Abstract
Study Objectives
Sleep slow waves behave like traveling waves and are thus a marker for brain connectivity. Across a night of sleep in adults, wave propagation is scaled down, becoming more local. Yet, it is unknown whether slow wave propagation undergoes similar across-night dynamics in childhood—a period of extensive cortical rewiring.
Methods
High-density electroencephalography (EEG; 128 channels) was recorded during sleep in three groups of healthy children: 2.0–4.9 years (n = 11), 5.0–8.9 years (n = 9) and 9.0–16.9 years (n = 9). Slow wave propagation speed, distance, and cortical involvement were quantified. To characterize across-night dynamics, the 20% most pronounced (highest amplitude) slow waves were subdivided into five time-based quintiles.
Results
We found indications that slow wave propagation distance decreased across a night of sleep. We observed an interesting interaction of across-night slow wave propagation dynamics with age (p < 0.05). When comparing the first and last quintiles, there was a trend level difference between age groups: 2- to 4.9-year-old children showed an 11.9% across-night decrease in slow wave propagation distance, which was not observed in the older two age groups. Regardless of age, cortical involvement decreased by 10.4%–23.7% across a night of sleep. No across-night changes were observed in slow wave speed.
Conclusions
Findings provide evidence that signatures of brain connectivity undergo across-night dynamics specific to maturational periods. These results suggest that across-night dynamics in slow wave propagation distance reflect heightened plasticity in underlying cerebral networks specific to developmental periods.
Keywords: brain connectivity, function of sleep, high-density EEG, neurodevelopmental marker, sleep regulation
Statement of Significance
Sleep is the most ideal setting for measuring maturational changes in brain activity and connectivity without influences of motivation and attention. Sleep slow waves propagate across the cortex with traveling patterns relating to age and intra-hemispheric brain myelin content. Here we examine the across-night dynamics of slow wave propagation in 29 healthy children and adolescents (2–16 years). Our results show an across-night decrease in slow wave distance most apparent in preschool-age children. The current findings indicate age-related differences in across-night dynamics of traveling wave propagation and propose specific electrophysiological brain markers for detecting heightened developmental plasticity.
Introduction
Sleep is an ideal state for measuring brain activity, connectivity and the development of the cerebral cortex because motivational, attentional and contextual influences are marginal. This is especially important during development when motivational and regulatory control systems undergo divergent trajectories [1, 2]. Brain markers assessed during sleep thus reliably reflect not only the static patterns of neural activation and connectivity but also the neuronal dynamics that occur across the course of sleeping or waking [3].
Sleep slow wave activity (0.5–4.5 Hz) is the most typical electrophysiological phenomenon of deep sleep. Slow wave activity is also a well-established marker of sleep need, as it is highest at sleep onset and decreases across the night [4]. The synaptic homeostasis hypothesis proposes that slow wave activity reflects synaptic strength and actively drives synaptic renormalization across the course of sleep [5]. Slow waves behave like traveling waves and their propagation dynamics can be simplified using the parameters traveling distance, speed, and cortical involvement [6]. Analogous to slow wave activity, the markers of slow wave propagation also experience an across-night reduction. For example, the cortical involvement of an ordinary slow wave shrinks from the evening to the morning hours by 5% in adults [7], reflecting decreased synchronization among neuronal units [3]. It is noteworthy that the magnitude of across-night changes is generally more pronounced in developing individuals compared to adults, as evidenced in animal [8, 9] and human data [10–13]. Yet, the magnitude of these dynamics, and whether their manifestation relates to specific developmental periods, remains unknown. To our knowledge this article is the first to report across-night dynamics in travelling patterns of sleep slow waves in children, extending previous insights into the development of inter- and intra-hemispheric sleep electroencephalographic (EEG) connectivity [12, 14], slow wave morphology [13, 15], and the link between regional sleep and behavioral development [11, 16].
