Abstract
The apolipoprotein (APOE) ε4 allele has been proposed as an example of an antagonistic pleiotropy gene, conferring a beneficial effect on cognition in early life and a detrimental impact on cognition during later years. However, findings on the cognitive associations of the ε4 allele in younger persons are mixed. This PRISMA conforming study aimed to investigate APOE genotype (e4/non-e4) associations across seven cognitive domains (intelligence/achievement, attention/working memory, executive functioning, memory, language, processing speed and visuospatial abilities) in younger humans using a meta-analytic approach. Of 689 records reviewed, 29 studies (34 data-points) were selected for the quantitative synthesis. Participants’ ages ranged from 2-40. Results showed that young ε4 carriers did not statistically differ from non-ε4 carriers across any cognitive domains. Overall, findings do not provide compelling support for an antagonistic pleiotropic effect of the ε4 allele across the lifespan.
Keywords: Apolipoprotein E, Alzheimer’s disease, Cognition, Neuropsychology, Executive functions, PRISMA
1. Introduction
The link between the apolipoprotein (APOE) ε4 allele and Alzheimer’s disease (AD) is well established in the literature (Farrer et al., 1997; Saunders et al., 1993). Presence of the APOE ε4 allele confers a three- to four-fold increased risk of developing Alzheimer’s disease (AD; Saunders et al., 1993) and has been linked to neuropathological changes associated with AD, including beta-amyloid plaques (Morris et al., 2010; Serrano-Pozo et al., 2015; Strittmatter et al., 1993) and neurofibrillary tangles (Namba et al., 1991). Furthermore, presence of the ε4 allele in healthy non-demented older adults is associated with poorer cognitive performance (Bondi et al., 1995; Caselli et al., 2004; Small et al., 2004), reduced grey matter volume in regions associated with AD (Den Heijer et al., 2002; Scarmeas and Stern, 2006; Soininen et al., 1995), and differences in cerebral activity during resting and task-based functional magnetic resonance imaging (fMRI; e.g., Bondi et al., 2005; Bookheimer et al., 2000; Tuminello and Han, 2011 for review) compared to non-demented older adults without the allele.
In recent years, there has been increased interest in understanding the effect of the APOE ε4 allele on cognition in different age groups, including children and young adults. Findings support differential effects of the ε4 allele on cognition based on the age group under investigation. Compared to healthy older adults in whom cognitive deficits have been consistently reported in ε4 carriers (Small et al., 2004), differences between ε4 and non-ε4 carriers in middle age are reduced or null (Salvato, 2015 for review). Conversely, in young adults and children, some studies report ε4 carriers outperforming non-ε4 carriers on cognitive tasks (Han and Bondi, 2008 for review).
Based on findings suggesting differential cognitive effects of ε4 allele possession throughout the lifespan, Han and Bondi (2008) along with others (Alexander et al., 2007; Jochemsen et al., 2012; Rusted et al., 2013) proposed the antagonistic pleiotropy hypothesis of APOE ε4. Antagonistic pleiotropy is a theory of senescence in which “individual loci/alleles have different effects on fitness at different ages” (Albin, 1993; Williams, 2001). Specifically, these alleles are thought to have a positive, beneficial effect on fitness in early life and a negative, detrimental impact on fitness during later years in the context of aging (Albin, 1993). Han and Bondi (2008) suggested that ε4 is one such allele, conferring advantages on cognitive tasks early in life but resulting in cognitive and neural disadvantages in late life. Although this is theoretically compelling, findings regarding cognition in younger ε4 carriers are mixed. While some studies provide support for better cognition in young ε4 carriers compared to non-ε4 carriers (Bloss et al., 2010; Puttonen et al., 2003; Schultz et al., 2008; Wright et al., 2003; Yu et al., 2000), other studies fail to find support (Deary et al., 2003; Dennis et al., 2010; Filbey et al., 2006; Jorm et al., 2007; Luciano et al., 2009; Richter-Schmidinger et al., 2011) and some even report poorer cognitive performances in young ε4 carriers (Acevedo et al., 2010; Bloss et al., 2008; Calderon-Garciduenas et al., 2016).
Mixed findings regarding cognitive effects of the ε4 allele in younger persons likely relate to methodological variability between research studies. In a review of the literature, Tuminello and Han (2011) discuss the implications of some studies including high-risk groups in their samples and suggest that accounting for additional variables that can affect cognition is an important factor that can impact study results. For example, other AD risk factors such as family history of AD (e.g., see Bloss et al., 2008) and presence of other AD-related genes (e.g., Green et al., 2014) may interact with APOE genotype to impact cognition. Additionally, studies vary with regards to their definition of young ε4 and non-ε4 carriers, with some examining very wide age ranges (e.g., Stening et al., 2016; Suri et al., 2015) and others including restricted ranges (Bunce et al., 2011, 2014; Dell’Acqua et al., 2015). This can potentially be an important source of variability if the ε4 allele exerts a beneficial effect on cognition during a restricted time period in early life (Tuminello and Han, 2011).
An additional source of variability between studies is classification of ε4 and non-ε4 participants. While some studies exclude ε4 carriers who also possess the ε2 allele (e.g., Calderon-Garciduenas et al., 2016; Dennis et al., 2010; Filbey et al., 2006; Jorm et al., 2007), others do not (e.g., Acevedo et al., 2010; Luciano et al., 2009; Marchant et al., 2010; Puttonen et al., 2003; Richter-Schmidinger et al., 2011; Schultz et al., 2008; Wright et al., 2003; Yu et al., 2000). The ε2 allele has been associated with reduced cognitive decline among healthy older persons (Farrer et al., 1997; Shinohara et al., 2016), reduced clinical and pathological progression in AD (Serrano-Pozo et al., 2015), and increased longevity and survival among older adults (Corder et al., 1996). It is therefore considered a protective factor against AD. The presence of both ε2 and ε4 alleles may have either opposing influences or synergistic effects in young age, depending on the role of the ε4 allele on cognition in young age. Thus, differential inclusion of ε2-ε4 heterozygotes may produce variability in findings across studies.
A final source of variability between studies relates to the specific neuropsychological tests and cognitive domains under investigation. One possibility is that the ε4 allele confers benefits in some domains of cognition but not in others due to a differential influence of the allele on underlying neural systems. Han and Bondi (2008) suggest that benefits on cognitive tasks in young ε4 carriers may be mediated by increased recruitment of frontal-executive neural networks. This is supported by imaging work implicating the frontal-executive system as a focus of compensatory recruitment in healthy older ε4 carriers (Bondi et al., 2005; Han and Bondi, 2008; Kukolja et al., 2010; Seidenberg et al., 2009; Tuminello and Han, 2011; Wierenga et al., 2010) and studies that provide evidence for increased recruitment of frontal systems in young ε4 carriers (Filbey et al., 2010, 2006). However, findings regarding frontal involvement in young and older ε4 carriers are mixed (Trachtenberg et al., 2012; Tuminello and Han, 2011). For example, some studies of young ε4 carriers do not support increased frontal system recruitment in young ε4 carriers, instead finding evidence for increased recruitment of task-related regions (e.g., Dennis et al., 2010; Filippini et al., 2009; Tuminello and Han, 2011 for review). To the degree that the ε4 allele results in increased recruitment of neural networks underlying specific cognitive functions, performance differences between ε4 and non-ε4 persons may arise for some cognitive domains but not others.
A recent meta-analysis by Ihle and colleagues (Ihle et al., 2012) sought to integrate findings across studies reporting on associations between APOE ε4 and cognition in younger persons. The authors did not find an association between presence of the ε4 allele and cognition in persons between the ages of 5 and 35. Based on a potential association between the ε4 allele and frontal-executive networks (Han and Bondi, 2008), the authors also conducted post-hoc analyses to investigate whether tasks requiring increased executive demands would moderate the association between possession of the ε4 allele and performance on cognitive measures. Findings were non-significant. The authors conclude that the antagonistic pleiotropy hypothesis of APOE ε4 should be treated with caution.
Findings of Ihle et al. (2012) are informative and important. However, for several reasons, an updated meta-analysis is needed. Most relevant is the fact that new studies have been published since the 2012 meta-analysis. Additionally, although Ihle et al. investigated moderating effects of executive demands, the authors did not specifically examine other cognitive domains which may reveal associations with the ε4 allele and acknowledge this as a limiting factor in their study. Finally, Ihle and colleagues also analyzed studies that included ε2-ε4 heterozygote participants, which can introduce confounds due to well-established protective factors of the ε2 allele. Thus, the aim of the present meta-analysis is to update and extend findings of Ihle et al. with these considerations in mind. To this end, we embarked on a systematic literature review of studies that report associations between cognition and APOE in younger persons (infancy to age 40) and quantitatively integrated these findings using meta-analytical techniques across seven cognitive domains.
