Abstract
Establishing a non-destructive method for spatially assessing advanced glycation end-products (AGEs) is a potentially useful step toward investigating the mechanistic role of AGEs in bone quality. To test the hypothesis that the shape of the Amide I in the Raman Spectroscopy (RS) analysis of bone matrix changes upon AGE accumulation, we incubated paired cadaveric cortical bone in ribose or glucose solutions and in control solutions for 4 and 16 weeks, respectively, at 37 ˚C. Acquiring 10 spectra per bone with a 20X objective and a 830 nm laser, RS was sensitive to AGE accumulation (confirmed by biochemical measurements of pentosidine and fluorescent AGEs). Hyp/Pro ratio increased upon glycation using either 0.1 M ribose, 0.5 M ribose, or 0.5 M glucose. Glycation also decreased the Amide I sub-peak ratios (cm−1) 1668/1638 and 1668/1610 when directly calculated using either second derivative spectrum or local maxima of difference spectrum, though the processing method (e.g., averaged spectrum vs. individual spectra) to minimize noise influenced detection of differences for the ribose-incubated bones. Glycation however did not affect these sub-peak ratios including the matrix maturity ratio (1668/1690) when calculated using indirect sub-band fitting. The Amide I sub-peak ratios likely reflected changes in the collagen I structure.
Keywords: Raman spectroscopy, advanced glycation end-product, type 1 collagen, bone quality, pentosidine, glucosepane, post-translation modifications, deconvolution, spectral processing
1. Introduction
As the primary component of the organic matrix, hydrated type I collagen confers plasticity to the otherwise brittle mineral phase of bone. Post-translational modifications (PTMs) to the triple helical collagen form crosslinks within and between neighboring collagen molecules and fibrils, thereby further influencing the structural integrity or deformation mechanics of the organic matrix. To date, there are no non-destructive tools or tools with minimal sample preparation to assess collagen structure or collagen integrity in bone, even though age- and disease-related changes to collagen likely affect fracture risk. Raman spectroscopy (RS) has the ability to assess collagen characteristics of bone (1), and recent advances in the transcutaneous acquisition of Raman signals from bone in vivo using fiber-optic probes makes clinical assessment of the organic matrix possible (2–4). To help with the translation toward bone diagnostic assessment, establishing the sensitivity of RS to PTMs that affect the secondary structure of collagen 1 could be useful.
Important to the fibril formation of the helical tropocollagen, enzymatic crosslinking is mediated by the PTMs of lysyl oxidase (i.e., collagen hydroxylation) (5) and involve lysine and/or hydroxylysine residues initially forming immature divalent crosslinks (6). Overtime, a portion of immature divalent crosslinks converts into mature trivalent crosslinks such as pyridinoline (PYD), pyrrololine (PYL), deoxypyridinoline (DPD), and deoxypyrrololine (DPL) (5). PYD and DPD, extracted using acid hydrolysis (destructive technique), are commonly used as an indicator of collagen maturity when assessed in bone (7–9).
Non-enzymatic crosslinking of collagen also occurs and initially involves the Maillard reaction between lysine residues and reducing sugars. Subsequently, the adducted sugar moiety can react with either lysine or arginine side chains, thus completing crosslink formation and result in the formation of an advanced glycation end-product (AGE). Common crosslinks formed through this mechanism are pentosidine (PEN) and glucosepane (GLU) (10, 11). AGE crosslinks can also form via oxidative degradation of sugars and formation of reactive dicarbonyl species. Glyoxallysine dimer (GOLD) and methylglyoxallysine dimer (MOLD) crosslinks are derived through this mechanism (12). Non-crosslinking AGE modifications also form within collagen involving reactive carbonyl compounds and sugar adduction. The most common of these AGEs are methylglyoxal and glyoxal imidazolone adducts to arginine residues (MG-H1 and G-H1, respectively) and carboxymethyllysine (CML), which can form through dicarbonyl reaction with lysine residues or via oxidative cleavage of the lysine-adducted sugar moiety (13–15). AGEs are naturally fluorescent (e.g., PEN, crossline, pyrropyridine) or non-fluorescent (e.g., CML, GLU, PYR, GOLD and MOLD). Because PEN structure can withstand acid hydrolysis, it is a well-established measurement of AGE crosslinks in connective tissues along with the non-specific measurement of fluorescence of hydrolysates (5, 9).
While enzymatic crosslinks stabilize the collagen matrix in bone and their disruption in lathyrism (due to the toxin β-aminopropionitrile) (16) and in Cu deficiency (17) degrade mechanical properties of bone, accumulation of AGE crosslinks with aging is negatively associated with post-yield mechanical properties (18–21). Moreover, when bovine cortical bone was incubated in high concentrations of ribose (0.6 M to 1 M) to study the effects of AGE accumulation on mechanical properties of cortical bone, the subsequent in vitro glycation of the organic matrix increased PEN amount or AGE fluorescence in cortical bone and significantly reduced either bone toughness (22, 23) or bending and compressive yield strength (24). The latter study involved fetal bovine bone with a low degree of mineralization. An in vitro glycation study of human cortical bone (0.6 M ribose for 7 days) also reported a significant reduction in crack growth toughness but not crack initiation toughness (25). As further evidence that AGEs affect fracture risk, there are several clinical studies that found higher circulating PEN in patients with osteoporosis (26, 27) and with type 2 diabetes (28, 29) compared to controls.
Mechanistically, AGE accumulation in the bone matrix is thought to impede collagen fibril deformation (brittle effect), and this loss in energy dissipation reduces the fracture resistance of bone (30). The causal relationship is supported by recent work in which the introduction of AGEs into collagen fibrils reduced their failure strain, post-yield strain, and stress relaxation (31). To the best of our knowledge, associations between non-crosslinking AGEs and mechanical properties of human cortical bone have not been reported, but higher levels of circulating CML were significantly associated with higher risk of fracture in cohort of women and men (68 to 107 years old) (32). There is still much to be learned about how AGE accumulation lowers the fracture resistance of bone.
With respect to the composition and organization of the bone matrix, RS is sensitive to the organic, mineral and water phases of bone (1, 33, 34). There are several common RS parameters widely used to quantify bone chemical characteristics (1, 35) such as the mineral-to-matrix ratio (ν1PO4/Proline, ν1PO4/Amide I, ν2PO4/Amide III), carbonate substitutions into the lattice structure of hydroxyapatite (CO3/ν1PO4), crystallinity (line-width of the ν1PO4 band), and the matrix maturity (ratio of the Amide I peak to the right shoulder: 1660/1683 cm−1 (36), 1664/1685 cm−1 (37), or 1660/1690 cm−1 (38)). More recently, two rodent studies, one involving chronic kidney disease (39) and the other involving type 1 diabetes (40), reported different RS measurements of PEN normalized to the Amide I or CH2-wag, respectively.
Previous studies also found that Raman sub-peaks of the Amide I band acquired from bone are sensitive to a disruption of enzymatic crosslinking (16), mechanical damage (41–43), thermal denaturation (42, 44), degree of mineralization (45), and ionizing radiation (46, 47). However, to date, the effect of AGE accumulation on Amide I sub-peak ratios, as determined by RS, has not been investigated. Accordingly, since AGEs can alter the collagen structure (48–51), we hypothesized that glycation of the organic matrix affects sub-peak ratios of the Amide I band. In testing this hypothesis, we assessed whether changes in the previously reported RS measurements of PEN in rodent bone (39, 40) could be detected in human cortical bone. Moreover, since there are no clear guidelines on how to identify sub-peaks and to filter the inherent noise in Raman spectra, we investigated the effect of peak identification/fitting techniques and smoothing/de-noising techniques on differences in sub-peak ratios across glycation groups. Similar to a recent Fourier transform infrared (FTIR) spectroscopy study involving incubation of adolescent human cortical bone in ribose (52), we incubated adult human cortical bone in ribose or glucose solutions for 4 weeks or 16 weeks, respectively, as well as in buffered saline control solutions at 37 ˚C.
2. Material and Methods
2.1. Bone sample preparation and study design
Fresh frozen human cadaveric femurs from 10 donors (5 males and 5 females, aged 46–60 years old, mean ± standard deviation (SD): 55 ± 5 years) were obtained from the Musculoskeletal Transplant Foundation (Edison, NJ), the Vanderbilt Donor Program (Nashville, TN), and the National Disease Research Interchange (Philadelphia, PA). From each of the ten donors - a cortical bone strip, approximately 70 mm in length and 2.8 mm in thickness, was extracted from the medial quadrant of the distal mid-shaft using a low speed, water-irrigated, diamond-embedded circular saw (South Bay Technologies, San Clemente, California). The strip was machined along the endosteal and periosteal sides to create a beam sample with a width of 5 mm. After grinding (EXAKT grinding system, Oklahoma City, OK) and polished (Vibromet II, Buehler, Lake Bluff, IL) one radial surface with silica carbide papers and polishing with alumina solutions, each beam specimen was further cut length-wise into twelve individual bone specimens (nominal dimensions: 5.0 mm x 5.0 mm x 2.5 mm).
