Abstract
Background
Evaluating the progression of hepatic fibrosis in chronic hepatitis C (CHC) is critical, and identifying a predictive biomarker for fibrosis will be helpful for implementing personalized surveillance of hepatocellular carcinoma after the elimination of hepatitis C virus by antiviral therapy. This study aimed to investigate the association of circulating let-7a-5p levels with severity of hepatic fibrosis.
Methods
We analyzed circulating let-7a-5p levels in serum and serum-derived extracellular vesicles (EVs) in 84 Japanese CHC patients who underwent a liver biopsy by quantitative real-time polymerase chain reaction, and investigated the association of its levels with histological hepatic fibrotic stage, liver stiffness, and several hepatic fibrotic markers.
Results
The levels of let-7a-5p in serum and EVs were significantly lower in patients with liver cirrhosis. Additionally, the serum let-7a-5p level correlated significantly with hepatic fibrotic markers, Mac-2 binding protein glycan isomer (M2BPGi), fibrosis-4 (FIB-4) index, aspartate aminotransferase-to-platelet ratio index (APRI), and liver stiffness, evaluated by transient elastography. Furthermore, the serum let-7a-5p level was superior to M2BPGi, FIB-4, and APRI and was comparable to liver stiffness in discriminating liver cirrhosis.
Conclusions
These results provide evidence that circulating let-7a-5p in serum may serve as a surrogate marker for severity of hepatic fibrosis in CHC.
Keywords: extracellular vesicles, hepatitis C virus, hepatic fibrosis, let-7, microRNA
Chronic hepatitis C virus (HCV) infection poses a significant risk for progressive hepatic fibrosis, subsequent liver cirrhosis (LC), and hepatocellular carcinoma (HCC). Recently developed, interferon-free oral regimens combining direct-acting antiviral agents (DAAs) have achieved remarkably high rates of HCV eradication. However, a risk of HCC development remains after the elimination of HCV, and an advanced state of hepatic fibrosis is the major risk factor. Therefore, evaluating the progression of hepatic fibrosis in chronic hepatitis C (CHC) is critical, and identifying a predictive biomarker for fibrosis will be helpful for implementing personalized treatment and surveillance of disease progression and HCC. In the current clinical practice, hepatic fibrosis indexes, such as the fibrosis-4 (FIB-4) index and aspartate aminotransferase (AST)-to-platelet ratio index (APRI) score, are widely used as a noninvasive hepatic fibrotic markers in CHC [1, 2]. Recently, wisteria floribunda agglutinin–positive Mac-2 binding protein (WFA+-M2BP) was developed as a novel serum biomarker that shows significant correlation with liver fibrosis in CHC patients [3]. In addition, many studies have shown that liver stiffness evaluated by transient elastography (TE) reflects liver fibrosis [4].
MicroRNAs (miRNAs) are involved in various biological phenomena, such as cell development, differentiation, proliferation, apoptosis, and metabolism [5], and also play roles in the pathogenesis of inflammation, fibrogenesis, and carcinogenesis in liver diseases [6]. Expression profiling of circulating miRNAs in serum, plasma, and other body fluids holds promise for identifying novel, noninvasive markers of disease progression and severity. Previous studies have revealed that several circulating miRNAs are associated with chronic HCV infection, hepatic necroinflammatory activity, and progression of fibrosis in CHC [7–11]. However, it is difficult to reproduce a previously reported association between a specific miRNA and disease progression in an independent cohort. On the other hand, several studies have reproduced the results that circulating miR-122 levels were correlated with elevated aminotransferases and pathological hepatic necroinflammatory activities in CHC patients [8, 12, 13]. Using a prospectively followed and well-characterized HCV-infected blood donor cohort in the United States, we recently revealed that the levels of several let-7 members in plasma and plasma-derived extracellular vesicles (EVs) were correlated with progression of hepatic fibrosis in the natural course of chronic HCV infection [14]. However, the number of patients, especially those with significant hepatic fibrosis, was limited in that study. Therefore, the association between circulating let-7 levels and hepatic fibrosis should be validated in independent cohorts to implement let-7 as a biomarker for hepatic fibrosis.
