Abstract
With the growing recognition of the extent and prevalence of human cerebellar disorders, an understanding of developmental programs that build the mature cerebellum is necessary. In this chapter we present an overview of the basic epochs and key molecular regulators of the developmental programs of cerebellar development. These include early patterning of the cerebellar territory, the genesis of cerebellar cells from multiple spatially distinct germinal zones, and the extensive migration and coordinated cellular rearrangements that result in the formation of the exquisitely foliated and laminated mature cerebellum. This knowledge base is founded on extensive analysis of animal models, particularly mice, due in large part to the ease of genetic manipulation of this important model organism. Since cerebellar structure and function are largely conserved across species, mouse cerebellar development is highly relevant to humans and has led to important insights into the developmental pathogenesis of human cerebellar disorders. Human fetal cerebellar development remains largely undescribed; however, several human-specific developmental features are known which are relevant to human disease and underline the importance of ongoing human fetal research.
Keywords: cerebellum, development, malformation, mouse, human, signaling, histogenesis, foliation, neuronal specification
INTRODUCTION
Human genetic studies and novel imaging techniques have led to an increased recognition of a diversity of cerebellar-related malformations and disorders, and a growing understanding of the repertoire of brain regions and functions influenced by cerebellar activity. An understanding of the developmental processes that generate the mature cerebellum is therefore essential to contextualize human cerebellar disease.
Although adult cerebellar morphology and basic cerebellar circuitry have been described for more than 100 years (Lugaro, 1894; Ramon y Cajal, 1909–1911), the molecular and cellular mechanisms which drive cerebellar development have only more recently begun to be elucidated (Leto et al., 2016). Much of our current understanding of the cellular and molecular basis of human cerebellar development and disease has come from analyses of mutant mice and other model vertebrates based on the conservation of cerebellar lamination, foliation, and histogenesis across evolution.
Several features of the cerebellum have made it particularly amenable to experimental manipulation in mice. The cerebellum has relatively simple architecture, with stereotyped cell morphologies, lamination, and circuitry. Although the cerebellum is involved in a number of nonmotor functions (Koziol et al., 2014), spontaneous and targeted mouse mutants with cerebellar dysfunction are relatively easy to identify based on their abnormal motor phenotypes. As a result, more than 800 ataxic mouse strains representing mutant alleles in more than 450 genes (www.informatics.jax.org) provide a rich resource for analysis of cerebellar developmental biology and physiology. Additionally, with the rapid evolution of genome engineering technologies, including CRISPR/Cas9 (Singh et al., 2015; Joyner, 2016), it is now easier than ever to generate mouse models to dissect the basic biology of cerebellar development and directly model human cerebellar disease.
In this chapter, we will discuss three major overlapping stages of cerebellar development with a focus on the mouse literature. First, we will review how the cerebellar territory of the neural tube is defined and patterned by the expression of a series of transcription factors and signaling molecules. We will then describe how three germinal zones arise within this territory and undergo extensive proliferation to give rise to cell types of the adult cerebellum, followed by an overview of how these cells then undergo migration and acquire their appropriate positions within the cerebellum as they initiate their differentiation programs. The establishment of cerebellar efferents and afferents and the cerebellar circuit itself is beyond the scope of this review and is covered in detail elsewhere in this book.
Our current mouse-centric knowledge base of cerebellar development has enabled a multitude of insights into the pathogenesis of human cerebellar disease. However, mice are not human. We therefore have chosen to end our review by highlighting the limited literature available specifically describing human fetal cerebellar development. We will emphasize species similarities and differences that may be relevant to disease and underline the importance of ongoing human fetal research.
OVERVIEW OF CEREBELLAR DEVELOPMENT
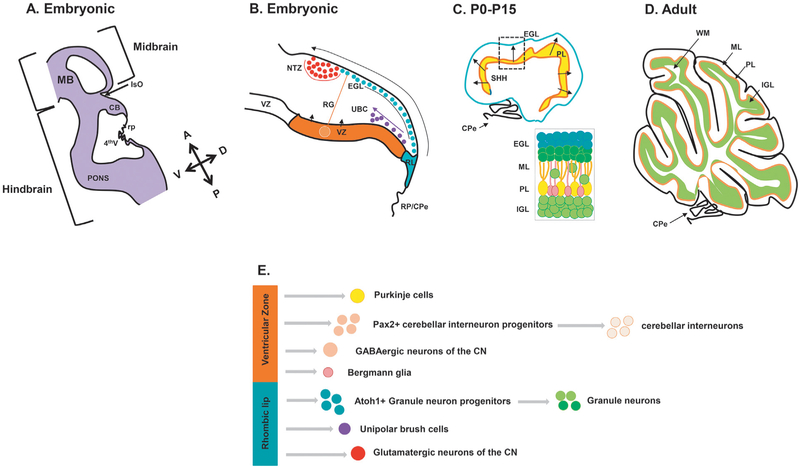
The spatial complexities of cerebellar morphogenesis and the interrelatedness of cellular populations can be difficult to conceptualize. Therefore, before diving into the details of cerebellar developmental biology, Figure 2.1 provides an overview of the major epochs of development.
Fig. 2.1.
(A) Schematic representation of an e12.5 mouse cerebellum (CB) sectioned along the sagittal plane. The cerebellum is derived from the dorsal region rhombomere 1 under the influence of signaling factors from the isthmic organizer (IsO) and roof plate (RP). 4th V, fourth ventricle; MB, midbrain. (B) The developing cerebellum has two zones of neurogenesis, the ventricular zone (VZ) and the rhombic lip (RL). The cerebellar ventricular zone that consists of a lining radial glia (RG) gives rise to all cerebellar GABAergic neurons and interneurons. GABAergic cerebellar nuclei neurons are produced first, followed by Purkinje cells and PAX2-expressing cerebellar interneuron progenitors. Bergmann glia are also derived from the cerebellar ventricular zone. The rhombic lip on the other hand gives rise to the three major glutamatergic neuronal subtypes that populate the cerebellum. Firstly, cerebellar nuclei projection neurons migrate from the rhombic lip into the nuclear transitory zone (NTZ) over the anlage as the rostral migratory stream. As embryonic development proceeds, granule neuron progenitors (GNPs) next migrate out of the rhombic lip between embryonic days 12.5 and 16. These cell progenitors migrate tangentially under the pial surface to establish the external granule layer (EGL) of the developing cerebellum in an anterior-to-posterior manner. The rhombic lip also gives rise to unipolar brush cells (UBC) that migrate into the cerebellar anlage. (C) The EGL is a secondary germinal zone, or transit amplifying center. The EGL is composed of two sublayers – a proliferating external zone and an inner differentiating zone. Proliferation of GNPs takes place during postnatal days P0–P14. This proliferation is largely driven by the mitogen sonic hedgehog (SHH) secreted from Purkinje cells which have formed the Purkinje layer (PL) under the EGL. (D) Proliferation of GNPs in the EGL is responsible for the dramatic size increase of the postnatal mouse cerebellum. As granule neurons exit the cell cycle, they migrate tangentially within the inner EGL and then exit the EGL, migrating radially inward to settle below the developing Purkinje cell layer to form the internal granule layer (IGL), resulting in the final laminar arrangement of the mature cerebellum. (E) Schematic representation of the multiple cell types that arise in the cerebellar ventricular zone and rhombic lip. CN, cranial nerve; CPe, choroid plexus epithelium; ML, medal/lateral; WM, white matter.
The cerebellum is a derivative of the anteriormost dorsal hindbrain and development starts as soon as the mid/hindbrain boundary is established at neural plate stages. The isthmic organizer (IsO), a transient embryonic signaling center that is a derivative of this boundary, secretes several molecules to define the cerebellar territory along the anterior–posterior axis of the developing neural tube (Sato and Joyner, 2009; Harada et al., 2016). The roof of the hindbrain over the fourth ventricle roof plate is another signaling center required to establish the dorsal–ventral extent of the cerebellum within the anterior hindbrain (Chizhikov et al., 2006). In overlapping waves of neurogenesis, from e10.5 in the mouse until late mouse embryogenesis, progenitors in the cerebellar ventricular zone give rise to GABAergic neurons of the cerebellar nuclei, and Purkinje cells in addition to cerebellar interneuron progenitors, which migrate into the developing cerebellar anlage (Butts et al., 2014b; Green and Wingate, 2014).
By e10.5 a secondary germinal zone, the cerebellar rhombic lip, is established at the junction of the cerebellar ventricular zone and dorsal roof plate. The glutamatergic neurons of the cerebellar nuclei emerge from this zone and migrate over the top of the anlage to form the nuclear transitory zone, which is a staging zone for cerebellar nuclei assembly. By e11.5, large numbers of granule neuron progenitors (GNPs) emerge from the rhombic lip to migrate over the anlage to form the external granule layer (EGL), which resides on the pial surface of the developing anlage, but under the developing meninges. Within the EGL, GNPs divide extensively. In mice, peak EGL proliferation occurs around postnatal day (P) 7 in mice and is complete by P15. Exponential GNP proliferation in the EGL drives cerebellar growth and foliation. GNP differentiation occurs continually from P0 to P14. As granule neurons exit the cell cycle, they migrate tangentially within the inner EGL and then exit the EGL migrating radially inward to settle below the developing Purkinje cell layer to form the internal granule layer (IGL), resulting in the final laminar arrangement of the mature cerebellum (Millen and Gleeson, 2008; Butts et al., 2014a; Marzban et al., 2014; Leto et al., 2016).
