Abstract
Objectives:
We investigated the association of personal, reproductive, and familial characteristics with bilateral oophorectomy performed for nonmalignant indications in a US population.
Study design:
In an established cohort study, we used the records-linkage system of the Rochester Epidemiology Project (REP http://www.rochesterproject.org) to identify 1,653 premenopausal women who underwent bilateral oophorectomy in Olmsted County, Minnesota between 1988 and 2007 for a nonmalignant indication. Each woman was matched by age (±1 year) to a population-based referent woman who had not undergone bilateral oophorectomy as of the index date. We used case-control analyses to investigate several characteristics associated with bilateral oophorectomy. Odds ratios and their 95% confidence intervals were adjusted for ethnicity, education, and income.
Results:
In the overall analyses, infertility was more common in women who underwent bilateral oophorectomy than in the controls, whereas use of oral contraceptives, a history of breast feeding, and fibrocystic breast disease were less common. The women who underwent bilateral oophorectomy weighed more than controls, had a higher body mass index and were older at menarche. The associations were more pronounced for women who underwent the bilateral oophorectomy before age 46 years, and some associations were different for women with or without a benign ovarian indication. Reported family histories of uterine and other cancers were more common in women without a benign ovarian indication.
Conclusions:
We identified a number of personal, reproductive, and familial characteristics that were associated with bilateral oophorectomy over a 20-year period. Our historical findings may help inform decision-making about oophorectomy in the future.
Keywords: Bilateral oophorectomy, Family history, Reproductive history, Oral contraceptives, Smoking, Body mass index
1. Introduction
Bilateral oophorectomy continues to be performed in isolation or more commonly with hysterectomy in women before the age of natural menopause [1,2]. In the majority of cases, these surgeries are performed to treat nonmalignant gynecological symptoms or conditions. In addition, a large number of bilateral oophorectomies are performed at the time of a hysterectomy without a specific ovarian indication. For example, recent data from California suggest that approximately 38% of women undergo bilateral oophorectomy at the time of a hysterectomy in the absence of a documented ovarian condition [2]. This practice reflects the unresolved controversy about the advantages and disadvantages of removing healthy ovaries in premenopausal women for the prevention of ovarian and breast cancer [3–9]. In addition to a family history of ovarian cancer, intraoperative events, surgeons’ preferences, women’s preferences and past experiences, and social, reproductive, and familial factors may be involved in the decision to remove healthy ovaries [10,11]. We recently reported on the association between adverse childhood or adult experiences and the risk of bilateral oophorectomy [10,11]. However, other characteristics associated with bilateral oophorectomy have not been investigated extensively.
We conducted a case-control study to investigate the personal, reproductive, and familial characteristics associated with bilateral oophorectomy in the Mayo Clinic Cohort Study of Oophorectomy and Aging 2 (MOA-2). We report a series of case-control analyses contrasting premenopausal women who underwent bilateral oophorectomy to their respective age-matched controls in a geographically defined US population with a special focus on women who underwent oophorectomy at younger ages and on women who did not have a specified ovarian indication.
2. Methods
2.1. Study population
The overall study design and the clinical characteristics of the women included in the MOA-2 study were reported elsewhere [12–14]. In brief, MOA-2 included a cohort of premenopausal women who underwent bilateral oophorectomy for a nonmalignant indication, and a corresponding cohort of age-matched referent women. Both cohorts were representative of the geographically defined population of Olmsted County, Minnesota (USA) for the 20-year period 1988–2007. All data collection was through the records-linkage system of the Rochester Epidemiology Project (REP) that has been described elsewhere [15–18]. The women originally sampled to serve as exposed and referent women for the cohort analyses were re-labeled as cases and controls to be used in the case-control analyses reported here.
2.2. Data collection
A physician (LGR) and a trained nurse abstractor reviewed the medical records of all women who received a surgical code for unilateral or bilateral oophorectomy. For those women confirmed to have undergone surgery, detailed information about surgical characteristics was abstracted (e.g., indication for the surgery, pathology of the removed ovaries, and pathology of the removed uterus, if applicable). In addition, for both women with oophorectomy and their age-matched controls, the complete medical records were reviewed to collect an extensive series of demographic, social, and reproductive history data, and information about adult life characteristics and family history of cancer. Only characteristics documented before the index date were considered in the case-control analyses. Data were abstracted and recorded using an electronic data entry application. The application provided real time data checks (e.g., range of valid values), and comprehensive data checks were performed regularly during abstraction. To increase the consistency of the data collected, the two data abstractors followed a manual of instructions providing definitions and examples for the characteristics of interest. The manual was updated iteratively during the data collection phase. Information about income was derived from the 2000 United States Census (Summary File 3) [19]. Each woman was assigned the median household income for the census block group in which she lived at the index date.
2.3. Statistical analysis
Cases and controls were compared using conditional logistic regression models for matched pairs, and the associations were measured using odds ratios and 95% confidence intervals. Because sociodemographic characteristics were considered possible confounding variables [12], analyses for personal, reproductive, and familial characteristics were adjusted for race (white; non-white), education (≤12; 13–16; >16 years), and household income (quartiles: <$42,000; $42,000–56,999; $57,000–71,999; ≥$72,000). We conducted a set of analyses including the complete sample, and three sets of analyses stratified by age at the index date (≤45 years and 46–49 years), by indication (benign ovarian condition and no ovarian indication), and by calendar year (1988–1997 and 1998–2007). We also conducted a set of sensitivity analyses for the overall sample after excluding 165 case-control pairs in which the control had undergone hysterectomy before the index date and 24 pairs in which the case had not undergone hysterectomy as of the index date. All analyses were conducted using SAS v.9.4 (SAS Institute), and tests of statistical significance were conducted at the two-tailed alpha level of 0.05.
2.4. Ethical approval
All study procedures and ethical aspects were approved by the institutional review boards of both Mayo Clinic and Olmsted Medical Center. Because the data collection was historical, women did not need to provide a study-specific informed consent but rather a general consent to use their medical records for research (Minnesota legal requirements) [16,17].
3. Results
Supplementary Table 1 shows the results of case-control analyses for race, education, and income overall and in strata by age at oophorectomy and by indication for the oophorectomy. Non-white race was significantly less common in cases than controls overall and in all stratified analyses. However, the numbers for non-white women were small. Cases had significantly fewer years of education than controls overall, in the age stratum ≤45 years, and in women with a benign indication (with a dose-effect trend). Finally, cases had significantly lower income in women with age ≤45 years and in women with a benign indication (with a dose-effect trend).
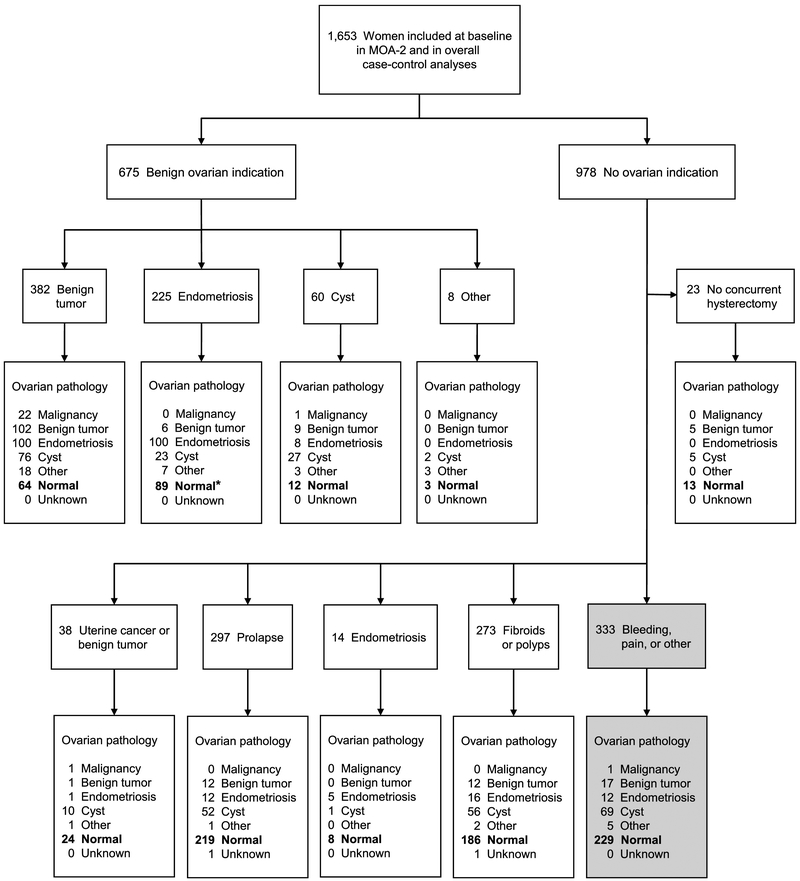
Figure 1 provides details about the indications for the oophorectomy and the pathological findings in the ovaries removed. Of the 1,653 pairs of ovaries removed for any indication, 847 (51.2%) were found to be healthy at pathological examination (bolded numbers). Interestingly, 333 women underwent removal of their ovaries and uterus in the absence of any recognized ovarian or uterine condition (shaded boxes in right lower corner). The only indication in these women was excessive bleeding or abdominal pain.
Fig. 1.
