Abstract
Background:
The health effects of smoking marijuana are not well-understood.
Purpose:
To examine the association between marijuana use and respiratory symptoms, pulmonary function, and obstructive lung disease among adolescents and adults.
Data Sources:
PubMed, Embase, PsycINFO, MEDLINE, and the Cochrane Library from 1 January 1973 to 30 April 2018.
Study Selection:
Observational and interventional studies published in English that reported pulmonary outcomes of adolescents and adults who used marijuana.
Data Extraction:
Four reviewers independently extracted study characteristics and assessed risk of bias. Three reviewers assessed strength of evidence. Studies of similar design with low or moderate risk of bias and sufficient data were pooled.
Data Synthesis:
Twenty-two studies were included. A pooled analysis of 2 prospective studies showed that marijuana use was associated with an increased risk for cough (risk ratio [RR], 2.04 [95% CI, 1.02 to 4.06]) and sputum production (RR, 3.84 [CI, 1.62 to 9.07]). Pooled analysis of cross-sectional studies (1 low and 3 moderate risk of bias) showed that marijuana use was associated with cough (RR, 4.37 [CI, 1.71 to 11.19]), sputum production (RR, 3.40 [CI, 1.99 to 5.79]), wheezing (RR, 2.83 [CI, 1.89 to 4.23]), and dyspnea (RR, 1.56 [CI, 1.33 to 1.83]). Data on pulmonary function and obstructive lung disease were insufficient.
Limitation:
Few studies were at low risk of bias, marijuana exposure was limited in the population studied, cohorts were young overall, assessment of marijuana exposure was not uniform, and study designs varied.
Conclusion:
Low-strength evidence suggests that smoking marijuana is associated with cough, sputum production, and wheezing. Evidence on the association between marijuana use and obstructive lung disease and pulmonary function is insufficient.
Primary Funding Source:
None. (PROSPERO: CRD42017059224)
Approximately 13.3% of U.S. adults use marijuana (1), and rates are rising. Use by young adults (aged 18 to 29 years) doubled from 10.5% in 2002 to 21.2% in 2014 (2), and an estimated 7000 persons start using marijuana each day. Smoking remains the main method of consumption (3). The increasing prevalence of marijuana use, especially by smoking and vaping (4), raises concerns about effects on pulmonary health.
Similarities between marijuana and tobacco smoke are concerning from a public health perspective. Marijuana cigarettes are believed to contain particulate matter, toxic gases, reactive oxygen species, and polycyclic aromatic hydrocarbons (5) at a concentration possibly 20 times that of tobacco smoke. Studies have shown that marijuana is associated with histopathologic changes in bronchial inflammation that are similar to changes seen with smoking tobacco (6). In addition, tetrahydrocannabinol may have adverse immunomodulatory effects (6, 7) that could lead to infections and cancer.
Marijuana's high particulate content and toxins suggest that long-term use may lead to chronic respiratory symptoms and adverse health effects, such as obstructive lung disease. Widespread use and increasing social acceptance mean that a better understanding of its health effects is needed. We did a systematic review and meta-analysis to examine whether marijuana use is associated with respiratory symptoms, obstructive lung disease, and changes in pulmonary function.
Methods
Our review was consistent with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement (8). The protocol was registered with PROSPERO (CRD42017059224). This review focuses on the association between marijuana use and pulmonary symptoms and function; our protocol also included review of the association between marijuana and respiratory tract neoplasms, which is not reported here.
Data Sources and Searches
We did a systematic literature review using several online databases (PubMed, Embase, PsycINFO, MEDLINE, and the Cochrane Library) from 1 January 1973 to 30 April 2018. We chose 1973 as the start date because Oregon decriminalized possession of marijuana in that year (9) (Part 1 of the Supplement, available at Annals.org).
Study Selection
Two reviewers (M.G. and D.R.) independently screened all titles and abstracts. We included observational studies (cohort, case–control, and cross-sectional) and interventional studies (randomized controlled and experimental) that were published in English and involved participants older than 12 years who had at least 30 days of lifetime marijuana use. This cutoff was chosen to ensure meaningful marijuana exposure. We excluded studies reporting only outcomes after short-term exposure in a laboratory setting and those including fewer than 10 marijuana users. Our search was augmented by author and reference tracking to identify additional articles. The same 2 investigators independently reviewed the full text of all titles and abstracts that passed the initial screen, and disagreements were resolved by discussion or by a third reviewer (S.K.). Interrater reliability on 80 randomly selected abstracts for the 2 primary reviewers was excellent (Cohen κ, 0.81) (Part 2 of the Supplement).
Data Extraction and Quality Assessment
For each included study, 4 reviewers independently extracted data on all outcomes, which were categorized as symptoms, obstructive lung disease, pulmonary function, or other respiratory outcomes. They also extracted data on design (observational or experimental), study population, participant age, exposure route, average marijuana use, percentage of marijuana-only users, confounders (such as tobacco use, occupational exposure, or respiratory disease), exposure duration, funding source, and baseline variables.
Risk of bias (ROB) in individual studies was assessed independently by 4 reviewers (M.G., D.R., S.K., and D.K.) at both study and outcome levels using either the Cochrane Risk of Bias Tool for outcomes in trial studies (10) or the Newcastle-Ottawa Scale for outcomes in observational studies (11) (Part 3 of the Supplement). We rated studies as having low ROB if they had robust assessment and adjustment for tobacco use, had sufficient follow-up for outcomes to occur, provided detail on exposure assignment (for example, marijuana-only smokers vs. marijuana and tobacco smokers), and quantified marijuana use.