INITIAL DEFINITION OF THE CEREBELLAR TERRITORY BY TRANSIENT EMBRYONIC SIGNALING CENTERS
The cerebellar territory emerges from the anteriormost segment (rhombomere 1) of the hindbrain.
The mid/hindbrain boundary is the first segmental division of the developing neural plate and forms due to activation of a gene cascade at neural plate stages culminating in juxtaposed expression of two key transcription factors: orthodenticle homeobox 2 (Otx2) and gastrulation brain homeobox 2 (Gbx2). Otx2 is expressed in the forebrain and midbrain, with its posterior limit at the presumptive mid/hindbrain boundary. Concurrently, Gbx2 is expressed in the posterior central nervous system, with an anterior boundary at the presumptive mid/hindbrain boundary. Loss of Otx2 shifts the mid/hindbrain boundary anteriorly, enlarging the cerebellar anlage at the expense of posterior midbrain tissue and loss of Gbx2 shifts the mid/hindbrain boundary posteriorly, causing an expansion of the midbrain at the expense of cerebellar tissue. The establishment of juxtaposed Otx2 and Gbx2 results in formation of a transient signaling center called the IsO straddling the mid/hindbrain boundary. The IsO secretes fibroblast growth family 8 and WNT1, which are required for cell survival and pattern the adjacent tissue from e8 to 11.5 in mice (Sato and Joyner, 2009; Harada et al., 2016).
The posterior limit of the cerebellum is defined by Hoxa2, which is expressed in the caudal central nervous system with its anterior boundary at the rhombomere 1/2 boundary. Loss of Hoxa2 results in a caudal enlargement of the cerebellum at the expense of more posterior hindbrain structures (Gavalas et al., 1997). Ectopic Hoxa2 expression in rhombomere 1 suppresses the specification of cerebellar neurons (Eddison et al., 2004). Hoxa2 expression is normally excluded from rhombomere 1 via repression by fibroblast growth family 8 from the IsO (Irving and Mason, 2000; Mason et al., 2000).
Much less is known regarding the mechanisms which define the dorsal nature of the cerebellum; however, the dorsal roof plate, another transient signaling center, clearly plays a role. The roof plate forms on the dorsal midline of the early neural tube and expresses bone morphogenetic protein (BMP) and WNT-secreted factors. In rhombomere 1, roof plate-derived Wnt expression is required to drive early cerebellar anlage ventricular zone proliferation, while secreted BMP gene expression is required to induce the cerebellar rhombic lip and correctly pattern expression of pancreatic transcription factor (Ptf1a) in the ventricular zone of the nascent cerebellar anlage (Mishima et al., 2009; Millen et al., 2014; Yamada et al., 2014). Loss of Ptf1a leads to transformation of cerebellar ventricular zone fates into more ventral brainstem fates (Millen et al., 2014). To date, Ptf1a is the sole known gene defining the ventral boundary of the cerebellar territory of rhombomere 1, although the molecular cascades that precisely regulate this ventral limit of Ptf1a cerebellar expression remain unknown.
THE CEREBELLAR RHOMBIC LIP GIVES RISE TO CEREBELLAR GLUTAMATERIC NEURONS
The cerebellar rhombic lip, which forms adjacent to the fourth ventricle roof plate in rhombomere 1, is one of two primary progenitor zones in the developing cerebellum. Fate-mapping studies have demonstrated that the cerebellar rhombic lip gives rise to cells that populate extra-cerebellar regions of the hindbrain, including the pontine nuclei (Machold and Fishell, 2005; Wang et al., 2005; Rose et al., 2009). It also gives rise to the three major glutamatergic neuronal subtypes that populate the cerebellum. These lineages are derived in overlapping waves of neurogenesis. Beginning at ~e10.5 and terminating at ~e12.5, cerebellar nuclei projection neurons migrate from the rhombic lip into the aforementioned nuclear transitory zone over the anlage as the rostral migratory stream. As embryonic development proceeds, GNPs next migrate out of the rhombic lip between e12.5 and e16. These cell progenitors migrate tangentially under the pial surface to establish the EGL of the developing cerebellum in an anterior to posterior manner. Additionally, between e13.5 and the early neonatal period, the rhombic lip gives rise to unipolar brush cells (UBCs), which migrate through the developing white matter in the nascent cerebellum and eventually settle in the IGL (Englund et al., 2006).
BMP signaling from the roof plate and choroid plexus is essential to induce the rhombic lip and the expression of the bHLH transcription factor gene atonal homologue 1 (Atoh1) (Alder et al., 1996; Chizhikov et al., 2006,2010). Atoh1 is expressed in all rhombic lip derivatives as they exit the rhombic lip and Atoh1 is currently the only gene known to be required to generate all derivatives of the rhombic lip (Wang et al., 2005; Rose et al., 2009). In early Atoh1-expressing cells have many downstream gene targets, and a molecular cascade involving paired box protein 6 (PAX6), NEUROD, T-box brain 1 (TBR1), and TBR2 proteins is variably expressed within each rhombic lip lineage contributing to cell type-specific morphologies and migratory pathways (Englund et al., 2006). For example, PAX6 is expressed in all rhombic lip derivatives. It is not required to generate rhombic lip-derived cerebellar nuclei neurons, but rather is needed for their survival as they transit to the nuclear transitory zone over the pial surface (Yeung et al., 2016). PAX6 is also not required to generate GNPs that form the EGL but regulates their migration and the migration of granule neurons as they differentiate from GNPs (Swanson and Goldowitz, 2011). In contrast, PAX6 is required to generate UBCs (Yeung et al., 2016). Although ATOH1 is required for all rhombic lip lineages, there is no evidence that ATOH1 actually regulates rhombic lip-derivative identity. There is, however, emerging evidence that cell fate identity is imparted within the rhombic lip itself, prior to ATOH1 expression. Although the mechanisms directing rhombic lip neurogenesis remain largely unknown, Lmx1a and Wls have been shown to be involved in rhombic lip maintenance and cell fate determination (Chizhikov et al., 2010; Yeung et al., 2014, 2016; Yeung and Goldowitz, 2017).
External granule layer development and cerebellar foliation
Given their sheer number, cerebellar granule neurons represent perhaps the most important rhombic lip derivative. Indeed, of all cerebellar cell types, the development of this population is the most studied. GNPs continue to proliferate as they exit the rhombic lip, migrating over the cerebellar anlage to form the EGL. Rhombic lip exit is at least partially directed by SDF1a – a chemotaxic signal secreted from the overlying pia and posterior fossa mesenchyme and received by the CXCR4 receptor expressed by GNPs (Hartmann et al., 1998a; Zou et al., 1998; Reiss et al., 2002; Zhu et al., 2002; Vilz et al., 2005; Tiveron and Cremer, 2008; Yu et al., 2010; Haldipur etal., 2017). The anterior limit of migration at the mid/hindbrain boundary is at least partially controlled by UNC5H3 (Ackerman et al., 1997; Leonardo et al., 1997). Temporal fate mapping of rhombic lip progenitors using inducible cre recombinase under the control of an Atoh1 promoter shows that GNPs generated the earliest tend to populate the anterior lobes of the adult cerebellum, whilst the distribution of the cells exiting the rhombic lip progressively becomes more caudal as development progresses (Machold and Fishell, 2005).
The EGL is a secondary germinal zone, or transit amplifying center. The EGL is composed of two sublayers – a proliferating external zone and an inner differentiating zone. Proliferating GNPs undergo a dramatic clonal expansion, which in mice takes place during postnatal days P0–P14. This proliferation is largely driven by the mitogen sonic hedgehog (SHH) secreted from Purkinje cells which form the Purkinje cell plate under the EGL (Wallace, 1999; De Luca et al., 2016), although other factors, including Wnt 5a (Subashini et al., 2017), insulin-like growth factor (IGF1) (Fernandez et al., 2010), NOTCH2 (Solecki et al., 2001), and SDF1a (Klein et al., 2001), have also been implicated as GNP mitogens. GNPs are maintained in their proliferative niche by the signaling protein SDF1a which is secreted from the overlying developing meninges, which activates the CXCR4 receptor in GNPs and regulates cell adhesion (Hartmann et al., 1998a, b; Zou et al., 1998; Reiss et al., 2002; Zhu et al., 2002; Vilz et al., 2005; Tiveron and Cremer, 2008; Yu et al., 2010; Huang et al., 2014; Haldipur et al., 2017).