Flow chart of the indications for the bilateral oophorectomy and of the pathological findings in the ovaries removed. *In 89 women who underwent bilateral oophorectomy with an endometriosis indication, the ovaries were normal. However, in 45 of these women (50.6%), endometriosis was found elsewhere.
Table 1 shows the results of case-control analyses for personal characteristics in the overall sample after adjusting for race, education, and income. Infertility, higher weight (with a dose-effect trend), and higher body mass index (BMI; with a dose-effect trend) were more common in women who underwent bilateral oophorectomy. By contrast, older age at menarche (with a dose-effect trend), any use of oral contraceptives, longer use of oral contraceptives (with a dose-effect trend), history of breast feeding, and fibrocystic breast disease were less common in women who underwent bilateral oophorectomy (Table 1; all ages, all indications). The results were similar in a set of sensitivity analyses in which we removed controls who had undergone hysterectomy and cases who had not undergone hysterectomy as of the index date (data not shown).
Table 1.
Case-control analyses in the overall sample (all ages, all indications).
| Characteristica | Bilateral Oophorectomy (n=1,653) |
Control Women (n=1,653) |
Adjusted Odds Ratio (95% Cl)b |
p Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Age at menarchec | |||||||
| <12 | 147 | 9.8 | 135 | 10.0 | 1.00 (reference) | -- | |
| 12 | 548 | 36.7 | 458 | 34.0 | 1.17(0.87, 1.58) | 0.30 | |
| 13 | 572 | 38.3 | 488 | 36.3 | 1.06(0.79, 1.42) | 0.70 | |
| >13 | 226 | 15.1 | 265 | 19.7 | 0.79(0.57, 1.10) | 0.16 | |
| Oral contraceptive use (ever vs. never)d | 841 | 50.9 | 1,000 | 60.5 | 0.65 (0.57, 0.75) | <0.001 | |
| Years of oral contraceptive usec,e | |||||||
| 0 or <0.5 | 812 | 51.3 | 653 | 42.1 | 1.00 (reference) | -- | |
| 0.5–4.9 | 402 | 25.4 | 349 | 22.5 | 0.90(0.75, 1.08) | 0.26 | |
| ≥5.0 | 369 | 23.3 | 550 | 35.4 | 0.51 (0.43, 0.61) | <0.001 | |
| Gravidityc | |||||||
| 0d | 253 | 15.3 | 227 | 13.7 | 1.00 (reference) | -- | |
| 1 | 164 | 9.9 | 179 | 10.8 | 0.82(0.62, 1.09) | 0.17 | |
| 2 | 496 | 30.0 | 481 | 29.1 | 0.91 (0.73, 1.14) | 0.40 | |
| ≥3 | 740 | 44.8 | 766 | 46.3 | 0.86(0.70, 1.07) | 0.17 | |
| Twin or multiple gestationd | 42 | 2.5 | 33 | 2.0 | 1.25(0.79, 1.98) | 0.34 | |
| Pregnancy lossd | 550 | 33.3 | 531 | 32.1 | 1.07(0.92, 1.25) | 0.36 | |
| Induced abortiond,f | 92 | 5.6 | 118 | 7.1 | 0.77(0.58, 1.02) | 0.07 | |
| Spontaneous abortion or still birthd | 488 | 29.5 | 454 | 27.5 | 1.13(0.96, 1.32) | 0.13 | |
| Parityc | |||||||
| 0d | 313 | 18.9 | 279 | 16.9 | 1.00 (reference) | -- | |
| 1 | 194 | 11.7 | 216 | 13.1 | 0.80(0.62, 1.04) | 0.09 | |
| 2 | 619 | 37.4 | 595 | 36.0 | 0.91 (0.74, 1.11) | 0.35 | |
| ≥3 | 527 | 31.9 | 563 | 34.1 | 0.83(0.67, 1.02) | 0.08 | |
| Breast feeding | 493 | 40.8 | 655 | 51.2 | 0.68(0.56, 0.81) | <0.001 | |
| lnfertilityd | 292 | 17.7 | 164 | 9.9 | 1.95(1.59, 2.40) | <0.001 | |
| Fibrocystic breast diseased | 1,083 | 65.5 | 1,128 | 68.2 | 0.85(0.72, 1.00) | 0.047 | |
| Smoking statusc | |||||||
| Neverd | 897 | 54.3 | 957 | 57.9 | 1.00 (reference) | -- | |
| Former | 393 | 23.8 | 377 | 22.8 | 1.06(0.90, 1.26) | 0.49 | |
| Current | 363 | 22.0 | 319 | 19.3 | 1.15(0.96, 1.39) | 0.13 | |
| Pack-yearsc,e | |||||||
| 0 | 897 | 54.6 | 957 | 59.1 | 1.00 (reference) | -- | |
| 0.1–5.9 | 180 | 10.9 | 167 | 10.3 | 1.11 (0.88, 1.40) | 0.39 | |
| ≥6.0 | 567 | 34.5 | 495 | 30.6 | 1.16(0.99, 1.36) | 0.06 | |
| Height (cm)c,e | |||||||
| <161.0 | 509 | 30.8 | 529 | 32.1 | 1.00 (reference) | -- | |
| 161.0–166.9 | 602 | 36.4 | 579 | 35.2 | 1.04(0.87, 1.23) | 0.67 | |
| ≥167.0 | 542 | 32.8 | 538 | 32.7 | 1.01 (0.85, 1.21) | 0.92 | |
| Weight (kg)c,e | |||||||
| <64.0 | 455 | 27.5 | 573 | 35.0 | 1.00 (reference) | -- | |
| 64.0–78.9 | 556 | 33.6 | 564 | 34.5 | 1.23(1.03, 1.46) | 0.02 | |
| ≥79.0 | 642 | 38.8 | 499 | 30.5 | 1.58(1.32, 1.89) | <0.001 | |
| Body mass index (kg/m2)c | |||||||
| <25.0 | 596 | 36.1 | 700 | 42.9 | 1.00 (reference) | -- | |
| 25.0–29.9 | 481 | 29.1 | 488 | 29.9 | 1.17(0.99, 1.39) | 0.07 | |
| ≥30.0 | 576 | 34.8 | 442 | 27.1 | 1.51 (1.27, 1.79) | <0.001 | |
Women with missing or unknown data were not included in the respective analysis for the following characteristics: 467 for age at menarche (160 cases and 307 controls), 171 for years of birth control use (70 cases and 101 controls), 817 for breast feeding status (444 cases and 373 controls), 43 for smoking pack-years (9 cases and 34 controls), and 23 women for height, weight, and/or body mass index (0 cases and 23 controls).
The odds ratios and confidence intervals for each characteristic were calculated using conditional logistic regression models (matched pairs) adjusted for years of education (≤12; 13–16; >16), race (white; non-white) and quartiles of household income (<$42,000; $42,000–56,999; $57,000–71,999; ≥$72,000). Women with unknown education (3 cases and 35 controls) were assigned to the ≤12 years group, and women with unknown income (6 cases and 2 controls) were assigned to the $42,000–56,999 group (second quartile).
Ordinal characteristics were also tested for dose-effect (i.e., linear) trends adjusted for years of education, race, and household income: age at menarche (p=0.02), years of oral contraceptive use (p<0.001), gravidity (p=0.31), parity (p=0.18), smoking status (p=0.13), pack-years (p=0.06), height (p=0.93), weight (p<0.001), and body mass index (p<0.001).
For these characteristics, women with missing or unknown data were included in the no exposure category.
Years of oral contraceptive use, smoking pack-years, height, and weight were stratified using tertiles calculated from the overall sample (case-control groups pooled).
We may have underestimated induced abortions performed outside of the medical care facilities included in the REP.