Data Synthesis and Analysis
Meta-analyses were done separately for prospective cohort and cross-sectional studies if each design had 2 or more studies with low or moderate ROB. In the symptoms category, we collected data on marijuana users and nonusers (nonmarijuana and nontobacco users) for the following 4 specific symptoms: cough, sputum production, wheezing, and dyspnea. For binary outcomes (such as cough), we extracted risk ratios (RRs) or calculated them (with 95% CIs) when adequate data were provided. For continuous outcomes (such as pulmonary function test indices), we extracted means and SDs for marijuana smokers and nonsmokers and calculated mean differences with 95% CIs. When several studies examined the same cohort (that is, similar participants over a similar period) we included only data from the study with the longest follow-up for each outcome. We present a narrative synthesis of data for which pooled analysis was not possible.
We pooled data using a random-effects model. We used the Paule–Mandel method (12) to estimate τ2 and the Knapp–Hartung method (13) to adjust for small sample sizes. Statistical analysis was done using R software (package “meta”), version 3.3.3. Heterogeneity was evaluated using forest plots and the I2 statistic; I2 values of 25%, 50%, and 75% were considered evidence of low, moderate, and high heterogeneity, respectively (14).
Three reviewers (M.G., S.K., and D.K.) discussed the overall strength of evidence for each outcome and graded it as insufficient, low, moderate, or high on the basis of methods outlined by the Agency for Healthcare Research and Quality (15).
Role of the Funding Source
This study was not funded.
Results
Literature Search
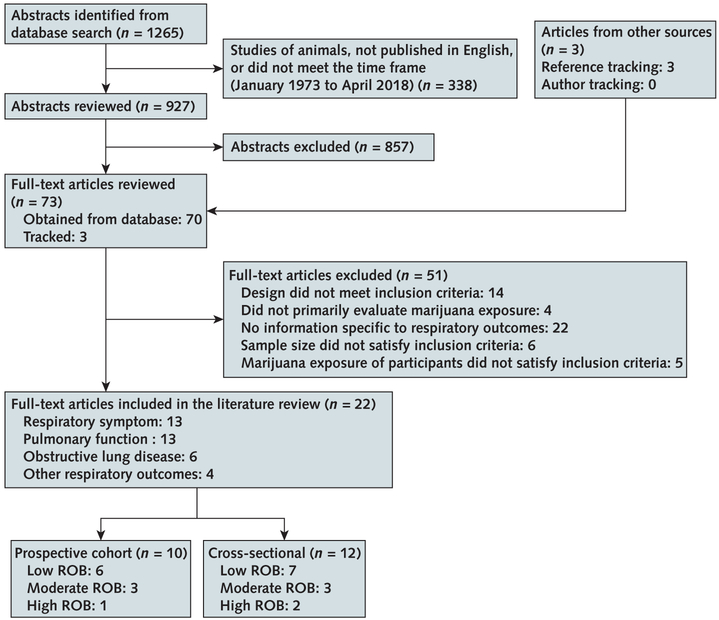
Initial searches across databases identified 1265 abstracts; we selected 927 for further evaluation and 70 of those for full-text review. We identified 3 additional articles by reference tracking, yielding 73 potentially relevant articles, of which 22 met inclusion criteria. Of these, 3 were rated as having high ROB (Figure 1). Supplement Tables 2 to 4 (available at Annals.org) list all articles meeting inclusion criteria; studies with sufficient data for metaanalysis are referenced in Figures 2 and 3.
Figure 1. Evidence search and selection.
Some studies were assigned >1 outcome label and are counted twice. ROB = risk of bias.
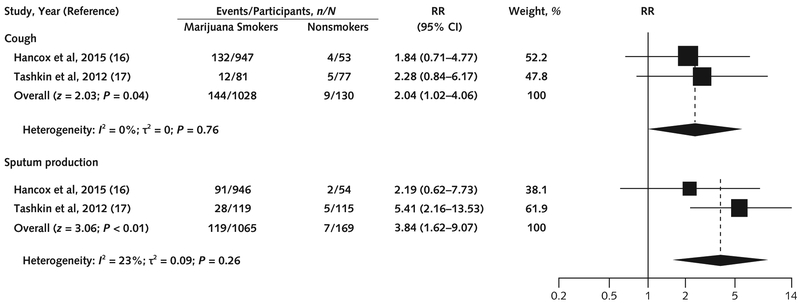
Figure 2. Association between marijuana use and cough and sputum production in prospective cohort studies.
We used the Paule–Mandel method for estimating τ2. The squares indicate RRs from primary studies, and size reflects the statistical weight of the studies. The horizontal lines indicate 95% CIs. The diamonds represent the subtotal and overall RR and 95% CI. The vertical solid line shows the line of no effect (RR = 1). The pooled RR was not significant after the Knapp–Hartung small-sample adjustment for cough (P = 0.10) and sputum production (P = 0.20). RR = risk ratio.
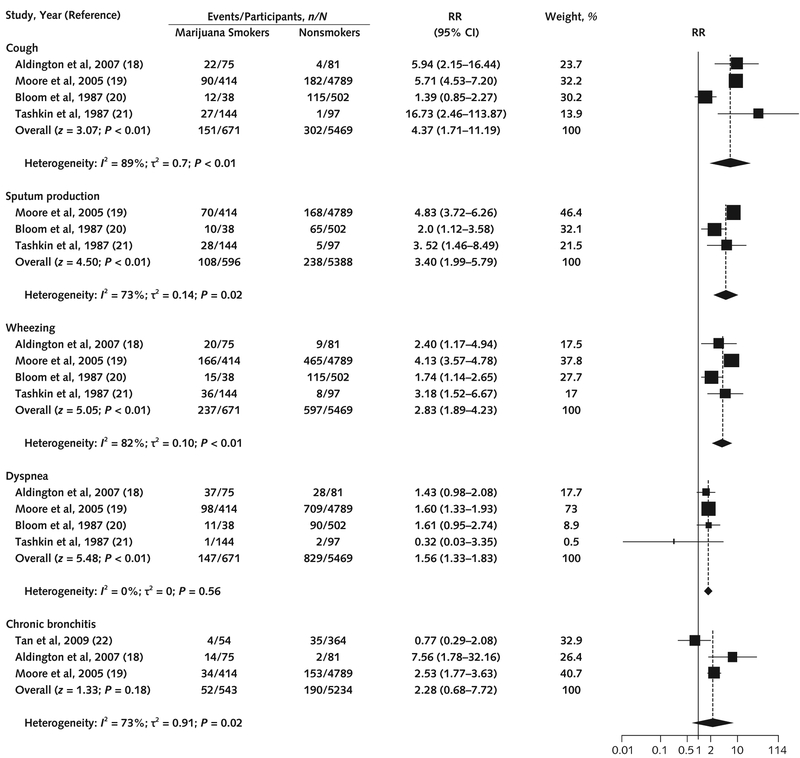
Figure 3. Association between marijuana use and cough, sputum production, wheezing, dyspnea, and chronic bronchitis in cross-sectional studies.