Proliferation of GNPs in the EGL is responsible for the dramatic size increase of the postnatal mouse cerebellum. Since the size of the posterior fossa does not dramatically increase, increased cerebellar size is accommodated within the posterior fossa by folding along the anterior/posterior axis. In mice, the circumference of the cerebellar medial anterior/posterior axis increases 17.6-fold between E17.5 and P14 compared with only a 2.2-fold increase in the mediolateral axis (Legue et al., 2015). Intriguingly, the basic pattern of cerebellar foliation is conserved across evolution. Foliation is initiated by the formation of multiple “anchoring centers” at stereotypic places across the anlage which will form the base of each fissure separating two folia (Sudarov and Joyner, 2007). The patterning mechanisms that position these anchoring centers are incompletely defined, but are related to underlying Purkinje cell patterning and clustering and at least partially involve function of the homeobox engrailed genes (En1/2) (Sillitoe and Joyner, 2007; Sudarov and Joyner, 2007). In the mouse cerebellar vermis, four anchoring centers give rise to the five cardinal lobules. Anchoring centers are first characterized by localized increase in proliferation and inward thickening of the granule cell precursors and subsequent coordinated Bergmann glial fiber rearrangement. Anterior/posterior-oriented cell division with the EGL drives the outward and preferential anterior/posterior expansion of cerebellar folia, while differential timing of granule neuron production in each lobule is responsible for the overall shaping of the mature cerebellar form (Sillitoe and Joyner, 2007; Legue et al., 2016).
The mechanisms responsible for cell cycle exit and subsequent differentiation of GNPs into granule neurons are poorly understood. As GNPs exit the cell cycle, they move to the inner layer of the EGL where they undergo a brief period of tangential migration (Komuro et al., 2001), which transitions into glial-guided radial migration along the radial glial fibers of the Bergmann glia (Rakic, 1971; Edmondson and Hatten, 1987; Hatten, 1999). These migratory phases are associated with dramatic morphologic transformations. Outer EGL proliferative GNPs are round with multiple short processes. In the inner EGL, newly differentiating granule neurons reorganize their cytoskeleton reorganized to form two short horizontal processes that elongate during their journey to the molecular layer. After a brief latency at the molecular layer, they acquire an elongated spindle shape and a vertical process (Komuro et al., 2001). It is currently understood that upregulated activity of the Par cell polarity complex is coupled to cell cycle exit, as well as changes in cellular adhesion and the initiation of migration. Zinc finger and homeobox transcription factor-1 (Zeb1), a master regulator of epithelial polarity, is highly expressed in unpolarized GNPs and controls neuronal differentiation by transcriptionally repressing polarity genes in GNPs. ZEB1 expression itself is maintained by SHH expression. Posttranslational ubiquitination of Par complex protein PARD3A via Siah, a Par complex-interacting E3 ubiquitin ligase expressed in the EGL, also regulates Par complex activity in the EGL (Famulski and Solecki, 2013; Ramahi and Solecki, 2014; Singh and Solecki, 2015; Singh et al., 2016).
Since an imbalance between GNP proliferation and differentiation can lead to either cerebellar hypoplasia or tumor formation, it is unsurprising that redundant systems regulate GNP differentiation. Several pathways are also thought to provide negative regulation of GNP proliferation, including BMP4, WNT3, and the anaphase-promoting complex/cyclosome (APC/CCDH1) ubiquitin ligase (Leto et al., 2016). WNT3 suppresses GNP growth through a noncanonic WNT signaling pathway, activating prototypic mitogen-activated protein kinases (MAPKs), the RAS-dependent extracellular signal-regulated kinases 1/2 (ERK1/2) and ERK5, instead of the classical β-catenin pathway (Anne et al., 2013). WNT3 also inhibits GNP proliferation by downregulating proliferative target genes of the mitogen Shh and the bHLH transcription factor ATOH1 (Anne et al., 2013). CK1δ is another novel regulator of GNP expansion as a loss of casein kinase (CK) 1δ or treatment of GNPs with a highly selective small-molecule CK1δ inhibitor inhibits GNP expansion. CK1δ is targeted for proteolysis via APC/CCdh1 ubiquitin ligase, and conditional deletion of the APC/CCdh1 activator, Cdh1 in cerebellar GNPs results in higher levels of CK1δ, suggesting an important role for the APC/CCdh1 complex in GNP cell cycle exit (Penas et al., 2015).
Finally, epigenetic modification of SHH downstream effectors, the Gli genes, are contributors to GNP cell cycle exit (Zanin et al., 2016). For example, activation of the p75 neurotrophin receptor p75NTR prevents deacetylation of acetylated gene-regulatory regions surrounding Gli1 and Gli2 by inhibiting histone deacetylases such as HDAC1, thereby ensuring that they remain transcriptionally inactive (Canettieri et al., 2010). Indeed, dramatic chromatin reorganization is also required to execute the entire granule neuron differentiation program as these neurons migrate through the molecular layer and establish their connectivity in the IGL (Zhu et al., 2016). A thorough discussion of this extensive literature however is beyond the scope of this review.
Glutamatergic cerebellar nuclei and UBCs
Unlike GNPs, both glutamatergic cerebellar nuclei neurons and UBCs exit the rhombic lip as postmitotic newly differentiating neurons. Cerebellar nuclei neurons exit the rhombic lip early to migrate over the anlage to form the nuclear transitory zone. Recent work has determined that these rhombic lip-derived cerebellar nuclei neurons are defined by a temporal transcriptional program (Leto et al., 2016). LHX9+ cells are born first and are destined to form the lateral nucleus (projecting to midbrain and thalamus), followed by a TBR1+ medial (fastigial) group, which sends axons to the hindbrain via the fasciculus uncinatus, or hook bundle (Green and Wingate, 2014). The progressive deposition of cells in more dorsal (ultimately medial) positions reflects a decreasing sensitivity to netrin signaling from ventral midline in migrating cells (Alcantara et al., 2000; Gilthorpe et al., 2002). Netrin receptors are also responsible for determining the laterality of the projections of cerebellar nuclei axons (Tamada et al., 2008), which extend seamlessly from the leading processes of migrating cells (Gilthorpe et al., 2002). Target selection (rostral or caudal central nervous system) appears to be a property ofLHX9 versus TBR1 expression (Green and Wingate, 2014).
UBCs are late derivatives exiting the rhombic lip directly into the cerebellar anlage and remained unrecognized until 1994 (Mugnaini and Floris, 1994; Englund et al., 2006). Other than the requirement of PAX6 in the rhombic lip for their generation (Yeung et al., 2016), little is known of their molecular drivers of specification, migration, or differentiation (Leto et al., 2016).
THE CEREBELLAR VENTRICULAR ZONE GIVES RISE TO ALL CEREBELLAR GABAERGIC NEURONS
The other major primary progenitor zone of the developing cerebellum is the cerebellar ventricular zone which gives rise to all cerebellar GABAergic neurons and interneurons, including in overlapping periods of neurogenesis from e11.5 to e14.5 (Sudarov et al., 2011). GABAergic cerebellar nuclei neurons are produced first, followed by Purkinje cells, then PAX2-expressing cerebellar inhibitory interneuron progenitors (PIPs), which will continue to divide within the presumptive cerebellar white matter, to eventually produce the interneurons of the cerebellar cortex, including basket, stellate, Golgi, Lugaro, globular, and candelabrum neurons. Bergmann glia are also derived from the cerebellar ventricular zone.
The mechanisms that specify each derivative of the ventricular zone are only just beginning to be elucidated. Fate-mapping studies have clearly demonstrated that Ptf1a expression defines the cerebellar ventricular zone and Ptf1a itself is required to generate all GABAergic derivatives (Hoshino et al., 2005; Yamada et al., 2014). Further, a complex interaction between PTF1a and ATOH1 maintains ventricular zone versus rhombic lip identity at the interface of these two progenitor ventricular zones (Pascual et al., 2007; Yamada et al., 2014). Additional recent fate-mapping studies have identified two spatially and temporally distinct progenitor zones within the PTF1a+ ventricular zone. Expression of oligodendrocyte-specific bHLH transcription factor (Olig2) defines Purkinje cell progenitors, while PIPs are derived from a PTF1a+ subzone-expressing homeodomain-containing transcription factor, GSX1. At e12.5 in the developing mouse embryo, OLIG2+ Purkinje cell progenitors comprise a predominant portion of the PTF1a+ ventricular zone domain. By e14.5, GSX1 has swept across the ventricular zone and PIPs become the output of the ventricular zone. GSX1 inhibits Olig2 expression and acts as a brake for temporal identity transition and there is some evidence that Olig gene expression is required for the Purkinje cell progenitors identity (Seto et al., 2014). However, the molecular regulatory details of these dramatic changes in gene expression and subsequent changes in ventricular zone output remain to be determined.
Purkinje cell development
As detailed above, Purkinje cells are specified within the PTF1a+ OLIG2+ ventricular zone. As they exit the ventricular zone starting at e10.5 in mice, they cease proliferation and initiate migration towards the nascent EGL to form a multilayered structure called the Purkinje cell plate. Early-born Purkinje cells, predominantly born in the posterior region of the ventricular zone, are the first cells to migrate to the nascent Purkinje cell plate by e14.5. These cells utilize a tangential migratory pathway at first, similar to that used by cells derived from the rhombic lip. However, at e13.5, these cells abruptly change orientation upon sending their trailing processes into a cortical region where protein reelin is abundant, secreted from newly arriving GNPs in the forming EGL. This quick, reelin-dependent transition from tangential to radial migration guides the nascent Purkinje cells to the Purkinje cell plate (Miyata et al., 2010; Leto et al., 2016). Later-born Purkinje cells, as well as cells produced during the early phase but in an anterior region, must migrate further than their earlier-born counterparts, utilizing a radial glial scaffold away from the ventricular zone. The role of reelin in the migration of these later-born cells to the Purkinje cell plate has yet to be elucidated.