Sleep undergoes numerous transitions throughout development that also involve changes in sleep depth [17, 18] and in EEG connectivity [12, 14]. Slow wave activity reaches a maximum during childhood and declines throughout adolescence [17]. Slow wave activity also undergoes topographical changes that parallel the maturational trajectory of cortical thickness [19]. Furthermore, slow wave morphology is transformed across development. For example, prepubertal children exhibit a steeper slope of slow waves compared to adolescents, suggesting increased synaptic strength [20, 21]. Previous studies reported an increase in EEG long-range connectivity (coherence calculated from bipolar EEG channels) across preschool-age [12] or adolescence [14]. Coherence also exhibits across-night dynamics, including a decrease in intra-hemispheric connectivity and an increase in inter-hemispheric connectivity, as investigated at preschool age [12].
Slow wave propagation transforms throughout maturation, such that traveling distance increases by 0.2 cm per year across childhood [6] (based on a subset of data presented here). It is further known that slow wave propagation parameters are linked to intra-hemispheric white matter microstructure [6]. Accordingly, slow wave propagation patterns are a promising proxy for not only developmental but also diurnal processes of brain connectivity. Yet, it is unknown whether (1) slow wave propagation undergoes across-night dynamics in childhood and adolescence, and whether (2) these changes differ with age showing maturational trajectories. Consequently, the identification of sleep-related cerebral markers may not only identify cornerstones in maturational brain modifications but also promote insight into the role of sleep for cognitive development. Here, we quantified high-density sleep EEG (hdEEG) in children and adolescents and examined the propagation of slow waves and their dynamics across the night.
Materials and Methods
Participants
Twenty-nine healthy children (12 females) between 2 and 16 years participated in the study. Participants were recruited via newspapers, website advertising, flyers, and personal contact at community events. Before participation, children were screened via telephone interview and questionnaires for health problems and current medication use as well as personal and family history of sleep disorders, psychosis, bipolar disorder, narcolepsy, physical and developmental disabilities, head injury and chronic diseases. All participants included in the study were in excellent health, had no history of these disorders or were currently using medications. Furthermore, participants were excluded for travel beyond two time zones within 2 months of the study, caffeine use, daily/nightly co-sleeping, pre-term or post-term delivery, or low birth weight. Sleep was stabilized according to habitual bed and wake times for 5 or more days before assessments (compliance was confirmed with actigraphy and parent-reported sleep diaries). Ethics approval was obtained from local Institutional Review Boards (Brown University, the University of Colorado Boulder, the University of Zurich), and study procedures were consistent with the declaration of Helsinki. Written parental consent and child assent (when appropriate) were obtained after explanation of the study.
EEG recording
Results on a subset of these participants were published previously [6]. Sleep assessments were performed at families’ homes in 14 participants (2.0–5.7 years, eight males) in the Providence, RI area (United States) and in nine participants (5.5–11.3 years, six males) in the Boulder, CO area (United States). Six participants (11.0 - 16.4 years, 3 males) were recorded in the sleep laboratory of the University Children’s Hospital Zurich (Zurich, Switzerland). Sleep assessment times were scheduled to individual bedtimes. Participants were awakened in the morning to allow for school participation or other obligations. The procedures were consistent across sites. Sleep quality did not significantly change across geographic sites (sleep efficiency: one-way analysis of variance [ANOVA] p = 0.47; sleep latency: one-way ANOVA p = 0.22). High-density (hd) EEG (128 channels; Electrical Geodesics Inc., EGI, Eugene, OR) was used for monitoring one night of sleep scheduled to habitual bedtimes. Signals were obtained with a sampling frequency of 500 Hz and referenced to the vertex for direct visualization (NetStation, version 4.5.1). Impedances were below 50 kΩ.
For sleep scoring, the signal was band-pass filtered (0.5–50 Hz), down-sampled to 128 Hz and poor-quality channels were excluded. Artifacts were semi-automatically rejected for 20-second segments (as described in [22]). Sleep stages were visually scored for 20-second epochs according to the AASM Manual for the Scoring of Sleep and Associated Events [23]. Sleep quality was good with a relatively short sleep latency (24.4 ± 16.9 minutes) and a high sleep efficiency (88.8 ± 4.7%).