In humans, there are 12 let-7 family members (let-7a-1, -2, -3; let-7b; let-7c; let-7d; let-7e; let-7f-1, -2; let-7g; let-7i; miR-98) located at 8 different chromosome loci, and these miRNAs have an identical seed sequence, a highly conserved region of nucleotides 2 to 8, which is important for target recognition [15].
Here, we investigated the association of circulating let-7 levels with histological hepatic fibrotic stage, and liver stiffness which was evaluated by TE, a novel glyco-biomarker, Mac-2 binding protein glycan isomer (M2BPGi), and other fibrotic markers using serum samples in Japanese CHC cohorts.
METHODS
Study Population and Design
We enrolled 84 Japanese CHC patients who underwent a liver biopsy; the clinical characteristics at the time of liver biopsy are shown in Table 1. No participants were infected with hepatitis B virus or HIV or had other liver diseases such as autoimmune hepatitis, nonalcoholic steatohepatitis, and primary biliary cirrhosis. Written informed consent was obtained from all individual participants, and the study protocol conformed to the ethics guidelines of the Declaration of Helsinki and was approved by the institutional ethics review committees.
Table 1.
Clinical Characteristics of 84 Chronic Hepatitis C Patients at Liver Biopsy
| Total | METAVIR F1-3 | METAVIR F4 | P Value | |
|---|---|---|---|---|
| (n = 84) | (n = 59) | (n = 25) | F1-3 vs F4 | |
| Gender, male/female | 48/36 | 36/23 | 12/13 | NS |
| Age, y | 63 (54–69) | 62 (52–69) | 64 (55–71) | NS |
| AST, IU/L | 38 (25–54) | 32 (24–49) | 51 (36–64) | .005 |
| ALT, IU/L | 37 (25–58) | 35 (23–53) | 42 (34–88) | .027 |
| γ-GTP, IU/L | 33 (20–51) | 28 (19–50) | 39 (27–52) | NS |
| Total bilirubin, mg/dL | 0.8 (0.6–1.0) | 0.8 (0.6–0.9) | 0.9 (0.7–1.3) | NS |
| Albumin, g/dL | 3.9 (3.6–4.0) | 3.9 (3.7–4.0) | 3.7 (3.5–3.9) | .020 |
| Prothrombin time, % | 84 (76–94) | 89 (82–96) | 75 (71–80) | <.001 |
| Platelet count, ×109/L | 155 (122–205) | 163 (143–214) | 118 (91–140) | <.001 |
| APRI | 0.82 (0.40–1.22) | 0.60 (0.36–0.95) | 1.21 (0.88–2.02) | <.001 |
| FIB-4 | 2.69 (1.47–3.74) | 1.85 (1.32–2.99) | 3.82 (2.87–5.61) | <.001 |
| M2BPGi, COI | 1.67 (0.97–3.09) | 1.25 (0.84–2.16) | 3.27 (1.95–6.11) | <.001 |
| Liver stiffness, kPa | 7.9 (5.4–14.8) | 6.9 (4.9–9.5) | 17.6 (12.5–25.7) | <.001 |
| HCV RNA, log IU/mL | 6.4 (5.9–6.9) | 6.5 (5.8–6.9) | 6.3 (6.0–6.8) | NS |
| HCV genotype 1/2/3 | 60/23/1 | 39/19/1 | 21/4/0 | NS |
| Pathology (METAVIR score) | ||||
| F1/2/3/4 | 20/20/19/25 | 20/20/19/0 | 0/0/0/25 | ‒ |
| A0/1/2/3 | 0/26/56/2 | 0/26/32/1 | 0/0/24/1 | ‒ |
Data from the liver biopsy are expressed as numbers for categorical data and medians (first–third quartiles) for noncategorical data. Categorical variables were compared between METAVIR F1-3 and F4 by the chi-square test, and noncategorical variables were compared using the Mann-Whitney U test.
Abbreviations: γ GTP, γ-glutamyl transpeptidase; ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; COI, cutoff index; FIB-4, fibrosis-4; kPa, kilopascals; M2BPGi, Mac-2 binding protein glycan isomer; NS, not significant.