2.2. In Vitro Glycation Process
The bone segments from each donor were randomly assigned to three groups: control solution of phosphate buffered saline (PBS), 0.1 M sugar (D-ribose or D-glucose, Sigma Aldrich, St. Louis, MO), and 0.5 M sugar. The pH range was adjusted between 7.0 and 7.5, and the buffered solution was supplemented with 0.02% sodium azide as a biocide. Two bone samples per group (one for RS and one for AGE measurement) were incubated in 15 ml of solution while on a rotary mixer within an oven (Thermo Fisher Scientific, Waltham, MA) at 37°C for either 4 weeks (ribose) or 16 weeks (glucose). Sentinel specimens were incubated under the same conditions and used to monitor the pH range during each of the experiments. Following incubation, the specimens were stored frozen at −20 °C in PBS without sugar until Raman spectral assessment.
2.3. Raman Spectroscopy: Data Acquisition and Parametric Analysis
All Raman spectra were obtained from 10 points randomly distributed over the polished surface per bone sample using a confocal Raman system (Renishaw InVia Raman microscope) with a holographic grating (1200 lines/mm) providing ~1 cm−1 spectral resolution and a 830 nm laser source (Renishaw). Each spectrum was obtained as the average of 10 consecutive spectra with each collected for 5 seconds using a 20X objective (NA=0.40), focusing the laser into a ~2.5 µm spot on each sample. Daily silicon and laser power measurements (~35 mW) before and after data collection ensured wavenumber calibration and light throughput, respectively. Background fluorescence was removed from all spectra using fourth order polynomial fitting algorithm (53).
2.3.1. Effect of pre-processing spectra
After establishing that processing the averaged Raman spectrum per bone was more sensitive to sugar-mediated changes in the Amide I band than processing individual spectra per bone (Supplemental Tables 1 and 2), averaged spectra were processed to further minimize signal noise in the spectra with either a Savitzky-Golay (S-G) filter or a proprietary de-noising (D-n) algorithm provided by the LabSpec software (Horiba Jobin Yvon, Edison, NJ). The proprietary de-nosing algorithm of LabSpec software involves a self-adapting frame size to select an optimal wavenumber range for fitting the spectral data with a second-order polynomial. Furthermore, we investigated the effect of applying a second- or fourth-order S-G with different window sizes or heights (11, 15 or 21), which basically dictate the number of data samples processed at a time (54).
2.3.2. Effect of sub-peak identification method
Identification of sub-peak ratios within Amide I as a marker of collagen structure (42, 55) involved 3 methods – second derivative, local maxima of difference spectrum (LMDS) and band fitting (deconvolution) – to determine which one is most sensitive to sugar-mediated changes in the shape of the Amide I band. Since the second derivative amplifies noise, the D-n spectra were further processed with a fourth-order S-G filter (window size of 21). The LMDS involved a subtraction of each intensity value from the intensity value of its neighbor, and then the peak locations were found as the local maxima of the difference spectrum (Fig. 1a). The sub-peak locations were also determined as the local minima (<0) of the second derivative spectrum (Fig. 1a). Then, the ratio of peak heights at ~1670/1640, ~1670/1690 and ~1670/1610 (cm−1) of original Amide I intensities (normalized to ν1PO4 at ~960 cm−1) were directly calculated using each method (Fig. 1a). Lastly, four sub-peaks in the Amide I band were fitted using Gaussian-Lorentzian mixed functions (Fig. 1b). At the beginning, each peak was centered on the 4 points that were identified by the second derivative analysis, and the Gauss/Lorentzian mixture was set to 50%/50%. Then, a non-linear constrained optimization algorithm in MATLAB determined the final position of each peak within a ±5 cm−1 window as well as the final mixture for each peak that minimized the weighted root mean square error between the experimental data and the sum of the 4 sub-peaks. The sub-peak ratios of interest were then calculated as the ratio of the area of sub-peaks (Fig. 1b). Lastly, to check whether the additional S-G filtering affected identification of the 4 peaks, we further ran the second derivative analysis while varying the window size from 25 down to 5 (Supplementary Figure 1) on the spectra from ribose experiment. We observed only 4 peaks under Amide I regardless of the filtering process until the window size of 9. Between 9 and 5, the number of identified peaks changed from 11 to 34. These peaks appear to be the result of noise, and so we determined the sub-peak ratios for the 4 fitted sub-bands.
Figure 1.
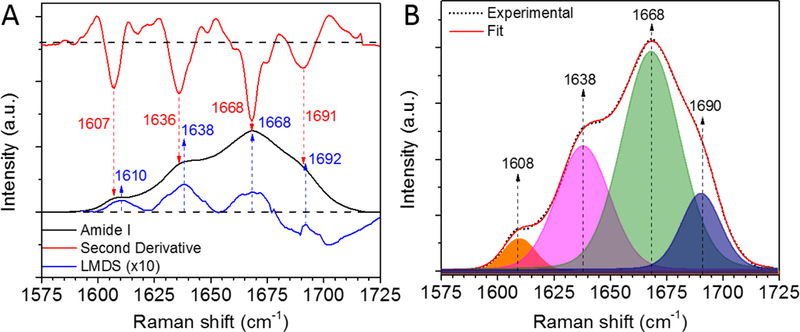
Sub-band analysis of Amide I. To determine which one is most sensitive to sugar-mediated changes in the shape of the Amide I band, identification of sub-peak peaks involved 3 methods: (A) second derivative and local maxima of difference spectrum (LMDS) and (B) band fitting (deconvolution). Before the sub-band analysis, each spectrum was de-noised using a proprietary de-noising (D-n) algorithm provided by the LabSpec software to ensure no artificial peaks due to the noise were identified. LMDS intensity was multiplied by 10 for clarity.
To determine how sugar incubation affects the Raman properties of bone, the following ratios of peak intensities were also calculated from processed spectra: 1) mineral-to-matrix ratio (ν1PO4 at ~960 cm−1 per Proline at ~855 cm−1, ν1PO4 per Amide III at ~1247 cm−1 and ν1PO4 per Amide I at ~1668 cm−1) as a measurement mineralization, 2) type B carbonate substitution (CO3 at ~1072 cm−1 per ν1PO4) as a way to assess mineral crystal distortion, 3) Hydroxyproline/Proline ratio (Hyp at ~877 cm−1 per Pro at ~855 cm−1 or at 922 cm−1) as an indicator of post-translational modifications (45), and 4) PEN-associated ratio (peak at ~1362 cm−1 per CH2-wag at ~1453 cm−1 or peak at ~1495 cm−1 per CH2-wag) (39, 40) and CML-associated ratio (peak at ~1150 cm−1 per CH2-wag) (40). The inverse of the full-width at half maximum of the ν1PO4 peak (at ~960 cm−1) was also determined as a measure of the degree of the crystal lattice order.
2.3.3. Surface-enhanced Raman spectroscopy of crosslinks and bone
Since the location of Raman peaks arising from pentosidine or glucosepane were not prominent (Fig. 2 and 3), we used surface-enhanced Raman spectroscopy (SERS) as an alternate technique for identifying these AGEs. Briefly, ~10 µl of a pentosidine standard (0.2 pmol/L) or a glucosepane standard (0.725 mM) was directly deposited onto nano-sponge, gold-SERS substrates coating a microscope slide (RA-SERS-AU, Ocean Optics, Dunedin, FL, USA). Laser power was set to ~ 30 mW. Ten spectra were randomly collected from the SERS active area after pentosidine or glucosepane standard was dried. Each Raman data was obtained as the average of 3 consecutive spectra from the same point (3 sec acquisition time and average of 3) using a 20X objective (NA=0.40). We also collected SERS-bone spectrum from bone powder (<1 mg) mixed with 20 mL HPLC-grade water. SERS-bone spectra were further acquired from each of the incubation groups (N=3). As described above, ten bone spectra, each being the average of 3 consecutive data collection from the same point (improved the reproducibility of SERS spectrum), were randomly collected from the SERS active area.
Figure 2.