2. Methods
2.1. Literature search
This meta-analysis followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Liberati et al., 2009; Moher et al., 2009). In accordance with PRISMA guidelines, the project was registered with PROSPERO, the international prospective register of systematic reviews (registration number: CRD42017079478). The literature search was conducted on October 13, 2017 with no imposed date restriction. The search term “(APOE or Apolipoprotein e) and (cognition or cognitive function or neuropsychology or neuropsychological tests)” was applied to PubMed and PsychINFO databases, with APOE used as a common abbreviation for apolipoprotein E. Age limits were selected in the search engine menu in order to restrict results to the age range of interest. For PubMed, child (birth to 18) and adult, ages 19–44, were selected. For PsychInfo, we selected 30 s, young adulthood, adolescence, childhood, school age, preschool, infancy, and neonatal.
2.2. Inclusion/Exclusion criteria
Inclusion criteria were as follows: 1) human subject research, 2) participant age range of less than or equal to 40 years of age, 3) non-clinical samples (i.e., not meeting criteria for a medical or mental health condition that could impair cognition), 4) report of at least one neuropsychological or cognitive outcome measure, and 5) report of cognitive outcomes stratified by ε4 and non-ε4 groups. We excluded studies that 1) focused on animal research, 2) focused on another topic (e.g., brain injury, cancer), 3) were duplicate studies, 4) did not report data separately for APOE ε4 and non-ε4 groups, 5) included ε2-ε4 heterozygotes, 6) were not empirical, peer-reviewed research articles (e.g., dissertation, books, abstract only, conference presentations, case studies) or 6) lacked cognitive outcomes. The decision to exclude APOE-ε2 carriers from the ε4 group was made due to the potential confound of protective effects that are conferred by the allele (Farrer et al., 1997).
2.3. Data extraction and risk of bias
Two authors (GHW and SDH) independently reviewed all individual titles and abstracts of citations yielded from the search. Disagreements that arose for citations were discussed until an agreed-upon decision was reached. Full-text articles were downloaded and reviewed whenever there was a question regarding one of the selection criteria. Risk of bias was assessed at the study level for selective reporting, incomplete outcome data, quality of experimental design (e.g., inadequate sample size per genotype group, demographic considerations, IRB approval for research procedures), and undue influence of funding sources. Studies judged to indicate a potential risk of bias were excluded from review. Each article was reviewed by GHW and SDH for potential risk of bias with disagreements settled through discussion.
2.4. Data analysis
Outcomes in the present study were neuropsychological or cognitive test scores (e.g., experimental designs, fMRI tasks) stratified by genotype group (ε4 vs. non-ε4). The ε4 group included homozygotes of the ε4 allele as well as more commonly occurring ε3/ε4 heterozygotes. The non-ε4 group consisted of ε3 homozygotes, ε2 homozygotes, and ε2/3 heterozygotes. Tests were grouped into the following seven categories to assess for any specific effects by domain: achievement/intelligence, attention/working memory, executive functioning, language, memory, processing speed, and visuospatial abilities. To examine whether ε4 and non-ε4 groups differ across each of the seven domains, Hedges’ g (Hedges, 1981) were calculated with random effects models, which assume that the true effect size might differ from study to study. Thus, results are weighted based on study sample size, allowing inferences to be extended beyond the studies included in the meta-analysis (Hedges and Vevea, 1998). Statistical analyses were conducted using Comprehensive Meta Analysis (CMA) software, version 3.3.070 (Biostat, Englewood, NJ). Forest plots were visualized using CMA, and results were deemed significant at an alpha of p < .05. Follow-up analyses were conducted excluding studies that included sub-groups of ε4 and non-ε4 carriers with an additional risk factor for AD or cognitive impairment (e.g., positive family history of AD). As a quality control measure, if fewer than five studies were available for a particular analysis, the analysis was not conducted due to lack of adequate data. This was not the case for any of the analyses.
Heterogeneity, which refers to the variability or diversity of studies included in a systematic review, can impact the robustness and generalizability of the results (Higgins et al., 2003; Thompson, 1994). Heterogeneity was considered via statistical calculation of Q, Tau, Tau2, and I2. Q provides a measure of absolute heterogeneity of effects with a corresponding p-value (Cochran, 1954). Tau and Tau2 provide measures of the standard deviation and variance of true effects respectively (Borenstein et al., 2010), and provide a basis for comparison across studies. I2 refers to a ratio of true effect variance to observed error variance (Higgins et al., 2003).
Table 1 lists the measures extracted from articles that were used in the present review according to each cognitive domain. Per meta-analysis convention, if more than one measure was reported in a single study for a given cognitive domain, outcomes were pooled and the mean effect size was used. If a study further subdivided ε4 and non-ε4 participants into subgroups based on a common factor (e.g., sex), each subgroup was considered to be a separate data point.
Table 1.
List of cognitive measures by domain.
| Domain | Abbreviation | Test |
|---|---|---|
| Achievement/Intelligence | ||
| CAT-6 Language | California Achievement Test-6 - Language | |
| CAT-6 Math | California Achievement Test-6 - Math | |
| CAT-6 Reading | California Achievement Test-6 - Reading | |
| CAT-6 Spelling | California Achievement Test-6 - Spelling | |
| CDIIT Global Score | Comprehensive Development Inventory for Infants and Toddlers - Global Score | |
| HAWIE-R IQ | Hamburg Wechsler Intelligence Scale Revised Test - Full Scale IQ | |
| IQ | Unknown sourcea | |
| MWTB-IQ | Mehrfachwahl Wortschatz Intelligenz Test B - IQ | |
| NART | National Adult Reading Test - Total Words | |
| SRA Numeric ability | SRA Test of Educational Ability - Spanish, Numeric Ability | |
| SRA Reasoning ability | SRA Test of Educational Ability - Spanish, Reasoning Ability | |
| SRA Verbal ability | SRA Test of Educational Ability - Spanish, Verbal Ability | |
| WAIS Full-Scale iQ | Wechsler Adult Intelligence Scale (unspecified edition) - Full Scale IQ | |
| WISC-III Full-Scale IQ | Wechsler Intelligence Scale for Children Third Edition - Full Scale IQ | |
| WISC-IV Perceptual Reasoning Index | Wechsler Intelligence Scale for Children Fourth Edition - Perceptual Reasoning Index | |
| WISC-IV Verbal Comprehension Index | Wechsler Intelligence Scale for Children Fourth Edition - Verbal Comprehension Index | |
| WISC-R Performance IQ | Wechsler Intelligence Scale for Children Revised - Performance IQ | |
| WISC-R Verbal IQ | Wechsler Intelligence Scale for Children Revised - Verbal IQ | |
| Attention/Working Memory | ||
| 2-back | n-back task - 2-back accuracy | |
| 3-back | n-back task - 3-back accuracy | |
| CANTAB Spatial Span Length | Cambridge Neuropsychological Test Automated Battery - Spatial Span Length | |
| CANTAB Spatial Working Memory | Cambridge Neuropsychological Test Automated Battery - Spatial Working Memory | |
| IGD Working Memory Score | Inventory for Memory Diagnostics - Working Memory Score | |
| PMT cards sorted | Prospective Memory Task - number of cards sorted | |
| RVIP detections | Rapid Visual Information Processing Task - mean detections per minute | |
| RVIP false alarms | Rapid Visual Information Processing Task - false alarms | |
| TMT part A | Trail Making Test - Part A | |