Early- and late-born Purkinje cells have distinct molecular identities essential to the formation of the sagittally striped topographic map of the mature cerebellum (Brochu et al., 1990; Armstrong et al., 2000; Sarna et al., 2006; White and Sillitoe, 2013). Indeed, the functional topographic map of the mature cerebellum is organized in medial/lateral stripes of afferents and efferents that directly relate to clusters of Purkinje cells with shared expression patterns of genes, arrayed in medial/lateral stripes. This mediolateral Purkinje cell code is established during development and is at least partially related to Purkinje cell birth date (Fujita et al., 2012). One particularly well-known marker of Purkinje cell molecular heterogeneity is zebrinII (ZII) – named for the interspersed zebra-like sagittal arrays of ZII+ and ZII− seen in the mature cerebellum. Early-born Purkinje cells become ZII+ and late-born Purkinje cells remain ZII− (Larouche et al., 2006; Namba et al., 2011).
Beginning around e14.5, the cerebellar plate begins to reorganize itself, forming more than 50 distinct clusters by e18.5. These clusters are composed of either ZII+ or ZII− Purkinje cells. The mechanisms driving cluster formation are not understood; however, differential expression of cell-cell adhesion molecules, especially cadherins, has been promoted as possible organizing molecules (Vibulyaseck et al., 2017). Clusters transform to sagittal stripes as the cerebellum expands along the anterior/posterior axis due to GNP proliferation and inward migration of granule neurons, changing the multicellular Purkinje cell layer into a Purkinje cell monolayer residing on top of the IGL. The relationship between embryonic Purkinje cell clusters and mature Purkinje cell stripes is highly complicated. One cluster may give rise to a singular stripe or multiple stripes, or combine with other clusters to form a singular stripe. Although we have molecular markers for these Purkinje cell clusters from early to mature stages, the mechanisms that pattern and define these clusters in early development remain incompletely understood, although the Engrailed genes (En1 and En2) are involved (Sillitoe and Joyner, 2007; Sillitoe et al., 2010; Fujita et al., 2012).
As the Purkinje cell layer transforms into a monolayer, each Purkinje cell initiates neurite formation around P4–P5 in mice, resulting in the iconic and elaborate flat dendritic arbor and extended single axon of the mature Purkinje cell. Neurite-patterning mechanisms also remain largely unknown. There is evidence that intrinsic programs may drive initiation and some aspects of Purkinje cell dendrite patterning (Sotelo and Dusart, 2009; Tanaka, 2009, 2015; Shih et al., 2015). However, Purkinje cell development is clearly also influenced by GNPs and granule neurons. They provide essential Purkinje cell trophic factors. In addition, granule neuron defects nonautonomously cause delays in Purkinje cell dendrite formation and disruptions of the planarity of Purkinje cell dendritic trees (Baptista et al., 1994; Hirai and Launey, 2000; Ohashi et al., 2014).
INHIBITORY INTERNEURON DEVELOPMENT
In contrast to Purkinje cells which are born directly from the ventricular zone, cerebellar inhibitory interneurons are derived from proliferative precursors that delaminate into the developing anlage, otherwise called the prospective white matter (Leto et al., 2016). These precursors remain mitotically active, with peak proliferation during the first postnatal week in mice (Leto et al., 2006, 2012). Heterotopic/heterochronic transplantation experiments indicate that these interneuron progenitors maintain full developmental potential up to the end of cerebellar development and acquire mature phenotypes under the influence of environmental cues present in the prospective white matter. Furthermore, the final fate choice occurs in postmitotic cells, rather than dividing progenitors (Leto et al., 2009). Fates are acquired in an inside-out manner, with cerebellar nuclei-resident interneurons born first and molecular layer-resident basket and stellate cells born last.
The molecular details of the cell autonomous and non-autonomous effectors of PIP differentiation remain largely obscure. SHH disseminated by Purkinje cell axons in the prospective white matter is an important mitotic driver for PIPs in the prospective white matter (De Luca et al., 2015; Fleming and Chiang, 2015). Purkinje cells are also important for the terminal differentiation and morphogenesis of cerebellar interneurons. For example, the complexity of basket/stellate cell axonal arborizations and their positioning on Purkinje cells is critically dependent on neurofascin and also Semaphorin (SEMA3A)/neuropilin-1-mediated signaling between Purkinje cells and differentiating medial/lateral interneurons (Ango et al., 2004; Buttermore et al., 2012; Cioni et al., 2013; Leto et al., 2016). Additionally, granule neuron-dependent signals are known to direct the survival and growth of early postmigratory basket/stellate interneurons (Mertz et al., 2000; Konno et al., 2014).
Gliogenesis
The ventricular zone is the origin of all cerebellar astrocytes, a subpopulation of oligodendrocytes, and Bergmann glia. Notch signaling, which begins at e10.5 in the developing mouse cerebellar anlage, is essential to promote the astrocytic cell fate. Ablation of Notch activity in the nascent cerebellum leads to precocious neural differentiation with reduced astrocyte formation. Constitutive Notch activation leads to the overproduction of astrocytes along with a concomitant decrease in neurons. Astrocyte production is also regulated by Achaete-scute homolog 1 (ASCL1), another protein transiently expressed in the ventricular zone between e10.5 and e13.5. Ablation of Ascl1 results in increased numbers of astrocytes, while overexpression of Ascl1 suppresses astrocytic cell fate and promotes GABAergic interneuronal cell fates. Although a small number of oligodendrocytes are derived from the cerebellar ventricular zone, targeted in utero electroporation experiments have pointed to an extracerebellar source for most of these cells (Grimaldi et al., 2009). Mouse fate-mapping studies have determined that most originate from the Olig2-expressing neuroepithelial domain in the ventral rhombomere 1 (Hashimoto et al., 2016). Chick quail chimeras and in ovo transplants implicate a region of the mesencephalic ventricular zone as the origin of the majority of avian cerebellar oligodendrocytes (Mecklenburg et al., 2011), indicating potential species differences.
Bergmann glia are an essential cell type in the developing cerebellum. They are generated directly from the radial glia that line the cerebellar ventricular zone from e13.5, after Purkinje cells are born (Sudarov and Joyner, 2007). Initially, the ventricular zone radial glia form the scaffold upon which Purkinje cells migrate to the Purkinje cell plate. However, after Purkinje cells are generated, radial glia lose their apical attachment and their cell bodies migrate through the cerebellar anlage radially upwards, terminating their journey within the Purkinje cell layer. While the protein PTPN11 has been shown to aid in the transformation of radial glia into Bergmann glia (Li et al., 2014; Heng et al., 2017), their arrangement within the Purkinje cell layer is influenced by the interaction of Bergmann glial-expressed Notch with delta/Notch-like EGF-related receptor (DNER) on Purkinje cells (Eiraku et al., 2005).
Bergmann glia possess multiple ascending processes that traverse the molecular layer and EGL. As already discussed, granule cells migrate inward from the EGL to the IGL along these processes. Bergmann glia have also been shown to direct migration of molecular layer interneurons (Ango et al., 2008). The Bergmann glial end feet hook on to the basement membrane of the posterior fossa mesenchyme. Aberrations in end feet attachment can drastically affect Bergmann glial function which in turn can lead to abnormalities in cerebellar lamination and foliation (Moore et al., 2002; Qu and Smith, 2005; Satz et al., 2008). In rodents, the number of Bergmann glial fibers increases significantly during the first 2 postnatal weeks, particularly during peak proliferation and migration of granule cell progenitors, suggesting that GNPs play a role in Bergmann glial fiber formation. The molecular identity of signals from granule cells to Bergmann glia however remains largely unexplored. However, it is known that the differentiation and maintenance of Bergmann glia are heavily dependent on Purkinje cell secretion of SHH. Blocking SHH function results in reduced Bergmann glial survival. Conversely, Purkinje cell survival is also dependent on Bergmann glia (Wang et al., 2011), yet the molecular regulators interactions of this interaction remain unknown.
COMPLEX INTERRELATIONSHIPS BETWEEN MULTIPLE CELLULAR POPULATIONS ARE REQUIRED FOR NORMAL DEVELOPMENT
In the sections above, we have generally discussed the genesis and initial differentiation of each cerebellar cell type in isolation. This is an artificial reflection of the true nature of cerebellar development. Indeed, we have briefly outlined several complex interactions between multiple developing cells that are essential for normal cerebellar development. These include interdependencies between developing Purkinje cells, interneurons, granule neurons, and Bergmann glial cells for generation, survival, and histogenesis. There remain however two notable complex interrelationships that are worthy of separate discussion as they are active areas of ongoing research.
Signaling from the choroid plexus and posterior fossa mesenchyme
The cerebellar territory is established under the influence of the IsO and roof plate, both extracerebellar structures. The central nervous system-derived roof plate differentiates into the choroid plexus epithelium and is juxtaposed by vascular elements derived from head mesenchyme. Genetic loss-of-function studies have demonstrated that the choroid vascular elements are a source of SHH operating through a transventricular route to maintain the last phase of proliferative cerebellar ventricular zone from e13.5 (Huang et al., 2010).