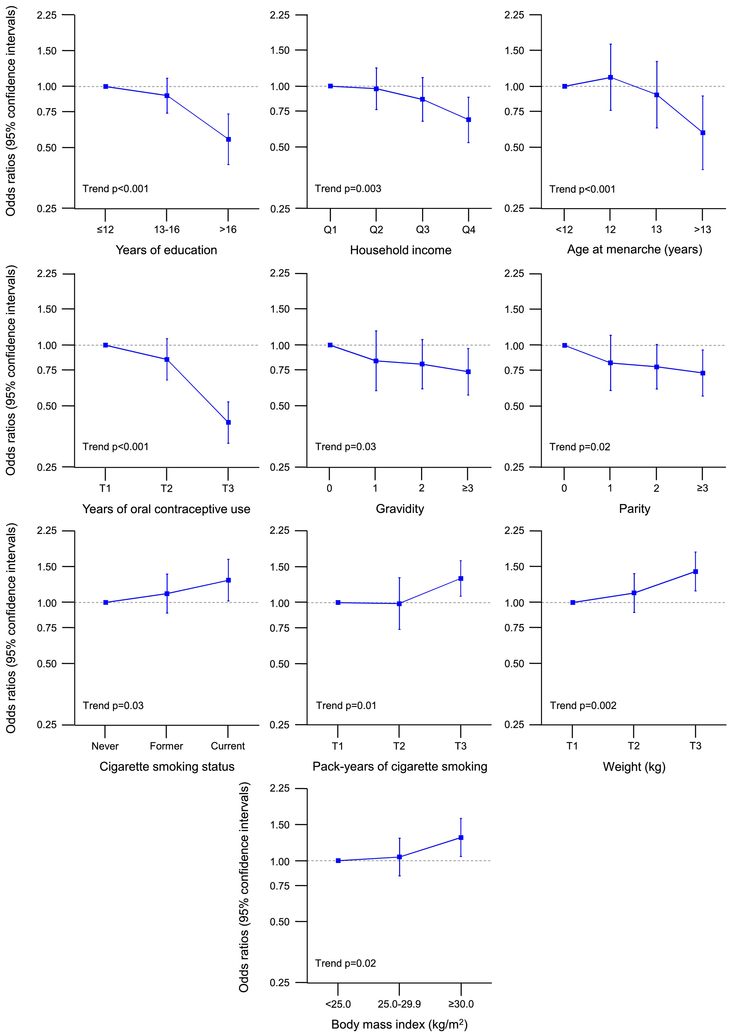
Table 2 shows case-control analyses for bilateral oophorectomy stratified by age at the time of oophorectomy (or index date; all indications). The median age at oophorectomy was 41 years (IQR, 38–44) for the ≤45 year stratum and 47 years (IQR, 47–48) for the 46–49 year stratum. The associations were more pronounced for the younger stratum, and some characteristics were significantly associated with bilateral oophorectomy only in the younger stratum. In particular, any oral contraceptive use, older age at menarche (with a dose-effect trend), ≥3 pregnancies (with a dose-effect trend), ≥3 live births (with a dose-effect trend), any induced abortion, breast feeding, and fibrocystic breast disease were less common, whereas infertility and smoking (with a dose-effect trend) were more common in cases than in controls only in the younger age stratum. By contrast, higher weight and higher BMI (with a dose-effect trend) were more common and longer use of oral contraceptives were less common in both age strata. Figure 2 shows the dose-effect trend analyses in women who underwent bilateral oophorectomy before age 46 years (only significant trends are shown).
Table 2.
Case-control analyses stratified by age at oophorectomy (or index date; all indications).
| Age ≤45 Years | Age 46–49 Years | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | Bilateral Oophorectomy (n=1,031) |
Control Women (n=1,031) |
Adjusted Odds Ratio (95% Cl)b |
p Value | Bilateral Oophorectomy (n=622) |
Control Women (n=622) |
Adjusted Odds Ratio (95% Cl)b |
p Value | ||||||
| N | % | N | % | N | % | N | % | |||||||
| Age at menarchec | ||||||||||||||
| <12 | 102 | 10.6 | 81 | 9.7 | 1.00 (reference) | -- | 45 | 8.5 | 54 | 10.5 | 1.00 (reference) | -- | ||
| 12 | 350 | 36.3 | 262 | 31.5 | 1.11 (0.76, 1.61) | 0.60 | 198 | 37.4 | 196 | 38.2 | 1.30(0.78,2.15) | 0.31 | ||
| 13 | 377 | 39.1 | 312 | 37.5 | 0.91 (0.62, 1.32) | 0.62 | 195 | 36.9 | 176 | 34.3 | 1.30(0.80,2.12) | 0.29 | ||
| >13 | 135 | 14.0 | 178 | 21.4 | 0.59 (0.39, 0.90) | 0.01 | 91 | 17.2 | 87 | 17.0 | 1.30(0.75,2.24) | 0.35 | ||
| Oral contraceptive use (ever vs. never)d | 481 | 46.7 | 633 | 61.4 | 0.54(0.45, 0.65) | <0.001 | 360 | 57.9 | 367 | 59.0 | 0.91 (0.72, 1.16) | 0.46 | ||
| Years of oral contraceptive usec,e | ||||||||||||||
| 0 or <0.5 | 550 | 54.2 | 398 | 41.5 | 1.00 (reference) | -- | 262 | 46.1 | 255 | 43.0 | 1.00 (reference) | -- | ||
| 0.5–4.9 | 258 | 25.4 | 215 | 22.4 | 0.85(0.67, 1.07) | 0.17 | 144 | 25.4 | 134 | 22.6 | 1.03(0.75, 1.42) | 0.86 | ||
| ≥5.0 | 207 | 20.4 | 346 | 36.1 | 0.42 (0.33, 0.52) | <0.001 | 162 | 28.5 | 204 | 34.4 | 0.72 (0.54, 0.96) | 0.03 | ||
| Gravidityc | ||||||||||||||
| 0d | 182 | 17.7 | 151 | 14.6 | 1.00 (reference) | -- | 71 | 11.4 | 76 | 12.2 | 1.00 (reference) | -- | ||
| 1 | 118 | 11.4 | 117 | 11.3 | 0.84(0.60, 1.17) | 0.30 | 46 | 7.4 | 62 | 10.0 | 0.79(0.48, 1.32) | 0.37 | ||
| 2 | 299 | 29.0 | 297 | 28.8 | 0.80(0.61, 1.06) | 0.13 | 197 | 31.7 | 184 | 29.6 | 1.10(0.74, 1.63) | 0.63 | ||
| ≥3 | 432 | 41.9 | 466 | 45.2 | 0.74(0.57, 0.96) | 0.02 | 308 | 49.5 | 300 | 48.2 | 1.11 (0.76, 1.61) | 0.59 | ||
| Twin or multiple gestationd | 22 | 2.1 | 19 | 1.8 | 1.14(0.61, 2.14) | 0.67 | 20 | 3.2 | 14 | 2.3 | 1.36(0.68,2.72) | 0.38 | ||
| Pregnancy lossd | 344 | 33.4 | 343 | 33.3 | 0.99(0.82, 1.20) | 0.92 | 206 | 33.1 | 188 | 30.2 | 1.18(0.92, 1.50) | 0.20 | ||
| Induced abortiond, f | 62 | 6.0 | 88 | 8.5 | 0.66 (0.46, 0.93) | 0.02 | 30 | 4.8 | 30 | 4.8 | 1.03(0.61, 1.73) | 0.93 | ||
| Spontaneous abortion or still birthd | 303 | 29.4 | 287 | 27.8 | 1.07(0.88, 1.30) | 0.51 | 185 | 29.7 | 167 | 26.8 | 1.18(0.91, 1.52) | 0.20 | ||
| Parityc | ||||||||||||||
| 0d | 224 | 21.7 | 185 | 17.9 | 1.00 (reference) | -- | 89 | 14.3 | 94 | 15.1 | 1.00 (reference) | -- | ||
| 1 | 140 | 13.6 | 142 | 13.8 | 0.82(0.60, 1.12) | 0.21 | 54 | 8.7 | 74 | 11.9 | 0.78(0.49, 1.26) | 0.32 | ||
| 2 | 375 | 36.4 | 383 | 37.1 | 0.78(0.61, 1.01) | 0.06 | 244 | 39.2 | 212 | 34.1 | 1.19(0.83, 1.70) | 0.34 | ||
| ≥3 | 292 | 28.3 | 321 | 31.1 | 0.73 (0.56, 0.95) | 0.02 | 235 | 37.8 | 242 | 38.9 | 1.04(0.73, 1.50) | 0.81 | ||
| Breast feeding | 280 | 37.4 | 434 | 53.4 | 0.49 (0.38, 0.63) | <0.001 | 213 | 46.2 | 221 | 47.2 | 1.02(0.76, 1.37) | 0.88 | ||
| Infertilityd | 214 | 20.8 | 98 | 9.5 | 2.56(1.96, 3.34) | <0.001 | 78 | 12.5 | 66 | 10.6 | 1.17(0.82, 1.65) | 0.39 | ||
| Fibrocystic breast diseased | 1568 | 55.1 | 619 | 60.0 | 0.78 (0.64, 0.95) | 0.01 | 515 | 82.8 | 509 | 81.8 | 1.04(0.78, 1.39) | 0.81 | ||
| Smoking statusc | ||||||||||||||
| Neverd | 544 | 52.8 | 611 | 59.3 | 1.00 (reference) | -- | 353 | 56.8 | 346 | 55.6 | 1.00 (reference) | -- | ||
| Former | 222 | 21.5 | 212 | 20.6 | 1.11 (0.89, 1.38) | 0.37 | 171 | 27.5 | 165 | 26.5 | 0.99(0.75, 1.31) | 0.96 | ||
| Current | 265 | 25.7 | 208 | 20.2 | 1.29(1.02, 1.63) | 0.04 | 98 | 15.8 | 111 | 17.8 | 0.87(0.63, 1.21) | 0.41 | ||
| Pack-yearsc,e | ||||||||||||||
| 0 | 544 | 53.0 | 611 | 60.3 | 1.00 (reference) | -- | 353 | 57.1 | 346 | 57.1 | 1.00 (reference) | -- | ||
| 0.1–5.9 | 104 | 10.1 | 115 | 11.4 | 0.99(0.74, 1.32) | 0.93 | 76 | 12.3 | 52 | 8.6 | 1.38(0.92,2.05) | 0.12 | ||
| ≥6.0 | 378 | 36.8 | 287 | 28.3 | 1.31 (1.07, 1.60) | 0.008 | 189 | 30.6 | 208 | 34.3 | 0.91 (0.70, 1.18) | 0.47 | ||
| Height (cm)c,e | ||||||||||||||
| <161.0 | 307 | 29.8 | 313 | 30.5 | 1.00 (reference) | -- | 202 | 32.5 | 216 | 34.8 | 1.00 (reference) | -- | ||
| 161.0–166.9 | 378 | 36.7 | 358 | 34.9 | 1.04(0.83, 1.30) | 0.71 | 224 | 36.0 | 221 | 35.6 | 1.02(0.77, 1.34) | 0.91 | ||
| ≥167.0 | 346 | 33.6 | 354 | 34.5 | 0.97(0.77, 1.21) | 0.79 | 196 | 31.5 | 184 | 29.6 | 1.06(0.78, 1.42) | 0.71 | ||
| Weight (kg)c,e | ||||||||||||||
| <64.0 | 315 | 30.6 | 376 | 36.7 | 1.00 (reference) | -- | 140 | 22.5 | 197 | 32.2 | 1.00 (reference) | -- | ||
| 64.0–78.9 | 331 | 32.1 | 350 | 34.2 | 1.11 (0.89, 1.39) | 0.34 | 225 | 36.2 | 214 | 35.0 | 1.49(1.11,2.01) | 0.008 | ||
| ≥79.0 | 385 | 37.3 | 298 | 29.1 | 1.42(1.14, 1.77) | 0.002 | 257 | 41.3 | 201 | 32.8 | 1.93(1.41,2.63) | <0.001 | ||
| Body mass index (kg/m2)c | ||||||||||||||
| <25.0 | 409 | 39.7 | 452 | 44.4 | 1.00 (reference) | -- | 187 | 30.1 | 248 | 40.6 | 1.00 (reference) | -- | ||
| 25.0–29.9 | 284 | 27.5 | 302 | 29.6 | 1.04(0.84, 1.29) | 0.71 | 197 | 31.7 | 186 | 30.4 | 1.45(1.08, 1.94) | 0.01 | ||
| >30.0 | 338 | 32.8 | 265 | 26.0 | 1.30(1.05, 1.61) | 0.02 | 238 | 38.3 | 177 | 29.0 | 1.94(1.45,2.60) | <0.001 | ||
Women with missing or unknown data were not included in the respective analysis for the following characteristics: 467 for age at menarche (67 cases age ≤45 and 198 controls; 93 cases age 46–49 and 109 controls), 171 for years of birth control use (16 cases age ≤45 and 72 controls; 54 cases age 46–49 and 29 controls), 817 for breast feeding status (283 cases age ≤45 and 219 controls; 161 cases age 46–49 and 154 controls), 43 for smoking pack-years (5 cases age ≤45 and 18 controls; 4 cases age 46–49 and 16 controls), and 23 women for height, weight and/or body mass index (0 cases age ≤45 and 12 controls; 0 cases age 46–49 and 11 controls).