Slow wave detection
EEG was pre-processed offline using NetStation (version 4.5.1) and MATLAB (Mathworks, Natick, MA, version R2012a). Data was filtered using a 0.5–40 Hz bandpass filter, rejection of channels with artifacts and re-referencing to the mastoids. The algorithm published in Siclari et al. (2014) was used for slow wave detection and adapted for use in children and with 128 channels. At each time point, the third most negative sample (2.5% of all channels) was used to create a single negative reference envelope for detecting local and global slow waves. Specific detection criteria were applied in order to target stereotyped high-amplitude waves in this pediatric sample. Relatedly, to account for maturational effects due to slow wave morphology (amplitude, slope [20, 24]), we included only the top 20% of waves (i.e. with largest amplitudes) in each participant for analysis. We determined the timing of any local maxima that occurred within ± 200 ms of the reference peak, had an amplitude of at least 25% of the peak and was within 10 ms of at least one other detected channel peak; we then chose the maxima that occurred most closely to the voltage peak in each electrode. Streamlines of the slow wave propagation were calculated with a three-dimensional gradient (two for direction, one for timing).
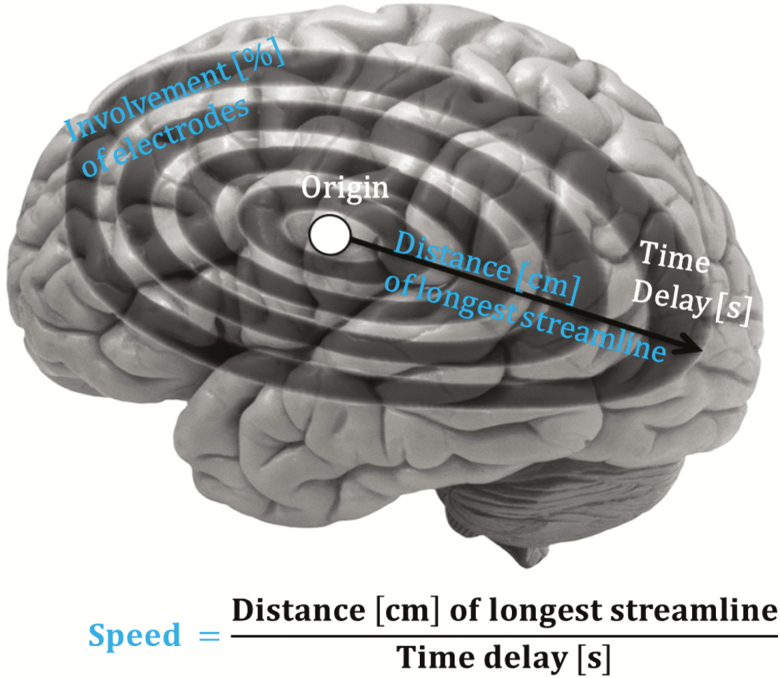
Consistent with our previous work in children [6], we focused on slow wave parameter speed, distance and cortical involvement (Figure 1). Slow wave distance was calculated as the length on the scalp of the longest streamline in cm. Slow wave speed incorporated scalp distance in cm and the longest streamline time delay (i.e. estimated distance divided by time). Cortical involvement quantified the percentage of electrodes in which the slow wave was detected relative to the total number of electrodes.
Figure 1.
Slow wave propagation metrics. Slow wave propagation was quantified with three key metrics: slow wave distance, slow wave speed and cortical involvement.
Wave quintiles were calculated for a quantification of across-night dynamics. This approach was utilized in each participant in order to account for individual differences in numbers of waves. The number of detected waves was identified, and slow waves were assigned to the first (1%–20%), second (21%–40%), third (41%–60%), fourth (61%–80%), or fifth (81%–100%) quintile. Per quintile, we calculated the median of all included slow waves. The median was used because the propagation metrics can occur with skewed distribution [6].