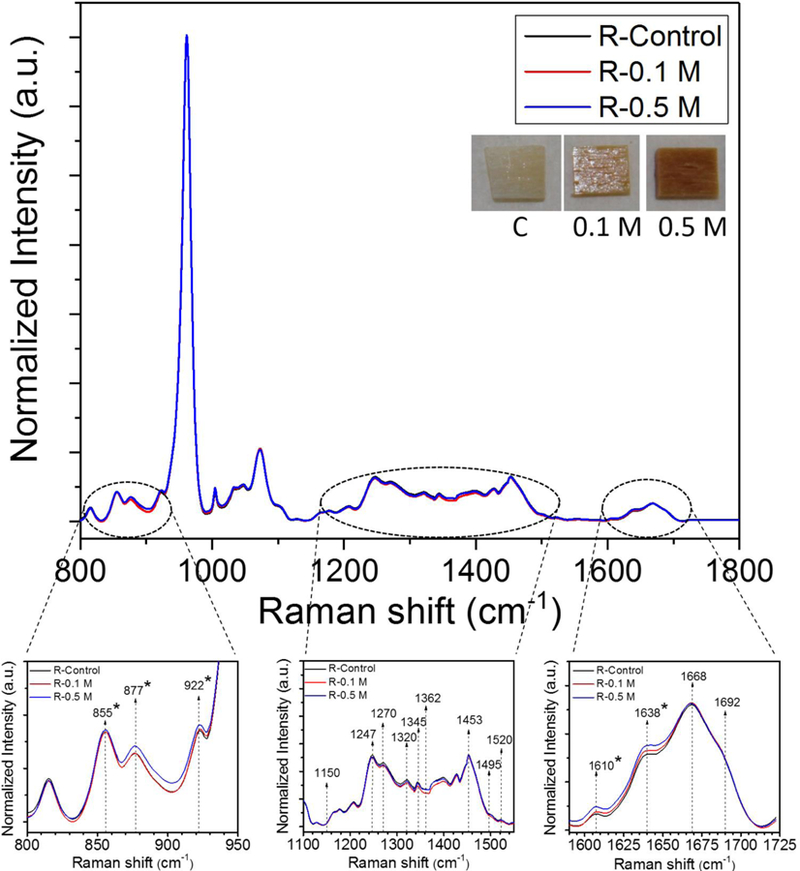
Comparison of the averaged Raman spectra across ribose incubated groups. Raman spectra were normalized to phosphate peak located at ~ 960 cm-1. * indicates significant differences in Raman intensities between control and 0.5 M ribose incubation groups as determined by by Mann-Whitney U tests at each wavenumber.
Figure 3.
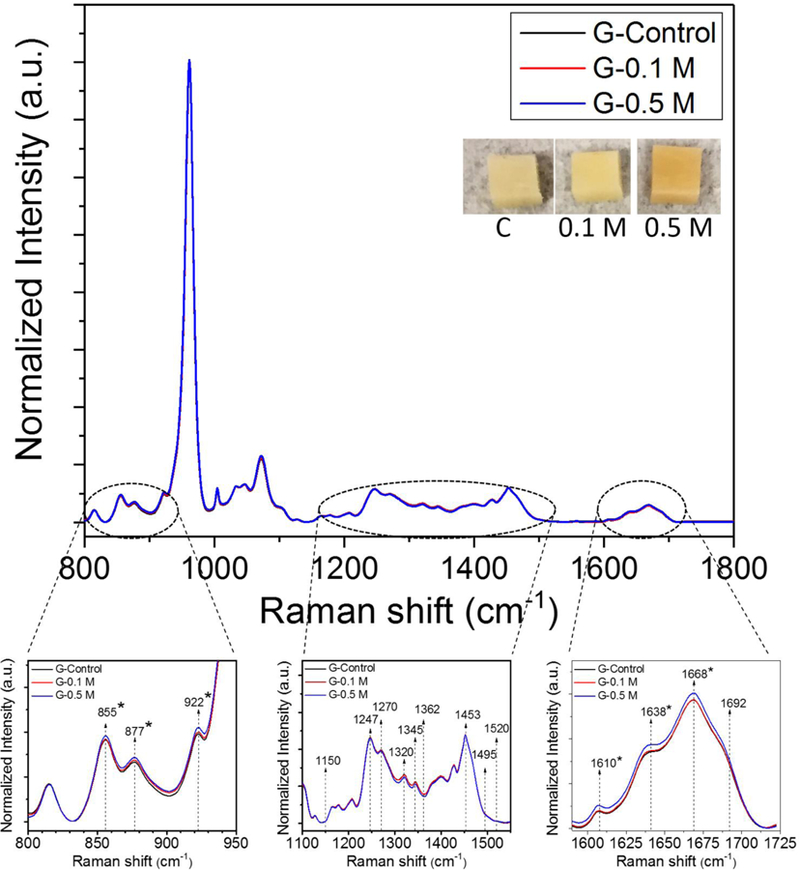
Comparison of the averaged Raman spectra across glucose incubated groups. Raman spectra were normalized to phosphate peak located at ~ 960 cm-1. * indicates significant differences in Raman intensities between control and 0.5 M ribose incubation groups as determined by Mann-Whitney U tests at each wavenumber.
2.4. High Performance Liquid Chromatography
From each paired sample, a ~2.5 mm x 2.5 mm corner section was cut for high performance liquid chromatography (HPLC). Bone segments were demineralized in 20% ethylenediaminetetraacetic acid (EDTA) at 4 °C. Then, these bone segments were hydrolyzed in 6 N HCl with 4.5 mM alpha-amino-N-butyric acid (α-ABA) for ~20 hours. After removal of the acid and filtration through 0.2 µm syringe filter, a portion of the re-suspended hydrolysate was used to measure hydroxyproline (to give mole of collagen) via the chromatogram generated by a UV detector (Beckman Coulter 168 Detector, Brea, CA) and following our previously published HPLC method (56).
To determine crosslink concentrations, another portion of the re-suspended hydrolysate was diluted in acetonitrile-heptafluorobutyric acid solution (10% v/v) with 0.25 µg/ml of pyridoxine (PYR) as the internal standard. Samples were injected along with standards consisting of PYR and varying concentrations of pyridinoline (PYD), deoxypyridinoline (DPD) and pentosidine (PEN) into a silica-based, reversed-phase C18 column (Waters Spherisorb® 5μm ODS2, Milford, MA)(57). A programmable fluorescence detector (Waters 2475 Multi λ Fluorescence Detector) was used to generate chromatograms. Moles of each crosslink per sample were divided by the corresponding moles of collagen.
2.5. Measurement of Fluorescence AGEs
A portion of the hydrolyzed bone sample (~1 mg) prepared as described for HPLC was suspended in 500 µL of 0.1 N H2SO4. A standard curve was prepared using quinine sulfate suspended in 0.1 N H2SO4. Fluorescence was measured with an excitation wavelength of 370 nm and emission wavelength of 440 nm using a Synergy HT Microplate reader (BioTek Instruments Inc., Winooski, VT). Values for Fluorescence AGEs (fAGEs) were calculated based on the standard curve (58) and normalized to the collagen content determined by HPLC.
2.6. Statistical Analysis
All statistical analyses were done using GraphPad Prism (v6.0a, GraphPad Software, Inc., La Jolla, CA). Data are presented as mean ± SD (Tables) or as box and whisker plots (Figures). The effect of incubation on RS, HPLC and fAGEs parameters was assessed by the non-parametric Kruskal-Wallis test, followed by Dunn’s correction for multiple comparisons with a family-wise significance level of 0.05. To find the target wavenumbers showing significant differences in Raman intensities between control and 0.5 M incubation groups, p-values as a function of wavenumber were generated by the non-parametric Mann-Whitney U test.
3. Results
3.1. Effect of pre-processing techniques on sensitivity of Raman spectroscopy to sugar-mediated changes in the Amide I band
Since the Amide I peak at 1667 cm−1 is weak compared to the predominant phosphate peak at ~ 960 cm−1 or other organic peaks such as the CH2-wag at 1453 cm−1 (Fig. 2 and 3), the Amide I sub-peak ratios are more influenced by noise in the spectrum than other standard Raman peak ratios. Because there is a paucity of information on whether different pre-processing techniques affect the Amide I sub-peak ratios, we first systematically investigated which of several common smoothing/de-noising algorithms were most sensitive to identifying sugar-mediated changes in the Amide I sub-peak ratios. Then, we assessed whether the method of calculating the sub-peak ratios affected the differences in the Amide I between the experimental groups for the de-noising (D-n) algorithm.
3.1.1. Different smoothing and de-noising filters
The 1668/1638 and 1668/1610 ratios were always significantly different between R-control and R-0.5 regardless of the pre-processing method used (Fig. 4). However, only the fourth order S-G, regardless of height, and D-n detected differences in 1668/1638 between R-control and R-0.1 and between R-0.1 and R-0.5. Increasing the height of the 4th order SG to 21 improved the ability to detect a significant difference in 1668/1610 between R-0.1 and R-0.5 (Fig. 4). Interestingly, the ability to detect differences in 1668/1638 and in 1668/1610 among glucose groups did not depend on filtering method (Fig. 5).
Figure 4.