The odds ratios and confidence intervals for each characteristic were calculated using conditional logistic regression models (matched pairs) adjusted for years of education (≤12; 13–16; >16), race (white; non-white) and quartiles of household income (<$42,000; $42,000–56,999; $57,000–71,999; ≥$72,000). Women with unknown education (2 cases age ≤45 and 23 controls; 1 case age 46–49 and 12 controls) were assigned to the ≤12 years group, and women with unknown income (5 cases age ≤45 and 2 controls; 1 case age 46–49 and 0 controls) were assigned to the $42,000–56,999 group (second quartile).
Ordinal characteristics were also tested for dose-effect (i.e., linear) trends adjusted for years of education, race, and household income: age at menarche (age ≤45 stratum p<0.001, age 46–49 stratum p=0.50), years of oral contraceptive use (age ≤45 stratum p<0.001, age 46–49 stratum p=0.03), gravidity (age ≤45 stratum p=0.03, age 46–49 stratum p=0.26), parity (age ≤45 stratum p=0.02, age 46–49 stratum p=0.41), smoking status (age ≤45 stratum p=0.03, age 46–49 stratum p=0.47), pack-years (age ≤45 stratum p=0.01, age 46–49 stratum p=0.54), height (age ≤45 stratum p=0.76, age 46–49 stratum p=0.72), weight (age ≤45 stratum p=0.002, age 46–49 stratum p<0.001), and body mass index (age ≤45 stratum p=0.02, age 46–49 stratum p<0.001).
For these characteristics, women with missing or unknown data were included in the no exposure category.
Years of oral contraceptive use, smoking pack-years, height, and weight were stratified using tertiles calculated from the overall sample (case-control groups pooled).
We may have underestimated induced abortions performed outside of the medical care facilities included in the REP.
Fig. 2.
Dose-effect trend analyses for women who underwent bilateral oophorectomy in the age ≤45 year stratum (all indications; Table 2 and Supplementary Table 1; only significant trends). The results are shown as odds ratios and 95% confidence intervals adjusted for race, education, and income (when applicable). Q1–Q4 = four quartiles; T1–T3 = three tertiles.
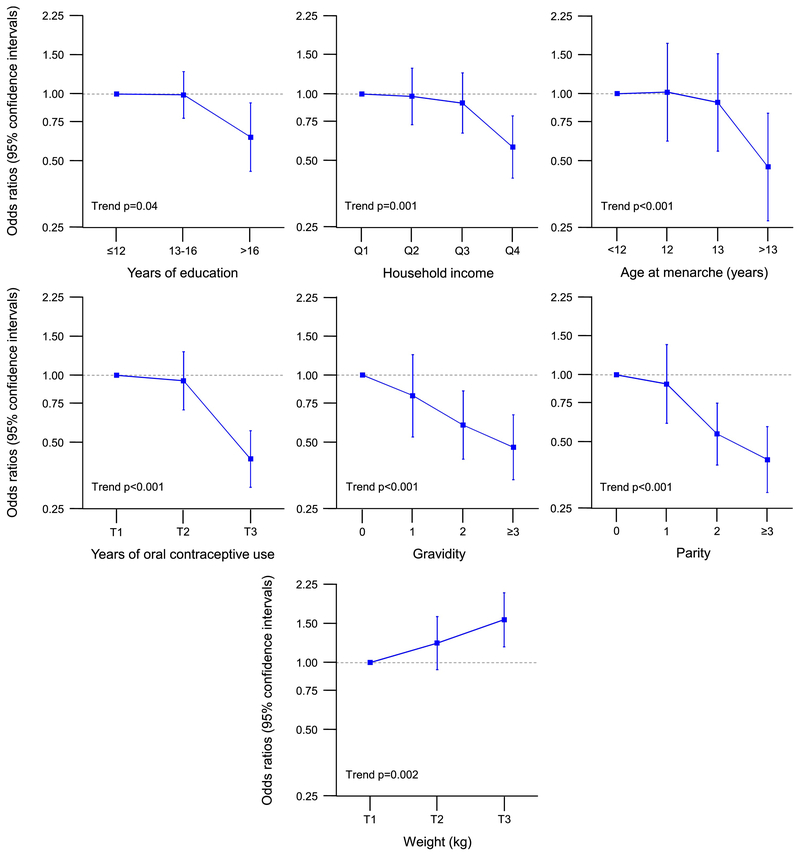
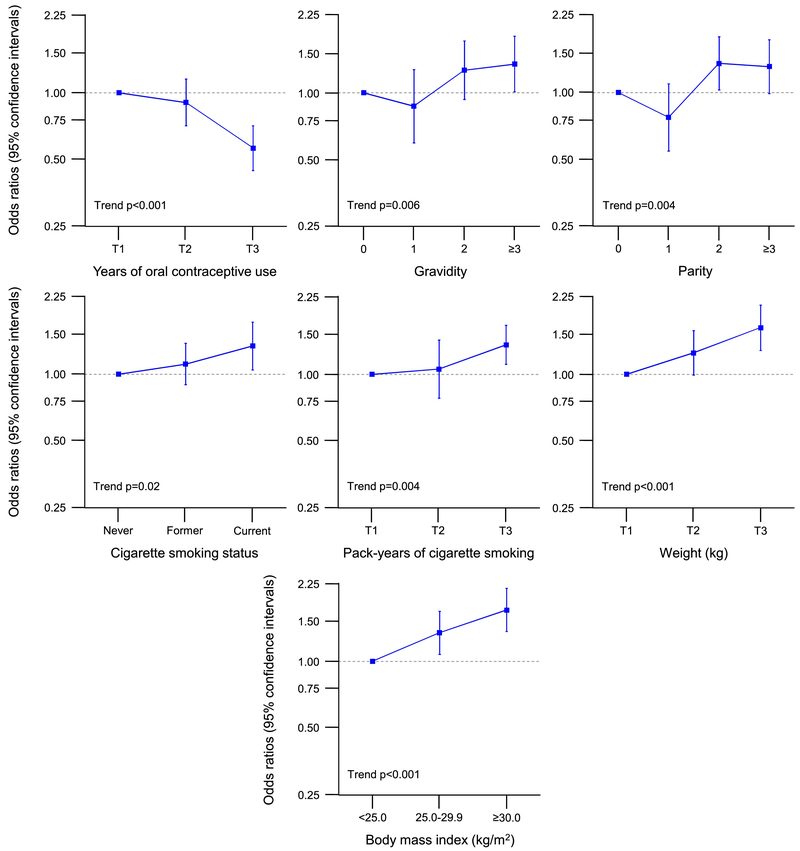
Table 3 shows case-control analyses for bilateral oophorectomy stratified by indication for the oophorectomy (all ages). Of the 675 women with a benign indication, 654 (96.9%) had a concurrent or preceding hysterectomy. Of the 978 women without an ovarian indication, 975 (99.7%) had a concurrent or preceding hysterectomy. Among 1,653 referent women, only 165 (10%) had concurrent or preceding hysterectomy. The women without an ovarian indication were similarly distributed in the younger (52.4%) and older stratum (47.7%) by age of bilateral oophorectomy. By contrast, women with an ovarian indication were more commonly in the younger age stratum (76.9%) than in the older age stratum (23.1%). Some results were different or in opposite directions in women who had a benign ovarian indication compared to women who did not have an ovarian indication for bilateral oophorectomy. Having ≥3 pregnancies was less common in women who underwent oophorectomy with a benign ovarian indication but more common in women without an ovarian indication. Infertility was more common in the benign indication stratum but not in the no indication stratum. Older age at menarche, history of breast feeding, and fibrocystic breast disease were less common in the benign condition stratum but not in the no indication stratum. Smoking and longer duration of smoking were more common in the no indication stratum but not in the benign indication stratum. By contrast, the associations for any use and longer use of oral contraceptives (less common) and higher weight (more common) were similar in the two indication strata (Table 3). Figure 3 shows the dose-effects trend analyses for women with a benign ovarian indication (all ages; only significant trends). Figure 4 shows the dose-effect trend analyses for women without an ovarian indication (all ages; only significant trends). Supplementary Table 2 shows case-control analyses for bilateral oophorectomy stratified by calendar year (all ages and all indications). The results were not noticeably different in the two strata.