Statistical analysis
Analysis was performed with MATLAB (Mathworks, version 2012a) and R (Version 3.2, R Development Core Team, Vienna, Austria 2016) [25]. Main results were analyzed using linear mixed-effects models to assess the dynamics across the night, the effect of age and their interaction. Age was included with 0 representing the youngest participant’s age (2.04 years). Mixed-effect models were selected because the main outcome variables (cortical involvement, slow wave distance and slow wave speed) were non-independent within participant (intraclass correlation coefficient [ICC] = 0.73, F(1,143) = 6.75, p = 0.01 for cortical involvement, ICC = 0.37, F(1,143) = 43, p < 0.00001 for slow wave distance, and ICC = 0.14, F(1,143) = 12.62, p = 0.0005 for slow wave speed). A latent growth model is recommended to examine overall changes [26]. A growth curve model was thus implemented using the R package nlme [27] and the function lme. Models were estimated using restricted maximum likelihood, and model comparisons were performed using maximum likelihood. Linear was the best fit for all models. With exception of the initial model, all models included head size and sex as covariates. When an MR image was available, head size was calculated as head circumference measured from the structural MR images (in 23 participants); otherwise, it was based on EEG net size (in six participants). To examine across-night trajectories, we implemented random-intercept-and-slope models. We applied both a random-intercept and a random-intercept-and-slopes model and performed comparisons with log-likelihood ANOVA. Subsequent analysis included only the better fitting model. A lag-1 autocorrelation was integrated if it significantly improved the model fit otherwise it was dropped. After establishing this basic model, age was added as predictor in a first step, and as an interaction-term in a second step. The model with the lowest Akaike information criterion (AIC) was chosen as the best fitting model. Normal distribution of the residuals of the final models was assessed visually using histograms. Pseudo R2 was calculated using the R package MuMIn [28] and the function r.squareGLMM.
Age groups
In the analyses where a main effect of age or an interaction with age was observed, participants were assigned to one of three age groups: 2.0–4.9 years (n = 11), 5.0–8.9 years (n = 9) or 9.0–16.9 years (n = 9, Table 1). This age subdivision was data-driven and reflected EEG milestones based upon the topographical maturation status of slow wave activity [29]. Specifically, slow wave activity demonstrates an inverted U-shape trajectory across the maturational period, with slow wave activity maxima attained between 5 and 9 years of age, contingent to cortical regions [18]. The age groups selected for analysis refer to the age before (2–4.9 years), during (5–8.9 years) or after (9–16.9 years) the time point (age) when slow wave activity maxima occur. To streamline across-night dynamics, we calculated the change (decrease or increase) from the first to the last quintile of slow waves in each participant. ANOVA was used for between-group comparison of the first/last quintile change, and Wilcoxon signed rank tests were performed whenever significant group differences were found. Based upon our previous examination of traveling slow waves in childhood [6], we refined the analysis and extended it to adolescence. To examine overall group differences, mean values across the whole night were calculated for each group and ANOVA was performed to examine group differences. When differences were found Tukey’s HSD post hoc tests were performed. The alpha level was set to p = 0.05.
Table 1.
Descriptive statistics (M ± SD; N) for participant age, sex and sleep characteristics by age group
| Group | 2–4.9 years | 5–8.9 years | 9.00–16.9 years | p |
|---|---|---|---|---|
| Age | 3.3 ± 1.1 | 6.7 ± 1.7 | 12.6 ± 2.5 | <0.001* |
| N (female) | 11 (4) | 9 (3) | 9 (5) | 0.58 |
| Time in bed (minutes) | 609.5 ± 65.6 | 601.6 ± 55.0 | 515.6 ± 64.6 | 0.005* |
| Total sleep time (minutes) | 540.8 ± 66.0 | 532.2 ± 67.1 | 467.7 ± 52.5 | 0.04 |
| Sleep latency (minutes) | 31.4 ± 19.8 | 19.7 ± 11.9 | 22.0 ± 14.4 | 0.24 |
| Sleep efficiency (%) | 88.6 ± 3.8 | 88.4 ± 6.3 | 90.9 ± 3.9 | 0.45 |
| Stage 1 (%) | 1.1 ± 0.4 | 1.6 ± 1.0 | 5.6 ± 4.2 | 0.02* |
| Stage 2 (%) | 33.9 ± 3.6 | 45.0 ± 6.6 | 50.9 ± 4.5 | <0.001* |
| Stage 3 (%) | 30.2 ± 7.4 | 29.5 ± 5.6 | 23.1 ± 4.8 | 0.04 |
| REM (%) | 34.8 ± 5.9 | 23.9 ± 8.1 | 20.4 ± 3.7 | <0.001* |
*Significant after False Discovery correction.