We used the Paule–Mandel method for estimating τ2. The squares indicate RRs from primary studies, and size reflects the statistical weight of the studies. The horizontal lines indicate 95% CIs. The diamonds represent the subtotal and overall RR and 95% CI. The vertical solid line shows the line of no effect (RR = 1). After the Knapp–Hartung small-sample adjustment, pooled RRs were significant for cough (P = 0.05), sputum production (P = 0.05), wheezing (P = 0.01), and dyspnea (P < 0.01) but not for chronic bronchitis (P = 0.31). RR = risk ratio.
Study Characteristics
We included 22 studies (10 prospective cohort and 12 cross-sectional). Methods of quantifying marijuana use varied (for example, monthly vs. weekly vs. daily use and joint-years vs. cigarette-years). Across all outcomes and studies, 1255 participants had more than 10 joint-years of exposure (equal of 1 joint a day for more than 10 years) and 756 marijuana-only smokers had more than 20 joint-years of exposure. We identified 13 distinct outcomes (Supplement Figure 1, available at Annals.org), of which 5 had sufficient supporting data in 2 or more studies and could be pooled in a meta-analysis.
Respiratory Symptoms
Four prospective observational studies (16, 17, 23, 24) and 7 cross-sectional studies (18–21, 25–27) examined the association between marijuana use and cough, sputum production, wheezing, or dyspnea. One prospective study with moderate ROB (24) followed a random sample of the population of Tucson, Arizona (n = 1802), in 4 sequential surveys from 1981 through 1988. Current marijuana smoking was associated with chronic cough (odds ratio [OR], 1.73 [95% CI, 1.21 to 2.47]), chronic sputum production (OR, 1.53 [CI, 1.08 to 2.18]), and wheezing (OR, 2.01 [CI, 1.50 to 2.70]). Although the study had strengths (robust exposure assessment and moderate length of follow-up), it presented limited data and could not be included in the pooled analysis. The other prospective study with moderate ROB (17) included participants from Los Angeles, California (n = 299), who had smoked a mean (± SE) of 3.0 ± 0.4 joints per day for 9.8 years. Baseline exposure assessment was adequate, but loss to follow-up was substantial (49%). Two prospective studies (low ROB) (16, 23) used the Dunedin Multidisciplinary Health and Development Study cohort of 1037 children born in Dunedin, New Zealand, in 1972 and 1973. Marijuana exposure and outcome data (self-reported respiratory symptoms and pulmonary function test results) were collected several times during a mean follow-up of 15 years. Participants smoked an average of once a week for a year. Data were included only from the study with longer follow-up (16), leaving 2 prospective studies for meta-analysis (16, 17). Compared with nonsmokers, marijuana users had increased risks for cough (RR, 2.04 [CI, 1.02 to 4.06]; risk difference [RD], 0.07 [CI, 0.02 to 0.13]) and sputum production (RR, 3.84 [CI, 1.62 to 9.07]; RD, 0.12 [CI, −0.02 to 0.25]). Heterogeneity between the pooled studies was low (Figure 2). Both found that quitting smoking marijuana led to a significant reduction in respiratory symptoms. The Dunedin Study (16) also examined wheezing and dyspnea. Marijuana use was associated with wheezing (OR, 1.55 [CI, 1.23 to 1.94]; P < 0.001), with a trend toward association with dyspnea (OR, 1.23 [CI, 0.97 to 1.56]; P = 0.086) (Supplement Table 2).
Seven cross-sectional studies (3 low ROB [21, 26, 27], 3 moderate [18–20], and 1 high [25]) examined the association between marijuana use and cough and wheezing; 5 of these also assessed sputum production (19–21, 25, 26) or dyspnea (18–21, 25). All studies included moderate to heavy marijuana users. Pooled analysis of cross-sectional studies with low or moderate ROB showed that marijuana use was associated with cough (RR, 4.37 [CI, 1.71 to 11.19]; RD, 0.18 [CI, 0.15 to 0.21]), sputum production (RR, 3.40 [CI, 1.99 to 5.79]; RD, 0.14 [CI, 0.1 to 0.17]), wheezing (RR, 2.83 [CI, 1.89 to 4.23]; RD, 0.22 [CI, 0.14 to 0.29]), and dyspnea (RR, 1.56 [CI, 1.33 to 1.83]; RD, 0.06 [CI, −0.01 to 0.13]) (Figure 3).
Obstructive Lung Disease
Six studies (1 prospective observational cohort [17] and 5 cross-sectional [18, 19, 22, 25, 27]) examined the association between marijuana exposure and chronic bronchitis. The prospective cohort study (moderate ROB) (17) recruited participants in 1983 to 1985. It included comprehensive baseline and outcome data collected in person (self-reported respiratory symptoms and pulmonary function test results) and several exposure assessments over a mean of 9.8 years but was limited by substantial loss to follow-up (49%). The study found that marijuana use (mean [± SE], 3.0 ± 0.4 joints per day) in healthy participants increased risk for bronchitis episodes (OR, 2.3 [CI, 1.2 to 4.4]; P = 0.011) compared with nonuse (Supplement Table 3).