Table 3.
Case-control analyses stratified by oophorectomy indication (all ages).
| Benign Indication | No Ovarian Indication | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | Bilateral Oophorectomy (n=675) |
Control Women (n=675) |
Adjusted Odds Ratio (95% Cl)b |
p Value | Bilateral Oophorectomy (n=978) |
Control Women (n=978) |
Adjusted Odds Ratio (95% Cl)b |
p Value | ||||||
| N | % | N | % | N | % | N | % | |||||||
| Age at menarchec | ||||||||||||||
| <12 | 54 | 8.8 | 47 | 8.6 | 1.00 (reference) | -- | 93 | 10.5 | 88 | 11.0 | 1.00 (reference) | -- | ||
| 12 | 227 | 37.2 | 178 | 32.4 | 1.02(0.61, 1.69) | 0.95 | 321 | 36.4 | 280 | 35.1 | 1.21 (0.83, 1.75) | 0.33 | ||
| 13 | 251 | 41.1 | 199 | 36.2 | 0.91 (0.55, 1.52) | 0.72 | 321 | 36.4 | 289 | 36.3 | 1.10(0.76, 1.59) | 0.61 | ||
| >13 | 79 | 12.9 | 125 | 22.8 | 0.47(0.27, 0.81) | 0.007 | 147 | 16.7 | 140 | 17.6 | 1.04(0.69, 1.57) | 0.84 | ||
| Oral Contraceptive use (ever vs. never)d |
317 | 47.0 | 404 | 59.9 | 0.58 (0.46, 0.73) | <0.001 | 524 | 53.6 | 596 | 60.9 | 0.71 (0.59, 0.85) | <0.001 | ||
| Years of oral contraceptive usec,e | ||||||||||||||
| 0 or <0.5 | 358 | 54.7 | 271 | 42.9 | 1.00 (reference) | -- | 454 | 48.9 | 382 | 41.5 | 1.00 (reference) | -- | ||
| 0.5–4.9 | 158 | 24.1 | 130 | 20.6 | 0.94(0.70, 1.28) | 0.71 | 244 | 26.3 | 219 | 23.8 | 0.90(0.71, 1.15) | 0.41 | ||
| ≥5.0 | 139 | 21.2 | 231 | 36.6 | 0.42(0.31, 0.56) | <0.001 | 230 | 24.8 | 319 | 34.7 | 0.56(0.45,0.71) | <0.001 | ||
| Gravidityc | ||||||||||||||
| 0d | 137 | 20.3 | 88 | 13.0 | 1.00 (reference) | -- | 116 | 11.9 | 139 | 14.2 | 1.00 (reference) | -- | ||
| 1 | 87 | 12.9 | 68 | 10.1 | 0.81 (0.52, 1.24) | 0.32 | 77 | 7.9 | 111 | 11.3 | 0.87(0.59, 1.27) | 0.47 | ||
| 2 | 205 | 30.4 | 205 | 30.4 | 0.59 (0.42, 0.85) | 0.004 | 291 | 29.8 | 276 | 28.2 | 1.26(0.93, 1.71) | 0.13 | ||
| ≥3 | 246 | 36.4 | 314 | 46.5 | 0.47 (0.34, 0.66) | <0.001 | 494 | 50.5 | 452 | 46.2 | 1.35(1.01, 1.80) | 0.04 | ||
| Twin or multiple qestationd | 16 | 2.4 | 14 | 2.1 | 1.09(0.52, 2.27) | 0.83 | 26 | 2.7 | 19 | 1.9 | 1.32(0.73,2.39) | 0.37 | ||
| Pregnancy lossd | 201 | 29.8 | 200 | 29.6 | 1.03(0.80, 1.31) | 0.83 | 349 | 35.7 | 331 | 33.8 | 1.11 (0.92, 1.35) | 0.28 | ||
| Induced abortiond, f | 35 | 5.2 | 50 | 7.4 | 0.67(0.42, 1.07) | 0.09 | 57 | 5.8 | 68 | 7.0 | 0.84(0.58, 1.21) | 0.35 | ||
| Spontaneous abortion or still birthd | 174 | 25.8 | 171 | 25.3 | 1.05(0.81, 1.36) | 0.70 | 314 | 32.1 | 283 | 28.9 | 1.18(0.97, 1.44) | 0.09 | ||
| Parityc | ||||||||||||||
| 0d | 167 | 24.7 | 108 | 16.0 | 1.00 (reference) | -- | 146 | 14.9 | 171 | 17.5 | 1.00 (reference) | -- | ||
| 1 | 104 | 15.4 | 74 | 11.0 | 0.91 (0.60, 1.37) | 0.65 | 90 | 9.2 | 142 | 14.5 | 0.77(0.54, 1.09) | 0.15 | ||
| 2 | 235 | 34.8 | 259 | 38.4 | 0.54(0.39, 0.75) | <0.001 | 384 | 39.3 | 336 | 34.4 | 1.35(1.03, 1.78) | 0.03 | ||
| ≥3 | 169 | 25.0 | 234 | 34.7 | 0.41 (0.29, 0.58) | <0.001 | 358 | 36.6 | 329 | 33.6 | 1.31 (0.99, 1.73) | 0.06 | ||
| Breast feeding | 165 | 34.1 | 278 | 52.1 | 0.40 (0.29, 0.55) | <0.001 | 328 | 45.2 | 377 | 50.5 | 0.89(0.70, 1.13) | 0.35 | ||
| Infertilityd | 166 | 24.6 | 55 | 8.1 | 3.77 (2.66, 5.33) | <0.001 | 126 | 12.9 | 109 | 11.1 | 1.17(0.89, 1.53) | 0.27 | ||
| Fibrocystic breast diseased | 352 | 52.1 | 420 | 62.2 | 0.59 (0.46, 0.75) | <0.001 | 731 | 74.7 | 708 | 72.4 | 1.14(0.91, 1.42) | 0.26 | ||
| Smoking statusc | ||||||||||||||
| Neverd | 376 | 55.7 | 386 | 57.2 | 1.00 (reference) | -- | 521 | 53.3 | 571 | 58.4 | 1.00 (reference) | -- | ||
| Former | 143 | 21.2 | 140 | 20.7 | 0.99(0.75, 1.32) | 0.96 | 250 | 25.6 | 237 | 24.2 | 1.11 (0.90, 1.38) | 0.34 | ||
| Current | 156 | 23.1 | 149 | 22.1 | 0.94(0.70, 1.26) | 0.67 | 207 | 21.2 | 170 | 17.4 | 1.34(1.05, 1.72) | 0.02 | ||
| Pack-yearsc, e | ||||||||||||||
| 0 | 376 | 56.0 | 386 | 58.1 | 1.00 (reference) | -- | 521 | 53.5 | 571 | 59.8 | 1.00 (reference) | -- | ||
| 0.1–5.9 | 78 | 11.6 | 65 | 9.8 | 1.22(0.84, 1.77) | 0.30 | 102 | 10.5 | 102 | 10.7 | 1.06(0.78, 1.43) | 0.73 | ||
| ≥6.0 | 217 | 32.3 | 213 | 32.1 | 0.90(0.70, 1.17) | 0.44 | 350 | 36.0 | 282 | 29.5 | 1.36(1.11, 1.66) | 0.003 | ||
| Height (cm)c, e | ||||||||||||||
| <161.0 | 189 | 28.0 | 207 | 30.8 | 1.00 (reference) | -- | 320 | 32.7 | 322 | 33.0 | 1.00 (reference) | -- | ||
| 161.0–166.9 | 255 | 37.8 | 259 | 38.6 | 1.07(0.81, 1.41) | 0.65 | 347 | 35.5 | 320 | 32.8 | 1.04(0.83, 1.30) | 0.72 | ||
| ≥167.0 | 231 | 34.2 | 205 | 30.6 | 1.20(0.90, 1.59) | 0.21 | 311 | 31.8 | 333 | 34.2 | 0.90(0.71, 1.14) | 0.38 | ||
| Weight (kg)c, e | ||||||||||||||
| <64.0 | 199 | 29.5 | 249 | 37.3 | 1.00 (reference) | -- | 256 | 26.2 | 324 | 33.4 | 1.00 (reference) | -- | ||
| 64.0–78.9 | 218 | 32.3 | 221 | 33.1 | 1.22(0.93, 1.61) | 0.15 | 338 | 34.6 | 343 | 35.4 | 1.25(0.99, 1.57) | 0.06 | ||
| ≥79.0 | 258 | 38.2 | 197 | 29.5 | 1.56(1.18, 2.07) | 0.002 | 384 | 39.3 | 302 | 31.2 | 1.62(1.28,2.05) | <0.001 | ||
| Body mass index (kg/m2)c | ||||||||||||||
| <25.0 | 275 | 40.7 | 294 | 44.3 | 1.00 (reference) | -- | 321 | 32.8 | 406 | 42.0 | 1.00 (reference) | -- | ||
| 25.0–29.9 | 177 | 26.2 | 197 | 29.7 | 0.97(0.74, 1.28) | 0.84 | 304 | 31.1 | 291 | 30.1 | 1.34(1.08, 1.68) | 0.009 | ||
| ≥30.0 | 223 | 33.0 | 173 | 26.1 | 1.31 (1.00, 1.71) | 0.051 | 353 | 36.1 | 269 | 27.8 | 1.70(1.36,2.13) | <0.001 | ||
Women with missing or unknown data were not included in the respective analysis for the following characteristics: 467 for age at menarche (64 cases with benign indication and 126 controls; 96 cases with no ovarian indication and 181 controls),171 for years of birth control use (20 cases with benign indication and 43 controls; 50 cases with no ovarian indication and 58 controls); 817 for breast feeding status (191 cases with benign indication and 141 controls; 253 cases with no ovarian indication and 232 controls); 43 for smoking pack-years (4 cases with benign indication and 11 controls; 5 cases with no ovarian indication and 23 controls); and 23 women for height, weight and/or body mass index (0 cases with benign indication and 11 controls; 0 cases with no ovarian indication and 12 controls).