Results
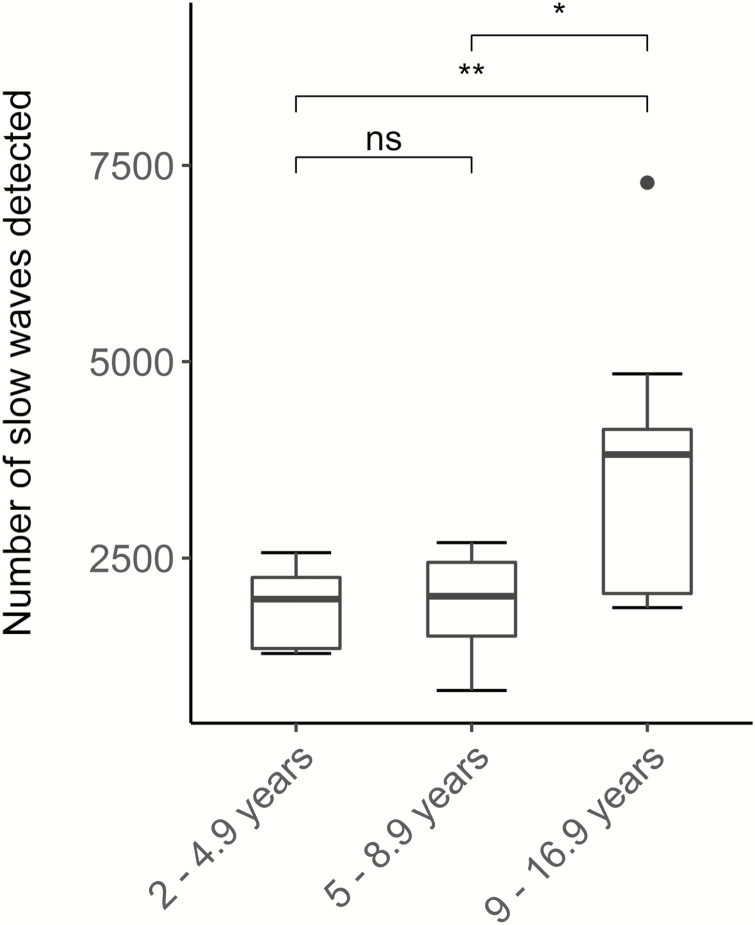
Between 813 and 7281 slow waves were detected among all whole-night recordings (Figure 2). The 9- to 16.9-year-old group showed significantly more of the targeted high amplitude slow waves (3765 ± 1708, M ± SD) compared to the younger age groups (1852 ± 476, 2- to 4.9-year-old children, 1908 ± 601, 5- to 8.9-year-old children, F(2,14.39) = 5.1, p = 0.02, Welch correction due to unequal variances). Consistent with this finding, the number of detected slow waves was positively correlated with age (r(27) = 0.63, p = 0.0002). Findings withstand removal of the outlier in the oldest age group (F(2, 13.59) = 5.55, p = 0.02 for ANOVA and r(26) = 0.72, p < 0.001) correlation. Because this participant was not identified as an outlier in any other measure, it was included for subsequent analyses.
Figure 2.
Number of slow waves detected in each age group: between 813 and 7281 waves were detected (813–4845 excluding the outlier). 9- to 16.9-year-olds exhibited significantly more slow waves compared to 2- to 4.9-year-olds or 5- to 8.9-year-old children (p = 0.02). *p < 0.05; **p < 0.01; N = 29; “ns” not significant, N=29.