In pooled data from the 3 cross-sectional studies (1 low ROB [22] and 2 moderate [18, 19]), the association between marijuana use and chronic bronchitis did not reach statistical significance (RR, 2.28 [CI, 0.68 to 7.72]; RD, 0.06 [CI, −0.04 to 0.16]) (Figure 3). One of the remaining studies (low ROB) (27) analyzed 1174 participants in SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) and found no association between current or former use of marijuana and chronic bronchitis (OR, 0.87 [CI, 0.59 to 1.31] or OR, 1.0 [CI, 0.79 to 1.26], respectively). However, the sample included only current and former tobacco smokers, limiting generalizability. The final study (high ROB) (25) examined chronic obstructive pulmonary disease and found that each additional joint-year of marijuana use increased its prevalence (absolute increase per additional joint-year, 0.3% [CI, 0.0% to 0.5%]). However, reported outcomes were for cannabis and tobacco users combined rather than cannabis users alone (Supplement Table 3).
Change in Pulmonary Function
Thirteen studies (6 prospective observational [23, 24, 28–31] and 7 cross-sectional [18–21, 27, 32, 33]) investigated the effect of marijuana exposure on pulmonary function. Seven were rated as having low ROB. Most analyzed 1 or 2 variables of pulmonary function and did not report all metrics, precluding meta-analysis (Supplement Table 4).
FEV1
Ten studies (4 prospective observational cohort [24, 28, 29, 31] and 6 cross-sectional [18, 20, 21, 27, 32, 33]) evaluated marijuana exposure and changes in FEV1. One prospective study (low ROB) (28) used data from the CARDIA (Coronary Artery Risk Development in Young Adults) Study cohort, which began in 1985 with 5115 men and women aged 18 to 30 years. Participants had repeated exposure assessments over 20 years, and baseline adjustment for confounding was adequate. Current marijuana use was not associated with FEV1 (adjusted mean difference, −18 mL [CI, −42 to 6.1 mL]; P = 0.32), which did not show a significant decrease compared with that of nonsmokers. However, exposure was minimal (2 to 3 episodes per month). The other prospective study (low ROB) (31) was a longitudinal study based in Los Angeles that included a young cohort (mean age, 33 years) with average use of more than 3.5 joints per day at cohort inception. Exposure was assessed several times over 8 years, and adjustment for confounders was adequate. Neither continuing nor intermittent marijuana smokers had significant decreases in FEV1 compared with non–marijuana smokers. The Tucson study (moderate ROB) (24) was done over 6 years in 4 sequential surveys. Among current marijuana smokers (4 to 5 marijuana cigarettes per week), researchers found significant reductions in FEV1 (P < 0.01) after 1 year of follow-up. The remaining low-ROB prospective study (Dunedin) (29) did not report median cumulative exposure but followed smokers (aged 18 to 32 years) over 10 years and found no change in FEV1.
Six cross-sectional studies (3 low ROB [21, 27, 32], 2 moderate [18, 20], and 1 high [33]) compared FEV1 in marijuana smokers versus nonsmokers. NHANES (National Health and Nutrition Examination Survey) (2007 to 2008 and 2009 to 2010) and SPIROMICS (both low ROB) (27, 32) had large sample sizes, lifetime exposure assessments, and adequate adjustment for key confounders; however, they reported FEV1 outcomes only for smokers of marijuana and tobacco together, limiting interpretation. The remaining 4 studies (1 low ROB [21], 2 moderate [18, 20], and 1 high [33]) found no effect on FEV1 but were limited by small sample sizes, young populations, and inadequate assessment of baseline characteristics (Supplement Table 4).
FVC
Six studies (21, 27–29, 32, 33) assessed the effect of marijuana on FVC; 2 were prospective observational cohorts (CARDIA and Dunedin) (28, 29), and 4 were cross-sectional (21, 27, 32, 33). In CARDIA (low ROB) (28), current marijuana use (>20 episodes in the past 30 days) was associated with higher FVC (adjusted mean difference, 20 mL [CI, −5.2 to 49 mL]; P = 0.03), as was lifetime marijuana use (>10 joint-years) (adjusted mean difference, 59 mL [CI, 12 to 107 mL]; P = 0.01). In the Dunedin Study (low ROB) (29), 1037 participants completed questionnaires about health, sociodemographics, and cannabis smoking history at ages 18, 21, 26, and 32 years. Cannabis dependence was nonsignificantly associated with higher FVC (adjusted mean difference, 17.5 mL [CI, −2.5 to 37.4 mL]; P = 0.087).
Four cross-sectional studies (3 low ROB [21, 27, 32] and 1 high [33]) examined the association between marijuana use and FVC. The large SPIROMICS study (low ROB) (27) reported higher FVC among current and former marijuana users (P < 0.001 for each). One study (32) based on NHANES (low ROB) with an average cumulative exposure of 15.8 joint-years reported an increase in FVC (mean, 0.07% [SD, 0.02%]; P = 0.004) for each additional joint-year smoked. The other 2 crosssectional studies (1 low ROB [21] and 1 high [33]) found no effect on FVC. The low-ROB study (21) had an average exposure of 50.4 joint-years (SD, 4.6), whereas the high-ROB study (33) was limited by inadequate exposure and a small sample of marijuana-only smokers (n = 50) (Supplement Table 4).
FEV1–FVC Ratio
Three prospective observational cohorts (2 low ROB [23, 29] and 1 moderate [30]) were based on 3 follow-up periods of the Dunedin Study. These studies measured exposure at ages 21, 26, and 32 years and had robust assessment of baseline characteristics and outcomes. The study (29) with the longest follow-up (10 years) showed no change in FEV1–FVC ratio (adjusted mean difference, −0.19 [CI, −0.42% to 0.04%]; P = 0.100). However, median cumulative marijuana exposure in the cohort was not reported, limiting interpretability. In the Tucson study (moderate ROB) (24), marijuana use (4 to 5 marijuana cigarettes per week) was associated with a significant reduction in FEV1–FVC ratio after 1 year of follow-up.