| WAIS-R Arithmetic | Wechsler Adult Intelligence Scale Revised - Arithmetic | |
| WAIS-R Digit Span | Wechsler Adult Intelligence Scale Revised - Digit Span | |
| WAIS-R Orientation | Wechsler Adult Intelligence Scale Revised - Orientation | |
| WISC-R Arithmetic | Wechsler Intelligence Scale for Children Revised - Arithmetic | |
| WISC-R Digit Span | Wechsler Intelligence Scale for Children Revised - Digit Span | |
| WMS-III Digits Backward | Wechsler Memory Scale Third Edition - Digits Backward | |
| WMS-III Letter Number Span | Wechsler Memory Scale, Third Edition - Letter Number Span | |
| WMS-III Letter Number Span | Wechsler Memory Scale, Third Edition - Spatial Span | |
| WMS-III Spatial Span | Wechsler Memory Scale Revised - Backward Digit Span | |
| WMS-R Concentration/Attention Score | Wechsler Memory Scale Revised - Concentration and Attention Score | |
| Executive Functioning | ||
| CA validity effect | Covert Attention Task Validity Effect | |
| CANTAB set-shifting errors | Cambridge Neuropsychological Test Automated Battery - Intra-extra dimensional set shifting errors | |
| COWAT | Controlled Oral Word Association Test | |
| DKEFS Design Fluency switching | Delis-Kaplan Executive Function System - Design Fluency, switching score | |
| DKEFS Letter Fluency | Delis-Kaplan Executive Function System - Letter Fluency | |
| DKEFS TMT switching | Delis-Kaplan Executive Function System - Trail Making Test, switching score | |
| Kramer Card Sorting | Kramer Card Sorting - number of correct concepts | |
| MSIT Incongruent RT | Multi-Source Interference Task - Incongruent Trials RT | |
| Nonverbal Fluency | Nonverbal Fluency | |
| Stroop interference condition | Stroop interference condition | |
| TMT part B | Trail Making Test - Part B | |
| Verbal Fluency-FAS | Verbal Fluency - letters F-A-S | |
| Verbal Fluency-S words | Verbal Fluency - S words | |
| WISC-IV Similarities | Wechsler Intelligence Scale for Children Fourth Edition - Similarities | |
| WISC-IV Matrix Reasoning | Wechsler Intelligence Scale for Children Fourth Edition - Matrix Reasoning | |
| WISC-R Similarities | Wechsler Intelligence Scale for Children Revised - Similarities | |
| WISC-R Mazes | Wechsler Intelligence Scale for Children Revised - Mazes | |
| Language | ||
| BNT | Boston Naming Test | |
| CDIIT Language | Comprehensive Development Inventory for Infants and Toddlers - Language | |
| Spot-the-Word | Spot-the-Word Task | |
| SRB Synonyms | Dureman–Sälde battery - Synonyms | |
| WAIS-R Information | Wechsler Adult Intelligence Scale Revised - Information | |
| WISC-IV Vocabulary | Wechsler Intelligence Scale for Children Revised - Vocabulary | |
| WISC-R Comprehension | Wechsler Intelligence Scale for Children Revised - Comprehension | |
| WISC-R Information | Wechsler Intelligence Scale for Children Revised - Information | |
| Memory | ||
| AVLT total learning | Auditory-Verbal Learning Test - total learning | |
| AVLT long-term recall | Auditory-Verbal Learning Test - long-term recall | |
| BVMT-R total learning | Brief Visuospatial Memory Test Revised - total learning | |
| CANTAB Paired Associate Learning | Cambridge Neuropsychological Test Automated Battery - paired associate learning | |
| CANTAB Pattern Recognition | Cambridge Neuropsychological Test Automated Battery - paired recognition memory | |
| CANTAB Spatial Recognition | Cambridge Neuropsychological Test Automated Battery - spatial recognition memory | |
| Complex Figure Test recall | Complex Figure Test - recall | |
| CVLT I immediate Recall | California Verbal Learning Test, immediate recall | |
| CVLT I delayed Recall | California Verbal Learning Test, delayed recall | |
| CVLT-German immediate recall | California Verbal Learning Test - German, immediate recall | |
| CVLT-German trial 1 immediate recall | California Verbal Learning Test - German, Trial 1 immediate recall | |
| CVLT-German short delay recall | California Verbal Learning Test - German, short delay recall | |
| CVLT-German delayed recall | California Verbal Learning Test - German, delayed recall | |
| CVLT-II Trials 1-5 | California Verbal Learning Test-II - Trials 1-5 total learning | |
| CVLT-II long delay free recall | California Verbal Learning Test-II - long delay free recall | |
| Episodic Memory Task immediate recall | Episodic Memroy Task - immediate written recall (created by authors)b | |
| fMRI Face-Name immediate retrieval | fMRI Face-Name paradigm - immediate retrieval | |
| fMRI Face-Name delayed retrieval | fMRI Face-Name paradigm - delayed retrieval | |
| fMRI neutral scenes encoded | fMRI Neutral Scenes - percent encoded | |
| fMRI neutral scenes retrieved | fMRI Neutral Scenes - percent retrieved | |
| fMRI Picture Encoding false alarms | fMRI Picture Encoding Task - subsequent item memory false alarm rate | |
| fMRI Picture Encoding hits | fMRI Picture Encoding Task - subsequent item memory hit rate | |
| fMRI post-scan memory test | fMRI post-scan memory test - global performance | |
| fMRI Spatial Memory drop error | fMRI Spatial Memory Paradigm - degree of drop error | |
| IGD learning ability | Inventory for Memory Diagnostics - learning ability score | |
| IGD delayed recall | Inventory for Memory Diagnostics - delayed recall score | |
| PMT detections | Prospective Memory Task - detections | |
| VAT cued recall | Verbal Associative Learning Test – cued recall | |
| VAT recognition | Verbal Associative Learning Test – recognition | |
| WMS Logical Memory I | Wechsler Memory Scale (unknown version) - Logical Memory I | |
| WMS Logical Memory II | Wechsler Memory Scale (unknown version) - Logical Memory II | |
| WMS Visual Reproduction I | Wechsler Memory Scale (unknown version) - Visual Reproduction I | |
| WMS Visual Reproduction II | Wechsler Memory Scale (unknown version) - Visual Reproduction II | |
| WMS-R Verbal Memory | Wechsler Memory Scale Revised – Verbal Memory score | |
| WMS-R Visual Memory | Wechsler Memory Scale Revised – Visual Memory score | |
| WMS-R Delayed Recall | Wechsler Memory Scale Revised – Delayed Recall score | |
| WMS-R General Memory | Wechsler Memory Scale Revised – General Memory score | |
| WMS-R Logical Memory I | Wechsler Memory Scale Revised - Logical Memory I | |
| WMS-R Logical Memory II | Wechsler Memory Scale Revised - Logical Memory II | |
| Processing Speed | ||
| CANTAB Choice RT | Cambridge Neuropsychological Test Automated Battery - Choice RT | |
| CANTAB Rapid Visual Info Processing | Cambridge Neuropsychological Test Automated Battery - Rapid Visual Information Processing Task | |
| Simple RT | Simple RT | |
| Choice RT | Choice RT | |
| Letter Digit Substitution Test | Letter Digit Substitution Test | |
| MSIT Congruent Trials RT | Multi-Source Interference Task - congruent trials RT | |
| Processing speed composite | Sorting Task and Visual Attention Task - RT, combined z-scores | |
| Symbol-Digit Modalities Test | Symbol-Digit Modalities Test | |
| WISC-R Coding | Wechsler Intelligence Scale for Children Revised - Coding | |
| Visuospatial Abilities | ||
| Complex Figure Test Copy | Complex Figure Test - copy | |
| Luria Mental Rotation | Luria Mental Rotation | |
| RCFT Copy | Rey Complex Figure Test - copy | |
| WAIS-R Block Design | Wechsler Adult Intelligence Scale Revised - Block Design | |
| WISC-R Block Design | Wechsler Intelligence Scale for Children Fourth Edition - Block Design | |
| WISC-R Block Design | Wechsler Intelligence Scale for Children Revised - Block Design | |
| WISC-R Object Assembly | Wechsler Intelligence Scale for Children Revised - Object Assembly | |
| WISC-R Picture Arrangement | Wechsler Intelligence Scale for Children Revised - Picture Arrangement | |
| WISC-R Picture Completion | Wechsler Intelligence Scale for Children Revised - Picture Completion | |
Authors (Green et al., 2014; Shaw et al., 2007) do not cite source of IQ score.
This task was created by Dowell et al. (2013).