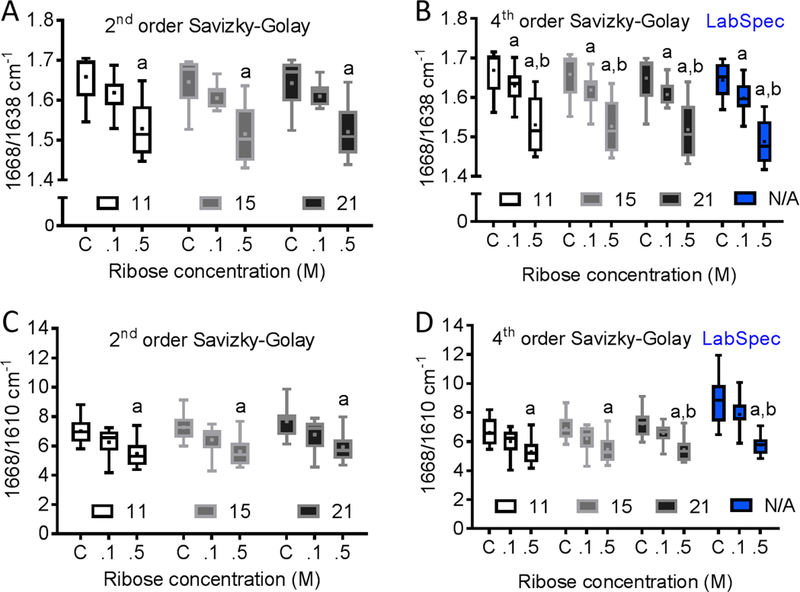
Effect of different de-nosing/smoothing techniques on sensitivity of Raman spectroscopy to ribose-mediated changes in the sub-peaks of the Amide I band. We used either 2nd or 4th order Savizky-Golay (S-G) filters with 11, 15 and 21 window sizes along with Lab-spec de-noising (D-n) algorithm. (A) Regardless of the window size used, 2nd order S-G detected differences in 1668/1638 ratio between R-control and R-0.5. (B) Regardless of the window size used, the 4th order S-G and D-n further detected differences in 1668/1638 ratio between R-control and R-0.1. (C) The 2nd order S-G also detected differences in 1668/1610 ratio between R-control and R-0.5. (D) Increasing the window size of the 4th order S-G to 21 improved the ability to detect a significant difference in 1668/1610 between R-0.1 and R-0.5.
Figure 5.
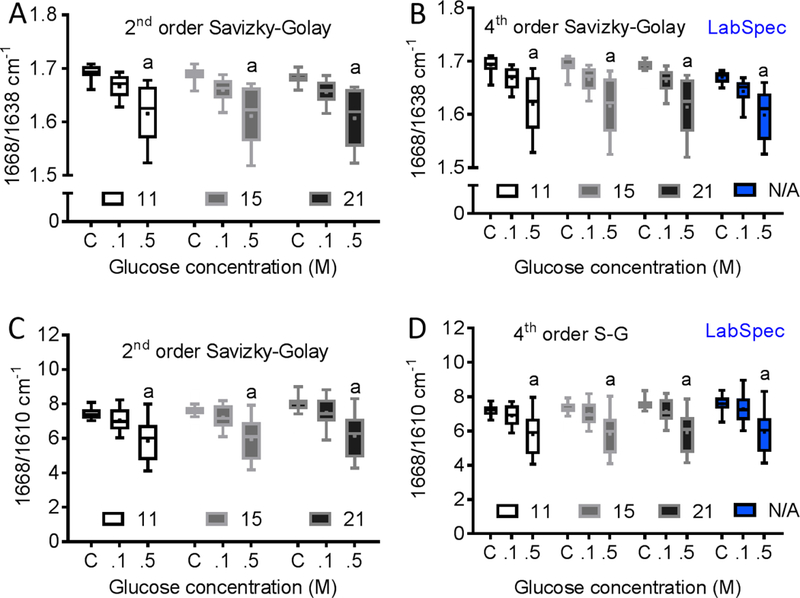
Effect of different de-nosing/smoothing techniques on sensitivity of Raman spectroscopy to glucose-mediated changes in the sub-peaks of Amide I band. We used either 2nd or 4th order Savizky-Golay (S-G) filters with 11, 15 and 21 window sizes along with Lab-spec de-noising (D-n) algorithm. Using different de-nosing/smoothing technique did not affect the ability to detect differences in (A–B) 1668/1638 and in (C–D) 1668/1610 among glucose groups.
3.1.2. Different methods for calculating Amide I sub-peak ratios
There are several ways to identify the sub-peak locations in the Amide I band (LDMS or second derivative spectra) and to calculate the sub-peak ratios (direct sub-peak intensity ratios or indirect sub-peak area ratios after band fitting with deconvolution). Based on second derivative analysis, the sub-peaks were located at ~1607, ~1636, ~1668 and ~1691 cm−1 (Fig. 1a). Using LDMS method, the same peaks were slightly shifted to locations at ~1610, ~1638, ~1668 and ~1692 cm−1, respectively (Fig. 1a). Although the values of the ratios calculated from these two methods were slightly different, both methods were sensitive to sugar-mediated changes in the Amide I band distinguishing statistically significant differences among the experimental groups for both ribose and glucose-treated bone samples (Supplemental Table 3 and 4). Constrained to ±5 cm−1 of the location identified by the second derivative spectra, the average locations for the band fitting technique (with mean ± SD of the final mixture as percent Gaussian) were ~1608 (60 ± 44%), ~1638 (56 ± 31%), ~1668 (83 ± 9%) and ~1690 cm−1 (92 ± 7%). Unlike the direct sub-peak intensity ratios, the derived sub-peak area ratios (1668/1638, 1668/1608, and 1668/1690) did not differentiate any sugar-mediated differences among the groups, indicating a limited ability of peak band fitting method to assess Amide I sub-peak ratios (Supplemental Table 3 and 4).
3.2. Sensitivity of Raman Parameters to sugar-mediated changes in human cortical bone
Comparing the averaged Raman spectra across groups per sugar showed that Proline- Hydroxyproline and Amide I bands were the primary regions of the Raman spectra that significantly changed with ribose and glucose incubation (p<0.05) (Fig. 2 and 3). None of the traditional Raman parameters including MMR (ν1PO4/Amide I, ν1PO4/Amide III, ν1PO4/Pro, ν1PO4/CH2), Type B carbonate substitution (CO3/ν1PO4) and crystallinity (1/FWHM ν1PO4) changed with ribose (Table 1) and glucose incubation (Table 2), indicating that the changes in the spectra are the result of non-enzymatic glycation. Thus, effects of glycation can be isolated from other RS parameters of bone matrix quality. In addition, there were no statistically significant changes in the previously identified PEN-associated ratio (~1495/ CH2 or ~1362/CH2) and CML-associated ratio (~1150/ CH2) with the incubation in either sugar (Table 1 and 2). The Hydroxyproline-to-Proline (Hyp/Pro) ratio was the only Raman parameter that changed with in vitro glycation (Tables 1 and 2). This ratio increased with both ribose (Table 1) and glucose incubation at the higher concentration (p<0.05) (Table 2). We did not observe any significant shift in Amide I sub-peak locations after glycation as well (Supplemental Fig. 2).
Table 1:
The summary of comparison of HPLC, Florescence AGE assay and Raman parameters across ribose-incubated groups. Significant p-values are in bold. N.S.: not significant.
| Parameters | Glycation Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Ribose | ||||||||
| R-Control | R-0.1 M | R-0.5 M | R-Control vs. R-0.1 M | R-Control vs. R-0.5 M | R-0.1 M vs. R-0.5 M | |||
| HPLC Parameters | PEN/collagen | mol/mol of collagen | 3.5±0.8 | 42.5±10.0 | 55.4±9.5 | p<0.0001 | p<0.0001 | p<0.0001 |
| DPD/collagen | mol/mol of collagen | 0.226±0.054 | 0.206±0.049 | 0.186±0.048 | N.S. | N.S. | N.S. | |
| PYD/collagen | mol/mol of collagen | 0.480±0.065 | 0.357±0.040 | 0.259±0.041 | N.S. | N.S. | N.S. | |
| Fluorescence AGE Assay | fAGEs | g/mol | 76.2±24.9 | 456.0±81.2 | 829.3±138.2 | p<0.0001 | p<0.0001 | p<0.0001 |
| Raman Parameters | Mineral-to- Matrix (MMR) | ν1PO4/Amide I | 26.93±2.10 | 26.56±1.55 | 26.72±3.12 | N.S. | N.S. | N.S. |
| ν1PO4/Amide III | 10.82±1.56 | 11.30±1.19 | 11.20±1.82 | N.S. | N.S. | N.S. | ||
| ν1PO4/CH2 wag | 10.76±1.35 | 10.99±1.02 | 10.91±1.56 | N.S. | N.S. | N.S. | ||
| ν1PO4/Proline | 16.67±1.30 | 16.88±0.75 | 16.35±1.22 | N.S. | N.S. | N.S. | ||
| Carbonate Substitution | CO3/ν1PO4 | 0.148±0.005 | 0.150±0.007 | 0.147±0.006 | N.S. | N.S. | N.S. | |
| Crystallinity | 1/FWHM(ν1PO4) | 0.063±0.007 | 0.062±0.001 | 0.063±0.006 | N.S. | N.S. | N.S. | |
| Hyp/Pro | 877/855 | 0.76±0.05 | 0.77±0.04 | 0.83±0.05 | N.S. | p=0.0287 | p=0.0288 | |
| 877/922 | 0.75±0.05 | 0.74±0.02 | 0.82±0.05 | N.S. | p=0.021 | p=0.020 | ||
| Raman-PEN | ~1362/CH2-wag | 0.518±0.112 | 0.468±0.081 | 0.512±0.172 | N.S. | N.S. | N.S. | |
| ~1495/CH2-wag | 0.164±0.048 | 0.149±0.040 | 0.156±0.064 | N.S. | N.S. | N.S. | ||
| Raman-CML | ~1150/CH2-wag | 0.048±0.021 | 0.045±0.013 | 0.049±0.023 | N.S. | N.S. | N.S. | |
Table 2:
The summary of comparison of HPLC, Florescence AGE assay and Raman parameters across glucose-incubated groups. Significant p-values are in bold. N.S.: not significant.