Six studies were cross-sectional (18–21, 27, 32). Two (19, 32) were based on 3 waves of NHANES (1988 to 1994, 2007 to 2008, and 2009 to 2010). One of these (2007 to 2008 and 2009 to 2010) (low ROB) (32) showed that more than 20 joint-years of marijuana exposure was associated with an FEV1–FVC ratio less than 70% (OR, 2.1 [CI, 1.1 to 3.9]; P = 0.02) compared with nonsmokers after adjustment for tobacco and other variables. The other NHANES study (moderate ROB) (19) found no definite relationship between marijuana use and an FEV1–FVC ratio less than 70%. However, average (± SE) cumulative exposure was 10.2 ± 0.84 days in the past month, and relatively few marijuana-only smokers were included, limiting interpretation of the findings. SPIROMICS (low ROB) (27) had a large sample (n = 1174) with a mean cumulative exposure of 30.1 joint-years (SD, 68.5). Current marijuana smoking was positively associated with FEV1–FVC ratio (P < 0.001) after adequate adjustment for key confounders. However, this study was limited by reporting results on marijuana and tobacco smokers together. Another study (moderate ROB) (18) recruited 339 participants through 2 populations (the Wellington Respiratory Survey and the greater Wellington area in New Zealand). Sample recruitment was not representative because marijuana smokers and nonsmokers came from different populations. Marijuana use (mean, 54.2 joint-years [SD, 75.3]) was associated with a marginally lower FEV1–FVC ratio (estimate of difference, −1.1% [CI, −2.6% to 0.1%]). The 2 remaining studies (20, 21) showed no association with reduced FEV1–FVC ratio, but both had limited generalizability. One was cross-sectional (low ROB) (21) and recruited users aged 25 to 49 years who had smoked at least 10 joints per week for at least 5 years. The other (moderate ROB) (20) included few marijuana-only smokers (n = 38 [3.8%]). It analyzed data on male current marijuana smokers, who had significantly lower FEV1–FVC ratios (P < 0.05) than nonsmokers.
Airway Resistance and Specific Conductance of Airways
Airway resistance and specific conductance of airways were examined in 1 prospective cohort (low ROB) (29) and 3 cross-sectional studies (1 low ROB [21], 1 moderate [18], and 1 high [33]). All studies reported a significantly higher airway resistance and decreased specific conductance of airways in marijuana smokers (Supplement Table 4).
Other Respiratory Outcomes
Two prospective observational studies (1 low ROB [34] and 1 high [35]) and 2 cross-sectional studies (low ROB) (36, 37) described other respiratory outcomes (such as upper respiratory tract infection, hospitalization, or airway reactivity). The 2 prospective studies (34, 35) followed participants over 8 years. Current marijuana use was associated with more outpatient visits for respiratory illnesses (such as cold, flu, or sore throat) (34) and respiratory problems (35), with no increased risk for hospital admission (34). The high-ROB study (35) was limited by inadequate adjustment for key confounders and lack of reporting on the nature of respiratory problems. The 2 cross-sectional studies (36, 37) examined the effect of methacholine challenge on airway reactivity among marijuana users compared with nonusers and found no difference (Supplement Table 5, available at Annals.org).
Strength of Evidence
Low-strength evidence suggests that smoking marijuana is associated with cough, sputum production, and wheezing. Evidence is insufficient on the association between daily marijuana use and changes in pulmonary function or development of obstructive lung disease (Table).
Table.
Strength of Evidence for Each Respiratory Symptom, Obstructive Lung Disease, Pulmonary Function, and Other Respiratory Outcomes
| Outcome | Study Type | Strength of Evidence |
Comments |
|---|---|---|---|
| Respiratory symptoms | |||
| Cough | 4 prospective observational studies (2 low* and 2 moderate ROB) and 7 cross-sectional studies (3 low, 3 moderate, and 1 high ROB) | Low | All prospective studies rated as low or moderate ROB and the pooled cross-sectional studies found an association between marijuana use and cough. |
| Sputum production | 4 prospective observational studies (2 low* and 2 moderate ROB) and 5 cross-sectional studies (2 low, 2 moderate, and 1 high ROB) | Low | Low- and moderate-ROB studies showed a consistent trend toward increased risk for sputum production with marijuana use. Pooled data showed that marijuana use was associated with an increased risk for sputum production. |
| Wheezing | 4 prospective observational studies (2 low* and 2 moderate ROB) and 7 cross-sectional studies (3 low, 3 moderate, and 1 high ROB) | Low | Three prospective studies (1 low and 2 moderate ROB) showed that marijuana use was associated with increased risk for wheezing. Pooled analysis of 4 cross-sectional studies (1 low and 3 moderate ROB) showed that marijuana use was associated with wheezing, but heterogeneity was high. Low- and moderate-ROB studies showed a consistent trend toward an association between marijuana use and wheezing. |
| Dyspnea | 2 prospective observational studies*(low ROB) and 5 cross-sectional studies (2 low, 2 moderate, and 1 high ROB) | Insufficient | The prospective studies were based on the same cohort, and the study with the longest follow-up found no effect on increased risk for dyspnea. This study was limited because the median marijuana exposure was not reported; however, the pooled analysis from cross-sectional studies showed that marijuana use was associated with increased risk for dyspnea. |
| Obstructive lung disease | 1 prospective observational cohort study (moderate ROB) and 5 cross-sectional studies (2 low, 2 moderate, and 1 high ROB) | Insufficient | One prospective study found an increased risk for chronic bronchitis during a long follow-up. Pooled analysis of 3 cross-sectional studies (1 low and 2 moderate ROB) did not show that marijuana use was associated with increased risk for chronic bronchitis. All studies showed a trend toward increased risk for chronic bronchitis from marijuana use; however, the body of available evidence was limited. |
| Pulmonary function | |||
| FEV1 | 4 prospective observational cohort studies (3 low and 1 moderate ROB) and 6 cross-sectional studies (3 low, 2 moderate, and 1 high ROB) | Insufficient | The low-ROB prospective studies found no effect on decrease of FEV1; however, the studies were limited by minimal exposure to marijuana and the young age of participants. The cross-sectional studies were limited by small sample size, a young population, and variable rigor in analysis; the experimental study was limited by lack of adjustment for key confounders. |