Similar to number of detected slow waves, an age effect was found in slow wave density: 9- to 16.9-year-olds showed significantly increased slow waves per minute of non-rapid eye movement (NREM) sleep compared to 2- to 4.9-year-olds or 5- to 8.9-year-old children (F(2,14.52) = 5.36, p = 0.02). There was no effect of sex in the number of detected slow waves (t(27) = −0.95, p = 0.35) and no correlation between number of slow waves and slow wave activity (F4 r(27) = −0.03, p = 0.86, C4 r(27) = −0.11, p = 0.58). As expected, sleep characteristics were associated with age (Table 1, [30–32]), including an age-related reduction of time spent in bed (F(2,26) = 6.58, p = 0.005) and rapid eye movement (REM) sleep (F(2,26) = 15.08, p < 0.001), and increases in stage 1 sleep (F(2,11.96) = 5.76, p = 0.02), and stage 2 sleep (F(2,26) = 30.63, p < 0.001). Age-related decreases at trend-level were observed in total sleep time (F(2,26) = 3.84, p = 0.04, n.s. after False Discovery Rate correction), and stage 3 sleep (F(2,26) = 3.79, p = 0.04, n.s. after False Discovery Rate correction).
Slow wave traveling distance decreases across the night only in 2- to 4.9-year-olds
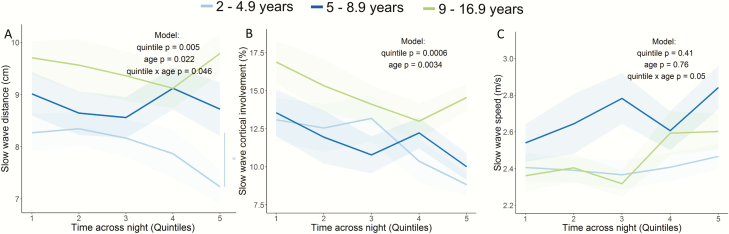
We investigated across-night changes in slow wave traveling distance. The random-intercept model accounting for time-lag-1 autocorrelation including the predictors quintile, age; their interaction yielded the best fit when correcting for head size. Main effects reached significance, indicating a decline in slow wave distance across the night (quintile t(114) = −2.87, p = 0.005, 95% CI [−0.41; −0.08]) and an age-related increase in slow wave distance, such that older participants exhibited larger propagation distance irrespective of quintile (age t(25) = 2.43, p = 0.02, 95% CI [0.02; 0.25]). Additionally, a significant interaction was observed (quintile × age t(114) = 2.02, p = 0.046, 95% CI [0.0005; 0.05]) indicating that across-night trajectories in traveling distance change with age (Table 2).
Table 2.
Parameters for the model explaining slow oscillation distance
| b ± SE | p | |
|---|---|---|
| Fixed effects | ||
| Intercept | 6.31 ± 4.15 | 0.13 |
| Quintile | −0.24 ± 0.08 | 0.005 |
| Age | 0.13 ± 0.06 | 0.02 |
| Quintile × age | 0.03 ± 0.01 | 0.046 |
| Head size | 0.04 ± 0.08 | 0.67 |
| Gender | 0.13 ± 0.36 | 0.72 |
| Random effects | ||
| Intercept | 0.81 | |
| Residual | 0.85 | |
| AIC | 425.13 | |
| Pseudo R2 (marginal; conditional) | 0.35; 0.66 | |
SE = standard error.
To streamline the reported age × quintile interaction, we quantified the percent change from the first to the last quintile of slow wave distance, which revealed a group difference at trend level (F(2,26) = 3.00, p = 0.07). Although a Wilcoxon signed rank test indicated a decrease in slow wave distance across the night in 2- to 4.9-year-old children (88.1 ± 3.7%, p = 0.014), no across-night dynamics were observed in the older age groups (Figure 3A).
Figure 3.
Across-night changes in slow wave propagation. (A) Slow wave distance decreased from the first to the last quintile in 2- to 4.9-year-old children (by 11.9%) but not in 5- to 8.9-year-old children and 9- to 16.9-year-olds. (B) Across-night decreased in cortical involvement across the night independent of age. (C) Across-night dynamics in slow wave speed. There was a trend for an age × quintile interaction; however, no group differences were found in changes from the first to the last quintile. The shaded area represents the SEM. *p < 0.05; n = 29.
Slow wave cortical involvement decreases across the night in all age groups
For cortical involvement, the linear random-intercept-and-slope model with quintile and age, but not their interaction, revealed the best fit. Adding time-lag-1 autocorrelation did not improve the model fit. Both main effects significantly predicted cortical involvement (quintile t(115) = −3.54, p = 0.0006, 95% CI [−1.3; −0.37], age t(25) = 3.03, p = 0.006, 95% CI [0.13; 0.67]), indicating a decrease of cortical involvement across the night and a general maturational difference (Table 3); however, we found no maturational effect on across-night changes, indicated by a lower fit with the inclusion of the interaction term.