3. Results
3.1. Study selection
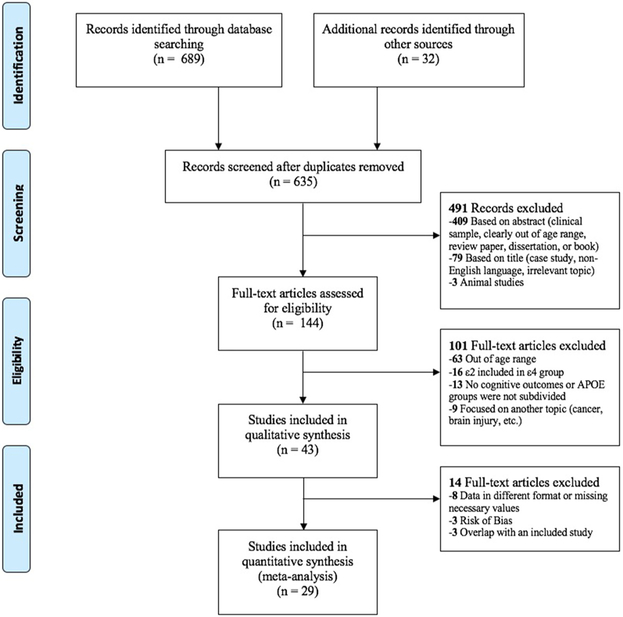
Search of databases yielded 689 records with an additional 32 records identified through other sources (e.g., reference lists of review articles). Following removal of duplicates, 635 records were screened by reviewing titles and abstracts. Of these, 79 were removed based on the title (e.g., case study, non-English language, irrelevant topic) and 412 were removed after reading the abstract (e.g., animal study, clinical sample, out of age range, review paper, dissertation, book). Thus, 144 full-text articles were assessed for eligibility and 101 were excluded following full-text review. Most of these studies (n = 63) were excluded for being outside of the pre-specified age range. These were studies in which the age range was unclear based on reading the abstract alone. Sixteen studies included ε2 carriers in the ε4 group, 13 did not report cognitive outcomes, and nine focused on APOE genotype in the context of a disease state or health condition (e.g., cancer, stroke, brain injury, cardiovascular disease). After these exclusions, 43 articles remained in the qualitative synthesis. Further assessment of records revealed three studies that met criteria for risk of bias, two due to small sample sizes per genotype group and one due to methodological concerns. Three studies were excluded because they reported identical data as other studies that were included in the quantitative synthesis. In these cases, the studies that provided more cognitive outcome data were selected. Ten articles reported data in a different format than the format necessary for the quantitative synthesis or were missing values (means and/or standard deviations) for relevant cognitive measures. In an attempt to include these studies, emails were sent to corresponding authors requesting data. Two authors responded by the date of preparation of this manuscript and were included in the final quantitative synthesis. In sum, 29 studies remained in the quantitative meta-analysis. Five of the 29 included studies subdivided genotype groups by other factors (e.g., sex) yielding a total of 34 data points to be included in the quantitative synthesis. Fig. 1 presents the flowchart for determining study inclusion into the meta-analysis.
Fig. 1.
PRISMA flow diagram.
3.2. APOE ε4 vs. APOE non-ε4
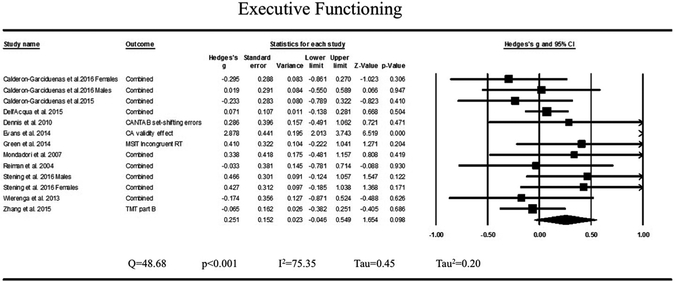
Demographic data per study are presented in Table 2. Comparing performance of ε4 and non-ε4 persons on measures across all domains combined revealed a summary effect size that did not significantly differ from zero (p = .98). Examining each of the seven domains separately also revealed no differences between groups for the domains of achievement/intelligence, attention/working memory, language, memory, processing speed, and visuospatial abilities (all ps ≥0.22). With regard to executive functioning, a marginal trend arose in which ε4 persons scored higher than non-ε4 persons (13 studies, Hg = .251, SEg = .152, 95% CI= −0.05– 0.56, p = .098). Heterogeneity was found to be in the high range for executive functioning (Q = 48.68, p < .001, I2 = 75.35, Tau = 0.45, Tau2 = 0.20). Fig. 2 presents the forest plot for the domain of executive functioning. Forest plots for all other domains can be visualized in Supplementary Materials (Figs. S1–S7).
Table 2.
Demographic data for participants in APOE ε4 versus APOE non-ε4 analysis.
| Study | Study Characteristics |
N |
Age |
Sex |
Education |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε4 | non-ε4 | ε4 |
non-ε4 |
ε4 | non-ε4 | ε4 |
non-ε4 |
Education Metric | |||||||
| Recruitment Age Range | Genotype Breakdown | M | SD | M | SD | %F | %F | M | SD | M | SD | ||||
| Alexopoulos et al. (2011) | College-aged | ε2/2, ε2/3 vs. ε3/4, ε4/4 | 16 | 17 | 24.20 | 4.10 | 24.70 | 3.20 | 56% | 71% | 16.56 | 2.07 | 17.35 | 2.69 | Years |
| Bloss et al. (2008) −familyhxa | 11-16 | ε2/3, 3/3 vs. ε3/4, ε4/4 | 24 | 85 | 13.42 | 1.40 | 13.42 | 1.22 | 50% | 56% | 16.46 | 2.17 | 16.35 | 2.09 | Mother-years |
| Bloss et al. (2008) +familyhxa | 11-16 | ε2/3, 3/3 vs. ε3/4, ε4/4 | 24 | 85 | 13.42 | 1.40 | 13.42 | 1.22 | 50% | 56% | 16.46 | 2.17 | 16.35 | 2.09 | Mother-years |
| Bloss et al. (2010) | 11-16 | ε3/3 vs. ε3/4, ε4/4 | 33 | 90 | 13.34 | 1.32 | 13.28 | 1.30 | 55% | 61% | 16.46 | 2.17 | 16.28 | 2.15 | Mother-years |
| Bunce et al. (2011) | 20-24 | ε3/3 vs. ε3/4, ε4/4 | 530 | 1291 | (20-24) | – | (20-24) | – | 50% | 53% | 14.56 | 1.59 | 14.56 | 1.55 | Years |
| Bunce et al. (2014) | 20-24 | ε2/2, ε2/3 vs. ε3/4, ε4/4 | 426 | 189 | (20-24) | – | (20-24) | – | – | – | – | – | – | – | – |
| Calderon-Garciduenas et al. (2016) Femalea | Children | ε3/3 vs. ε3/4 | 36 | 69 | 12.70 | 6.70 | 11.89 | 4.10 | 53% | 43% | 11.62 | 1.90 | 11.07 | 2.30 | Mother-years |
| Calderon-Garciduenas et al. (2016) Malesa | Children | ε3/3 vs. ε3/4 | 36 | 69 | 12.70 | 6.70 | 11.89 | 4.10 | 53% | 43% | 11.62 | 1.90 | 11.07 | 2.30 | Mother-years |
| Calderon-Garciduenas et al. (2015)) | Children | ε3/3 vs. ε3/4, ε4/4 | 22 | 28 | 13.62 | 4.80 | 13.36 | 5.00 | 50% | 43% | 11.62 | 1.74 | 11.07 | 2.12 | Mother-years |
| Dell’Acqua et al., 2015 | 14 year olds | ε3/3 vs. ε3/4 | 114 | 372 | 14.40 | 0.50 | 14.40 | 0.50 | 41% | 45% | – | – | – | – | – |
| Dennis et al. (2010) | Young Adult | ε2/3, ε3/3 vs. ε3/4, ε4/4 | 12 | 12 | 21.80 | 3.30 | 20.80 | 2.90 | 58% | 75% | – | – | – | – | – |
| Dowell et al. (2013) | 18-30 | ε3/3 vs. ε3/4, ε4/4 | 21 | 20 | 21.40 | 2.20 | 20.90 | 1.40 | 62% | 70% | – | – | – | – | – |
| Evans et al. (2014) | 18-30 | ε3/3 vs. ε3/4, ε4/4 | 21 | 20 | 21.40 | 2.20 | 20.90 | 1.40 | 62% | 70% | 15.10 | 0.20 | 15.10 | 0.30 | Years |
| Filippini et al. (2009) | 20-35 | ε3/3 vs. ε3/4, ε4/4 | 18 | 18 | 28.40 | 4.90 | 28.60 | 3.90 | 39% | 44% | 19.60 | 2.00 | 19.50 | 1.50 | Years |
| Green et al. (2014) CLU-C sampleb | College-aged | ε3/3 vs. ε3/4, ε4/4 | 23 | 16 | 24.00 | 5.49 | 25.25 | 5.87 | 17% | 6% | – | – | – | – | – |
| Jorm et al. (2007) | 20-24 | ε2/3, ε3/3 vs. ε3/4 | 517 | 1524 | (20-24) | – | (20-24) | – | – | – | – | – | – | – | – |
| Kunz et al. (2015) | 18-30 | ε3/3 vs. ε3/4 | 38 | 37 | 22.34 | 0.45 | 22.76 | 0.49 | 53% | 51% | 16.05 | 0.37 | 16.19 | 0.38 | Years |
| Matura et al. (2016) | 20-39 | ε3/3 vs. ε3/4, ε4/4 | 25 | 25 | 26.75 | 5.31 | 25.83 | 3.61 | 44% | 44% | 17.67 | 2.12 | 17.58 | 2.43 | Years |
| Matura et al. (2014) | 20-38 | ε3/3 vs. ε3/4, ε4/4 | 25 | 25 | 26.60 | 5.20 | 26.20 | 4.10 | 44% | 44% | 17.70 | 2.10 | 17.60 | 2.60 | Years |
| Mondadori et al. (2007) | Young Adult | ε2/3, ε3/3 vs. ε3/4, ε4/4 | 13 | 10 | 22.60 | 3.50 | 21.60 | 1.70 | 46% | 60% | 14.30 | 1.70 | 13.80 | 1.50 | Years |
| Ng et al. (2013) High Mercuryc | Age 2 | ε3/3 vs. ε3/4, ε4/4 | 14 | 59 | 2.00 | 0.00 | 2.00 | 0.00 | 50% | 39% | 50% | – | 57.6% | – | Mother %college edu |
| Ng et al. (2013) Low Mercuryc | Age 2 | ε3/3 vs. ε3/4, ε4/4 | 12 | 51 | 2.00 | 0.00 | 2.00 | 0.00 | 67% | 49% | 50% | – | 56.9% | – | Mother %college edu |
| Nichols et al. (2012) scanned cohort | 19-40 | ε3/3 vs. ε3/4 | 23 | 57 | 27.00 | 4.90 | 27.00 | 5.30 | 40% | 58% | 17.00 | 1.50 | 17.00 | 2.40 | Years |