The vascular elements of the choroid plexus are not the only posterior fossa mesenchymal derivatives influencing cerebellar development. More recently, it has become evident that nearly all aspects of cerebellar development are influenced by extracerebellar signaling from the developing meninges adjacent to the cerebellar anlage which is derived from head mesenchyme. Early experimental removal of the meninges during peak postnatal EGL proliferation causes fragmentation of the EGL and disruption of the radial fibers of the radial glial cells in hamster studies (Sievers et al., 1994). We have more recently shown that the developing meninges has a more extensive and much earlier influence on cerebellar development. Loss of FOXC1 – a transcription factor expressed exclusively in the mesenchyme adjacent to the early cerebellar anlage – causes dramatic cerebellar hypoplasia in mice and contributes to Dandy–Walker malformation in humans (Aldinger et al., 2009). Loss of this transcription factor nonautonomously disrupts cerebellar development because Foxc1 regulates the expression of a number of genes encoding secreted proteins that are required for normal cerebellar development. Most notable is SDF1a (Cxcl12). This ligand activates the CXCR4 receptor which is expressed by all cerebellar progenitor cells in the ventricular zone, rhombic lip, and EGL. Mutant analysis demonstrates that SDF1a-CXCR4 signaling is required for ventricular zone radial glial proliferation and survival in addition to maintenance and organization of radial glial fibers used by Purkinje cell to migrate to the Purkinje cell plate. Further, meningeal SDF1a is required to generate Bergmann glia and maintain Bergmann glial fiber organization (Haldipur et al., 2014). It is also required to maintain proliferation in the rhombic lip, attract rhombic lip-derived cerebellar neurons and GNPs away from the rhombic lip, and even direct the normal internal migratory route of UBCs (Haldipur et al., 2017). Given the vast influence of the meninges on multiple cerebellar developmental programs, it is clearly not valid to conceive of the cerebellum as an autonomously developing brain region.
Nucleogenesis of the cerebellar nuclei
Most of the focus of the cerebellar developmental field has been on development of the cerebellar cortex. The cerebellar nuclei of the cerebellum are clusters of gray matter lying within the white matter at the core of the cerebellum. They are, with the minor exception of the nearby vestibular nuclei, the sole sources of output from the cerebellum. From lateral to medial, the four deep cerebellar nuclei are the dentate, emboliform, globose, and fastigial nuclei. Some animals, including humans, do not have distinct emboliform and globose nuclei, instead having a single, fused interposed nucleus. Few, if any, mouse mutants have been described that specifically or preferentially affect the cerebellar nuclei. In combination with a lack of specific molecular markers and even a full description of the glutamatergic and GABAergic constituent neurons of the mature cerebellar nuclei, there is an extraordinary gap in our understanding of how mature cerebellar nuclei form. As discussed above, genetic lineage-mapping studies revealed that the neurons of the cerebellar nuclei are composed of two populations of distinct ventricular zone and rhombic lip lineal origin and that the glutamatergic rhombic lip-derived cerebellar nuclei neurons have some temporal encoding of their final positional fate within the nuclear transitory zone (Leto et al., 2016). However, it remains completely unknown how these two populations intercalate to form the mature cerebellar nuclei distribution and structure. Cerebellar nucleogenesis remains perhaps the biggest unexplored area of cerebellar development
HUMAN CEREBELLAR DEVELOPMENT
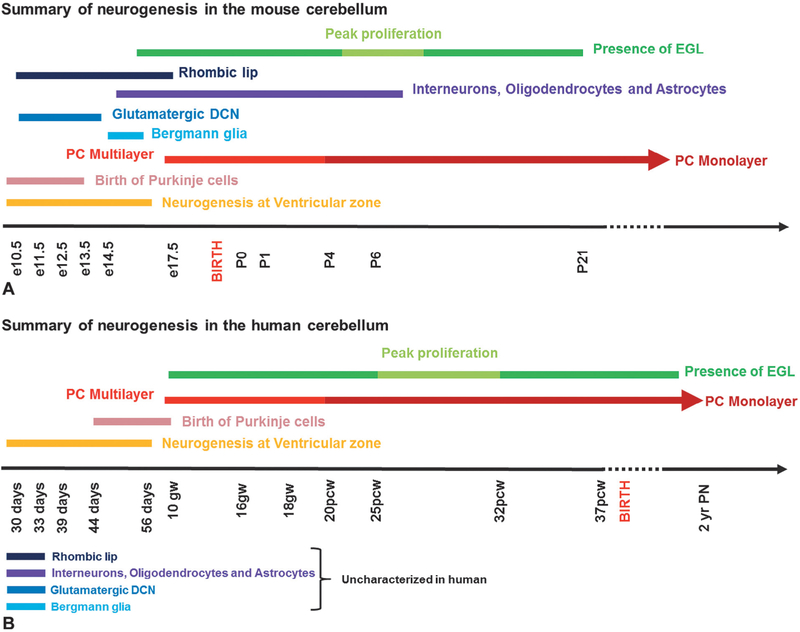
Cerebellar foliation, lamination, and neuronal morphology and circuitry are conserved from mice to humans, although there are some species-specific differences. For example, the cerebellar hemispheres are greatly expanded in humans, with a concomitant increase in the size of the dentate nucleus. Although foliation complexity is also increased in humans, the cardinal fissures present in mice underlie the folial pattern in humans. Additionally, cellular ratios are different across species. For example in mice there are 200 GNPs per Purkinje cell. In humans there are 3000 GNPs per Purkinje cell (Lange, 1975). Human cerebellar development however is highly protracted compared to mice (Fig. 2.2). In mice, the cerebellum develops over a period of 30–35 days, with peak EGL expansion, foliation, and IGL formation and Purkinje cell maturation occurring during the first 2 postnatal weeks (Marzban et al., 2014). In striking contrast, human cerebellar development extends from the early first trimester to final circuit maturity, which is achieved by the end of the second postnatal year. Despite this extended postnatal maturation period, a significant portion of human cerebellar development occurs in utero, including peak proliferation of GNPs and folia formation during the last trimester (Raaf, 1944; Rakic and Sidman, 1970). Regardless of these differences, available data indicate that principal developmental programs are conserved between humans and mice (Fig. 2.2). Here we overview published human cerebellar developmental data to familiarize the reader with the timing of human cerebellar development and some aspects of human cerebellar development that render it particularly sensitive to developmental insult.
Fig. 2.2.
Summary of neurogenesis in the developing (A) mouse and (B) human cerebellum. Human cerebellar development is highly protracted compared to mice. In mice, the cerebellum develops over a period of 30–35 days with the formation of gluta-matergic deep cerebellar nuclei (DCN), peak external granule layer (EGL) expansion, foliation, and internal granule layer formation and Purkinje cell (PC) maturation occurring during the first 2 postnatal weeks. In striking contrast, human cerebellar development extends from the early first trimester to final circuit maturity, which is achieved by the end of the second postnatal year. Also, a significant portion of human cerebellar development occurs in utero, including peak proliferation of granule neuron progenitors and folia formation during the last trimester.
Descriptions of human fetal cerebellar development are unsurprisingly rare with few molecular details. The earliest available data are from 8 gestational weeks (Larsell, 1947; Rakic and Sidman, 1970; Zecevic and Rakic, 1976; Haldipur et al., 2011, 2012). At this early stage, both zones of neurogenesis, the rhombic lip, and ventricular zone are visible. By 10–11 gestational weeks, a stream of cells can be seen present along the pial surface connecting to the rhombic lip, which presumably is the EGL, but cannot be confirmed due to lack of immunohistochemical data (Rakic and Sidman, 1970). The ventricular zone is thinner than that of the mouse at e11.5, indicating that Purkinje cells and PIPs were likely born prior to this stage. This is supported by the fact that a broad multilayered Purkinje cell layer extending from the ventricular zone to the nascent molecular layer is evident between 10 and 13 gestational weeks. Calbindin-expressing Purkinje cells remain as immature neurons until 16 gestational weeks, which constitutes their first stage of maturation. During this stage, Purkinje cells lack their characteristic dendritic morphology and remain mostly spindle-shaped and arranged as a multilayer. The second stage of Purkinje cell maturation occurs between the 16th and 28th gestational weeks, during which cerebellar volume increases as a result of the expansion of the EGL. At this stage, Purkinje cells begin to spread, resulting in decreased Purkinje cell plate thickness with a Purkinje cell monolayer achieved by 20–24 gestational weeks, although Purkinje cells remain structurally multipolar and stellate. In the third trimester, Purkinje cells enter the final stage of maturation initiating development of their characteristic extensive and flattened dendritic arbor and long axon (Rakic and Sidman, 1970; Zecevic and Rakic, 1976; Haldipur et al., 2011, 2012). This final maturation period is sixfold longer in humans versus mice (Zecevic and Rakic, 1976). The maturation of the human cerebellar Purkinje cell monolayer coincides with the phase during which a high number of neuron glia interactions take place as well as peak EGL proliferation, as evidenced by increased EGL thickness.
