographic determinants/exposure sources
Principal component (PC) and cluster analysis showed 6 components/5 clusters
Groundwater is a likely source of As, Mo, and W
Betel nut use is a likely source of Cd exposure
Air pollution may be a source of Sr, Ni, Cs, and Co exposure but needs confirmation
Acknowledgments
Funding Information: This work was supported by the National Institutes of Environmental Health Sciences at the National Institutes of Health [grant numbers P42ES010349 and 5P30ES009089] and an NIH Shared- Instrumentation Grant [S10-OD016384].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none
References:
- Abadin H, Ashizawa A, Stevens Y-W, Llados F, Diamond G, Sage G, Citra M, Quinones A, Bosch SJ, Swarts SG, 2007. Toxicological Profile for Lead U.S Public Heal. Serv. Agency Toxic Subst. Dis. Regist; 582 https://doi.org/doi:10.1201/9781420061888_ch106 [PubMed] [Google Scholar]
- Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H, Levy D, van Geen A, Howe G, Graziano J, 2006. Health Effects of Arsenic Longitudinal Study (HEALS): Description of a multidisciplinary epidemiologic investigation. J. Expo. Sci. Environ. Epidemiol 16, 191–205. 10.1038/sj.jea.7500449 [DOI] [PubMed] [Google Scholar]
- Al-Rmalli SW, Jenkins RO, Haris PI, 2011. Betel quid chewing elevates human exposure to arsenic, cadmium and lead. J. Hazard. Mater 190, 69–74. 10.1016/j.jhazmat.2011.02.068 [DOI] [PubMed] [Google Scholar]
- Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-zaman M, 2010. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376, 252–258. https://doi.org/10.1016/S0140-6736(10)60481- 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR, 2012. Toxicological profile for Manganese U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta, GA. [PubMed] [Google Scholar]
- ATSDR, 2003. Toxicological profile for Selenium U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry, Atlanta, GA. [PubMed] [Google Scholar]
- Begum BA, Biswas SK, Kim E, Hopke PK, Khaliquzzaman M, 2005. Investigation of Sources of Atmospheric Aerosol at a Hot Spot Area in Dhaka, Bangladesh. J. Air Waste Manage. Assoc 55, 227–240. 10.1080/10473289.2005.10464606 [DOI] [PubMed] [Google Scholar]
- Begum BA, Hopke PK, Markwitz A, 2013. Air pollution by fine particulate matter in Bangladesh. Atmos. Pollut. Res 4, 75–86. 10.5094/APR.2013.008 [DOI] [Google Scholar]
- Berglund M, Lindberg A-L, Rahman M, Yunus M, Grandér M, Lönnerdal B, Vahter M, 2011. Gender and age differences in mixed metal exposure and urinary excretion. Environ. Res 111, 1271–1279. 10.1016/j.envres.2011.09.002 [DOI] [PubMed] [Google Scholar]
- BGS/DPHE, 2001. Groundwater studies of Arsenic contamintation in Bangaldesh [WWW Document].
- Bhowmick S, Kumar A, Adhikari J, Chatterjee D, Chatterjee D, 2015. Assessment of toxic metals in groundwater and saliva in an arsenic affected area of West Bengal, India: A pilot scale study. Environ. Res 142, 328–336. 10.1016/j.envres.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Bolt AM, Mann KK, 2016. Tungsten: an Emerging Toxicant, Alone or in Combination. Curr. Environ. Heal. Reports 3, 405–415. 10.1007/s40572-016-0106-z [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2014. Urine Multi-Element ICP-DRC-MS
- Chanpiwat P, Himeno S, Sthiannopkao S, 2015. Arsenic and Other Metals’ Presence in Biomarkers of Cambodians in Arsenic Contaminated Areas. Int. J. Environ. Res. Public Health 12, 14285–14300. 10.3390/ijerph121114285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Zheng Y, Mortlock R, van Geen A, 2004. Rapid multi-element analysis of groundwater by high- resolution inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem 379, 512–518. 10.1007/s00216-004-2618-x [DOI] [PubMed] [Google Scholar]
- Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim- Tallaa L, Guha N, Freeman C, Galichet L, Wild CP, 2011. Preventable Exposures Associated With Human Cancers. JNCI J. Natl. Cancer Inst 103, 1827–1839. 10.1093/jnci/djr483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosselman KE, Navas-Acien A, Kaufman JD, 2015. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol 12, 627–642. 10.1038/nrcardio.2015.152 [DOI] [PubMed] [Google Scholar]
- Everitt BS, Landau S, Leese M, Stahl D, 2011. Ch. 4 Hierarchical Clustering, in: Cluster Analysis, Wiley Series in Probability and Statistics https://doi.org/doi:10.1002/9780470977811.ch4
- Flanagan PR, McLellan J, Haist J, Cherian G, Chamberlain M, Valberg LS, 1978. Increased dietary cadmium absorption human subjects with iron deficiency in mice and humans. Gastroenterology 74, 841– 846. [PubMed] [Google Scholar]