| FVC | 2 prospective observational cohort studies (low ROB) and 4 cross-sectional studies (3 low and 1 high ROB) | Insufficient | The prospective studies showed mixed results with marijuana use and an increase in FVC, and the 2 cross-sectional studies showed no effect of marijuana on FVC, but study quality and rigor in analysis varied. |
| FEV1-FVC ratio | 4 prospective observational cohort studies (2 low* and 2 moderate ROB) and 6 cross-sectional studies (3 low and 3 moderate ROB) | Insufficient | All low-ROB prospective studies were based on the same cohort study. The studies with the longest follow-up did not report average marijuana exposure in the cohort. The cross-sectional studies were limited by few marijuana-only smokers, variable rigor in analysis, and mixed findings. |
| Airway resistance and specific conductance of airways | 1 prospective cohort study (low ROB) and 3 cross-sectional studies (1 low, 1 moderate, and 1 high ROB) | Low | All relevant studies found that marijuana use was associated with increased airway resistance and decreased specific conductance of airways. |
| Other respiratory outcomes | 2 prospective observational studies (1 low and 1 high ROB) and 2 cross-sectional studies (low ROB) | Insufficient | The prospective studies were limited by inadequate marijuana exposure, inadequate adjustment for confounders, and many other methodological flaws with mixed findings. |
ROB = risk of bias.
Both studies were based on the same cohort.
Discussion
Because of increasing social acceptance and widespread use, understanding the health effects of smoking marijuana is important. Our review suggests that use (more than once per week for at least 1 year) is associated with cough, sputum production, and wheezing. Evidence on the association between daily use and obstructive lung disease and impaired pulmonary function testing is insufficient.
An English-language MEDLINE search returned 3 other reviews (38–40) that examined short- and long-term effects of smoking marijuana. A 2007 systematic review (38) noted an association between marijuana use and respiratory symptoms and outlined the need for more data on marijuana's association with pulmonary function. Two more recent studies (39, 40) also noted an association with increased respiratory symptoms, including cough, sputum production, and wheezing, but reported conflicting data on the association between long-term marijuana smoking and changes in pulmonary function. Our study confirmed these findings and built on the existing literature by assessing risk of bias, pooling data where feasible, and providing a clear picture of the gaps in evidence by rating the strength of the overall evidence.
Marijuana may be expected to cause respiratory symptoms. Its smoke contains particulate matter and compounds that induce oxidative stress and inflammation in the lung (41). Data comparing endobronchial biopsies from marijuana users versus nonusers support the clinical relevance of this type of effect from marijuana smoke exposure. Findings among marijuana users (26, 42, 43) are consistent with chronic airway inflammation and epithelial injury, including basal cell hyperplasia, goblet cell hyperplasia, and subepithelial inflammation, suggesting a mechanistic link between long-term marijuana use and respiratory symptoms.
Although our review found no relationship between marijuana use and impairment in spirometric indices, these data should be interpreted with caution because low-strength evidence suggests increased airway resistance with marijuana use, which can precede changes in lung function. Further, exposure in the included studies may have been insufficient to alter pulmonary function test results. Studies of long-term tobacco use suggest that changes to FEV1, become measurable only after 5 to 10 pack-years of smoking (36 500 to 73 000 cigarettes) (44–46). Our review included 243 marijuana users (131 marijuana-only users) with exposure greater than 20 joint-years (1 joint a day for 20 years = 7300 joints) across all prospective evaluations of lung function. These low exposure levels limit our ability to draw conclusions about the effect of daily marijuana use. Because obstructive lung disease develops in only about a third of long-term tobacco smokers (47), is usually not identified until after age 35 or 40 years, and increases in prevalence with age, large cohorts with middle-aged to older populations of heavier marijuana users may be necessary to identify effects on lung function and obstructive lung disease. On the other hand, given the psychoactive effects of tetrahydrocannabinol and its effect on overall function (48), few users may have heavy enough exposure to cause significant changes in pulmonary function testing. In other words, marijuana's effect on lung function may not be among its most important health outcomes in the long term.
Our review has important limitations. We excluded articles not published in English; thus, we may have overlooked relevant studies. Study populations were young, and marijuana exposure was limited in most prospective studies. Most studies inadequately assessed exposure, and some did not report effect size or details on exposure; this prevented meta-analysis for several outcomes. Although we report complete data for all analyses, meta-analyses of cross-sectional studies examining cough, sputum production, and wheezing were limited by heterogeneity. Heterogeneity was likely related to the lack of uniform assessment of marijuana use (for example, joint-year, times per week, or times in lifetime) and outcome ascertainment (for example, cough definition of most days for 3 consecutive months vs. >6 times a day). Our current understanding of the long-term health effects of marijuana could be improved by standardized assessment tools for marijuana use and studies with larger samples of marijuana-only users and longer follow-up times.
Low-strength evidence indicates that smoking marijuana is associated with cough, sputum production, and wheezing. Current understanding of marijuana's effect on pulmonary function tests and development of obstructive lung disease is insufficient and is limited by low exposure and young study populations. Given rapidly expanding use, we need large-scale longitudinal studies examining the long-term pulmonary effects of daily marijuana use.
Supplementary Material
Annals Teaching Tools.