| Parameters | Glycation Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Glucose | ||||||||
| G-Control | G-0.1 M | G-0.5 M | G-Control vs. G-0.1 M | G-Control vs. G-0.5 M | G-0.1 M vs. G-0.5 M | |||
| HPLC Parameters |
PEN/collagen | mol/mol of collagen | 2.9±1.1 | 3.1±1.1 | 4.2±1.2 | N.S. | p=0.010 | p=0.011 |
| DPD/collagen | mol/mol of collagen | 0.241±0.052 | 0.226±0.071 | 0.217±0.056 | N.S. | N.S. | N.S. | |
| PYD/collagen | mol/mol of collagen | 0.423±0.090 | 0.398±0.088 | 0.408±0.091 | N.S. | N.S. | N.S. | |
| Fluorescence AGE Assay | fAGEs | g/mol | 64.4±27.9 | 96.9±83.3 | 101.3±73.3 | N.S. | N.S. | N.S. |
| Raman Parameters | Mineral-to-Matrix | ν1PO4/Amide I | 27.63±2.03 | 28.07±2.74 | 26.52±2.37 | N.S. | N.S. | N.S. |
| ν1PO4/Amide III | 13.64±0.74 | 13.88±1.02 | 13.98±0.74 | N.S. | N.S. | N.S. | ||
| ν1PO4/CH2 wag | 13.26±0.64 | 13.43±1.00 | 13.59±0.89 | N.S. | N.S. | N.S. | ||
| ν1PO4/Proline | 17.67±1.03 | 17.41±0.50 | 16.99±0.81 | N.S. | N.S. | N.S. | ||
| Carbonate Substitution | CO3/ν1PO4 | 0.138±0.005 | 0.139±0.006 | 0.142±0.005 | N.S. | N.S. | N.S. | |
| Crystallinity | 1/FWHM(ν1PO4) | 0.066±0.003 | 0.065±0.001 | 0.064±0.001 | N.S. | N.S. | N.S. | |
| Hyp/Pro | 877/855 | 0.73±0.01 | 0.75±0.02 | 0.76±0.02 | N.S. | p=0.0069 | N.S. | |
| 877/922 | 0.68±0.02 | 0.72±0.01 | 0.75±0.04 | N.S. | p=0.0039 | N.S. | ||
| Raman-PEN | ~1362/CH2-wag | 0.335±0.016 | 0.331±0.037 | 0.295±0.049 | N.S. | N.S. | N.S. | |
| ~1495/CH2-wag | 0.077±0.008 | 0.077±0.015 | 0.070±0.014 | N.S. | N.S. | N.S. | ||
| Raman-CML | ~1150/CH2-wag | 0.030±0.005 | 0.031±0.009 | 0.027±0.010 | N.S. | N.S. | N.S. | |
3.3. Biochemical assessment of AGEs
We confirmed that the ribose incubation increased the concentration of pentosidine and fAGEs (Table 1). PYD and DPD normalized to the collagen content in bone did not differ between sugar groups and their respective control (Table 1). As expected for glucose incubation (favors glucosepane over pentosidine), there was small but higher concentration of PEN for G-0.1 and G-0.5 compared to G-control (Table 2). The fluorescence assay was not sensitive enough to detect a significant increase in fAGEs with incubation in glucose for 16 weeks (Table 2).
3.4. Surface-enhanced Raman spectroscopy of pentosidine and glucosepane in bone
Suspecting that a low signal for the AGEs-associated peaks precluded the detection of a significant difference in ~1362/CH2-wag and ~1495/CH2-wag for PEN and other possible GLU-associated peak ratios between control and the respective sugar incubation groups, we acquired Raman spectra from pentosidine (PEN) and glucosepane (GLU) standards, and bone powder using SERS gold nanosubstrates (Fig. 6a). Our results showed that both PEN and GLU (Fig 6a and Table 3) have several characteristic SERS peaks located in the range between 800 and 1700 cm−1 along with several peaks in common. Although PEN- and GLU-associated peaks were clearly observed on SERS bone spectrum (Figs. 6a and 6b, Table 3), these peaks were very weak and hardly detectable for non-SERS spectrum of bone (Fig. 6a and 6b). Among them, the peaks at ~ 821, 1122, 1192, 1309, 1360, 1431, 1521, 1575, 1600 and 1654 cm−1 were relatively unique to PEN (black in Fig. 6B and Table 3), while the peaks at ~ 834, 1134, 1172, 1210, 1297 and 1472 cm−1 were prominent in GLU (red in Fig. 6B and Table 3). SERS-spectra from the sugar-incubated groups compared to the control group showed that the intensities at wavenumber locations of the PEN- and GLU-associated peaks increased. With additional optimization of this technique, SERS may be useful to the measurement of AGE accumulation in bone.
Figure 6.
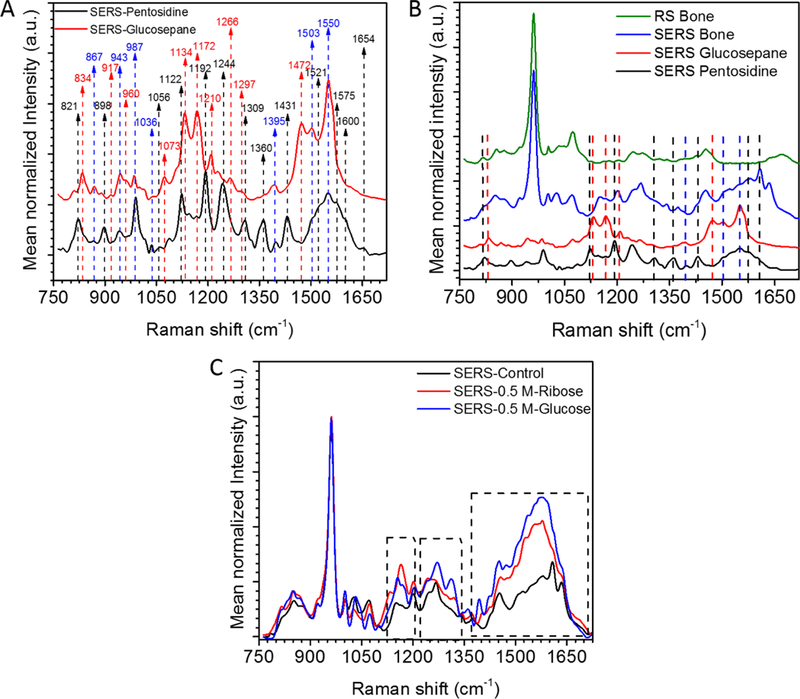
Surface-enhanced Raman spectroscopy (SERS) analysis of bone. (A) SERS spectra of pentosidine and glucosepane standards. (B) Comparison of the Raman spectrum of bone with SERS spectrum of bone, SERS spectrum of pentosidine, and SERS spectrum of glucosepane. Black dashed arrows indicate pentosidine-specific peaks; Red dashed arrows indicate glucosepane-specific peaks; and Blue dashed arrows indicate the peaks shared by both pentosidine and glucosepane. The Spectra are offset for clarity. (C) Comparison of the averaged SERS spectra of 0.5 M ribose and glucose groups with control group. Dashed frames indicate the regions that are sensitive to sugar-incubation. These regions overlap with pentosidine- and glucosepane-associated peak locations.