The human fetal EGL achieves its maximum thickness between the 20th and 32nd gestational week (Rakic and Sidman, 1970), indicating peak proliferation of GNPs. Indeed, the human cerebellum increases in size fivefold from gestational weeks 24 to 40 (Limperopoulos et al., 2005b; Volpe, 2009) due to extensive EGL proliferation, which also correlates with increased folial complexity. The human cerebellum is conspicuous by the lack of foliation prior to 11 gestational weeks. The primary fissure first appears around the 11th gestational week while the secondary fissure, seen around the 16th gestational week, becomes more prominent with age. Foliation between the 20th and 32nd gestational week increases dramatically as the cerebellum continues to increase in size and volume. Peak EGL thickness coincides with the secondary and tertiary phases of Purkinje cell maturation, during which the Purkinje cells coalesce beneath the EGL (Rakic and Sidman, 1970; Zecevic and Rakic, 1976). It is assumed that the increase in EGL thickness is a result of increased proliferation which is itself a result of SHH secretion by the Purkinje cell layer. However, studies indicate that, much like in the mouse, the nascent EGL can be a source of SHH (Haldipur et al., 2012). EGL thickness remains constant between 32 gestational weeks and birth.
The developing human cerebellar cortex has a unique transient layer – the lamina dissecans. The lamina dissecans is a cell-sparse region that is present between the maturing Purkinje cells and the nascent IGL between the 20th and 32nd gestational week. The lamina dissecans disappears after the 32nd gestational week. No function has been ascribed to this layer; however, electron microscopic studies indicate that the lamina dissecans shows the presence of many cellular processes which may indicate a site of incipient synapse formation (Rakic and Sidman, 1970).
At birth, the human cerebellar cortex has four layers: the EGL, the molecular layer, the Purkinje cell layer, and the IGL. The number of layers reduces from four to three in a matter of 12–24 months as the EGL gradually decreases in thickness as a result of decreased proliferation and migration of granule neurons into the IGL. By the end of the second postnatal year the EGL ceases to exist while the thickness of the molecular layer and length of the Purkinje layer increase with increased cerebellar volume (Raaf, 1944; Rakic and Sidman, 1970; Haldipur et al., 2011, 2012).
The well-understood developmental interdependence between cerebellar cell types together with the protracted nature of human cerebellar development make the human cerebellum particularly vulnerable to developmental disruption. A multitude of human cerebellar developmental disorders are recognized. A full discussion of these disorders and their presumed developmental pathogenesis is well beyond the scope of this chapter. The genetic landscape of these disorders has begun to be elucidated, which has led to key insights into their developmental pathogenesis (Aldinger and Doherty, 2016). However, it is important to note that not all cerebellar developmental malformations are genetic in nature. The protracted nature of human cerebellar development makes the human cerebellum particularly vulnerable to nongenetic developmental disruption and injury. During the third trimester, many critical events occur, including foliation, peak proliferation, and migration of GNPs; interneuron differentiation, late stages of Purkinje cell differentiation, and synapse formation all take place in utero (Rakic and Sidman, 1970; Zecevic and Rakic, 1976). Therefore it is perhaps not surprising that premature birth is a major risk factor for cerebellar injury and is associated with deficits in motor coordination and cognition and is also associated with reduced cerebellar volume (Limperopoulos et al., 2005b, 2007; Peralta-Carcelen et al., 2017). Preterm infants are prone to cerebellar hemorrhage, which often leads to cerebellar atrophy (Limperopoulos et al., 2005a). Additionally, prenatal or neonatal exposure to glucocorticoids (Heine et al., 2011), hypoxia (Darnall et al., 2017), and hyperoxia (Scheuer et al., 2017) can dramatically alter GNP proliferation and migration, contributing to cerebellar injury.
Cerebellar regions that interconnect with sensorimotor cortices are associated with motor impairments when damaged; disruption to posterolateral cerebellar regions that form circuits with association cortices impact long-term cognitive outcomes; and midline posterior vermal damage is associated with behavioral dysregulation and an autism-like phenotype (Stoodley and Limperopoulos, 2016). In addition to white-matter and circuit damage evidenced by diffusion tensor imaging studies (Brossard-Racine et al., 2017), postmortem analyses of preterm cerebella indicate a reduction in EGL thickness, increased packing density of GNPs, and increased Bergmann glial fiber density. SHH signaling is also reduced in the preterm cerebella (Haldipur et al., 2011). Together, these recent studies emphasize the need to fully define the mechanisms of genetic and environmental influence on perinatal cerebellar development and devise treatment paradigms that protect cerebellar developmental programs in preterm infants and other children at risk for significant cerebellar-related neurodevelopmental disorders.
CONCLUSIONS
There is a growing recognition of the extent and prevalence of human cerebellar developmental disorders/disruptions. To define disease pathogenesis, an understanding of basic mechanisms that build the cerebellum is required. Here, we have outlined the key developmental programs that generate the elegant and stereotypic morphologies of cerebellar neurons and the exquisitely laminated and foliated mature cerebellum. We have also emphasized several key areas of active research. While there has been extensive progress over the last two decades, much remains to be discovered. Most relevant to this audience, we note that most of our knowledge base is derived from the study of animal models, particularly mice. Very little is specifically known regarding normal or pathologic human cerebellum development. Although there is extensive conservation of cerebellar form and function, we do know there are important species-specific differences. A better appreciation of these phenomena will facilitate improved diagnosis and the development of treatment paradigms for these important neurodevelopmental disorders.
ACKNOWLEDGMENT
This work was supported by the following US National Institutes of Health grants to KJM: R01NS080390, R01NS050375, and R01NS095733.
References
- Ackerman SL, Kozak LP, Przyborski SA et al. (1997). The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 386: 838–842. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, De Castro F et al. (2000). Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development 127: 1359–1372. [DOI] [PubMed] [Google Scholar]
- Alder J, Cho NK, Hatten ME (1996). Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron 17: 389–399. [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Doherty D (2016). The genetics of cerebellar malformations. Semin Fetal Neonatal Med 21: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger KA, Lehmann OJ, Hudgins L et al. (2009). FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat Genet 41: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H et al. (2004). Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119: 257–272. [DOI] [PubMed] [Google Scholar]
- Ango F, Wu C, Van der Want JJ et al. (2008). Bergmann glia and the recognition molecule CHL1 organize GABAergic axons and direct innervation of Purkinje cell dendrites. PLoS Biol 6: e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anne SL, Govek EE, Ayrault O et al. (2013). WNT3 inhibits cerebellar granule neuron progenitor proliferation and medulloblastoma formation via MAPK activation. PLoS One 8: e81769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CL, Krueger-Naug AM et al. (2000). Constitutive expression of the 25-kDa heat shock protein Hsp25 reveals novel parasagittal bands of Purkinje cells in the adult mouse cerebellar cortex. J Comp Neurol 416: 383–397. [DOI] [PubMed] [Google Scholar]
- Baptista CA, Hatten ME, Blazeski R et al. (1994). Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron 12: 243–260. [DOI] [PubMed] [Google Scholar]
- Brochu G, Maler L, Hawkes R (1990). Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol 291: 538–552. [DOI] [PubMed] [Google Scholar]
- Brossard-Racine M, Poretti A, Murnick J et al. (2017). Cerebellar microstructural organization is altered by complications of premature birth: a case-control study. J Pediatr 182 (28–33): e1. [DOI] [PubMed] [Google Scholar]
- Buttermore ED, Piochon C, Wallace ML et al. (2012). Pinceau organization in the cerebellum requires distinct functions of neurofascin in Purkinje and basket neurons during postnatal development. J Neurosci 32: 4724–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts T, Green MJ, Wingate RJ (2014a). Development of the cerebellum: simple steps to make a ‘little brain. Development 141: 4031–4041. [DOI] [PubMed] [Google Scholar]
- Butts T, Hanzel M, Wingate RJ (2014b). Transit amplification in the amniote cerebellum evolved via a heterochronic shift in NeuroD1 expression. Development 141: 2791–2795. [DOI] [PubMed] [Google Scholar]
- Canettieri G, Di Marcotullio L, Greco A et al. (2010). Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol 12: 132–142. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Lindgren AG, Currle DS et al. (2006). The roof plate regulates cerebellar cell-type specification and proliferation. Development 133: 2793–2804. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Lindgren AG, Mishima Y et al. (2010). Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc Natl Acad Sci U S A 107: 10725–10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioni JM, Telley L, Saywell V et al. (2013). SEMA3A signaling controls layer-specific interneuron branching in the cerebellum. Curr Biol 23: 850–861. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Chen X, Nemani KV et al. (2017). Early postnatal exposure to intermittent hypoxia in rodents is proinflammatory, impairs white matter integrity, and alters brain metabolism. Pediatr Res 82: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Parmigiani E, Tosatto G et al. (2015). Exogenous Sonic hedgehog modulates the pool of GABAergic interneurons during cerebellar development. Cerebellum 14: 72–85. [DOI] [PubMed] [Google Scholar]
