Abstract
Quantitative autoradiography was used to visualize the anatomical distribution of adenosine receptors labeled by the carboxylic acid congener of 1,3-dipropylxanthine, [3H](8-(p-carboxymethyloxy)phenyl-1,3 dipropylxanthine) ([3H]XCC) in the rat brain. [3H]XCC was observed to specifically bind to rat brain sagittal sections in a heterogeneous pattern. Saturation experiments revealed that [3H]XCC binds with nanomolar affinity to 20-μm frozen tissue sections with the highest binding densities occurring in the hippocampus and cerebellum. Both the binding characteristics and regional receptor distribution obtained with [3H]XCC demonstrate the potential usefulness of this new ligand in the study of adenosine A1 receptors.
Keywords: Methylxanthine; Xanthine congener; 1,3-Dipropylxanthine; Adenosine; Receptor; Autoradiography; Rat
The methylxanthine (e.g. caffeine and theophylline) are widely used psychoactive agents which exert their central nervous system stimulation effects primarily through an antagonist action at adenosine receptors [12, 13]. However, the use of alkylxanthines and their derivatives to characterize central adenosine receptors has not been widespread. This is due primarily to: (1) the relatively low potency of these ligands for adenosine receptors [12, 13]; (2) their non-selective antagonist actions at both adenosine A1 and A2 receptors [12, 13]; (3) their low water solubility and high lipophilicity [6-8]; and (4) the low specific activity of the available radioligand, 1,3-diethyl-8-phenylxanthine (DPX) [6-8].
Recently, a functionalized congener approach to the design of 1,3-dipropylxanthine has been synthesized which overcomes many of the technical difficulties associated with the use of xanthines as radioligands [6-8]. In particular, a tritiated carboxylic acid congener of 1,3-dipropylxanthine (XCC) has been shown to bind specifically to rat brain homogenates with high affinity and apparent selectivity for adenosine A1 receptors [6, 7]. We now report that [3H]XCC exhibits a heterogeneous pattern of binding to rat brain sections, as revealed by quantitative autoradiography, that is consistent with the labeling of adenosine A1 receptors.
Adult male Sprague–Dawley (TAC:SD) rats weighing betwen 200–250 g were maintained in suspended metal cages with free access to food and water. Rats were housed in a humidity- and temperature-controlled colony room on a 12-h light/dark (lights on at 07.00 h) cycle. Rats were sacrificed with carbon dioxide and transcardially perfused with phosphate-buffered saline. Brains were quickly removed and frozen over powdered dry-ice. Twenty-μm sagittal sections were thaw-mounted onto gelatin coated slides and stored at −70°C.
On the day of the assay, tissue sections were warmed to room temperature and allowed to dry. Sections were preincubated in 50 mM Tris-HCl buffer (pH = 7.4) containing 2 IU/ml adenosine deaminase, at 37°C for 30 min. Saturation analysis of specific [3H]XCC (149 Ci/mmol) binding was then conducted by incubating tissue sections in 7 concentrations of ligand over the range of 0.03–10 nM. Non-specific binding was determined in the presence of 100 μM R-phenylisopropyladenosine (R-PIA). Incubations were conducted in 50 mM Tris-HCl buffer (pH=7.4) for 1 h at 23°C. Incubations were terminated by washing tissue sections four times for 5 min each in 50 mM Tris-HCl buffer, pH = 7.4, at 0°C. Tissue sections were then rapidly dried under a stream of warm air and apposed to tritium sensitive film (Ultrofilm, LKB, Bromma) for 21 days.
Quantitative analysis of the resulting autoradiographic images was performed using an EyeCom II Image Analysis System (Spatial Data Systems, Melbourne, FL). Grey values obtained from each region were converted to a measure of radioactivity (dpm) per milligram of brain tissue based upon internal tritium brain paste standards present on each sheet of film. Saturation parameters (Kd and Bmax) for each brain region were obtained by analysis with the iterative curve fitting program LUNDON I [10].
[3H](8-(p-Carboxymethyloxy)phenyl-1,3-dipropylxanthine ([3H]XCC) (149 Ci/mmol) was synthesized as previously described [7]. Adenosine deaminase (type III) was obtained from Boehringer-Mannheim (Indianapolis, IN) and R-PIA was obtained from Research Biochemicals (Wayland, MA). All other chemicals and reagents were obtained from Fisher Scientific (Springfield, NJ).
[3H]XCC bound specifically to rat brain sagittal sections in a saturable fashion with nanomolar affinity (Fig. 1). The average affinity (dissociation constant) of [3H]XCC across the brain areas examined was (Kd) = 3.5 ± 0.7 nM. At this concentration, specific binding, determined in presence of 100 μM R-PIA, amounted to approximately 90% of total binding. In all brain regions examined, [3H]XCC binding data were best fit to a one-site model [10].
Fig. 1.