Table 3:
Pentosidine (PEN) SERS-peak locations compared to previously reported PEN peaks in the literature along with the SERS-peak locations of glucosepane (GLU) and bone specimen. * reported peaks with tilde (~) in the table were read directly from the corresponding figures, these peaks were not reported in those papers.
| Glen et al., 2007 (68) | Iwasaki et al., 2011* (39) | Rubin et al., 2016* (40) |
Pereira et al., 2015 (69) | Soto et al., 2016 (70) | Schmidt et al., 2017* (52) | SERS PEN spectrum in the present study (cm−1) |
SERS GLU spectrum in the present study (cm−1) |
SERS Bone spectrum in the present study (cm−1) |
|---|---|---|---|---|---|---|---|---|
| Pawlak et al., 2008 (71) | ||||||||
| Raman-PEN (cm−1) |
Raman-PEN (cm−1) |
Raman-PEN (cm−1) |
PEN (Theoretical calculation) (cm−1) |
PEN (Theoretical calculation) (cm−1) |
FTIR-PEN (cm−1) |
|||
| 803 | ||||||||
| ~825 | 825 | 821 | 820 | |||||
| 838 | 830 | 835 | 834 | 831 | ||||
| 862 | 852 | ~850 | 867 | 867 | ||||
| 900 | 891 | 914 | 898 | 917 | ||||
| 930 | ~930 | |||||||
| 952 | 943 | 943 | ||||||
| 970 | 967 | 967 | 955 | 960 | ||||
| 995 | 990 | 987 | 987 | |||||
| 1013 | ||||||||
| 1030 | 1036 | 1036 | ||||||
| 1064 | ~1050 | 1056 | ||||||
| 1076 | 1068 | 1073 | ||||||
| 1130 | 1122 | 1134 | 1129 | |||||
| 1150, 1160 | ~1145 | 1155 | 1158 | ~1140 | 1157 | 1150 | ||
| ~1168 | 1172 | 1172 | ||||||
| ~1200 | 1191 | ~1200 | 1192 | |||||
| 1215 | 1210, 1227 | 1215 | 1210 | 1210 | ||||
| 1250 | ~1245 | 1254 | 1244 | |||||
| ~1260 | 1276 | 1276 | 1266 | |||||
| 1300 | 1305 | 1301,1305 | 1304 | 1309 | 1297 | 1304 | ||
| 1326 | ~1320 | 1318,1331 | ~1320 | 1327 | ||||
| 1360 | 1362 | 1365 | ~1350 | 1360 | 1360 | |||
| 1370 | ~1370 | 1374 | ||||||
| ~1389 | 1395 | 1395 | 1395 | |||||
| 1430 | ~1433 | 1436 | ~1440 | 1431 | 1431 | |||
| 1455 | 1463 | 1463 | ||||||
| ~1480 | 1472 | 1472 | ||||||
| ~1495 | ~1500 | 1503 | 1503 | 1503 | ||||
| 1521 | 1521 | |||||||
| 1549 | ~1540 | 1550 | 1550 | 1550 | ||||
| 1583 | 1575 | 1575 | ||||||
| ~1600 | 1600 | 1607 | ||||||
| 1638 | ||||||||
| ~1650 | 1654 | 1654 | ||||||
| 1668 | ||||||||
| ~1690 | 1690 | |||||||
| 1712 |
4. Discussion
AGE accumulation with aging and such diseases as diabetes is thought to contribute to deterioration in the fracture resistance of bone (18, 30). Thus, establishing a non-destructive method for the spatial assessment of AGEs in the organic matrix of bone could facilitate studies into the mechanism by which AGEs degrade bone quality. Unfortunately, the standard application of confocal Raman micro-spectroscopy to human cortical bone samples in the present study was unable to detect non-enzymatic glycation (NEG)-mediated changes in previously identified PEN-associated and CML-associated peaks in rodent bone (Tables 1 and 2). Nonetheless, when using direct quantification methods (LMDS or second derivative), not indirect sub-band fitting (deconvolution), RS was sensitive to AGE accumulation via the Amide I band. Namely, NEG with either ribose or glucose did not affect the so-called matrix maturity ratio (Amide I peak per Amide I shoulder at 1690–1692 cm−1), regardless of the technique to calculate the sub-peak ratio, but rather, NEG-mediated changes in the secondary structure of collagen I was observed in other sub-peak ratios (Amide I peak per Amide I shoulder at 1636–1638 cm−1 and Amide I peak per Amide I shoulder at 1607–1610 cm−1). As an alternative method to assess PEN and GLU in human bone, surface-enhanced RS (SERS) has the potential to detect differences in PEN- and GLU-associated peaks between glycated samples and control samples, albeit the bone had to be homogenized (destructive).
In addition to the changes in the Amide I sub-peak ratios, both ribose and glucose incubation at the higher concentration (0.5 M) increased the Hyp/Pro ratios (~877/855 or 877/922 cm−1) by 5–10% (Tables 1 and 2). In agreement with the present observation that these peaks increase with NEG (Figs. 2 and 3), the intensities of Hyp and Pro peaks were recently reported to increase with in vitro ribose and glucose incubation of type I collagen from rat tail tendons (59). The Hyp/Pro ratio was previously suggested as an indicator of PTMs of collagen I (45, 60) due to the enzymatic processing of proline (the hydroxylation of the proline residue). However, in vitro incubation of cadaveric bone in sugar is not expected to induce any hydroxylation processing of proline, but rather NEG targets lysine and arginine residues of collagen. As suggested by the in vitro glycated type I collagen study (59), the collagen molecules in bone may undergo a slight supra-molecular rearrangement upon in vitro NEG. Such a molecular change could alter the collagen birefringence such that inherent polarization bias of the Raman microscope becomes more sensitive to Hyp than Pro, causing an increase in the intensity of hydroxyproline peak relative to the proline peak.
Because C=O participates in hydrogen bonding with neighboring side chain groups (e.g., N-H) within the triple helix of collagen I (61), the location and shape of the Amide I reflects the modifications and disruptions to organic matrix of bone. However, organized into fibrils with crosslinking, collagen I does not fit the standard secondary structures of proteins (e.g., α helix, β sheet, β turn, coils or so on) (61). Thus, the Amide I band cannot simply be described by the sum of distinct sub-bands comprising these secondary structures. Instead, band-fitting (deconvolution) must be applied to Amide I band in an open-ended manner, and thus, it is an inherently subjective technique (62–64). While we used objective methods in our deconvolution of the Amide I (i.e., number of bands to fit and their relative location were based on the second derivative spectra and the shape of the bands could be any mixture of Lorentzian and Gaussian functions), the sub-band area ratios were not sensitive to NEG-mediated changes in the organic matrix.
A recent FTIR study of human cortical bone (a 14-year-old donor) identified an Amide I sub-peak area ratio (1678/1692) that changes with NEG and correlates with AGE fluorescence when pooling the control and ribose-incubated samples (52). In that study, with the guidance of second derivative spectra, seven sub-bands (1610, 1630, 1645, 1661, 1678, 1692, and 1702 cm−1) were fit to FTIR Amide I band, compared to the typical 4 bands fit to RS-derived Amide I (37). Also unlike the present SERS analysis of PEN (Fig. 6a and 6b), the several prominent peaks in the FTIR spectrum of PEN overlapped the Amide I band of bone. Thus, Amide I band from FTIR potentially reflects PEN content in bone, whereas the Amide I band from RS does not directly reflect the presence of PEN. Moreover, because the Amide I band is more prominent in FTIR than in RS (65), because the removal background fluorescent in RS influences deconvolution (64), and because some underlying low-intensity sub-bands of Amide I may not be properly resolved in RS (66), useful sub-peak ratios in FTIR related to collagen crosslinking (e.g., 1660/1690 and 1678/1692) are not necessarily quantifiable in RS.
In the earlier study by Rubin et. al (40), two AGE parameters (PEN and CML) were identified by RS and found to be different in cortical bone between non-diabetic and diabetic mice (type 1 diabetes at birth), whereas these parameters did not differ among the sugar groups in the present study of human cortical bone. Aside from the difference in species, there could be two reasons for this discrepancy. First, the identified peaks associated with PEN and CML in the previous study were relatively weak. Thus, extracting information from the identified peaks depends on data processing, which likely differed between the present study and the prior study. Second, the sensitivity to resolve subtle peaks depends on how the Raman signals are acquired. There were differences in the RS instruments (e.g., numerical aperture, laser wavelength and power, spectrograph) between the studies such that the spectral resolution and inherent signal-to-noise of the RS system in prior study was probably able to resolve the subtle differences in PEN and CML peaks, whereas our RS system apparently could not.
While there is an extensive literature on the assessment of bone composition using confocal RS (1, 35, 66), there is no consensus or guidelines for processing raw Raman spectra of bone before calculating peak ratios. In the present study, involving a 20X objective, a 830 nm laser, and a spectrograph with 1 cm−1 resolution, the averaged Raman spectrum per bone sample was more sensitive than the individual Raman spectra per sample in detecting NEG-mediated differences in Amide I sub-peak ratios (Supplemental Tables 1 and 2) because averaging Raman spectra reduced signal noise in the spectra (Supplemental Fig. 3A). The selected removal of remaining noise by the S-G filter depends on the order of the polynomial used to fit discrete spectral intensities and the fixed wavenumber range (window size) being fit (54); whereas, the proprietary de-nosing algorithm of LabSpec software involves a self-adapting window size to select an optimal wavenumber range for fitting the spectral data with a second-order polynomial. In the case of relatively noisy data such as the spectra observed for the ribose groups (Supplemental Fig. 3), increasing the order of the polynomial of the S-G improved sensitivity to NEG-related differences in 1668/1638 (Fig. 4A–B), while increasing the frame size of the S-G filter and the order of the polynomial improved sensitivity to difference in 1668/1610 (Fig. 4C–D). As for the less noisy data in the glucose groups (Supp. Fig 3B–D), detecting differences did not depend on filtering process (Fig. 5).