| Nichols et al. (2012) non-scanned cohort | 18-40 | ε3/3 vs. ε3/4 | 48 | 122 | (18-40) | – | (18-40) | – | 67% | 54% | – | – | – | – | – |
| O’Dwyer et al. (2012) | 20-38 | ε3/3 vs. ε3/4, ε4/4 | 22 | 22 | 26.90 | 5.30 | 26.70 | 4.00 | 41% | 41% | 17.00 | 4.30 | 16.80 | 4.50 | Years |
| Reiman et al. (2004) | 20-39 | ε2/3, ε3/3 vs. ε3/4 | 12 | 15 | 30.70 | 5.40 | 31.20 | 5.00 | 75% | 80% | 16.00 | 1.70 | 16.10 | 1.50 | Years |
| Ruiz et al. (2010) | 13-18.5 | ε2/3, ε3/3 vs. ε3/4 | 76 | 336 | NA | NA | NA | NA | 49% | 51% | – | – | – | – | – |
| Shaw et al. (2007) | < 21yo | ε3/3 vs. ε3/4, ε4/4 | 65 | 145 | NA | NA | NA | NA | 51% | 42% | – | – | – | – | – |
| Sinclair et al. (2015) | 18 | ε3/3 vs. ε3/4 | 542 | 1215 | 18.00 | 0.00 | 18.00 | 0.00 | 51% | 56% | 23.2% | – | 20.9% | – | Mother % w/ degree |
| Stening et al. (2016) Female | 19-35 | ε3/3 vs. ε3/4, ε4/4 | 16 | 39 | 23.20 | 3.20 | 23.60 | 3.60 | 100% | 100% | 14.60 | 1.90 | 14.80 | 1.90 | Years |
| Stening et al. (2016) Male | 19-35 | ε3/3 vs. ε3/4, ε4/4 | 19 | 36 | 25.30 | 3.90 | 23.60 | 2.80 | 0% | 0% | 16.10 | 1.80 | 14.70 | 1.30 | Years |
| Suri et al. (2015) | 20-40 | ε3/3 vs. ε3/4, ε4/4 | 18 | 17 | 23.88 | 4.75 | 24.11 | 4.96 | 56% | 53% | 17.05 | 2.18 | 17.50 | 2.89 | Years |
| Wierenga et al. (2013) | College-aged | ε3/3 vs. ε3/4, ε4/4 | 15 | 15 | 23.60 | 3.10 | 23.30 | 3.00 | 80% | 53% | 14.90 | 0.30 | 15.00 | 0.50 | Years |
| Zhang et al. (2015) | 16-39 | ε3/3 vs. ε3/4, ε4/4 | 47 | 200 | 26.90 | 5.90 | 27.44 | 6.40 | 55% | 47% | 11.30 | 3.75 | 11.02 | 3.59 | Years |
Note: n = study sample size; sd = standard deviation, M = mean, edu = education, %F = percent female; ε4= at least one Apolipoprotein ε4 allele; non-ε4 = Apolipoprotein ε2 and/or ε3 carriers.
Study reports cognitive data separately by subgroup but demographics are reported for full sample.
Study examines CLU-C and non-CLU-C; only CLU-C was considered because CLU-nonC ε4 group only included three participants.
Study of toddlers who were exposed to mercury while in the womb.
Fig. 2.
Forest plot for domain of executive functioning. Difference in means reflects APOE ε4 carriers minus APOE non-ε4 carriers. Rhombus midpoint is Hedges’ g and the left and right points span the lower and upper limit.
3.3. Follow-up analysis
To investigate whether inclusion of “high-risk” groups would impact study findings, we re-analyzed data excluding the positive family history sub-sample from Bloss et al. (2008), the high prenatal mercury exposure sub-sample from Ng et al. (2013), and data from Green et al. (2014) who investigated differences between ε4 and non-ε4 carriers who also had the CLU-C genotype. This was relevant for the combined domains analysis and for analyses of achievement/intelligence, executive functioning, language, processing speed, and visuospatial abilities. Findings remain non-significant across all domains combined, and the domains of achievement/intelligence, language, processing speed, and visuospatial abilities after excluding data points reflecting high-risk sub-samples. For executive functioning, only the Green et al. (2014) study was removed. In doing so, the summary effect is no longer marginal (12 studies, Hg = .241, SEg = .162, 95% CI= −0.08–0.56, p = 0.14). Heterogeneity remains high (Q = 47.86, p < .001, I2 = 77.02, Tau = 0.47, Tau2 = 0.22).
4. Discussion
This meta-analysis examined associations between cognition and presence of the APOE ε4 allele in younger persons. Findings were nonsignificant, suggesting that in children and young adults, ε4 and non-ε4 carriers perform similarly on cognitive tests. Findings from the present meta-analysis converge with a previous meta-analysis conducted by Ihle et al. (2012), providing further support against the antagonistic pleiotropic hypothesis of the ε4 allele as originally proposed by Han and Bondi (2008).
Examining cognitive differences between ε4 and non-ε4 persons separately across each of seven cognitive domains allowed for interrogation of associations between APOE ε4 and specific areas of functioning. Based on fMRI research suggesting functional differences in executive-frontal neural networks in young and older ε4 carriers (for review see Han and Bondi, 2008; Tuminello and Han, 2011), we postulated whether specific cognitive domains would show differences above others. Findings were non-significant across all domains assessed including achievement/intelligence, attention/working memory, language, memory, executive functioning, processing speed, and visuospatial abilities. In regard to executive functioning, a non-significant marginal difference arose such that ε4 carriers outperformed non-ε4 carriers on measures of executive functioning. This finding diverges from Ihle et al. (2012), who approached the question differently. In their meta-analysis, Ihle et al. subdivided tasks into those involving high executive demands and those involving low executive demands and did not find evidence of differences between ε4 and non-ε4 carriers on tasks with high executive demands.
Executive functioning reflects a range of abilities (e.g., set-shifting, inhibition, decision making) that are assessed with a variety of different cognitive tests, but all are believed to be frontally mediated. Han and Bondi (2008) speculated as part of their antagonistic pleiotropy hypothesis that “frontal-executive cognitive processes might mediate the APOE ε4 advantage in youth and the compensatory mechanisms invoked later in life.” The marginal finding of better scores on measures of executive functioning in young ε4 carriers relative to non-ε4 carriers aligns well with this aspect of the antagonistic pleiotropy hypothesis initially proposed by Han & Bondi. However, for several reasons, we now caution against interpreting this marginal finding as support for the antagonistic pleiotropy hypothesis. First, evidence for associations between the ε4 allele and frontal-executive neural networks is inconclusive. While a small number of fMRI studies support increased recruitment of frontal executive networks in young ε4 carriers (Filbey et al., 2010, 2006), a larger group of studies instead report increased neural recruitment of regions specific to the administered task (Dennis et al., 2010; Filippini et al., 2009; Tuminello and Han, 2011). In their literature review and reassessment of the antagonistic pleiotropy hypothesis, Tuminello and Han (2011) suggest that the preponderance of studies supporting increased frontal recruitment in younger ε4 carriers is likely due to utilization of frontally mediated tasks (e.g., Filbey et al., 2010 used a working memory task). The authors propose a revision of the antagonistic pleiotropy hypothesis to account for these findings, suggesting that compensatory neural recruitment in young ε4 carriers occurs in task-related regions rather than frontally-mediated regions. Thus, our finding of better performance in the domain of executive functioning but not in other cognitive domains does not seem to be supported by fMRI studies or the most recent revision of the antagonistic pleiotropy hypothesis (Tuminello and Han, 2011).