- De Luca A, Cerrato V, Fuca E et al. (2016). Sonic hedgehog patterning during cerebellar development. Cell Mol Life Sci 73: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddison M, Toole L, Bell E et al. (2004). Segmental identity and cerebellar granule cell induction in rhombomere 1. BMC Biol 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson JC, Hatten ME (1987). Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J Neurosci 7: 1928–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Tohgo A, Ono K et al. (2005). DNER acts as a neuron-specific Notch ligand during Bergmann glial development. Nat Neurosci 8: 873–880. [DOI] [PubMed] [Google Scholar]
- Englund C, Kowalczyk T, Daza RA et al. (2006). Unipolar brush cells of the cerebellum are produced in the rhombic lip and migrate through developing white matter. J Neurosci 26: 9184–9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulski JK, Solecki DJ (2013). New spin on an old transition: epithelial parallels in neuronal adhesion control. Trends Neurosci 36: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C, Tatard VM, Bertrand N et al. (2010). Differential modulation of Sonic-hedgehog-induced cerebellar granule cell precursor proliferation by the IGF signaling network. Dev Neurosci 32: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J, Chiang C (2015). The Purkinje neuron: a central orchestrator of cerebellar neurogenesis. Neurogenesis (Austin) 2: e1025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Morita N, Furuichi T et al. (2012). Clustered fine compartmentalization of the mouse embryonic cerebellar cortex and its rearrangement into the postnatal striped configuration. J Neurosci 32: 15688–15703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavalas A, Davenne M, Lumsden A et al. (1997). Role of Hoxa-2 in axon pathfinding and rostral hindbrain patterning. Development 124: 3693–3702. [DOI] [PubMed] [Google Scholar]
- Gilthorpe JD, Papantoniou EK, Chedotal A et al. (2002). The migration of cerebellar rhombic lip derivatives. Development 129: 4719–4728. [DOI] [PubMed] [Google Scholar]
- Green MJ, Wingate RJ (2014). Developmental origins of diversity in cerebellar output nuclei. Neural Dev 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi P, Parras C, Guillemot F et al. (2009). Origins and control of the differentiation of inhibitory interneurons and glia in the cerebellum. Dev Biol 328: 422–433. [DOI] [PubMed] [Google Scholar]
- Haldipur P, Bharti U, Alberti C et al. (2011). Preterm delivery disrupts the developmental program of the cerebellum. PLoS One 6: e23449.21858122 [Google Scholar]
- Haldipur P, Bharti U, Govindan S et al. (2012). Expression of Sonic hedgehog during cell proliferation in the human cerebellum. Stem Cells Dev 21: 1059–1068. [DOI] [PubMed] [Google Scholar]
- Haldipur P, Gillies GS, Janson OK et al. (2014). Foxc1 dependent mesenchymal signalling drives embryonic cerebellar growth. elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur P, Dang D, Aldinger KA et al. (2017). Phenotypic outcomes in mouse and human Foxc1 dependent Dandy-Walker cerebellar malformation suggest shared mechanisms. elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Sato T, Nakamura H (2016). Fgf8 signaling for development of the midbrain and hindbrain. Develop Growth Differ 58: 437–445. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Schulze M, Sievers J (1998a). Meningeal cells stimulate and direct the migration of cerebellar external granule cells in vitro. J Neurocytol 27: 395–409. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Ziegenhagen MW, Sievers J (1998b). Meningeal cells stimulate neuronal migration and the formation of radial glial fascicles from the cerebellar external granular layer. Neurosci Lett 244: 129–132. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Hori K, Owa T et al. (2016). Origins of oligodendrocytes in the cerebellum, whose development is controlled by the transcription factor, Sox9. Mech Dev 140: 25–40. [DOI] [PubMed] [Google Scholar]
- Hatten ME (1999). Central nervous system neuronal migration. Annu Rev Neurosci 22: 511–539. [DOI] [PubMed] [Google Scholar]
- Heine VM, Griveau A, Chapin C et al. (2011). A small-molecule smoothened agonist prevents glucocorticoid-induced neonatal cerebellar injury. Sci Transl Med 3: 105ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng X, Guo Q, Leung AW et al. (2017). Analogous mechanism regulating formation of neocortical basal radial glia and cerebellar Bergmann glia. elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Launey T (2000). The regulatory connection between the activity of granule cell NMDA receptors and dendritic differentiation of cerebellar Purkinje cells. J Neurosci 20: 5217–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Nakamura S, Mori K et al. (2005). Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron 47: 201–213. [DOI] [PubMed] [Google Scholar]
- Huang X, Liu J, Ketova T et al. (2010). Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci U S A 107: 8422–8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GJ, Edwards A, Tsai CY et al. (2014). Ectopic cerebellar cell migration causes maldevelopment of Purkinje cells and abnormal motor behaviour in Cxcr4 null mice. PLoS One 9: e86471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving C, Mason I (2000). Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression. Development 127: 177–186. [DOI] [PubMed] [Google Scholar]
- Joyner AL (2016). From cloning neural development genes to functional studies in mice, 30 years of advancements. Curr Top Dev Biol 116: 501–515. [DOI] [PubMed] [Google Scholar]
- Klein RS, Rubin JB, Gibson HD et al. (2001). SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development 128: 1971–1981. [DOI] [PubMed] [Google Scholar]
- Komuro H, Yacubova E, Rakic P (2001). Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci 21: 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno K, Matsuda K, Nakamoto C et al. (2014). Enriched expression of GluD1 in higher brain regions and its involvement in parallel fiber-interneuron synapse formation in the cerebellum. J Neurosci 34: 7412–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N et al. (2014). Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum 13: 151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange W (1975). Cell number and cell density in the cerebellar cortex of man and some other mammals. Cell Tissue Res 157: 115–124. [DOI] [PubMed] [Google Scholar]
- Larouche M, Che PM, Hawkes R (2006). Neurogranin expression identifies a novel array of Purkinje cell parasagittal stripes during mouse cerebellar development. J Comp Neurol 494: 215–227. [DOI] [PubMed] [Google Scholar]
- Larsell O (1947). The development of the cerebellum in man in relation to its comparative anatomy. J Comp Neurol 87: 85–129. [DOI] [PubMed] [Google Scholar]
- Legue E, Riedel E, Joyner AL (2015). Clonal analysis reveals granule cell behaviors and compartmentalization that determine the folded morphology of the cerebellum. Development 142: 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue E, Gottshall JL, Jaumouille E et al. (2016). Differential timing of granule cell production during cerebellum development underlies generation of the foliation pattern. Neural Dev 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo ED, Hinck L, Masu M et al. (1997). Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 386: 833–838. [DOI] [PubMed] [Google Scholar]
- Leto K, Carletti B, Williams IM et al. (2006). Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells. J Neurosci 26: 11682–11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto K, Bartolini A, Yanagawa Y et al. (2009). Laminar fate and phenotype specification of cerebellar GABAergic interneurons. J Neurosci 29: 7079–7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto K, Rolando C, Rossi F (2012). The genesis of cerebellar GABAergic neurons: fate potential and specification mechanisms. Front Neuroanat 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto K, Arancillo M, Becker EB et al. (2016). Consensus paper: cerebellar development. Cerebellum 15: 789–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Leung AW, Guo Q et al. (2014). Shp2-dependent ERK signaling is essential for induction of Bergmann glia and foliation of the cerebellum. J Neurosci 34: 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Benson CB, Bassan H et al. (2005a). Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics 116: 717–724. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Gauvreau K et al. (2005b). Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics 115: 688–695. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Gauvreau K et al. (2007). Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 120: 584–593. [DOI] [PubMed] [Google Scholar]
- Lugaro E (1894). Sulle connessioni tra gli elementi nervosi della corteccia cerebellare con considerazioni generali sul significato fisiologico dei rapporti tragli elementi nervosi. Riv Sper Freniatr Med Leg 20: 297–331. [Google Scholar]
- Machold R, Fishell G (2005). Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron 48: 17–24. [DOI] [PubMed] [Google Scholar]
- Marzban H, Del Bigio MR, Alizadeh J et al. (2014). Cellular commitment in the developing cerebellum. Front Cell Neurosci 8: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I, Chambers D, Shamim H et al. (2000). Regulation and function of FGF8 in patterning of midbrain and anterior hindbrain. Biochem Cell Biol 78: 577–584. [PubMed] [Google Scholar]
- Mecklenburg N, Garcia-Lopez R, Puelles E et al. (2011). Cerebellar oligodendroglial cells have a mesencephalic origin. Glia 59: 1946–1957. [DOI] [PubMed] [Google Scholar]
- Mertz K, Koscheck T, Schilling K (2000). Brain-derived neurotrophic factor modulates dendritic morphology of cerebellar basket and stellate cells: an in vitro study. Neuroscience 97: 303–310. [DOI] [PubMed] [Google Scholar]
- Millen KJ, Gleeson JG (2008). Cerebellar development and disease. Curr Opin Neurobiol 18: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen KJ, Steshina EY, Iskusnykh IY et al. (2014). Transformation of the cerebellum into more ventral brainstem fates causes cerebellar agenesis in the absence of Ptf1a function. Proc Natl Acad Sci USA 111: E1777–E1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Lindgren AG, Chizhikov VV et al. (2009). Overlapping function of Lmx1a and Lmx1b in anterior hindbrain roof plate formation and cerebellar growth. J Neurosci 29: 11377–11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Ono Y, Okamoto M et al. (2010). Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum. Neural Dev 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J et al. (2002). Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418: 422–425. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Floris A (1994). The unipolar brush cell: a neglected neuron of the mammalian cerebellar cortex. J Comp Neurol 339: 174–180. [DOI] [PubMed] [Google Scholar]