We previously found that ~1670/1640 ratio increased with thermal denaturation and mechanical damage (42), and an increase in this ratio was inversely associated with the toughness of bovine cortical bone (42). However, the current study showed that the same ratio decreased with increasing in AGEs accumulation (Fig. 4) suggesting that the observed NEG-mediated changes in the secondary structure of collagen I should increase toughness. There are three possible explanations for this unexpected outcome. First, others have observed decreases in mature-to-immature enzymatic crosslinks of bone collagen as measured by HPLC but also observed increases in the matrix maturity ratio as measured by RS (16) and FTIR (67), highlighting that these spectroscopic measurements are not absolute, but relative. Second, we incubated human cortical bone from middle age donors (46–60 years old, 55 ± 5 years) such that the organic matrix may already have experienced some age-related deterioration including a decrease in the packing density of collagen molecules. Upon glycation, the packing density of collagen molecules can increase (49), thereby decreasing the freedom of molecular rearrangement of collagen molecules. This may decrease the ratio of ~1670/1640. Lastly, NEG by ribose and glucose induce multiple AGEs, besides pentosidine and glucosepane, and the molecular structure of these AGEs (e.g., CML, MG-H1) may have overlapping Raman peaks in the Amide I band thereby causing decrease in this ratio.
The SERS analysis identified spectral differences among the groups, especially where the PEN- and GLU-associated peaks were located (Fig. 6), indicating a potential for SERS to assess AGEs in bone. However, SERS spectra are generally different than RS spectra of the bone, so interpretation of SERS data is not straightforward. Thus, it still needs technical optimization for quantification purposes.
This present study has some limitations. First, in vitro NEG does not necessarily reflect how AGEs affect the structure of type 1 collagen or NCPs in mineralizing bone in vivo. Second, we do not know at this point whether the observed NEG-induced changes to the secondary structure of collagen I, as determined by RS, affect mechanical properties of human cortical bone. Third, NEG-mediated AGE accumulation is non-specific, though differences in AGEs between ribose and glucose are expected, and we did not quantify other biochemical changes (e.g., glucosepane and CML).
In conclusion, being sensitive to sugar-mediated changes in the Amide I and hydroxyproline-to-proline ratio, Raman spectroscopy can assess the contribution of advanced glycation end-product accumulation to the secondary structure (helical nature) of collagen I structure, especially when using direct sub-peak identification methods, not sub-band fitting methods. However, NEG-sensitive sub-peak ratios, namely the Amide I peak normalized to either the peak at 1636–1638 cm−1 or the peak at 1608–1610 cm−1 are not necessarily a direct measures of non-enzymatic collagen crosslink concentrations or other AGEs. Lastly, the choice of the filtering algorithm to remove spectral noise can affect the ability to detect differences in Amide I sub-peak ratios especially if the signal quality of the spectra is low.
Supplementary Material
Acknowledgments
The presented work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (1I01BX001018) and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR063157). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding agencies. We thank Dr. Nick Ferrell for providing the glucosepane standard.
Footnotes
The authors do not have conflicts of interest pertaining to the present work. All authors have read the conflict of interest policy authorship statement of the journal.
References
- 1.Mandair GS, Morris MD. Contributions of Raman spectroscopy to the understanding of bone strength. BoneKEy reports 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matousek P, Draper ER, Goodship AE, Clark IP, Ronayne KL, Parker AW. Noninvasive Raman spectroscopy of human tissue in vivo. Applied spectroscopy 2006;60(7):758–63. [DOI] [PubMed] [Google Scholar]
- 3.Demers J-LH, Esmonde-White FW, Esmonde-White KA, Morris MD, Pogue BW. Next-generation Raman tomography instrument for non-invasive in vivo bone imaging. Biomedical optics express 2015;6(3):793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley K, Kerns JG, Vinton J, Gikas PD, Smith C, Parker AW, et al. Towards the in vivo prediction of fragility fractures with Raman spectroscopy. Journal of Raman Spectroscopy 2015;46(7):610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito M, Marumo K. Effects of collagen crosslinking on bone material properties in health and disease. Calcified tissue international 2015;97(3):242–61. [DOI] [PubMed] [Google Scholar]
- 6.Trackman PC. Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone. Matrix Biology 2016;52:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sroga GE, Vashishth D. Effects of Bone Matrix Proteins on Fracture and Fragility in Osteoporosis. Current Osteoporosis Reports 2012;10(2):141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karim L, Bouxsein ML. Effect of type 2 diabetes-related non-enzymatic glycation on bone biomechanical properties. Bone 2016;82:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pritchard JM, Willett TL. Pentosidine as a Biomarker for Poor Bone Quality and Elevated Fracture Risk. Biomarkers in Bone Disease 2017:355.
- 10.Sell D, Monnier V. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. Journal of Biological Chemistry 1989;264(36):21597–602. [PubMed] [Google Scholar]
- 11.Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross-link of the senescent human extracellular matrix relationship with diabetes. Journal of Biological Chemistry 2005;280(13):12310–5. [DOI] [PubMed] [Google Scholar]
- 12.Odani H, Shinzato T, Usami J, Matsumoto Y, Frye EB, Baynes JW, et al. Imidazolium crosslinks derived from reaction of lysine with glyoxal and methylglyoxal are increased in serum proteins of uremic patients: evidence for increased oxidative stress in uremia. FEBS letters 1998;427(3):381–5. [DOI] [PubMed] [Google Scholar]
- 13.Duran-Jimenez B, Dobler D, Moffatt S, Rabbani N, Streuli CH, Thornalley PJ, et al. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes 2009;58(12):2893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voziyan P, Brown KL, Chetyrkin S, Hudson B. Site-specific AGE modifications in the extracellular matrix: a role for glyoxal in protein damage in diabetes. Clinical chemistry and laboratory medicine 2014;52(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells-Knecht MC, Thorpe SR, Baynes JW. Pathways of formation of glycoxidation products during glycation of collagen. Biochemistry 1995;34(46):15134–41. [DOI] [PubMed] [Google Scholar]
- 16.McNerny E, Gong B, Morris MD, Kohn DH. Bone Fracture Toughness and Strength Correlate With Collagen Cross‐Link Maturity in a Dose‐Controlled Lathyrism Mouse Model. Journal of Bone and Mineral Research 2015;30(3):455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas J, Burns J, Abel E, Cresswell M, Strain J, Paterson C. Impaired mechanical strength of bone in experimental copper deficiency. Annals of nutrition and metabolism 1993;37(5):245–52. [DOI] [PubMed] [Google Scholar]
- 18.Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS One 2012;7(4):e35047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, et al. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 2005;37(6):825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X. Age‐related factors affecting the postyield energy dissipation of human cortical bone. Journal of Orthopaedic Research 2007;25(5):646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone 2002;31(1):1–7. [DOI] [PubMed] [Google Scholar]
- 22.Willett TL, Sutty S, Gaspar A, Avery N, Grynpas M. In vitro non-enzymatic ribation reduces post-yield strain accommodation in cortical bone. Bone 2013;52(2):611–22. [DOI] [PubMed] [Google Scholar]
- 23.Vashishth D, Gibson G, Khoury J, Schaffler M, Kimura J, Fyhrie D. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 2001;28(2):195–201. [DOI] [PubMed] [Google Scholar]
- 24.Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, et al. Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 2006;38(3):300–9. [DOI] [PubMed] [Google Scholar]