Laboratory and Histological Tests
Hematologic and blood chemistry tests were carried out using standard assays. Histological hepatic fibrosis stage, F0-4, and inflammatory activity grade, A0-3, were evaluated according to the METAVIR scoring system. Hepatic fibrosis indexes, the FIB-4 index and APRI score, were calculated as described previously: FIB-4 = (age [year] × AST [IU/L]) / (platelet count [109/L] ×√alanine aminotransferase (ALT) [IU/L]) and APRI = ((AST / ULN) / platelet count [109/L]) × 100 [1, 2]. Serum M2BPGi levels were measured using HISCL M2BPGi (Sysmex Corporation, Kobe, Japan). Liver stiffness was measured with TE (FibroScan, EchoSens, Paris, France) and was expressed in kilopascals (kPa).
Sampling Serum and Isolation of RNA
Peripheral blood was collected from each participant and centrifuged at 1500g for 5 minutes at room temperature. After serum separation, the samples were stored at –80°C until use. Total RNAs, including miRNAs in serum and EVs, were purified with miRNeasy Serum/Plasma Kits and exoRNeasy Serum/Plasma Midi Kits (QIAGEN, Hilden, Germany), respectively, following the manufacturer’s instructions with minor modifications. Specifically, we extracted total RNA from 200 μL of serum from each subject, to which 5.6 × 108 copies of Caenorhabditis elegans (cel)–miR-39 was added as spike-in RNA for later normalization; then total RNA was eluted from each column with 60 μL of nuclease-free water. Extracellular vesicles, including exosomes, were purified from 200 μL of serum, and 5.6 × 108 copies of synthetic cel-miR-39 miRNA mimic was added as spike-in control; then total RNA was eluted from each column with 60 μL of nuclease-free water. The concentration of total RNA was quantified using a NanoDrop 2000c (Thermo Fisher Scientific, Wilmington, DE).
miRNA Quantitation
In our previous study, the levels of let-7a/7c/7d-5p in plasma were correlated with the progression of hepatic fibrosis in CHC [14]. Of these let-7 members, let-7a-5p was the most abundantly expressed in plasma; therefore, we selected it as a representative of let-7 and examined its expression in the present study. In addition, we analyzed miR-122-5p levels as a potential marker, because it has been reported that circulating miR-122 levels are associated with hepatic inflammatory activity in CHC, as previously described [8, 12, 13]
Quantitative miRNA levels were determined using quantitative real-time polymerase chain reaction (qRT-PCR) with the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) and TaqMan MicroRNA Assay: hsa-miR-122-5p (assay ID 002245), hsa-let-7a-5p (assay ID 000377), cel-miR-39-3p (assay ID 000200) (Applied Biosystems). One microliter of total RNA which was extracted from serum, and 3 μL from EVs was subjected to reverse transcription with a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) and the respective TaqMan MicroRNA Assay reagents for the target molecules, in a total volume of 15 μL, followed by qRT-PCR in a total volume of 20 μL, according to the manufacturer’s protocol. Amplification was carried out as follows: 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 60 seconds. All reactions were carried out in duplicate. Cycle threshold (Ct) values were calculated using SDS Software, version 2.3 (Applied Biosystems). Expression levels of miRNAs were normalized to those of the spike-in cel-miR-39. The expression levels were determined by the 2-ΔCt method, in which ΔCt was calculated as ΔCt = Ct (miRNA of interest) – Ct (cel-miR-39-3p) and are represented by log2 scale for serum and log10 scale for EVs according to their levels, which made it easy for us to compare them visually in figures.
Statistical Analysis
Categorical variables were compared between groups by the chi-square test, and noncategorical variables were analyzed by the Mann-Whitney U test. Spearman’s rank correlation coefficient (rho) was used for the assessment of correlation between continuous variables. Receiver operating characteristic (ROC) curve analyses were carried out, and the area under the operating characteristic curve (AUROC) was calculated to evaluate the feasibility of using the let-7a-5p levels as markers for discriminating liver cirrhosis. Multivariate logistic regression analyses were performed to determine whether the let-7a-5 levels were independently associated with liver cirrhosis. In these analyses, liver cirrhosis was pathologically defined as F4 of the METAVIR scoring system. P values <.05 were considered significant in all tests. Statistical analyses were performed using R (version 3.1.1), EZR (version 1.35), which is based on R and R commander [16], and BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan).