- Forsyth JE, Saiful Islam M, Parvez SM, Raqib R, Sajjadur Rahman M, Marie Muehe E, Fendorf S, Luby SP, 2018. Prevalence of elevated blood lead levels among pregnant women and sources of lead exposure in rural Bangladesh: A case control study. Environ. Res 166, 1–9. 10.1016/j.envres.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbie SH, Ortega R, Maynard DM, Sarkar B, 2002. The Concentrations of Arsenic and Other Toxic Elements in Bangladesh’s Drinking Water. Environ. Health Perspect 110, 1147–1153. 10.1289/ehp.021101147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Gao B, Peng W, Xu D, Yin S, 2018. Assessing potential release tendency of As, Mo and W in the tributary sediments of the Three Gorges Reservoir, China. Ecotoxicol. Environ. Saf 147, 342–348. 10.1016/j.ecoenv.2017.08.036 [DOI] [PubMed] [Google Scholar]
- Gleason K, Shine JP, Shobnam N, Rokoff LB, Suchanda HS, Sharif O, Hasan I, Mostofa G, Amarasiriwardena C, Quamruzzaman Q, Rahman M, Kile ML, Bellinger DC, Christiani DC, Wright RO, Mazumdar M, 2014. Contaminated turmeric Is a potential source of lead exposure for children in rural Bangladesh. J Env. Public Heal 3–7. 10.1155/2014/730636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA, 1997. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488. 10.1038/41343 [DOI] [PubMed] [Google Scholar]
- Guttikunda SK, Begum BA, Wadud Z, 2013. Particulate pollution from brick kiln clusters in the Greater Dhaka region, Bangladesh. Air Qual. Atmos. Heal 6, 357–365. 10.1007/s11869-012-0187-2 [DOI] [Google Scholar]
- Hasan M, Salam A, Alam AMS, 2009. Identification and characterization of trace metals in black solid materials deposited from biomass burning at the cooking stoves in Bangladesh. Biomass and Bioenergy 33, 1376–1380. 10.1016/j.biombioe.2009.05.023 [DOI] [Google Scholar]
- Hawkesworth S, Wagatsuma Y, Kippler M, Fulford AJC, Arifeen SE, Persson L-A, Moore SE, Vahter M, 2013. Early exposure to toxic metals has a limited effect on blood pressure or kidney function in later childhood, rural Bangladesh. Int. J. Epidemiol 42, 176–185. 10.1093/ije/dys215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JH, Coker E, Barney Y, Shuey C, Lewis J, 2018. Spatial clustering of metal and metalloid mixtures in unregulated water sources on the Navajo Nation – Arizona, New Mexico, and Utah, USA. Sci. Total Environ 633, 1667–1678. 10.1016/j.scitotenv.2018.02.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotelling H, 1933. Analysis of a complex of statistical variables into Principal Components. J. Educ. Psychol 24, 417–441. [Google Scholar]
- Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, Dobson CB, Frelich J, Hoffman E, Yousuf J, Afroz S, Islam S, Christiani DC, 2007. Maternal Arsenic Exposure Associated With Low Birth Weight in Bangladesh. J. Occup. Environ. Med 49, 1097–1104. 10.1097/JOM.0b013e3181566ba0 [DOI] [PubMed] [Google Scholar]
- Islam S, 2015. Banglapedia: National Encyclopedia of Bangladesh [WWW Document].
- Jolliffe I, 2002. Introduction, in: Principal Component Analysis Springer New York, New York, NY, pp. 1–9. 10.1007/0-387-22440-8_1 [DOI] [Google Scholar]
- Kaiser HF, 1958. The varimax criterion for analytic rotation in factor analysis. Psychometrika 23, 187–200. [Google Scholar]
- Karri V, Schuhmacher M, Kumar V, 2016. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol 48, 203– 213. 10.1016/j.etap.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Khan MN, B. Nurs CZ, Mofizul Islam M, Islam MR, Rahman MM, 2017. Household air pollution from cooking and risk of adverse health and birth outcomes in Bangladesh: a nationwide population-based study. Environ. Heal 16, 57 10.1186/s12940-017-0272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SI, Ahmed AKM, Yunus M, Rahman M, Hore SK, Vahter M, Wahed MA, 2010. Arsenic and cadmium in food-chain in Bangladesh — An exploratory study. J Heal. Popul Nutr 6, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, Quamruzzaman Q, Rahman M, Christiani DC, 2015. Estimating effects of arsenic exposure during pregnancy on perinatal outcomes in a Bangladeshi cohort. Epidemiology 27, 1 10.1097/EDE.0000000000000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Houseman EA, Breton CV, Smith T, Quamruzzaman Q, Rahman M, Mahiuddin G, Christiani DC, 2007. Dietary Arsenic Exposure in Bangladesh. Environ. Health Perspect 115, 889–893. 10.1289/ehp.9462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A-L, Sohel N, Rahman M, Persson LA, Vahter M, 2010. Impact of smoking and chewing tobacco on arsenic-induced skin lesions. Env. Heal. Perspect 118, 533–538. 10.1289/ehp.0900728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm L, Jakobsson K, Lundh T, Zamir R, Shoeb M, Nahar N, Bergman A, 2011. Environmental exposure to POPs and heavy metals in urban children from Dhaka, Bangladesh. J Environ. Monit 2728– 2734. 10.1039/c1em10480b [DOI] [PubMed] [Google Scholar]