A second reason to caution against over-interpretation of this marginal finding relates to the high heterogeneity statistics of this analysis. High heterogeneity in meta-analyses suggests that the variability between studies is not due only to chance but also to the measurement of different effects across studies (Higgins et al., 2003; Thompson, 1994). This limits the generalizability of findings of a meta-analysis (Higgins et al., 2003; Thompson, 1994), although some have suggested that certain heterogeneity estimates are less reliable with smaller sample sizes of studies (Huedo-Medina et al., 2006; Ioannidis et al., 2007; von Hippel, 2015). One potential reason for high heterogeneity in this domain relates to the broad range of abilities that fall under executive functioning and the difficulty of isolating such abilities due to lower order processes that are also necessary for completing executive tasks (i.e., task impurity; Miyake and Friedman, 2012). Nevertheless, in light of high heterogeneity and a marginal trend towards significance, the executive functioning finding reported in the present meta-analysis should be interpreted with extreme caution and necessitates replication, ideally with a larger sample of studies, in order to clarify whether this finding may represent a true effect. A possibility that remains to be addressed is the notion that the ε4 effect is specific to one component of executive functioning. Future studies may consider further subdividing executive tasks into component processes to investigate this possibility.
Overall, the present findings do not support an antagonistic pleiotropic effect of the ε4 allele as it relates to cognition in younger age ranges. One possibility that the present study cannot rule out is a differential effect of the ε4 allele on cognition later in the lifespan. Exaggerated cognitive decline in older ε4 carriers compared to non-ε4 carriers is well-established in the literature (see Tuminello and Han, 2011 for review). However, one study reported higher cognitive performances in oldest-old ε4 carriers compared to oldest-old non-ε4 carriers (Carrion-Baralt et al., 2009). Additionally, other studies do not find exaggerated cognitive decline in ε4 compared to non-ε4 carriers when examining oldest-old persons as is typically found in young-old carriers (Juva et al., 2000; Kozauer et al., 2008; Welsh-Bohmer et al., 2009), suggesting that the effect of ε4 on cognition is age specific. As a result of such findings, some have suggested that the ε4 allele may exhibit antagonistic pleiotropy effects particularly in young-old and old-old age (Carrion-Baralt et al., 2009; Tuminello and Han, 2011), with a negative impact on cognition in young-old individuals and a positive impact in the oldest old. Future research can examine this in greater detail.
Visual examination of forest plots highlights the variable effect sizes found across studies within each cognitive domain assessed, and estimated heterogeneity parameters confirm this observation (see Supplemental Materials). Qualitatively, studies differed across many factors and this may have contributed to the high level of inconsistency between studies. One source of variability between studies is the specific age range under investigation. While some studies imposed a very restrictive age range (e.g., 20–24 years old, Bunce et al., 2011, 2014; age 14, Dell’Acqua et al., 2015), others examined a much wider age range of young ε4 and non-ε4 carriers (e.g., 20–40 years old, Suri et al., 2015; 18–30 years old, Kunz et al., 2015). Tuminello and Han (2011) suggest that pleiotropic effects of the ε4 allele in younger persons may be restricted to a narrow age range, and thus assessing associations across a wide range of ages may contribute to inconsistencies between studies. To further explore this possibility, we examined the impact of age on differences between ε4 and non-ε4 groups for all measures combined. We did this by calculating a weighted average of the average ages provided for ε4 and non-ε4 groups. Five studies were excluded because they provided age ranges, rather than averages, in their sample characteristics. Age did not explain a significant portion of the variance in cognitive differences between ε4 and non-ε4 carriers (p = 0.99), arguing against pleiotropic effects specific to narrower age ranges. Due to small numbers of studies within each cognitive domain, we could not investigate age as a moderator for each cognitive domain separately due to the propensity of Type 1 errors in meta-regression analyses (Higgins and Thompson, 2004).
A second source of variability relates to the specific allele composition of ε4 and non-ε4 carriers assessed. Some studies included ε2 hetero- or homozygotes in their non-ε4 group (e.g., (Alexopoulos et al., 2011; Bloss et al., 2008; Dennis et al., 2010) while others only included ε3 homozygotes (e.g., Bloss et al., 2010; Matura et al., 2016, 2014; Wierenga et al., 2013). Although we excluded studies that included ε2-ε4 participants, we opted not to exclude studies that included ε2 carriers in their non-ε4 group due to the already small number of studies meeting criteria for inclusion in the meta-analysis. Differences between studies with regard to the non-ε4 groups may contribute to variability in findings across studies, especially given the protective effect on cognition associated with the ε2 allele (Corder et al., 1996; Farrer et al., 1997; Serrano-Pozo et al., 2015; Shinohara et al., 2016). Relatedly, we included both ε3-ε4 and ε4-ε4 participants in the ε4 group, also potentially introducing a source of variability to the findings. Examining a dose-response effect of the ε4 allele was not possible due to the small number of studies under consideration in the present meta-analysis.
A final important source of variability between studies is the decision by some studies to include high-risk subgroups of ε4 and non-ε4 carriers (e.g., family history of AD, Bloss et al., 2008; presence of CLU-C genotype, Green et al., 2014; prenatal mercury exposure, Ng et al., 2013). Inclusion of other factors that can contribute to cognitive differences between groups makes interpretation of associations between ε4 and cognition difficult (Tuminello and Han, 2011). Excluding the three studies (Bloss et al., 2008; Green et al., 2014; Ng et al., 2013) that included high-risk groups did not change the outcome of the majority of analyses. However, excluding Green et al. (2014) from the executive functioning analysis reduced the marginal effect to a null effect, further warranting cautious interpretation of this marginal finding.
High heterogeneity across studies is one limitation of this meta-analysis. Other limitations include the small number of studies meeting inclusionary criteria, highlighting the fact that studies examining the cognitive effects of the ε4 allele in healthy young persons are few and far between. A second related limitation is the lack of power to fully assess for moderating variables such as age and sex. This should be a consideration for future meta-analyses that aim to examine the relationship of the ε4 allele with cognition in younger persons. A final limitation is the wide age range considered in the study, ranging from toddlers to 40 year olds. This was unavoidable given the already small number of studies under consideration.
Findings from the meta-analysis largely do not support the ε4 allele as a pleiotropic gene, replicating findings of an earlier report by Ihle et al. (2012). The present meta-analysis also extends findings of Ihle et al. by showing that differences between young ε4 and non-ε4 carriers were null across all cognitive domains assessed including achievement/intelligence, attention/working memory, language, memory, processing speed, and visuospatial abilities. A marginal trend arose in which ε4 carriers outperformed non-ε4 carriers on measures of executive functioning, but further replication is needed in light of high heterogeneity between studies and a small number of studies considered. Importantly, high variability between studies reported in the present meta-analysis highlights the need for more research in this area, particularly with greater consistency in the parameters implemented across studies.
Supplementary Material
Acknowledgments
This work is supported by National Institute on Aging [grant number R01AG055430] awarded to SDH; and the Department of Family Medicine of the University of Southern California, as well as National Institute on Aging [grant numbers R21AG055034, P01AG052350, P50AG005142] and Alzheimer’s Association [grant number AARG-17-532905] awarded to DAN; National Institute on Aging [K24 AG026431 and R01 AG049810] to MWB; and the Department of Psychology, University of Southern California. Authors acknowledge the Department of Family Medicine of University of Southern California for support of this work.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.neubiorev.2018.08.009.
*Studies included in the meta-analysis are denoted with an asterisk.