Saturation isotherm of [3H]XCC binding in CA1, region of rat hippocampus. Sections were incubated with varying concentrations of ligand in 50 mM Tris HCl, pH ±7.4, in the presence or absence of 100 μM R-PIA, for 1 h at 23°C. Data were obtained from triplicate determinations from 3 animals. Filled squares represent specific binding. Open squares represent total binding and filled circles indicate non-specific binding. The inset is a Scatchard plot of these data.
[3H]XCC exhibited a heterogeneous pattern of binding in rat brain sections (Figs. 2 and 3 and Table I). Receptor density was highest in the molecular layer of the cerebellum and the CA1 region of the hippocampus. Moderate concentrations of [3H]XCC binding sites were observed in CA3 and dentate gyrus regions of the hippocampus, dorsal thalamic nuclei, cerebral cortex, and the granular layer of the cerebellum. Relatively low concentrations of [3H]XCC binding sites were observed in the caudate nucleus. Lastly, [3H]XCC exhibited a dissociation constant of 2–4 nmol in all brain regions examined except in the molecular layer of the cerebellum, where there was a slight but significant decrease in receptor affinity.
Fig. 2.
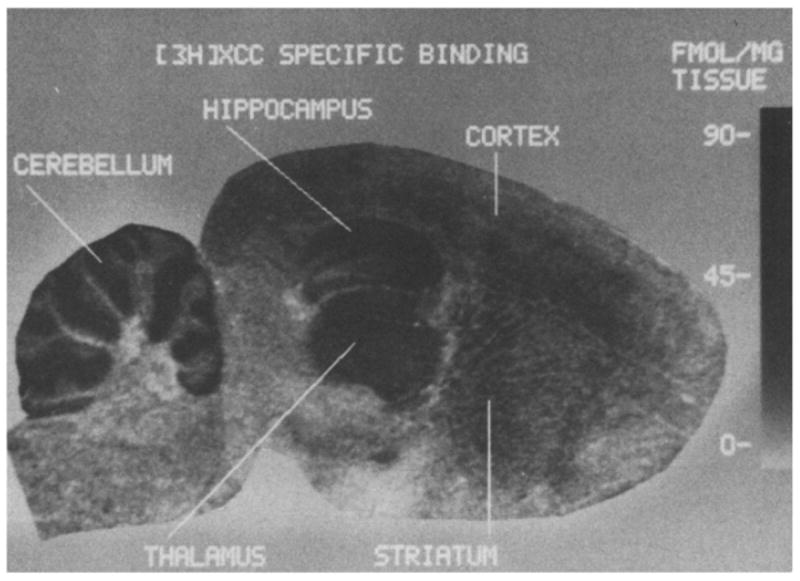
Autoradiogram of specific [3H]XCC (4 nM) binding in a rat brain sagittal section. The density of [3H]XCC binding sites is indicated by the gray-value concentration scale to the right of the figure. Specific [3H]XCC binding was revealed by digital subtraction autoradiography in which the image of specific binding was obtained through subtraction of the linearized non-specific binding image from the total binding image [1, 4].
Fig. 3.
Linearized autoradiographic image of non-specific [3H]XCC binding in a rat brain sagittal section.
TABLE 1.
REGIONAL BINDING PARAMETERS FOR [3H]XCC FROM RAT SAGITTAL SECTIONS
| Region | Kd (nM) | Bmax (fmol/mg tissue) |
|---|---|---|
| Caudate nucleus | 2,14 (0.19) | 19 (5) |
| Cerebral cortex | ||
| Lamina IV | 2.96 (0,70) | 24 (5) |
| Hippocampus | ||
| CA1 | 3.71 (0.87) | 76 (12)* |
| CA3 | 2.16 (0.19) | 38 (2) |
| Dentate gyrus | 2.97 (0.08) | 33 (1) |
| Thalamus | ||
| Dorsolateral nuclei | 2.13 (0.24) | 38 (5) |
| Cerebellum | ||
| Molecular layer | 7.70 (1.00)* | 95 (8)* |
| Granular layer | 3.98 (0.72) | 33 (1) |
Values represent the mean (± 1 S.E.M.) dissociation constant (Kd) and number of binding sites (Bmax) for 3 animals.
Indicates values significantly different from CA3 region of the hippocampus (P < 0.05, Student’s t-test).
It is interesting to note that in the present study both the affinity of [3H]XCC for A1 receptors and the degree of specific binding were higher than previously reported in homogenate preparations [7]. The present autoradiographic data demonstrate that [3H]XCC binds with high specificity and affinity to a heterogeneously distributed population of receptors in the rat brain, and indicate that [3H]XCC may provide an important advance in the use of radiolabeled adenosine antagonists in the study of adenosine receptor systems. Preliminary pharmacological characterization of [3H]XCC binding to rat brain sections indicates a binding profile consistent with the labeling of adenosine A1 receptors (data not shown). Purinergic agonists demonstrated nanomolar potency in the inhibition of [3H]XCC binding with the following rank order of potency: R-PIA ≥ cyclopentyladenosine > 5′-N-ethylcarboxamidoadenosine ≥ 2-chloroadenosine > S-PIA. Stereospecificity of [3H]XCC binding was indicated by a 20-fold difference in the potency between R-PIA and S-PIA.