Annals provides content and resources in formats that will assist you in your teaching activities. Teaching tools provided include:
Annals for Educators alerts: Tips from the editors on ways to use selected articles from each issue to help you in your teaching activities.
In the Clinic Slide Sets: PowerPoint slide sets that summarize key points from each In the Clinic issue.
On Being a Doctor Teaching Modules: Materials developed to support teaching and learning about the experiences of being a physician as represented in these popular essays.
ACP resources, such as the Physician Educators' Special Interest Group, High Value Care Curriculum, and other resources for medical educators.
For these resources, please visit www.annals.org/public/teachingtools.aspx.
Acknowledgments
Grant Support: This project was not directly supported by any funds. Dr. Keyhani was supported by grant R01HL130484-01A1 from the National Heart, Lung, and Blood Institute. Dr. Korenstein's contribution to this project was supported in part by a Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center from the National Cancer Institute.
Footnotes
Note: Dr. Keyhani had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18-0522.
Reproducible Research Statement: Study protocol: Available at www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017059224. Statistical code: Available from Ms. Vali (marzieh.vali@va.gov). Data set: See Supplement (available at Annals.org). Additional data are available from Dr. Ghasemiesfe (mehrnaz.ghasemiesfe@va.gov).
Contributor Information
Mehrnaz Ghasemiesfe, University of California, San Francisco, and San Francisco Veterans Affairs Medical Center, San Francisco, California.
Divya Ravi, The Wright Center for Graduate Medical Education, Scranton, Pennsylvania.
Marzieh Vali, Northern California Institute for Research and Education, San Francisco Veterans Affairs Medical Center, San Francisco, California.
Deborah Korenstein, Memorial Sloan Kettering Cancer Center, New York, New York.
Mehrdad Arjomandi, University of California, San Francisco, and San Francisco Veterans Affairs Medical Center, San Francisco, California.
James Frank, University of California, San Francisco, and San Francisco Veterans Affairs Medical Center, San Francisco, California.
Peter C. Austin, University of Toronto, Toronto, Ontario, Canada.
Salomeh Keyhani, University of California, San Francisco, and San Francisco Veterans Affairs Medical Center, San Francisco, California.
References
- 1.Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954–64. [PMID: ] doi: 10.1016/S2215-0366(16)30208-5 [DOI] [PubMed] [Google Scholar]
- 2.Prevalence of marijuana use among U.S. adults doubles over past decade [press release]. Bethesda: National Institutes of Health; 21 October 2015. [Google Scholar]
- 3.Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R. National estimates of marijuana use and related indicators—National Survey on Drug Use and Health, United States, 2002-2014. MMWR Surveill Summ. 2016;65:1–28. [PMID: ] doi: 10.15585/mmwr.ss6511a1 [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health. NIH's 2017 Monitoring the Future survey shows both vaping and marijuana are more popular than traditional cigarettes or pain reliever misuse. 2017. Accessed at www.drugabuse.gov/related-topics/trends-statistics/monitoring-future on 14 December 2017.
- 5.Hoffmann D, Brunnemann KD, Gori GB, Winder EL. On the carcinogenicity of marijuana smoke In: Runeckles VC, ed. Recent Advances in Phytochemistry. Boston: Springer-Verlag; 1975:63–81. [Google Scholar]
- 6.Tashkin DP, Baldwin GC, Sarafian T, Dubinett S, Roth MD. Respiratory and immunologic consequences of marijuana smoking. J Clin Pharmacol. 2002;42:71S–81S. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 7.Tashkin DP, Gliederer F, Rose J, Chang P, Hui KK, Yu JL, et al. Tar, CO and delta 9THC delivery from the 1st and 2nd halves of a marijuana cigarette. Pharmacol Biochem Behav. 1991;40:657–61. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 9.Blachly PH. Effects of decriminalization of marijuana in Oregon. Ann N Y Acad Sci. 1976;282:405–15. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. ; Cochrane Bias Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928 [PMID: ] doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute. 2014. Accessed at www.ohri.ca/programs/clinical_epidemiology/oxford.asp on 29 September 2017. [Google Scholar]
- 12.Paule RC, Mandel J. Consensus values and weighting factors. J Res Natl Bur Stand. 1982;87:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20:3875–89. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkman ND, Lohr KN, Ansari M, McDonagh M, Balk E, Whitlock E, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Methods Guide for Comparative Effectiveness Reviews. (Prepared by the RTI-UNC Evidence-based Practice Center under contract no. 290-2007-10056-I.) AHRQ publication no. 13(14)-EHC130-EF. Rockville: Agency for Healthcare Research and Quality; November 2013. Accessed at https://ahrq-ehc-application.s3.amazonaws.com/media/pdf/methods-guidance-grading-evidence_methods.pdf on 12 October 2017. [Google Scholar]
- 16.Hancox RJ, Shin HH, Gray AR, Poulton R, Sears MR. Effects of quitting cannabis on respiratory symptoms. Eur Respir J. 2015;46:80–7. [PMID: ] doi: 10.1183/09031936.00228914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tashkin DP, Simmons MS, Tseng CH. Impact of changes in regular use of marijuana and/or tobacco on chronic bronchitis. COPD. 2012;9:367–74. [PMID: ] doi: 10.3109/15412555.2012.671868 [DOI] [PubMed] [Google Scholar]