References1
- Acevedo SF, Piper BJ, Craytor MJ, Benice TS, Raber J, 2010. Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children. Pediatr. Res. 67, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, 1993. Antagonistic pleiotropy, mutation accumulation, and human genetic disease. Genetica 91, 279–286. [DOI] [PubMed] [Google Scholar]
- Alexander DM, Williams LM, Gatt JM, Dobson-Stone C, Kuan SA, Todd EG, Schofield PR, Cooper NJ, Gordon E, 2007. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol. Psychol. 75, 229–238. [DOI] [PubMed] [Google Scholar]
- *Alexopoulos P, Richter-Schmidinger T, Horn M, Maus S, Reichel M, Sidiropoulos C, Rhein C, Lewczuk P, Doerfler A, Kornhuber J, 2011. Hippocampal volume differences between healthy young apolipoprotein E epsilon2 and epsilon4 carriers. J. Alzheimers Dis. 26, 207–210. [DOI] [PubMed] [Google Scholar]
- *Bloss CS, Delis DC, Salmon DP, Bondi MW, 2008. Decreased cognition in children with risk factors for Alzheimer’s disease. Biol. Psychiatry 64, 904–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bloss CS, Delis DC, Salmon DP, Bondi MW, 2010. APOE genotype is associated with left-handedness and visuospatial skills in children. Neurobiol. Aging 31, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Thal LJ, Saitoh T, 1995. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology 45, 2203–2206. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG, 2005. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW, 2000. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 343 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR, 2010. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1, 97–111. [DOI] [PubMed] [Google Scholar]
- *Bunce D, Anstey KJ, Burns R, Christensen H, Easteal S, 2011. Does possession of apolipoprotein E varepsilon4 benefit cognitive function in healthy young adults? Neuropsychologia 49, 1693–1697. [DOI] [PubMed] [Google Scholar]
- *Bunce D, Bielak AA, Anstey KJ, Cherbuin N, Batterham PJ, Easteal S, 2014. APOE genotype and cognitive change in young, middle-aged, and older adults living in the community. J. Gerontol. A Biol. Sci. Med. Sci. 69, 379–386. [DOI] [PubMed] [Google Scholar]
- *Calderon-Garciduenas L, Mora-Tiscareno A, Franco-Lira M, Zhu H, Lu Z, Solorio E, Torres-Jardon R, D’Angiulli A, 2015. Decreases in short term memory, IQ, and altered brain metabolic ratios in urban apolipoprotein epsilon4 children exposed to air pollution. J. Alzheimers Dis. 45, 757–770. [DOI] [PubMed] [Google Scholar]
- *Calderon-Garciduenas L, Jewells V, Galaz-Montoya C, van Zundert B, Perez-Calatayud A, Ascencio-Ferrel E, Valencia-Salazar G, Sandoval-Cano M, Carlos E, Solorio E, Acuna-Ayala H, Torres-Jardon R, D’Angiulli A, 2016. Interactive and additive influences of Gender, BMI and Apolipoprotein 4 on cognition in children chronically exposed to high concentrations of PM2.5 and ozone. APOE 4 females are at highest risk in Mexico City. Environ Res 150, 411–422. [DOI] [PubMed] [Google Scholar]
- Carrion-Baralt JR, Melendez-Cabrero J, Schnaider Beeri M, Sano M, Silverman JM, 2009. The neuropsychological performance of nondemented Puerto Rican nonagenarians. Dement. Geriatr. Cogn. Disord. 27, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG, 2004. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology 62, 1990–1995. [DOI] [PubMed] [Google Scholar]
- Cochran WG, 1954. The combination of estimates from different experiments. Biometrics 10, 101–129. [Google Scholar]
- Corder EH, Lannfelt L, Viitanen M, Corder LS, Manton KG, Winblad B, Basun H, 1996. Apolipoprotein E genotype determines survival in the oldest old (85 years or older) who have good cognition. Arch. Neurol. 53, 418–422. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whalley LJ, Clair DS, Breen G, Leaper S, Lemmon H, Hayward C, Starr JM, 2003. The influence of the ε4 allele of the apolipoprotein E gene on childhood IQ, nonverbal reasoning in old age, and lifetime cognitive change. Intelligence 31, 85–92. [Google Scholar]
- *Dell’Acqua F, Khan W, Gottlieb N, Giampietro V, Ginestet C, Bouls D, Newhouse S, Dobson R, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Conrod P, Flor H, Frouin V, Garavan H, Gowland P, Heinz A, Lemaitre H, Nees F, Paus T, Pausova Z, Rietschel M, Smolka MN, Strohle A, Gallinat J, Westman E, Schumann G, Lovestone S, Simmons A, consortium, I, 2015. Tract based spatial statistic reveals no differences in white matter microstructural organization between carriers and non-carriers of the APOE varepsilon4 and varepsilon2 alleles in young healthy adolescents. J. Alzheimers Dis. 47, 977–984. [DOI] [PubMed] [Google Scholar]
- Den Heijer T, Oudkerk M, Launer L, Van Duijn C, Hofman A, Breteler M, 2002. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology 59, 746–748. [DOI] [PubMed] [Google Scholar]
- *Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R, 2010. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement. 6, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dowell NG, Ruest T, Evans SL, King SL, Tabet N, Tofts PS, Rusted JM, 2013. MRI of carriers of the apolipoprotein E e4 allele-evidence for structural differences in normal-appearing brain tissue in e4+ relative to e4− young adults. NMR Biomed. 26, 674–682. [DOI] [PubMed] [Google Scholar]
- *Evans S, Dowell NG, Tabet N, Tofts PS, King SL, Rusted JM, 2014. Cognitive and neural signatures of the APOE E4 allele in mid-aged adults. Neurobiol. Aging 35, 1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM, 1997. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA 278, 1349–1356. [PubMed] [Google Scholar]
- Filbey FM, Slack KJ, Sunderland TP, Cohen RM, 2006. Functional magnetic resonance imaging and magnetoencephalography differences associated with APOEepsilon4 in young healthy adults. Neuroreport 17, 1585–1590. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Chen G, Sunderland T, Cohen RM, 2010. Failing compensatory mechanisms during working memory in older apolipoprotein E-epsilon4 healthy adults. Brain Imaging Behav. 4, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Filippini N, Macintosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE, 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U. S. A. 106, 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Green AE, Gray JR, Deyoung CG, Mhyre TR, Padilla R, Dibattista AM, William Rebeck G, 2014. A combined effect of two Alzheimer’s risk genes on medial temporal activity during executive attention in young adults. Neuropsychologia 56, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Bondi MW, 2008. Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimers Dement. 4, 251–254. [DOI] [PubMed] [Google Scholar]
- Hedges LV, 1981. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 6, 107–128. [Google Scholar]
- Hedges LV, Vevea JL, 1998. Fixed-and random-effects models in meta-analysis. Psychol. Methods 3, 486. [Google Scholar]
- Higgins JP, Thompson SG, 2004. Controlling the risk of spurious findings from meta-regression. Stat. Med. 23, 1663–1682. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG, 2003. Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J, 2006. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 11, 193–206. [DOI] [PubMed] [Google Scholar]
- Ihle A, Bunce D, Kliegel M, 2012. APOE epsilon4 and cognitive function in early life: a meta-analysis. Neuropsychology 26, 267–277. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Patsopoulos NA, Evangelou E, 2007. Uncertainty in heterogeneity estimates in meta-analyses. BMJ 335, 914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen HM, Muller M, van der Graaf Y, Geerlings MI, 2012. APOE epsilon4 differentially influences change in memory performance depending on age. The SMART-MR study. Neurobiol. Aging 33 (832), e815–822. [DOI] [PubMed] [Google Scholar]
- *Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S, 2007. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology 21, 1–8. [DOI] [PubMed] [Google Scholar]
- Juva K, Verkkoniemi A, Viramo P, Polvikoski T, Kainulainen K, Kontula K, Sulkava R, 2000. APOE epsilon4 does not predict mortality, cognitive decline, or dementia in the oldest old. Neurology 54, 412–415. [DOI] [PubMed] [Google Scholar]
- Kozauer NA, Mielke MM, Chan GK, Rebok GW, Lyketsos CG, 2008. Apolipoprotein E genotype and lifetime cognitive decline. Int. Psychogeriatr. 20, 109–123. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Eggermann T, Zerres K, Fink GR, 2010. Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE epsilon4 carriers. Neuroscience 168, 487–497. [DOI] [PubMed] [Google Scholar]
- *Kunz L, Schroder TN, Lee H, Montag C, Lachmann B, Sariyska R, Reuter M, Stirnberg R, Stocker T, Messing-Floeter PC, Fell J, Doeller CF, Axmacher N, 2015. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science 350, 430–433. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D, 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6, e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Gow AJ, Harris SE, Hayward C, Allerhand M, Starr JM, Visscher PM, Deary IJ, 2009. Cognitive ability at age 11 and 70 years, information processing speed, and APOE variation: the Lothian Birth Cohort 1936 study. Psychol. Aging 24, 129–138. [DOI] [PubMed] [Google Scholar]