- 25.Poundarik AA, Wu P-C, Evis Z, Sroga GE, Ural A, Rubin M, et al. A direct role of collagen glycation in bone fracture. Journal of the mechanical behavior of biomedical materials 2015;52:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T. Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab 2008;26(1):93–100. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S, Kuroda T, Saito M, Shiraki M. Urinary pentosidine improves risk classification using fracture risk assessment tools for postmenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2011;26(11):2778–84. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism 2009;94(7):2380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism 2008;93(3):1013–9. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann EA, Schaible E, Bale H, Barth HD, Tang SY, Reichert P, et al. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proceedings of the National Academy of Sciences 2011;108(35):14416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fessel G, Li Y, Diederich V, Guizar-Sicairos M, Schneider P, Sell DR, et al. Advanced glycation end-products reduce collagen molecular sliding to affect collagen fibril damage mechanisms but not stiffness. PloS one 2014;9(11):e110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barzilay JI, Bůžková P, Zieman SJ, Kizer JR, Djoussé L, Ix JH, et al. Circulating Levels of Carboxy‐Methyl‐Lysine (CML) Are Associated With Hip Fracture Risk: The Cardiovascular Health Study. Journal of Bone and Mineral Research 2014;29(5):1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unal M, Akkus O. Raman spectral classification of mineral- and collagen-bound water’s associations to elastic and post-yield mechanical properties of cortical bone. Bone 2015;81:315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unal M, Yang S, Akkus O. Molecular spectroscopic identification of the water compartments in bone. Bone 2014;67:228–36. [DOI] [PubMed] [Google Scholar]
- 35.Morris MD, Mandair GS. Raman assessment of bone quality. Clinical Orthopaedics and Related Research® 2011;469(8):2160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oest ME, Gong B, Esmonde-White K, Mann KA, Zimmerman ND, Damron TA, et al. Parathyroid hormone attenuates radiation-induced increases in collagen crosslink ratio at periosteal surfaces of mouse tibia. Bone 2016;86:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, McNerny EG, Terajima M, Raghavan M, Romanowicz G, Zhang Z, et al. Loss of BMP signaling through BMPR1A in osteoblasts leads to greater collagen cross-link maturation and material-level mechanical properties in mouse femoral trabecular compartments. Bone 2016;88:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahata M, Maher JR, Juneja SC, Inzana J, Xing L, Schwarz EM, et al. Mechanisms of bone fragility in a mouse model of glucocorticoid‐treated rheumatoid arthritis: Implications for insufficiency fracture risk. Arthritis & Rheumatology 2012;64(11):3649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasaki Y, Kazama JJ, Yamato H, Fukagawa M. Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone 2011;48(6):1260–7. [DOI] [PubMed] [Google Scholar]
- 40.Rubin MR, Paschalis EP, Poundarik A, Sroga GE, McMahon DJ, Gamsjaeger S, et al. Advanced glycation endproducts and bone material properties in type 1 diabetic mice. PloS one 2016;11(5):e0154700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carden A, Rajachar R, Morris MD, Kohn D. Ultrastructural changes accompanying the mechanical deformation of bone tissue: a Raman imaging study. Calcified Tissue International 2003;72(2):166–75. [DOI] [PubMed] [Google Scholar]
- 42.Unal M, Jung H, Akkus O. Novel Raman Spectroscopic Biomarkers Indicate That Postyield Damage Denatures Bone’s Collagen. Journal of Bone and Mineral Research 2016;31(5):1015–25. [DOI] [PubMed] [Google Scholar]
- 43.Flanagan CD, Unal M, Akkus O, Rimnac CM. Raman spectral markers of collagen denaturation and hydration in human cortical bone tissue are affected by radiation sterilization and high cycle fatigue damage. Journal of the Mechanical Behavior of Biomedical Materials 2017;75:314–21. [DOI] [PubMed] [Google Scholar]
- 44.Lozano L, Pena-Rico M, Heredia A, Ocotlan-Flores J, Gomez-Cortes A, Velazquez R, et al. Thermal analysis study of human bone. Journal of materials science 2003;38(23):4777–82. [Google Scholar]
- 45.Buckley K, Matousek P, Parker AW, Goodship AE. Raman spectroscopy reveals differences in collagen secondary structure which relate to the levels of mineralisation in bones that have evolved for different functions. Journal of Raman Spectroscopy 2012;43(9):1237–43. [Google Scholar]
- 46.Gong B, Oest ME, Mann KA, Damron TA, Morris MD. Raman spectroscopy demonstrates prolonged alteration of bone chemical composition following extremity localized irradiation. Bone 2013;57(1):252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barth HD, Zimmermann EA, Schaible E, Tang SY, Alliston T, Ritchie RO. Characterization of the effects of x-ray irradiation on the hierarchical structure and mechanical properties of human cortical bone. Biomaterials 2011;32(34):8892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka S, Avigad G, Brodsky B, Eikenberry EF. Glycation induces expansion of the molecular packing of collagen. J Mol Biol 1988;203(2):495–505. [DOI] [PubMed] [Google Scholar]
- 49.Bai P, Phua K, Hardt T, Cernadas M, Brodsky B. Glycation alters collagen fibril organization. Connective tissue research 1992;28(1–2):1–12. [DOI] [PubMed] [Google Scholar]
- 50.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mechanisms of ageing and development 1998;106(1):1–56. [DOI] [PubMed] [Google Scholar]
- 51.Wilson SL, Guilbert M, Sulé-Suso J, Torbet J, Jeannesson P, Sockalingum GD, et al. A microscopic and macroscopic study of aging collagen on its molecular structure, mechanical properties, and cellular response. The FASEB Journal 2014;28(1):14–25. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt F, Zimmermann E, Campbell G, Sroga G, Püschel K, Amling M, et al. Assessment of collagen quality associated with non-enzymatic cross-links in human bone using Fourier-transform infrared imaging. Bone 2017;97:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lieber CA, Mahadevan-Jansen A. Automated method for subtraction of fluorescence from biological Raman spectra. Applied spectroscopy 2003;57(11):1363–7. [DOI] [PubMed] [Google Scholar]
- 54.Savitzky A, Golay MJ. Smoothing and differentiation of data by simplified least squares procedures. Analytical chemistry 1964;36(8):1627–39. [Google Scholar]
- 55.Paschalis E, Verdelis K, Doty S, Boskey A, Mendelsohn R, Yamauchi M. Spectroscopic characterization of collagen cross‐links in bone. Journal of Bone and Mineral Research 2001;16(10):1821–8. [DOI] [PubMed] [Google Scholar]
- 56.Granke M, Makowski AJ, Uppuganti S, Does MD, Nyman JS. Identifying novel clinical surrogates to assess human bone fracture toughness. Journal of Bone and Mineral Research 2015;30(7):1290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Creecy A, Uppuganti S, Merkel AR, O’Neal D, Makowski AJ, Granke M, et al. Changes in the Fracture Resistance of Bone with the Progression of Type 2 Diabetes in the ZDSD Rat. Calcified tissue international 2016;99(3):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang S, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone 2007;40(4):1144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guilbert M, Said G, Happillon T, Untereiner V, Garnotel R, Jeannesson P, et al. Probing non-enzymatic glycation of type I collagen: a novel approach using Raman and infrared biophotonic methods. Biochimica et Biophysica Acta (BBA)-General Subjects 2013;1830(6):3525–31. [DOI] [PubMed] [Google Scholar]
- 60.Olejnik C, Falgayrac G, During A, Cortet B, Penel G. Doses effects of zoledronic acid on mineral apatite and collagen quality of newly-formed bone in the rat’s calvaria defect. Bone 2016;89:32–9. [DOI] [PubMed] [Google Scholar]
- 61.Shoulders MD, Raines RT. Collagen structure and stability. Annual review of biochemistry 2009;78:929–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Surewicz WK, Mantsch HH, Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry 1993;32(2):389–94. [DOI] [PubMed] [Google Scholar]
- 63.Meier RJ. On art and science in curve-fitting vibrational spectra. Vibrational spectroscopy 2005;39(2):266–9. [Google Scholar]
- 64.Maddams W The scope and limitations of curve fitting. Applied Spectroscopy 1980;34(3):245–67. [Google Scholar]
- 65.Gamsjaeger S, Robins SP, Tatakis DN, Klaushofer K, Paschalis EP. Identification of pyridinoline trivalent collagen cross-links by Raman microspectroscopy. Calcified tissue international 2017;100(6):565–74. [DOI] [PubMed] [Google Scholar]
- 66.Paschalis E, Gamsjaeger S, Klaushofer K. Vibrational spectroscopic techniques to assess bone quality. Osteoporosis International 2017:1–17. [DOI] [PubMed]
- 67.Paschalis E, Tatakis D, Robins S, Fratzl P, Manjubala I, Zoehrer R, et al. Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone 2011;49(6):1232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glenn JV, Beattie JR, Barrett L, Frizzell N, Thorpe SR, Boulton ME, et al. Confocal Raman microscopy can quantify advanced glycation end product (AGE) modifications in Bruch’s membrane leading to accurate, nondestructive prediction of ocular aging. The FASEB journal 2007;21(13):3542–52. [DOI] [PubMed] [Google Scholar]
- 69.Pereira L, Soto C, Santos LD, Favero P, Martin A. Confocal Raman spectroscopy as an optical sensor to detect advanced glycation end products of the skin dermis. Sensor Letters 2015;13(9):791–801. [Google Scholar]
- 70.Soto CAT, Pereira L, dos Santos L, Rajasekaran R, Fávero P, Martin AA. DFT: B3LYP/3–21G theoretical insights on the confocal Raman experimental observations in skin dermis of healthy young, healthy elderly, and diabetic elderly women. Journal of biomedical optics 2016;21(12):125002-. [DOI] [PubMed] [Google Scholar]
- 71.Pawlak AM, Glenn JV, Beattie JR, McGarvey JJ, Stitt AW. Advanced glycation as a basis for understanding retinal aging and noninvasive risk prediction. Annals of the New York Academy of Sciences 2008;1126(1):59–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