Table 3.
Parameters for the model explaining slow oscillation cortical involvement
| b ± SE | p | |
|---|---|---|
| Fixed effects | ||
| Intercept | 11.62 ± 11.13 | 0.3 |
| Quintile | −0.84 ± 0.24 | 0.0006 |
| Age | 0.4 ± 0.13 | 0.006 |
| Head size | 0.01 ± 0.21 | 0.95 |
| Gender | −0.22 ± 0.97 | 0.82 |
| Random effects | ||
| Intercept | 18.34 | |
| Quintile | 0.73 | |
| Residual | 8.9 | |
| AIC | 798.05 | |
| Pseudo R2 (marginal; conditional) | 0.2; 0.6 | |
SE = standard error.
First-to-last quintile percentage changes confirmed the model results by indicating no group differences in the across-night decrease of slow wave cortical involvement (76.3 ± 10.9% in the 2- to 4.9-year-old group, 81.2 ± 10.3% in 5- to 8.9-year-old children, 89.6 ± 7.2% in 9- to 16.9-year-old group; p = 0.63, Figure 3B). Thus, cortical involvement decreases across the night, regardless of age.
Slow wave speed exhibits no distinct maturational changes throughout the night
The final model for slow wave speed was a linear random-intercept model that included quintile, age, and their interaction without autocorrelation. Main effects did not reach significance; however, the interaction between quintile and age showed a significant trend (t(114) = 1.97, p = 0.05, 95% CI [−0.00002; 0.01]) indicating no overall across-night change in slow wave speed, yet variation with age (Table 4). No group difference was found regarding the change in slow wave speed from the first to the last quintile (p = 0.19, Figure 3C).
Table 4.
Parameters for the model explaining slow oscillation speed
| b ± SE | p | |
|---|---|---|
| Fixed effects | ||
| Intercept | 2.72 ± 1.18 | 0.02 |
| Quintile | 0.02 ± 0.02 | 0.41 |
| Age | 0.008 ± 0.01 | 0.62 |
| Quintile × age | 0.005 ± 0.003 | 0.05 |
| Head size | −0.008 ± 0.02 | 0.74 |
| Gender | 0.11 ± 0.10 | 0.28 |
| Random effects | ||
| Intercept | 0.25 | |
| Residual | 0.2 | |
| AIC | 55.59 | |
| Pseudo R2 (marginal; conditional) | 0.10; 0.65 | |
SE = standard error.
Age-related changes in whole night slow wave propagation
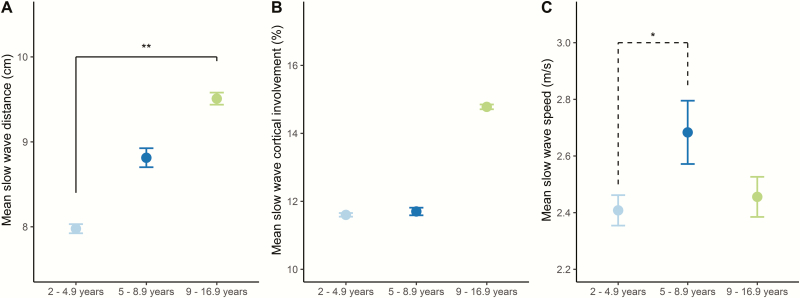
To examine the main effect of age, we then compared whole night mean data between age groups. A significant effect of age was found in slow wave distance (F(2,26) = 5.22, p = 0.01). Post-hoc tests revealed a difference between 2- to 4.9-year-old children and 9- to 16.9-year-olds (Figure 4A), confirming earlier results [6].
Figure 4.
Slow wave whole night mean data. (A) 9- to 16.9-year-olds exhibited significantly longer slow wave propagation distance than 2- to 4.9-year-old children. (B) No significant difference was apparent between age groups in slow wave cortical involvement, yet a trend was observed for increased cortical involvement in 9- to 16.9-year-olds compared to 5- to 8.9-year-old children. (C) Slow wave speed differed between 2- to 4.9-year-olds and 5- to 8.9-year-old children at trend level. Mean ± SEM is shown. *p = 0.05; **p < 0.01; N = 29.