- 18.Aldington S, Williams M, Nowitz M, Weatherall M, Pritchard A, McNaughton A, et al. Effects of cannabis on pulmonary structure, function and symptoms. Thorax. 2007;62:1058–63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore BA, Augustson EM, Moser RP, Budney AJ. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33–7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloom JW, Kaltenborn WT, Paoletti P, Camilli A, Lebowitz MD. Respiratory effects of non-tobacco cigarettes. Br Med J (Clin Res Ed). 1987;295:1516–8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tashkin DP, Coulson AH, Clark VA, Simmons M, Bourque LB, Duann S, et al. Respiratory symptoms and lung function in habitual heavy smokers of marijuana alone, smokers of marijuana and tobacco, smokers of tobacco alone, and nonsmokers. Am Rev Respir Dis. 1987;135:209–16. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 22.Tan WC, Lo C, Jong A, Xing L, Fitzgerald MJ, Vollmer WM, et al. ; Vancouver Burden of Obstructive Lung Disease (BOLD) Research Group. Marijuana and chronic obstructive lung disease: a population-based study. CMAJ. 2009;180:814–20. [PMID: ] doi: 10.1503/cmaj.081040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor DR, Poulton R, Moffitt TE, Ramankutty P, Sears MR. The respiratory effects of cannabis dependence in young adults. Addiction. 2000;95:1669–77. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24.Sherrill DL, Krzyzanowski M, Bloom JW, Lebowitz MD. Respiratory effects of non-tobacco cigarettes: a longitudinal study in general population. Int J Epidemiol. 1991;20:132–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 25.Macleod J, Robertson R, Copeland L, McKenzie J, Elton R, Reid P. Cannabis, tobacco smoking, and lung function: a cross-sectional observational study in a general practice population. Br J Gen Pract. 2015;65:e89–95. [PMID: ] doi: 10.3399/bjgp15X683521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fligiel SE, Roth MD, Kleerup EC, Barsky SH, Simmons MS, Tashkin DP. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest. 1997;112:319–26. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 27.Morris MA, Jacobson SR, Kinney GL, Tashkin DP, Woodruff PG, Hoffman EA, et al. Marijuana use associations with pulmonary symptoms and function in tobacco smokers enrolled in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS). Chronic Obstr Pulm Dis. 2018;5:46–56. [PMID: ] doi: 10.15326/jcopdf.5.1.2017.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307:173–81. [PMID: ] doi: 10.1001/jama.2011.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hancox RJ, Poulton R, Ely M, Welch D, Taylor DR, McLachlan CR, et al. Effects of cannabis on lung function: a population-based cohort study. Eur Respir J. 2010;35:42–7. [PMID: ] doi: 10.1183/09031936.00065009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor DR, Fergusson DM, Milne BJ, Horwood LJ, Moffitt TE, Sears MR, et al. A longitudinal study of the effects of tobacco and cannabis exposure on lung function in young adults. Addiction. 2002;97:1055–61. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 31.Tashkin DP, Simmons MS, Sherrill DL, Coulson AH. Heavy habitual marijuana smoking does not cause an accelerated decline in FEV1 with age. Am J Respir Crit Care Med. 1997;155:141–8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 32.Kempker JA, Honig EG, Martin GS. The effects of marijuana exposure on expiratory airflow. A study of adults who participated in the U.S. National Health and Nutrition Examination Study. Ann Am Thorac Soc. 2015;12:135–41. [PMID: ] doi: 10.1513/AnnalsATS.201407-333OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tashkin DP, Calvarese BM, Simmons MS, Shapiro BJ. Respiratory status of seventy-four habitual marijuana smokers. Chest. 1980;78:699–706. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 34.Polen MR, Sidney S, Tekawa IS, Sadler M, Friedman GD. Health care use by frequent marijuana smokers who do not smoke tobacco. West J Med. 1993;158:596–601. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- 35.Brook JS, Stimmel MA, Zhang C, Brook DW. The association between earlier marijuana use and subsequent academic achievement and health problems: a longitudinal study. Am J Addict. 2008;17:155–60. [PMID: ] doi: 10.1080/10550490701860930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tashkin DP, Simmons MS, Chang P, Liu H, Coulson AH. Effects of smoked substance abuse on nonspecific airway hyperresponsiveness. Am Rev Respir Dis. 1993;147:97–103. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 37.Tashkin DP, Simmons M, Clark V. Effect of habitual smoking of marijuana alone and with tobacco on nonspecific airways hyperreactivity. J Psychoactive Drugs. 1988;20:21–5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 38.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221–8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribeiro LI, Ind PW. Effect of cannabis smoking on lung function and respiratory symptoms: a structured literature review. NPJ Prim Care Respir Med. 2016;26:16071 [PMID: ] doi: 10.1038/npjpcrm.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinasek MP, McGrogan JB, Maysonet A. A systematic review of the respiratory effects of inhalational marijuana. Respir Care. 2016;61:1543–51. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 41.Sarafian TA, Magallanes JA, Shau H, Tashkin D, Roth MD. Oxidative stress produced by marijuana smoke. An adverse effect enhanced by cannabinoids. Am J Respir Cell Mol Biol. 1999;20:1286–93. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 42.Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP. Airway inflammation in young marijuana and tobacco smokers. Am J Respir Crit Care Med. 1998;157:928–37. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 43.Barsky SH, Roth MD, Kleerup EC, Simmons M, Tashkin DP. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J Natl Cancer Inst. 1998;90:1198–205. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 44.Tashkin DP, Shapiro BJ, Lee YE, Harper CE. Subacute effects of heavy marihuana smoking on pulmonary function in healthy men. N Engl J Med. 1976;294:125–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 45.Saqib M, Mahmud T, Bokhari SNH, Aasim M. Correlation between amount of smoking and decline in lung function among urban male population. Proceedings: Official Journal of the Shaikh Zayed Postgraduate Medical Institute. 2011;25:67–71. [Google Scholar]
- 46.Ashley F, Kannel WB, Sorlie PD, Masson R. Pulmonary function: relation to aging, cigarette habit, and mortality. Ann Intern Med. 1975;82:739–45. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 47.Laniado-Laborín R Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int J Environ Res Public Health. 2009;6:209–24. [PMID: ] doi: 10.3390/ijerph6010209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogeberg O Correlations between cannabis use and IQ change in the Dunedin cohort are consistent with confounding from socioeconomic status. Proc Natl Acad Sci U S A. 2013;110:4251–4. [PMID: ] doi: 10.1073/pnas.1215678110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.