- Namba K, Sugihara I, Hashimoto M (2011). Close correlation between the birth date of Purkinje cells and the longitudinal compartmentalization of the mouse adult cerebellum. J Comp Neurol 519: 2594–2614. [DOI] [PubMed] [Google Scholar]
- Ohashi R, Sakata S, Naito A et al. (2014). Dendritic differentiation of cerebellar Purkinje cells is promoted by ryanodine receptors expressed by Purkinje and granule cells. Dev Neurobiol 74: 467–480. [DOI] [PubMed] [Google Scholar]
- Pascual M, Abasolo I, Mingorance-Le Meur A et al. (2007). Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proc Natl Acad Sci U S A 104: 5193–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penas C, Govek EE, Fang Y et al. (2015). Casein kinase 1delta is an APC/C(Cdh1) substrate that regulates cerebellar granule cell neurogenesis. Cell Rep 11: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta-Carcelen M, Carlo WA, Pappas A et al. (2017). Behavioral problems and socioemotional competence at 18 to 22 months of extremely premature children. Pediatrics (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q, Smith FI (2005). Neuronal migration defects in cerebellum of the Largemyd mouse are associated with disruptions in Bergmann glia organization and delayed migration of granule neurons. Cerebellum 4: 261–270. [DOI] [PubMed] [Google Scholar]
- Raaf JWKaJ (1944). A study of the external granular layer in the cerebellum. The disappearance of the external granular layer and the growth of the molecular and internal granular layers in the cerebellum. Am J Anat 75: 151–172. [Google Scholar]
- Rakic P (1971). Guidance of neurons migrating to the fetal monkey neocortex. Brain Res 33: 471–476. [DOI] [PubMed] [Google Scholar]
- Rakic P, Sidman RL (1970). Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J Comp Neurol 139: 473–500. [DOI] [PubMed] [Google Scholar]
- Ramahi JS, Solecki DJ (2014). The PAR polarity complex and cerebellar granule neuron migration. Adv Exp Med Biol 800: 113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S (1909–1911). Histologie du système nerveux de l’homme et des vertébrés, Maloine, Paris. [Google Scholar]
- Reiss K, Mentlein R, Sievers J et al. (2002). Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience 115: 295–305. [DOI] [PubMed] [Google Scholar]
- Rose MF, Ahmad KA, Thaller C et al. (2009). Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc Natl Acad Sci U S A 106: 22462–22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna JR, Marzban H, Watanabe M et al. (2006). Complementary stripes of phospholipase Cbeta3 and Cbeta4 expression by Purkinje cell subsets in the mouse cerebellum. J Comp Neurol 496: 303–313. [DOI] [PubMed] [Google Scholar]
- Sato T, Joyner AL (2009). The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures. Development 136: 3617–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz JS, Barresi R, Durbeej M et al. (2008). Brain and eye malformations resembling Walker-Warburg syndrome are recapitulated in mice by dystroglycan deletion in the epi-blast. J Neurosci 28: 10567–10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer T, Sharkovska Y, Tarabykin V et al. (2017). Neonatal hyperoxia perturbs neuronal development in the cerebellum. Mol Neurobiol. 10.1007/s12035-017-0612-5. [DOI] [PubMed] [Google Scholar]
- Seto Y, Nakatani T, Masuyama N et al. (2014). Temporal identity transition from Purkinje cell progenitors to GABAergic interneuron progenitors in the cerebellum. Nat Commun 5: 3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih EK, Sekerkova G, Ohtsuki G et al. (2015). The spontaneous ataxic mouse mutant Tippy is characterized by a novel Purkinje cell morphogenesis and degeneration phenotype. Cerebellum 14: 292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers J, Pehlemann FW, Gude S et al. (1994). A time course study of the alterations in the development of the hamster cerebellar cortex after destruction of the overlying meningeal cells with 6-hydroxydopamine on the day of birth. J Neurocytol 23: 117–134. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Joyner AL (2007). Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol 23: 549–577. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Vogel MW, Joyner AL (2010). Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J Neurosci 30: 10015–10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Solecki DJ (2015). Polarity transitions during neurogenesis and germinal zone exit in the developing central nervous system. Front Cell Neurosci 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Schimenti JC, Bolcun-Filas E (2015). A mouse geneticist’s practical guide to CRISPR applications. Genetics 199: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Howell D, Trivedi N et al. (2016). Zeb1 controls neuron differentiation and germinal zone exit by a mesenchymal-epithelial-like transition. elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki DJ, Liu XL, Tomoda T et al. (2001). Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron 31: 557–568. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Dusart I (2009). Intrinsic versus extrinsic determinants during the development of Purkinje cell dendrites. Neuroscience 162: 589–600. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Limperopoulos C (2016). Structure-function relationships in the developing cerebellum: evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med 21: 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subashini C, Dhanesh SB, Chen CM et al. (2017). Wnt5a is a crucial regulator of neurogenesis during cerebellum development. Sci Rep 7: 42523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarov A, Joyner AL (2007). Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarov A, Turnbull RK, Kim EJ et al. (2011). Ascl1 genetics reveals insights into cerebellum local circuit assembly. J Neurosci 31: 11055–11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson DJ, Goldowitz D (2011). Experimental Sey mouse chimeras reveal the developmental deficiencies of Pax6-null granule cells in the postnatal cerebellum. Dev Biol 351: 1–12. [DOI] [PubMed] [Google Scholar]
- Tamada A, Kumada T, Zhu Y et al. (2008). Crucial roles of Robo proteins in midline crossing of cerebellofugal axons and lack of their up-regulation after midline crossing. Neural Dev 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M (2009). Dendrite formation of cerebellar Purkinje cells. Neurochem Res 34: 2078–2088. [DOI] [PubMed] [Google Scholar]
- Tanaka M (2015). The dendritic differentiation of Purkinje neurons: unsolved mystery in formation of unique dendrites. Cerebellum 14: 227–230. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Cremer H (2008). CXCL12/CXCR4 signalling in neuronal cell migration. Curr Opin Neurobiol 18: 237–244. [DOI] [PubMed] [Google Scholar]
- Vibulyaseck S, Fujita H, Luo Y et al. (2017). Spatial rearrangement of Purkinje cell subsets forms the transverse and longitudinal compartmentalization in the mouse embryonic cerebellum. J Comp Neurol 525: 2971–2990. [DOI] [PubMed] [Google Scholar]
- Vilz TO, Moepps B, Engele J et al. (2005). The SDF-1/CXCR4 pathway and the development of the cerebellar system. Eur J Neurosci 22: 1831–1839. [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2009). Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol 24: 1085–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VA (1999). Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol 9: 445–448. [DOI] [PubMed] [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY (2005). Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 48: 31–43. [DOI] [PubMed] [Google Scholar]
- Wang X, Imura T, Sofroniew MV et al. (2011). Loss of adenomatous polyposis coli in Bergmann glia disrupts their unique architecture and leads to cell nonautonomous neurodegeneration of cerebellar Purkinje neurons. Glia 59: 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JJ, Sillitoe RV (2013). Development of the cerebellum: from gene expression patterns to circuit maps. Wiley interdisciplinary reviews. Dev Biol 2: 149–164. [DOI] [PubMed] [Google Scholar]
- Yamada M, Seto Y, Taya S et al. (2014). Specification of spatial identities of cerebellar neuron progenitors by ptf1a and atoh1 for proper production of GABAergic and glutamatergic neurons. J Neurosci 34: 4786–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung J, Goldowitz D (2017). Wls expression in the rhombic lip orchestrates the embryonic development of the mouse cerebellum. Neuroscience 354: 30–42. [DOI] [PubMed] [Google Scholar]
- Yeung J, Ha TJ, Swanson DJ et al. (2014). Wls provides a new compartmental view of the rhombic lip in mouse cerebellar development. J Neurosci 34: 12527–12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung J, Ha TJ, Swanson DJ et al. (2016). A novel and multivalent role of Pax6 in cerebellar development. J Neurosci 36: 9057–9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Huang H, Li HF (2010). Stromal cell-derived factor-1 promotes migration of cells from the upper rhombic lip in cerebellar development. J Neurosci Res 88: 2775–2786. [DOI] [PubMed] [Google Scholar]
- Zanin JP, Abercrombie E, Friedman WJ (2016). Proneurotrophin-3 promotes cell cycle withdrawal of developing cerebellar granule cell progenitors via the p75 neurotrophin receptor. elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Rakic P (1976). Differentiation of Purkinje cells and their relationship to other components of developing cerebellar cortex in man. J Comp Neurol 167: 27–47. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang XC et al. (2002). Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci 5: 719–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Girardo D, Govek EE et al. (2016). Role of Tet1/3 genes and chromatin remodeling genes in cerebellar circuit formation. Neuron 89: 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M et al. (1998). Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393: 595–599. [DOI] [PubMed] [Google Scholar]