The odds ratios and confidence intervals for each characteristic were calculated using conditional logistic regression models (matched pairs) adjusted for years of education (≤12; 13–16; >16), race (white; non-white) and quartiles of household income (<$42,000; $42,000–56,999; $57,000–71,999; ≥$72,000). Women with unknown education (3 cases with benign indication and 15 controls; 0 cases with no ovarian indication and 20 controls) were assigned to the ≤12 years group, and women with unknown income (3 cases with benign indication and 2 controls; 3 cases with no ovarian indication and 0 controls) were assigned to the $42,000–56,999 group (second quartile).
Ordinal characteristics were also tested for dose-effect (i.e., linear) trends adjusted for years of education, race, and household income: age at menarche (benign indication stratum p<0.001, no ovarian indication stratum p=0.72), years of oral contraceptive use (benign indication stratum p<0.001, no ovarian indication stratum p<0.001), gravidity (benign indication stratum p<0.001, no ovarian indication stratum p=0.006), parity (benign indication stratum p<0.001, no ovarian indication stratum p=0.004), smoking status (benign indication stratum p=0.69, no ovarian indication stratum p=0.02), pack-years (benign indication stratum p=0.51, no ovarian indication stratum p=0.004), height (benign indication stratum p=0.20, no ovarian indication stratum p=0.37), weight (benign indication stratum p=0.002, no ovarian indication stratum p<0.001), and body mass index (benign indication stratum p=0.06, no ovarian indication stratum p<0.001).
For these characteristics, women with missing or unknown data were included in the no exposure category.
Years of oral contraceptive use, smoking pack-years, height, and weight were stratified using tertiles calculated from the overall sample (case-control groups pooled).
We may have underestimated induced abortions performed outside of the medical care facilities included in the REP.
Fig. 3.
Dose-effect trend analyses for women who underwent bilateral oophorectomy with a benign ovarian indication (all ages; Table 3 and Supplementary Table 1; only significant trends). The results are shown as odds ratios and 95% confidence intervals adjusted for race, education, and income (when applicable). Q1–Q4 = four quartiles; T1–T3 = three tertiles.
Fig. 4.
Dose-effect trend analyses for women who underwent bilateral oophorectomy without an ovarian indication (all ages; Table 3 and Supplementary Table 1; only significant trends). The results are shown as odds ratios and 95% confidence intervals adjusted for race, education, and income (when applicable). T1–T3 = three tertiles.
Table 4 shows the case-control analyses for family history of cancer stratified by indication for the oophorectomy (all ages). Women with a benign ovarian indication had an increased frequency of reported family history of ovarian (first-degree relatives) and colorectal cancer (any relative). Women without a benign ovarian indication had an increased frequency of reported family history of uterine cancer (first-degree relatives), and other cancers (first-degree relatives).
Table 4.
Case-control analyses for family history of cancer stratified by oophorectomy indication (all ages).
| Benign Indication | No Ovarian Indication | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Type | Bilateral Oophorectomy (n=675) |
Control Women (n=675) |
Adjusted Odds Ratio (95% Cl)a |
p Value | Bilateral Oophorectomy (n=978) |
Control Women (n=978) |
Adjusted Odds Ratio (95% Cl)a |
p Value | ||||||
| N | % | N | % | N | % | N | % | |||||||
| Breast | ||||||||||||||
| Noneb | 531 | 78.7 | 536 | 79.4 | 1.00 (reference) | -- | 762 | 77.9 | 766 | 78.3 | 1.00 (reference) | -- | ||
| First degree | 85 | 12.6 | 84 | 12.4 | 0.99(0.72, 1.38) | 0.98 | 140 | 14.3 | 145 | 14.8 | 0.94(0.73, 1.21) | 0.65 | ||
| Other relative | 59 | 8.7 | 55 | 8.1 | 1.16(0.79, 1.72) | 0.45 | 76 | 7.8 | 67 | 6.9 | 1.10(0.78, 1.54) | 0.59 | ||
| Ovarian | ||||||||||||||
| Noneb | 622 | 92.1 | 645 | 95.6 | 1.00 (reference) | -- | 905 | 92.5 | 927 | 94.8 | 1.00 (reference) | -- | ||
| First degree | 33 | 4.9 | 16 | 2.4 | 1.89(1.03, 3.48) | 0.04 | 46 | 4.7 | 36 | 3.7 | 1.27(0.81, 1.99) | 0.30 | ||
| Other relative | 20 | 3.0 | 14 | 2.1 | 1.24(0.62, 2.48) | 0.55 | 27 | 2.8 | 15 | 1.5 | 1.81 (0.96,3.41) | 0.07 | ||
| Uterine | ||||||||||||||
| Noneb | 651 | 96.4 | 636 | 94.2 | 1.00 (reference) | -- | 916 | 93.7 | 939 | 96.0 | 1.00 (reference) | -- | ||
| First degree | 14 | 2.1 | 35 | 5.2 | 0.39(0.21, 0.72) | 0.003 | 50 | 5.1 | 28 | 2.9 | 1.83(1.15,2.92) | 0.01 | ||
| Other relative | 10 | 1.5 | 4 | 0.6 | 2.25 (0.67, 7.54) | 0.19 | 12 | 1.2 | 11 | 1.1 | 1.12(0.49,2.55) | 0.79 | ||
| Other cancers by relative (any other type) | ||||||||||||||
| Noneb | 416 | 61.6 | 500 | 74.1 | 1.00 (reference) | -- | 637 | 65.1 | 690 | 70.6 | 1.00 (reference) | -- | ||
| First degree | 176 | 26.1 | 114 | 16.9 | 1.76(1.34, 2.31) | <0.001 | 247 | 25.3 | 194 | 19.8 | 1.30(1.06, 1.61) | 0.01 | ||
| Other relative | 83 | 12.3 | 61 | 9.0 | 1.47(1.02, 2.11) | 0.04 | 94 | 9.6 | 94 | 9.6 | 1.02(0.75, 1.37) | 0.92 | ||
| Other cancers by type (any relative) | ||||||||||||||
| Other gynecologicc | 7 | 1.0 | 3 | 0.4 | 2.16(0.55, 8.48) | 0.27 | 8 | 0.8 | 5 | 0.5 | 1.58(0.51,4.86) | 0.43 | ||
| Male specificd | 38 | 5.6 | 38 | 5.6 | 0.94(0.58, 1.52) | 0.79 | 53 | 5.4 | 49 | 5.0 | 1.04(0.70, 1.56) | 0.84 | ||
| Lung | 53 | 7.9 | 36 | 5.3 | 1.41 (0.90, 2.19) | 0.13 | 72 | 7.4 | 54 | 5.5 | 1.36(0.95, 1.96) | 0.10 | ||
| Kidney | 8 | 1.2 | 0 | 0.0 | -- | -- | 3 | 0.3 | 9 | 0.9 | 0.32(0.09, 1.19) | 0.09 | ||
| Liver or biliary duct | 11 | 1.6 | 12 | 1.8 | 0.96(0.42, 2.21) | 0.93 | 14 | 1.4 | 7 | 0.7 | 1.99(0.80,4.94) | 0.14 | ||
| Colorectal | 83 | 12.3 | 41 | 6.1 | 2.26(1.51, 3.39) | <0.001 | 92 | 9.4 | 90 | 9.2 | 1.00(0.74, 1.35) | 1.00 | ||
| Other gastrointestinale | 10 | 1.5 | 11 | 1.6 | 0.85(0.34, 2.13) | 0.73 | 16 | 1.6 | 27 | 2.8 | 0.61 (0.33, 1.14) | 0.12 | ||
| Pancreas | 10 | 1.5 | 11 | 1.6 | 0.94(0.39, 2.26) | 0.89 | 18 | 1.8 | 12 | 1.2 | 1.44(0.69,3.01) | 0.33 | ||
| Hematologicf | 16 | 2.4 | 26 | 3.9 | 0.63(0.34, 1.20) | 0.16 | 50 | 5.1 | 34 | 3.5 | 1.46(0.94,2.28) | 0.09 | ||
| Sking | 31 | 4.6 | 23 | 3.4 | 1.32(0.76, 2.29) | 0.32 | 36 | 3.7 | 37 | 3.8 | 0.94(0.59, 1.50) | 0.81 | ||
| Miscellaneoush | 31 | 4.6 | 22 | 3.3 | 1.36(0.77, 2.41) | 0.29 | 52 | 5.3 | 38 | 3.9 | 1.31 (0.85,2.02) | 0.23 | ||
| Unknown type | 19 | 2.8 | 12 | 1.8 | 1.38(0.66, 2.88) | 0.39 | 8 | 0.8 | 11 | 1.1 | 0.68(0.27, 1.72) | 0.42 | ||
The odds ratios and confidence intervals for each cancer were calculated using conditional logistic regression models (matched pairs) adjusted for years of education (≤12; 13–16; >16), race (white; non-white), and quartiles of household income (<$42,000; $42,000–56,999; $57,000–71,999; ≥$72,000). Women with unknown education (3 cases with benign indication and 15 controls; 0 cases with no ovarian indication and 20 controls) were assigned to the ≤12 years group, and women with unknown income (3 cases with benign indication and 2 controls; 3 cases with no ovarian indication and 0 controls) were assigned to the $42,000–56,999 group (second quartile).