RESULTS
Correlations of let-7a and miR-122 Levels in Serum With Clinical Features
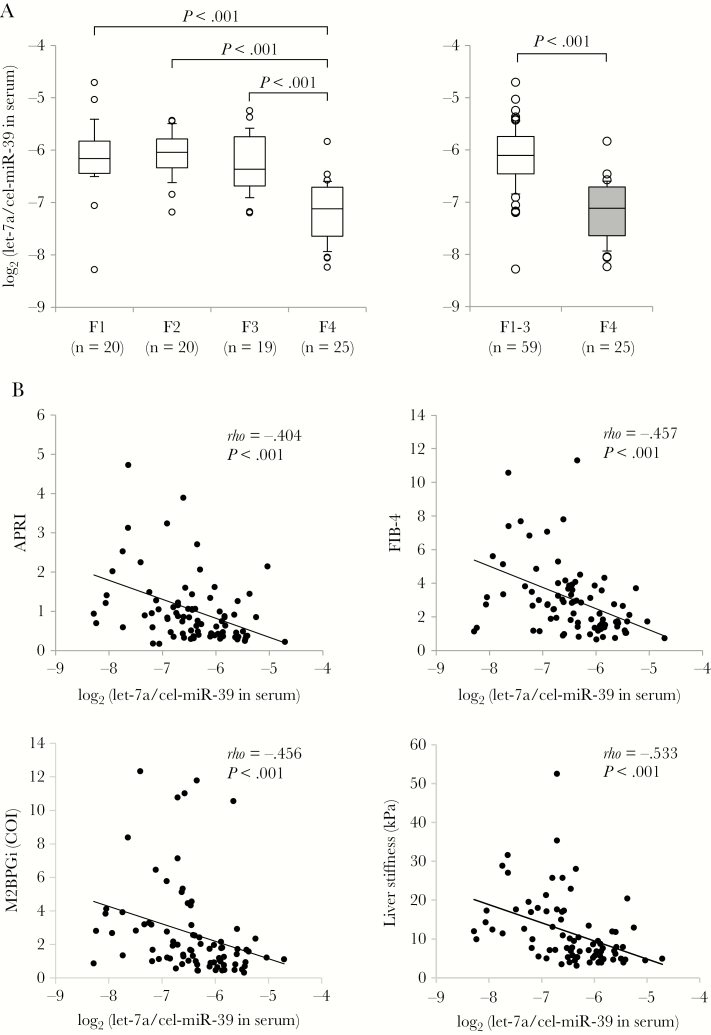
The levels of let-7a-5p and miR-122-5p in serum were significantly correlated with those in serum-derived EVs (let-7a-5p: rho = .457; P < .001; miR-122-5p: rho = .801; P < .001) (Supplementary Figure 1). The let-7a-5p levels in serum were significantly lower in the F4 stage compared with F1, F2, and F3, respectively, as well as the combined F1-3 (P < .001, for each comparison) (Figure 1A). Meanwhile, the let-7a-5p in EVs showed almost the same results, but the differences between F4 and F1-3 were weaker than those in serum (Supplementary Figure 2A). The levels of let-7a-5p in serum were inversely correlated with the levels of APRI (rho = –.404; P < .001), FIB-4 (rho = –.457; P < .001), M2BPGi (rho = –.456; P < .001), and liver stiffness (rho = –.533; P < .001) (Figure 1B). Meanwhile, the levels of let-7a-5p in EVs had statistically significant but weak correlations with the levels of FIB-4 and M2BPGi (Supplementary Figure 2B). Although the serum let-7a-5p levels were lower in patients with METAVIR A2-3 than those with A1, there were no correlations between let-7a-5p levels and ALT in serum or EVs (Supplementary Figure 3). Thus, let-7a-5p levels in serum and serum-derived EVs were associated with severity of fibrosis but not inflammatory activity in the liver.
Figure 1.
Correlations between serum let-7a-5p levels and hepatic fibrosis severity. A, The levels of let-7a-5p in serum were compared between each group using the Mann-Whitney U test. B, Correlations between serum let-7a-5p levels and hepatic fibrotic markers using Spearman’s rank correlation coefficient (rho). Abbreviations: APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; COI, cutoff index; FIB-4, fibrosis-4; kPa, kilopascals; M2BPGi, Mac-2 binding protein glycan isomer.