- Mohajerin TJ, Neal AW, Telfeyan K, Sasihharan SM, Ford S, Yang N, Chevis DA, Grimm DA, Datta S, White CD, Johannesson KH, 2014. Geochemistry of Tungsten and Arsenic in Aquifer Systems: A Comparative Study of Groundwaters from West Bengal, India, and Nevada, USA. Water, Air, Soil Pollut 225, 1792 10.1007/s11270-013-1792-x [DOI] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA, 2013. The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem. Environ. Health Perspect 121, 295–302. 10.1289/ehp.1205875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickson R, McArthur J, Burgess W, Ahmed KM, Ravenscroft P, Rahmanñ M, 1998. Arsenic poisoning of Bangladesh groundwater. Nature 395, 338–338. 10.1038/26387 [DOI] [PubMed] [Google Scholar]
- Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M, 2016. Environmental Metals and Cardiovascular Disease in Adults: A Systematic Review Beyond Lead and Cadmium. Curr. Environ. Heal. Reports 3, 416–433. 10.1007/s40572-016-0117-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon DE, Mussmann GV, Eckdahl SJ, Moyer TP, 1991. Total arsenic in urine: palladium-persulfate vs nickel as a matrix modifier for graphite furnace atomic absorption spectrophotometry. Clin Chem 37, 1575– 1579. [PubMed] [Google Scholar]
- Pang Y, Peng RD, Jones MR, Francesconi KA, Goessler W, Howard BV, Umans JG, Best LG, Guallar E, Post WS, Kaufman JD, Vaidya D, Navas-Acien A, 2016. Metal mixtures in urban and rural populations in the US: The Multi-Ethnic Study of Atherosclerosis and the Strong Heart Study. Environ. Res 147, 356–364. 10.1016/j.envres.2016.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszkowski E, Neubauer K, Thomas R, 1998. An overview of clinical applications by inductively coupled plasma mass spectrometry. At. Spectrosc 19, 111–115. [Google Scholar]
- Scheer JJ, Findenig S, Goessler W, Francesconi KAA, Howard BV, Umans JGG, Pollak J, Tellez- Plaza M, Silbergeld EKK, Guallar E, Navas-Acien A, 2012. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal. Methods 4, 406 10.1039/c2ay05638k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikder A, Khanom S, Hossain MF, Parveen Z, 2016. Accumulation of Zn, Cu, Fe, Mn and Pb due to brick manufacturing in agricultural soils and plants. Dhaka Univ J Biol Sci 25, 75–81. [Google Scholar]
- Smith AH, Lingas EO, Rahman M, 2000. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull. World Health Organ 78, 1093–103. https://doi.org/10.1590/S0042- 96862000000900005 [PMC free article] [PubMed] [Google Scholar]
- Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA, 2014. Metal pollutants and cardiovascular disease: Mechanisms and consequences of exposure. Am. Heart J 168, 812–822. 10.1016/j.ahj.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroh A, 1993. Determination of Pb and Cd in Whole Blood using Isotope Dilution ICP-MS. At. Spectrosc 14, 141–143. [Google Scholar]
- Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M, 2007. Gender differences in the disposition and toxicity of metals. Environ. Res 104, 85–95. 10.1016/j.envres.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Vahter ME, Li L, Nermell B, Rahman A, El Arifeen S, Rahman M, Persson LA, Ekström E-C, 2006. Arsenic exposure in pregnancy: a population-based study in Matlab, Bangladesh. J. Health. Popul. Nutr 24, 236–45. [PubMed] [Google Scholar]
- van Geen A, Zheng Y, Versteeg R, Stute M, Horneman A, Dhar R, Steckler M, Gelman A, Small C, Ahsan H, Graziano JH, Hussain I, Ahmed KM, 2003. Spatial variability of arsenic in 6000 tube wells in a 25 km 2 area of Bangladesh. Water Resour. Res 39, 1–16. 10.1029/2002WR001617 [DOI] [Google Scholar]
- Vesey DA, 2010. Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicol. Lett 198, 13–19. 10.1016/j.toxlet.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Wright RO, Baccarelli A, 2007. Metals and Neurotoxicology. J. Nutr 137, 2809–2813. 10.1093/jn/137.12.2809 [DOI] [PubMed] [Google Scholar]
- Yongming H, Peixuan D, Junji C, Posmentier E, 2006. Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci. Total Environ 355, 176–186. 10.1016/j.scitotenv.2005.02.026 [DOI] [PubMed] [Google Scholar]
- Yunus F, Khan S, Chowdhury P, Milton AH, 2016. A review of groundwater arsenic contamination in Bangladesh: The millennium development goal era and beyond. Int. J. Environ. Res. Public Health 13, 1–18. 10.3390/ijerph13020215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