Includes women with unknown family history of cancer (14 cases with benign indication and 0 controls; 4 cases with no ovarian indication and 3 controls).
Cervical, fallopian tube, or vulvar cancer.
Prostate or testicular cancer.
Appendix, esophageal, or stomach cancer.
Leukemia, lymphoma or myeloma.
Melanoma or non-melanoma skin cancer.
Bladder, bone, brain, oral, thyroid, or other types of cancer.
4. Discussion
4.1. Principal findings
Our study identified a number of personal, reproductive, and familial characteristics that may have influenced the decision to undergo a surgery resulting in bilateral oophorectomy. These characteristics have not been previously investigated in large epidemiologic studies. Infertility, higher weight, and higher body mass index were more common, whereas older age at menarche, use of oral contraceptives, history of breast feeding, and fibrocystic breast disease were less common in cases than controls. The associations were more extreme for bilateral oophorectomies performed at ages ≤45 years compared to 46–49 years, and were different in women with or without a benign ovarian indication. Family histories of ovarian and colorectal cancers were more common in women who underwent bilateral oophorectomy with a benign ovarian indication compared with referent women, whereas family histories of uterine cancer or other cancers were more common in women who underwent bilateral oophorectomy without a benign ovarian indication compared with referent women.
4.2. Comparison with previous studies
Our findings are consistent with the findings from some previous studies. A study that compared women who underwent hysterectomy with bilateral oophorectomy to women who underwent hysterectomy alone in the state of New York reported an association with family history of breast or ovarian cancer, and with a personal history of breast cancer, ovarian cyst, or endometriosis. In addition, both race (lower rate in African American and Hispanic women) and insurance status were associated with the performance of bilateral oophorectomy [20].
Another study conducted in Michigan showed that family history of cancer and personal history of endometrial hyperplasia, endometriosis, and cervical dysplasia were associated with bilateral oophorectomy [21]. A study in California showed higher risk of bilateral oophorectomy without an ovarian indication in Hispanic or African American women. The study also reported higher risk in urban hospitals and in hospitals with low California Medicaid utilization rates [2].
Some of the associations observed in our study for bilateral oophorectomy were consistent with the associations observed in a study of ovarian cancer. A European study showed an association between oral contraceptive use and greater number of full-term pregnancies with lower risk of ovarian cancer [22]. In our study, oral contraceptive use was associated with reduced risk of bilateral oophorectomy, and a greater number of pregnancies was associated with reduced risk of bilateral oophorectomy performed at age ≤45 years, or performed for a benign ovarian indication. These findings provide additional evidence that oral contraceptive use may have positive long-term effects.
4.3. Possible explanation of findings
A total of 675 women (40.8%) who underwent bilateral oophorectomy had a benign ovarian condition listed as the indication for the surgery (Figure 1) [12]. For these women, the associations that we observed might in part be interpreted as risk or protective factors for the specific ovarian conditions that prompted the surgery (benign tumor, cyst, endometriosis, or other benign ovarian condition). For many of these women, the removal of both ovaries was not needed to control the benign ovarian conditions, and 25% of these women had normal ovaries at pathology. More conservative practices may be considered for these women in the future.
A total of 978 women (59.2%) who underwent bilateral oophorectomy did not have any specified ovarian indication (Figure 1) [12]. Women without a benign ovarian condition were historically considered to have “prophylactic”, “elective”, or “incidental” bilateral oophorectomy. In most of these women, the presumed healthy ovaries were removed at the time of a hysterectomy that was performed for another gynecological indication. Therefore, the risk and protective factors that we observed might in part relate to the uterine conditions or symptoms that prompted the hysterectomy (most commonly, excessive bleeding, pelvic pain, fibroids, or prolapse), or to intraoperative-events, surgeons’ preferences, and women’s preferences and past experiences. For example, higher body mass index is associated with an increased risk of excessive bleeding that may be an indication for hysterectomy, in turn possibly leading to a decision to also remove the ovaries at the same time.
Only 46 women (4.7%) without an ovarian indication were recorded to have a positive family history for ovarian cancer in first-degree relatives (mothers, sisters, or daughters), and family history was not recorded by the surgeon as an indication for the oophorectomy in these women. Similarly, a total of 36 controls (3.7%) were also recorded to have a positive family history. Therefore, family history of ovarian cancer was not significantly different in cases and controls. The associations with family history of uterine cancer and other cancers suggest that a concern of women about the risk of cancer in general may have played a role in electing to remove their presumed healthy ovaries, even in the absence of a documented increased risk of ovarian cancer in their families.
Based on current knowledge and guidelines, these 978 women with no ovarian condition and no documented increased risk of malignancy had no clear indication for removing their presumed healthy ovaries [13,14]. The historical practice of bilateral oophorectomy for the prophylaxis of ovarian or breast cancer even in women at average risk of ovarian cancer, and the lack of awareness of the multiple long-term sequelae of bilateral oophorectomy, might have led the gynecologists to offer the oophorectomy as an option. However, women’s preferences and previous life experiences related to sexuality and reproduction may also have played a role in the decision. For example, women who had a higher number of pregnancies or live births were more likely to undergo the removal of presumed healthy ovaries, even though contraceptive methods were widely available during the study period. As shown in our previous study [10,11], some women had undergone prior abdominal surgeries (e.g., appendectomy, tubal resection, or Cesarean section), and requested the oophorectomy in the belief that it might definitively eliminate pain or other distress. These women may have been unaware or in denial of the possible psychological and emotional origins of their pain and distress, and the gynecologists may have underestimated the possible long-term harmful consequences of bilateral oophorectomy [10,11].
4.4. Strengths
Our case-control study has a number of strengths. First, details about the surgical procedure, prior risk factors, and conditions present at the index date were abstracted from the medical records included in a records-linkage system without direct involvement of the women included in the study (recall bias was minimized). Second, the non-participation was minimized because the data collection was historical and women did not need to provide a study-specific informed consent, but only a general research authorization (as per Minnesota legal requirements) [16,17].
Third, control women comprised an unrestricted sample representative of the general population rather than women who underwent hysterectomy with ovarian conservation. We elected not to use hysterectomy with ovarian conservation as a control group because hysterectomy itself may be associated with similar risk or protective factors [23,24]. Finally, the population studied included all race and ethnicity groups regardless of socioeconomic status, insurance status, and health care delivery setting [17].