The levels of miR-122-5p in serum and EVs were not correlated with histological findings of fibrosis and inflammation (Supplementary Figure 4) but were significantly correlated with ALT levels (serum: rho = .727; P < .001; EVs: rho = .653; P < .001) (Supplementary Figure 5).
Discrimination of Liver Cirrhosis by Serum let-7a Level and Other Markers of Hepatic Fibrosis
The ROC curve analysis indicated that the ability to discriminate cirrhosis by serum let-7a-5p level was superior to that by M2BPGi, APRI, or FIB-4 (AUROC, 0.892 for serum let-7a-5p; 0.800 for M2BPGi; 0.788 for APRI; 0.783 for FIB-4) and was comparable to liver stiffness (AUROC, 0.909 for liver stiffness). But let-7a-5p levels in EVs (AUROC, 0.681 for let-7a-5p in EVs) were not superior to those in serum (Table 2; Supplementary Figure 6). We set a cutoff value for each hepatic fibrotic marker when the sum of sensitivity and specificity reached the maximum for discriminating liver cirrhosis, indicating that the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of let-7a-5p in serum were generally superior to those of let-7a-5p in EVs, APRI, FIB-4, and M2BPGi. Notably, the accuracy of let-7a-5p in serum was the best in all the markers including liver stiffness (Table 2).
Table 2.
Receiver Operating Characteristic Curve Analysis for Discriminating Liver Cirrhosis
| AUROC | Cutoff | Sensitivity, % (No.) | Specificity, % (No.) | PPV, % (No.) | NPV, % (No.) | Accuracy, % (No.) | |
|---|---|---|---|---|---|---|---|
| log2 (let-7a-5p in serum) | 0.892 | –6.573 | 92 (23/25) | 80 (47/59) | 65 (22/34) | 94 (47/50) | 83 (70/84) |
| log10 (let-7a-5p in EVs) | 0.681 | –2.948 | 72 (18/25) | 61 (36/59) | 44 (18/41) | 84 (36/43) | 64 (54/84) |
| APRI | 0.788 | 0.859 | 80 (20/25) | 70 (41/59) | 51 (19/37) | 87 (41/47) | 73 (61/84) |
| FIB-4 | 0.783 | 2.745 | 84 (21/25) | 66 (39/59) | 50 (20/40) | 89 (39/44) | 71 (60/84) |
| M2BPGi, COI | 0.800 | 2.660 | 70 (16/23) | 83 (49/59) | 62 (16/26) | 88 (49/56) | 79 (65/82) |
| Liver stiffness, kPa | 0.909 | 9.9 | 96 (22/23) | 76 (45/59) | 61 (22/36) | 98 (45/46) | 82 (67/82) |
M2BPGi levels were not measured in 2 cases because of insufficiant sample volume. Transient elastgraphy for measuring liver stiffness was not performed in 2 cases.
Abbreviations: APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; AUROC, area under the receiver operating characteristic curve; COI, cutoff index; EVs, extracellular vesicles; FIB-4, fibrosis-4; M2BPGi, Mac-2 binding protein glycan isomer; NPV, negative predictive value; PPV, positive predictive value.
Next, we evaluated factors associated with liver cirrhosis using logistic regression models. Several factors were associated with liver cirrhosis by univariate analysis, as shown in Table 1. As described in the “Methods,” APRI is calculated by AST and platelet count, whereas FIB-4 is done by age, AST, ALT, and platelet count. Considering the correlations between these factors, we included the following variables: FIB-4, M2BPGi, serum let-7a-5p level, and liver stiffness for Analysis 1; APRI, M2BPGi, serum let-7a-5p level, and liver stiffness for Analysis 2. Serum let-7a-5p level and liver stiffness were independently associated with liver cirrhosis in each analysis (Table 3).
Table 3.