Several previous autoradiographic studies of rat brain adenosine receptors have used [3H]cyclohexyladenosine (CHA) to label adenosine A1 receptors [5, 9, 11]. The regional concentration of [3H]CHA binding sites is greatest in the stratum moleculare of the hippocampal CAI region and the molecular layer of the cerebellum. Moderate concentrations of [3H]CHA binding are found in the dentate gyrus, thalamus, cerebral cortex, striatum, and granular layer of the cerebellum. Low levels of [3H]CHA binding are found in the brainstem [5, 9, 11]. In the present study, the regional distribution of [3H]XCC binding sites was found to be highly consistent with the regional distribution of adenosine A1 receptors labeled by [3H]CHA [5]. Specifically, there is a significant positive correlation between the present regional distribution of [3H]XCC binding sites and two independent quantitative assessments of the distribution of adenosine A1 receptors labeled by [3H]CHA, r=0.85–0.87, P < 0.05 [9, 11].
A neuromodulatory role for adenosine in the central nervous system has received much support from the identification of purinergic cell surface receptors. However, the study of purinergic systems has been difficult due to the lack of selective ligands for adenosine A1 and A2 receptors. Both the binding characteristics and regional receptor densities obtained with [3H]XCC demonstrate the potential utility of this new ligand in the study of central adenosine A1 receptors. The high specific activity of [3H]XCC coupled with its apparent high affinity for adenosine receptors should provide a new tool for the study of peripheral adenosine systems as well. In addition, the recent identification of 8-cyclopentyl-1,3-dipropylxanthine [2] and PD 115,199 [3] as selective antagonists of adenosine A1 and A2 receptors, respectively, will allow a more rigorous evaluation of purinergic function in the mammalian brain.
References
- 1.Altar CA, O’Neil S, Walter R, Marshall J. Brain dopamine and serotonin receptor sites revealed by digital substraction autoradiography. Science. 1986;228:597–600. doi: 10.1126/science.2580352. [DOI] [PubMed] [Google Scholar]
- 2.Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LA, Hartman JD, Hays SJ, Huang CC. Binding of the A-1 selective antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn-Schmiedeberg’s Arch Pharmacol. 1987;335:59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- 3.Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LA, Hays SJ. PD 115, 199: an antagonist ligand for adenosine A-2 receptors, Naunyn-Schmiedeberg’s. Arch Pharmacol. 1987;335:64–69. doi: 10.1007/BF00165038. [DOI] [PubMed] [Google Scholar]
- 4.Goodman RR, Pasternack GW. Visualization of μ1 opiate receptors in rat brain by using a computerized autoradiographic substraction technique. Proc Natl Acad Sci USA. 1985;82:6667–6671. doi: 10.1073/pnas.82.19.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman RR, Snyder SH. Autoradiographic localization of adenosine receptors in rat brain using [-3H]cyclohexyladenosine. J Neurosci. 1982;2:1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson KA, Kirk LL, Padgett WL, Daly J. Functionalized congeners of 1,3-diakylxanthines: preparation of analogs with high affinity for adenosine receptors. J Med Chem. 1985;28:1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson KA, Ukena D, Daly J, Kirk KL. Synthesis of tritiated congeners of 1,3-dipropylxanthine having high affinity at adenosine receptors. J Label Comp Radiopharmacol. 1986;23:519–526. [Google Scholar]
- 8.Jacobson KA, Ukena D, Kirk, Daly J. [3H]Xanthine amine congener of 1,3-dipropyl-8-phenylxanthine: an antagonist radioligand for adenosine receptors. Proc Natl Acad Sci USA. 1986;83:4089–4093. doi: 10.1073/pnas.83.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KS, Reddington M. Autoradiographic evidence for multiple CNS binding sites for adenosine derivatives. Neuroscience. 1986;19:535–545. doi: 10.1016/0306-4522(86)90279-4. [DOI] [PubMed] [Google Scholar]
- 10.Lundeen JE, Gordon JH. Computer analysis of binding data. In: O’Brien RA, editor. Receptor Binding in Drug Research. Dekker; New York: 1985. pp. 31–49. [Google Scholar]
- 11.Snowhill EW, Williams M. [3H]Cyclohexyladenosine binding in rat brain: a pharmacological analysis using quantitative autoradiography. Neurosci Lett. 1986;68:41–46. doi: 10.1016/0304-3940(86)90226-0. [DOI] [PubMed] [Google Scholar]
- 12.Snyder SH. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- 13.Williams M. Purinergic receptors in the CNS. In: Boulton AA, Baker GB, Hrina P, editors. Neuromethods. Vol. 4. Receptor Binding, Hurnana; Clifton, NJ: 1986. pp. 365–13. [Google Scholar]