4.5. Limitations
First, because the characteristics considered were abstracted from medical records in a records-linkage system, absence of information for some characteristics was considered evidence that the characteristic was not present. On the other hand, we could not impute the value for some other missing characteristics, such as age at menarche, length of oral contraceptive use, and history of breast feeding. These missing values may have introduced a bias because, in general, controls had more missing values than cases (Table 1, footnote a). Second, the oophorectomies took place over 20 years, from 1988 through 2007, and surgical practices and estrogen use have changed over time. However, we conducted a set of secondary analyses stratified by decade of the surgery (1988–1997 vs. 1998–2007) and found similar associations for most of the characteristics. Third, because income was derived from census data for only one point in time and at the census block group level, some misclassification of income may have occurred. However, the year 2000 was approximately the central year of the study period, and the methods used were identical for women with and without bilateral oophorectomy (non-differential misclassification).
Finally, our study focused on a single geographically defined US population, and the observed associations may differ in other populations. However, the demographic and socioeconomic characteristics of our population are similar to those of the upper Midwest and of a large segment of the entire United States population [16,18]. Replication of this study in other populations in the USA and worldwide will allow for useful comparisons.
5. Conclusions
We identified a number of personal, reproductive, and familial characteristics that were associated with bilateral oophorectomy over a 20-year period. Some of these characteristics (e.g., family history of cancer) may have influenced the decision of the women to undergo and of the gynecologist to perform the bilateral oophorectomy. Some other characteristics (e.g., age at menarche or breast feeding) may not have been considered in the decision making. Understanding the characteristics that were associated with the practice in the past is important for decision-making about bilateral oophorectomy in the future. Mounting research evidence suggests that bilateral oophorectomy should be limited to the treatment of ovarian malignancy or to the prevention of cancer when women carry a genetic variant known to increase their risk of cancer significantly (e.g., variants of the BRCA-1 or BRCA-2 genes) [9,13,14]. For other women, the risks of endocrine disruption appear to exceed the benefits, as discussed in detail in our previous publications from MOA-2 [13,14].
Supplementary Material
Highlights.
Infertility was more common in women who underwent bilateral oophorectomy than in controls.
Women who underwent bilateral oophorectomy weighed more and had a higher body mass index than controls.
Use of oral contraceptives, a history of breast feeding, and fibrocystic breast disease were less common in women who underwent bilateral oophorectomy than in controls, and they were older at menarche than controls.
A family history of cancer was more common in women who underwent bilateral oophorectomy.
Approximately 51% of women in this population-based surgical series were found to have healthy ovaries at pathological examination.
Acknowledgement
We thank Ms. Deborah C. Olson for her abstraction of information from medical records and Ms. Robin M. Adams for her assistance in typing and formatting the manuscript.
Funding
The MOA-2 used the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health (grants R01 AG034676 and R01 AG052425). However, the content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. This study was also supported by funds from the Mayo Clinic Research Committee (to WAR). WAR was partly supported by the National Institutes of Health (P50 AG044170, U01 AG006786, and P01 AG004875). VMM was partly supported by the National Institutes of Health (P50 AG044170). MMM was partly supported by the National Institutes of Health (R01 AG049704, P50 AG044170, U01 AG006786, and RF1 AG055151). KK was partly supported by the National Institutes of Health (P50 AG044170).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical approval
All study procedures and ethical aspects were approved by the institutional review boards of both Mayo Clinic and Olmsted Medical Center. Because the data collection was historical, women did not need to provide a study-specific informed consent but rather a general consent to use their medical records for research.
Provenance and peer review
This article has undergone peer review.
Conflict of interest
The authors declare that they have no conflict of interest.
Research data (data sharing and collaboration)
There are no linked research data sets for this paper. Data will be made available on request.
References
- [1].Laughlin-Tommaso SK, Stewart EA, Grossardt BR, Gazzuola Rocca L, Rocca WA, Incidence, time trends, laterality, indications, and pathological findings of unilateral oophorectomy before menopause, Menopause 21(5) (2014) 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mahal AS, Rhoads KF, Elliott CS, Sokol ER, Inappropriate oophorectomy at time of benign premenopausal hysterectomy, Menopause 24(8) (2017) 947–953. [DOI] [PubMed] [Google Scholar]
- [3].Berek JS, Chalas E, Edelson M, Moore DH, Burke WM, Cliby WA, Berchuck A, Prophylactic and risk-reducing bilateral salpingo-oophorectomy: recommendations based on risk of ovarian cancer, Obstet. Gynecol 116(3) (2010) 733–743. [DOI] [PubMed] [Google Scholar]
- [4].Jacoby VL, Grady D, Wactawski-Wende J, Manson JE, Allison MA, Kuppermann M, Sarto GE, Robbins J, Phillips L, Martin LW, O’Sullivan MJ, Jackson R, Rodabough RJ, Stefanick ML, Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study, Arch. Intern. Med 171(8) (2011) 760–768. [DOI] [PubMed] [Google Scholar]
- [5].Vitonis AF, Titus-Ernstoff L, Cramer DW, Assessing ovarian cancer risk when considering elective oophorectomy at the time of hysterectomy, Obstet. Gynecol 117(5) (2011) 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Plusquin C, Fastrez M, Vandromme J, Rozenberg S, Factors affecting gynaecologists’ decision to perform prophylactic oophorectomy concomitantly with hysterectomy: a Belgian survey, Maturitas 70(4) (2011) 391–394. [DOI] [PubMed] [Google Scholar]
- [7].Evans EC, Matteson KA, Orejuela FJ, Alperin M, Balk EM, El-Nashar S, Gleason JL, Grimes C, Jeppson P, Mathews C, Wheeler TL, Murphy M, Salpingo-oophorectomy at the time of benign hysterectomy: a systematic review, Obstet. Gynecol 128(3) (2016) 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parker WH, Broder MS, Berek JS, Manson JE, Salpingo-oophorectomy at the time of benign hysterectomy: a systematic review, Letter, Obstet. Gynecol 129(1) (2017) 202. [DOI] [PubMed] [Google Scholar]
- [9].Rocca WA, Faubion SS, Stewart EA, Miller VM, Salpingo-oophorectomy at the time of benign hysterectomy: a systematic review, Letter, Obstet. Gynecol 129(1) (2017) 202–203. [DOI] [PubMed] [Google Scholar]
- [10].Gazzuola Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Stewart EA, Rocca WA, Adverse childhood or adult experiences and risk of bilateral oophorectomy: a population-based case-control study, BMJ Open 7(5) (2017) e016045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gazzuola Rocca L, Smith CY, Stewart EA, Rocca WA, Adverse childhood experiences and adult abuse are predictors of hysterectomy and oophorectomy, Maturitas 106 (2017) 95–96. [DOI] [PubMed] [Google Scholar]
- [12].Rocca WA, Gazzuola Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Stewart EA, Mielke MM, Kantarci K, Miller VM , Cohort profile: the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2) in Olmsted County, Minnesota (USA), BMJ Open 7(11) (2017) e018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rocca WA, Gazzuola-Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Kirkland JL, Stewart EA, Miller VM, Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study, Mayo Clin. Proc 91(11) (2016) 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rocca WA, Gazzuola Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Kirkland JL, Stewart EA, Miller VM, Bilateral oophorectomy and accelerated aging: cause or effect?, J. Gerontol. A. Biol. Sci. Med. Sci 72(9) (2017) 1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd, History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population, Mayo Clin. Proc 87(12) (2012) 1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA, Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project, Am. J. Epidemiol 173(9) (2011) 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, Rocca WA, Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system, Int. J. Epidemiol 41(6) (2012) 1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA, Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project, Mayo Clin. Proc 87(2) (2012) 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].United States Census Bureau, Census 2000, Summary File 3, Minnesota, Table P053. https://www.census.gov/census2000/sumfile3.html. (Accessed 2/25/2018).
- [20].Novetsky AP, Boyd LR, Curtin JP, Trends in bilateral oophorectomy at the time of hysterectomy for benign disease, Obstet. Gynecol 118(6) (2011) 1280–1286. [DOI] [PubMed] [Google Scholar]
- [21].Karp NE, Fenner DE, Burgunder-Zdravkovski L, Morgan DM, Removal of normal ovaries in women under age 51 at the time of hysterectomy, Am. J. Obstet. Gynecol 213(5) (2015) 716 e711–716. [DOI] [PubMed] [Google Scholar]
- [22].Tsilidis KK, Allen NE, Key TJ, Dossus L, Lukanova A, Bakken K, Lund E, Fournier A, Overvad K, Hansen L, Tjonneland A, Fedirko V, Rinaldi S, Romieu I, Clavel-Chapelon F, Engel P, Kaaks R, Schutze M, Steffen A, Bamia C, Trichopoulou A, Zylis D, Masala G, Pala V, Galasso R, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, van Duijnhoven FJ, Braem MG, Onland-Moret NC, Gram IT, Rodriguez L, Travier N, Sanchez MJ, Huerta JM, Ardanaz E, Larranaga N, Jirstrom K, Manjer J, Idahl A, Ohlson N, Khaw KT, Wareham N, Mouw T, Norat T, Riboli E, Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition, Br. J. Cancer 105(9) (2011) 1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Laughlin-Tommaso SK, Khan Z, Weaver AL, Schleck CD, Rocca WA, Stewart EA, Cardiovascular risk factors and diseases in women undergoing hysterectomy with ovarian conservation, Menopause 23(2) (2016) 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Laughlin-Tommaso SK, Khan Z, Weaver AL, Smith CY, Rocca WA, Stewart EA, Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study, Menopause 25(5) (2018) 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.