Logistic Regression Analysis of Factors Associated With Liver Cirrhosis
| Variables | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Analysis 1 | ||
| FIB-4 | 0.928 (0.641–1.340) | .691 |
| M2BPGi | 0.984 (0.687–1.410) | .928 |
| log2 (let-7a-5p/cel-miR-39) in serum | 0.140 (0.041–0.478) | .002 |
| Liver stiffness | 1.230 (1.060–1.430) | .007 |
| Analysis 2 | ||
| APRI | 0.956 (0.432–2.120) | .912 |
| M2BPGi | 0.960 (0.677–1.360) | .819 |
| log2 (let-7a-5p/cel-miR-39) in serum | 0.144 (0.043–0.482) | .002 |
| Liver stiffness | 1.220 (1.060–1.410) | .007 |
Different variables were selected in each analysis.
Abbreviations: APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; CI, confidence interval; FIB-4, fibrosis-4; M2BPGi, Mac-2 binding protein glycan isomer.
We also examined the ability to discriminate advanced hepatic fibrosis: F3/4 by the same methods as above, indicating that the AUROC values of liver stiffness and M2BPGi were superior to those of let-7a-5p level in serum (Supplementary Table 1). Although let-7a-5p levels in serum were significantly lower in F3/4 stage than F1/2 (P < .001) (Supplementary Figure 7), the let-7a-5p levels in F3 were significantly higher than those in F4, but not different from those in F1 or F2 (Figure 1). Taken together, we conclude that serum let-7a-5p level is a more suitable marker for liver cirrhosis.
DISCUSSION
As far as we know, very few circulating miRNAs have been validated for their association with disease progression in different CHC cohorts. Furthermore, a number of miRNAs were differentially expressed in serum and plasma because of blood coagulation [17]. Although the previous study analyzed miRNA expression using plasma samples from the HCV-infected blood donor cohort in the United States [14], the results were not validated in another cohort. The present study confirms that circulating let-7a-5p levels are correlated with the severity of hepatic fibrosis using serum samples from another CHC cohort with a different ethnicity. Notably, serum let-7a-5p level was superior to other hepatic fibrotic markers based on blood testing, M2BPGi, FIB-4, and APRI, and was comparable to liver stiffness by TE for discriminating liver cirrhosis. In addition, we found that measuring the let-7a-5p levels in serum-derived EVs was not superior to direct measurement in serum for discriminating liver cirrhosis, which was consistent with the previous results [14]. We also confirmed that circulating miR-122-5p levels in serum are correlated with inflammatory activity but not fibrosis in the liver [8, 12, 13]. In introducing serum let-7a-5p level as a diagnostic tool for liver fibrosis in clinical practice, we have several issues: (1) there are technical problems, such as sample collection, data normalization, and the standardization of the platform for miRNA expression analysis; (2) the cost of measuring miRNA expression levels is higher than that of hepatic fibrosis indexes such as FIB-4 and APRI, whereas the initial investment in the instrument for analyzing miRNA levels would be less than that of elastography; and (3) the results of the present study need to be validated in larger multicenter cohorts.
The cellular source of circulating miRNAs and the mechanism of regulation of miRNA expression are not well known. It is presumed that circulating let-7 is derived from various cellular sources, because members of the let-7 family are abundantly expressed in most cell types. We previously showed no correlations in let-7 expression levels between whole-liver tissue and plasma, and no associations between let-7 expression in the liver and hepatic fibrosis severity [14]. It would be difficult with the current technology to identify the specific source of circulating let-7 responsible for the different levels of let-7 in plasma/serum and EVs in CHC patients. However, the biological activities of let-7 in hepatic fibrogenesis and carcinogenesis should be elucidated in the future. Our bioinformatics analyses suggest that decreased levels of let-7 may activate hepatic profibrotic processes, which are associated with the TGF-β signaling pathway in hepatic stellate cells (HSCs) [14]. Indeed, a recent study indicated that let-7 regulates the activation of HSCs in alcoholic liver injury models and may contribute to the pathogenesis of alcoholic liver fibrosis, suggesting novel therapeutic approaches for human alcoholic diseases with let-7 [18]. Wang et al. revealed that let-7c-5p regulated ethanol-induced hepatic steatosis and apoptosis by targeting NLRC5 [19]. In addition, let-7 and its major regulator Lin28 are thought to be involved in regeneration, inflammation, fibrogenesis, and carcinogenesis in the liver, regardless of the etiology of liver diseases [20]. These findings, including ours, suggest that let-7 could be a promising therapeutic target for liver diseases.