- Marchant NL, King SL, Tabet N, Rusted JM, 2010. Positive effects of cholinergic stimulation favor young APOE epsilon4 carriers. Neuropsychopharmacology 35, 1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Matura S, Prvulovic D, Jurcoane A, Hartmann D, Miller J, Scheibe M, O’Dwyer L Oertel-Knochel V, Knochel C, Reinke B, Karakaya T, Fusser F, Pantel J, 2014. Differential effects of the ApoE4 genotype on brain structure and function. Neuroimage 89, 81–91. [DOI] [PubMed] [Google Scholar]
- *Matura S, Prvulovic D, Hartmann D, Scheibe M, Sepanski B, Butz M, Oertel-Knochel V, Knochel C, Karakaya T, Fusser F, Hattingen E, Pantel J, 2016. Age-related effects of the apolipoprotein E gene on brain function. J. Alzheimers Dis. 52, 317–331. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, 2012. The nature and organization of individual differences in executive functions: four general conclusions. Curr. Dir. Psychol. Sci. 21, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Boesiger P, Hock C, Nitsch RM, Papassotiropoulos A, Henke K, 2007. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb. Cortex 17, 1934–1947. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA, 2010. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 67, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K, 1991. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 541, 163–166. [DOI] [PubMed] [Google Scholar]
- *Ng S, Lin CC, Hwang YH, Hsieh WS, Liao HF, Chen PC, 2013. Mercury, APOE, and children’s neurodevelopment. Neurotoxicology 37, 85–92. [DOI] [PubMed] [Google Scholar]
- *Nichols LM, Masdeu JC, Mattay VS, Kohn P, Emery M, Sambataro F, Kolachana B, Elvevag B, Kippenhan S, Weinberger DR, Berman KF, 2012. Interactive effect of apolipoprotein e genotype and age on hippocampal activation during memory processing in healthy adults. Arch. Gen. Psychiatry 69, 804–813. [DOI] [PubMed] [Google Scholar]
- *O’Dwyer L, Lamberton F, Matura S, Scheibe M, Miller J, Rujescu D, Prvulovic D, Hampel H, 2012. White matter differences between healthy young ApoE4 carriers and non-carriers identified with tractography and support vector machines. PLoS One 7, e36024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttonen S, Elovainio M, Kivimaki M, Lehtimaki T, Keltikangas-Jarvinen L, 2003. The combined effects of apolipoprotein E polymorphism and low-density lipoprotein cholesterol on cognitive performance in young adults. Neuropsychobiology 48, 35–40. [DOI] [PubMed] [Google Scholar]
- *Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J, 2004. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc. Natl. Acad. Sci. U. S. A. 101, 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Schmidinger T, Alexopoulos P, Horn M, Maus S, Reichel M, Rhein C, Lewczuk P, Sidiropoulos C, Kneib T, Perneczky R, Doerfler A, Kornhuber J, 2011. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J. Neural. Transm. (Vienna) 118, 249–257. [DOI] [PubMed] [Google Scholar]
- *Ruiz JR, Castillo R, Labayen I, Moreno LA, Fuentes MG, Lamuno DG, Alvarez Granda JL, Lucia A, Ortega FB, Group AS, 2010. Individual and combined effects of ApoE and MTHFR 677C/T polymorphisms on cognitive performance in Spanish adolescents: the AVENA study. J. Pediatr. 156, 978–984 984 e971. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Evans SL, King SL, Dowell N, Tabet N, Tofts PS, 2013. APOE e4 polymorphism in young adults is associated with improved attention and indexed by distinct neural signatures. Neuroimage 65, 364–373. [DOI] [PubMed] [Google Scholar]
- Salvato G, 2015. Does apolipoprotein E genotype influence cognition in middle-aged individuals? Curr. Opin. Neurol. 28, 612–617. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. , 1993. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43, 1467–1472. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, 2006. Imaging studies and APOE genotype in persons at risk for Alzheimer’s disease. Curr. Psychiatry Rep. 8, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MR, Lyons MJ, Franz CE, Grant MD, Boake C, Jacobson KC, Xian H, Schellenberg GD, Eisen SA, Kremen WS, 2008. Apolipoprotein E genotype and memory in the sixth decade of life. Neurology 70, 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Antuono P, Zhang Q, Rao SM, 2009. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology 73, 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Qian J, Monsell SE, Betensky RA, Hyman BT, 2015. APOEepsilon2 is associated with milder clinical and pathological Alzheimer disease. Ann. Neurol. 77, 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, 2007. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 6, 494–500. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Kanekiyo T, Yang L, Linthicum D, Shinohara M, Fu Y, Price L, Frisch-Daiello JL, Han X, Fryer JD, Bu G, 2016. APOE2 eases cognitive decline during Aging: clinical and preclinical evaluations. Ann. Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Sinclair LI, Button KS, Munafo MR, Day IN, Lewis G, 2015. Possible association of APOE genotype with working memory in young adults. PLoS One 10, e0135894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L, 2004. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol. Aging 19, 592–600. [DOI] [PubMed] [Google Scholar]
- Soininen H, Partanen K, Pitkänen A, Hallikainen M, Hänninen T, Helisalmi S, Mannermaa A, Ryynänen M, Koiuisto K, Riekkinen P, 1995. Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E ε4 allele. Neurology 45, 391–392. [DOI] [PubMed] [Google Scholar]
- *Stening E, Persson J, Eriksson E, Wahlund LO, Zetterberg H, Soderlund H, 2016. Apolipoprotein E 4 is positively related to spatial performance but unrelated to hippocampal volume in healthy young adults. Behav. Brain Res. 299, 11–18. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD, 1993. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Suri S, Mackay CE, Kelly ME, Germuska M, Tunbridge EM, Frisoni GB, Matthews PM, Ebmeier KP, Bulte DP, Filippini N, 2015. Reduced cerebrovascular reactivity in young adults carrying the APOE epsilon4 allele. Alzheimers Dement. 11, 648–657 e641. [DOI] [PubMed] [Google Scholar]
- Thompson SG, 1994. Why sources of heterogeneity in meta-analysis should be investigated. BMJ 309, 1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg AJ, Filippini N, Mackay CE, 2012. The effects of APOE-epsilon4 on the BOLD response. Neurobiol. Aging 33, 323–334. [DOI] [PubMed] [Google Scholar]
- Tuminello ER, Han SD, 2011. The apolipoprotein e antagonistic pleiotropy hypothesis: review and recommendations. Int. J. Alzheimers Dis. 2011, 726197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel PT, 2015. The heterogeneity statistic I 2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 15, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh-Bohmer KA, Ostbye T, Sanders L, Pieper CF, Hayden KM, Tschanz JT, Norton MC, Cache Country Study G, 2009. Neuropsychological performance in advanced age: influences of demographic factors and Apolipoprotein E: findings from the Cache County Memory Study. Clin. Neuropsychol. 23, 77–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Stricker NH, McCauley A, Simmons A, Jak AJ, Chang YL, Delano-Wood L, Bangen KJ, Salmon DP, Bondi MW, 2010. Increased functional brain response during word retrieval in cognitively intact older adults at genetic risk for Alzheimer’s disease. Neuroimage 51, 1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wierenga CE, Clark LR, Dev SI, Shin DD, Jurick SM, Rissman RA, Liu TT, Bondi MW, 2013. Interaction of age and APOE genotype on cerebral blood flow at rest. J. Alzheimers Dis. 34, 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC, 2001. Pleiotropy, Natural Selection, and the Evolution of Senescence. Science’s SAGE KE 2001, 13. [Google Scholar]
- Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Bellinger D, Palazuelos E, Weiss ST, Hernandez-Avila M, 2003. Apolipoprotein E genotype predicts 24-month bayley scales infant development score. Pediatr. Res. 54, 819–825. [DOI] [PubMed] [Google Scholar]
- Yu YW, Lin CH, Chen SP, Hong CJ, Tsai SJ, 2000. Intelligence and event-related potentials for young female human volunteer apolipoprotein E epsilon4 and non-epsilon4 carriers. Neurosci. Lett. 294, 179–181. [DOI] [PubMed] [Google Scholar]
- *Zhang CC, Ren HY, Li ML, Wang Q, Deng W, Guo WJ, Lei W, Xiang B, Zhao LS, Ma XH, Yao YG, Li T, 2015. Apolipoprotein E gene polymorphisms associated with processing speed and executive functions in healthy Han Chinese. Neurosci. Bull. 31, 368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.