In conclusion, this study provides evidence that circulating let-7a-5p in serum may serve as a surrogate marker for the severity of hepatic fibrosis in CHC. Further studies are required to implement let-7 as a biomarker for hepatic fibrosis and determine its therapeutic potential in clinical practice.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was funded by the Research Program on Hepatitis from Japan Agency for Medical Research and Development (project code JP18fk0210001h0003 to Yasuhito Tanka) and by Japan Society for the Promotion of Science (grant number 17K09435 to Kentaro Matsuura).
Potential conflicts of interest. Yasuhito Tanaka has received Honoraria from Bristol-Myers Squibb Company, Chugai Pharmaceutical Co., Ltd., and Gilead Sciences and research funding from Bristol-Myers Squibb Company, Chugai Pharmaceutical Co., Ltd., Gilead Sciences, and Janssen Pharmaceutical K.K. Takashi Kumada has received remuneration for lectures at meetings from Bristol-Myers Squibb. Hidenori Toyoda has received remuneration for lectures at meetings from MSD K.K. and AbbVie GK. The other co-authors have no conflicts of interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation: A part of data in the present study were presented at American Association for the Study of Liver Diseases (AASLD); November 1–5, 2017; Washington D.C.
References
- 1. Sterling RK, Lissen E, Clumeck N, et al. APRICOT Clinical Investigators Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25. [DOI] [PubMed] [Google Scholar]
- 2. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–26. [DOI] [PubMed] [Google Scholar]
- 3. Kuno A, Ikehara Y, Tanaka Y, et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 2013; 3:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwok R, Tse YK, Wong GL, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease–the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther 2014; 39:254–69. [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281–97. [DOI] [PubMed] [Google Scholar]
- 6. Wang XW, Heegaard NH, Orum H. MicroRNAs in liver disease. Gastroenterology 2012; 142:1431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bala S, Tilahun Y, Taha O, et al. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J Transl Med 2012; 10:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bihrer V, Friedrich-Rust M, Kronenberger B, et al. Serum miR-122 as a biomarker of necroinflammation in patients with chronic hepatitis C virus infection. Am J Gastroenterol 2011; 106:1663–9. [DOI] [PubMed] [Google Scholar]
- 9. Bihrer V, Waidmann O, Friedrich-Rust M, et al. Serum microRNA-21 as marker for necroinflammation in hepatitis C patients with and without hepatocellular carcinoma. PLoS One 2011; 6:e26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shrivastava S, Petrone J, Steele R, et al. Up-regulation of circulating miR-20a is correlated with hepatitis C virus-mediated liver disease progression. Hepatology 2013; 58:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 2011; 53:209–18. [DOI] [PubMed] [Google Scholar]
- 12. Trebicka J, Anadol E, Elfimova N, et al. Hepatic and serum levels of miR-122 after chronic HCV-induced fibrosis. J Hepatol 2013; 58:234–9. [DOI] [PubMed] [Google Scholar]
- 13. van der Meer AJ, Farid WR, Sonneveld MJ, et al. Sensitive detection of hepatocellular injury in chronic hepatitis C patients with circulating hepatocyte-derived microRNA-122. J Viral Hepat 2013; 20:158–66. [DOI] [PubMed] [Google Scholar]
- 14. Matsuura K, De Giorgi V, Schechterly C, et al. Circulating let-7 levels in plasma and extracellular vesicles correlate with hepatic fibrosis progression in chronic hepatitis C. Hepatology 2016; 64:732–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol 2008; 18:505–16. [DOI] [PubMed] [Google Scholar]
- 16. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ge Q, Shen Y, Tian F, et al. Profiling circulating microRNAs in maternal serum and plasma. Mol Med Rep 2015; 12:3323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDaniel K, Huang L, Sato K, et al. The let-7/Lin28 axis regulates activation of hepatic stellate cells in alcoholic liver injury. J Biol Chem 2017; 292:11336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Q, Li M, Shen Z, et al. The long non-coding RNA MEG3/miR-let-7c-5p axis regulates ethanol-induced hepatic steatosis and apoptosis by targeting NLRC5. Front Pharmacol 2018; 9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDaniel K, Hall C, Sato K, et al. Lin28 and let-7: roles and regulation in liver diseases. Am J Physiol Gastrointest Liver Physiol 2016; 310:G757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.