Mean cortical involvement differed between age groups at the trend level (F(2,26) = 3.38, p = 0.05). Post-hoc tests correspondingly revealed a trend level difference between 5- to 8.9-year-old children and 9- to 16.9-year-olds (p = 0.07), yet not in other age groups (Figure 4B). This is again in line with previous reports on a lack of change in slow wave cortical involvement from the preschool to the school-age years.
No significant main effect of age group was found in the linear model explaining slow wave speed. Because the linear model does not account for nonlinear relationships, we explored whole night differences in slow wave speed. Mean slow wave speed revealed age group differences (p = 0.049), and post-hoc tests reached trend level significance for differences between 2- to 4.9-year-old and 5- to 8.9-year-old children (p = 0.05, Figure 4C).
Discussion
We investigated across-night dynamics of traveling slow waves during sleep in children and adolescents and reported three main findings. First, slow wave propagation distance decreases across the night, which interacts with age. A 12% reduction from the first to last quintile was only observed into the 2- to 4.9-year-old children, and not in children older than 5 years. Additionally, there was a general maturational increase in slow wave distance (unrelated to quintile). Second, cortical involvement decreases across the night in all age groups, yet exhibits generally increased values across age. Third, slow wave speed undergoes no maturation-specific across-night dynamics. Together, these data indicate transitional periods in the across-night dynamics of sleep slow waves that are specific to certain ages. We propose that these transitions represent important cornerstones in maturational brain processes. We discuss the bidirectional implications of these findings and propose that slow waves are markers for neurodevelopment that are possibly directly involved in human brain development processes.
Using high spatial resolution EEG, we consolidated complex topographical information into EEG features. With these measures, we quantified the stability and maturation of brain connectivity. Our findings extend previous knowledge by indicating that across-night dynamics in slow wave distance are most apparent in 2- to 4.9-year-old children. This observation may reflect plastic processes specific to preschool age, a period characteristic of early development with heightened plasticity and rapid learning [33, 34]. The across-night change was specifically apparent towards the morning (last quintile). Interestingly, while the oldest age group experienced a decline in preceding quintiles, there was an increase in the last quintile. The trend of change in all three wave metrics in the last percentile of the oldest group, may reflect an underlying neurophysiological process. Alternatively, decreasing stability of wave propagation measures towards the end of the sleep period is possible (even though variance does not increase). A third possible explanation is the influence of circadian effects [35] which were not controlled for in this paper. Divergence in age groups could have been influenced by maturational change in circadian sleep regulation, linked to typically delayed sleep phase and evening chronotype in adolescents [36]. Our previous findings suggest that slow wave distance relates to white matter myelin content in the corpus callosum [6]. In an exploratory approach with a subsample of participants, myelin water fraction was acquired with mcDESPOT magnetic resonance imaging (methods detailed in [6]). Results suggest a positive correlation between myelin water fraction in the corpus callosum with across-night changes in slow wave distance (first-to-last quintile) in 2- to 4.9-year-old children (r(26) = 0.72, p = 0.03, corrected for age). In other words, children with reduced callosal myelin show a larger decrease in slow wave distance over the course of the night. It remains speculative whether the decrease in slow wave distance is a development-specific function of sleep as a process of active rewiring of the developing brain.
We observed an age-related effect (i.e. group difference) in slow wave traveling distance indicating larger propagation distance in older participants, irrespective of across-night dynamics. Interestingly, this parameter also decreased across-night within the youngest age group. How can this phenomenon be explained? It is widely accepted that slow wave activity is linked to neuronal (synaptic) connectivity [5]. In developing rats, neuronal connectivity increases across a 24-hour-period despite opposite trends during the inherent period of sleep [8]. This finding indicates that the connectivity increase during the wake period generally outsizes its decrease during sleep. This imbalance within a 24-hour window is associated with a net connectivity increase—a phenomenon restricted to the maturational period. It is unclear, whether similar mechanisms hold true in humans. Despite a reduction in slow wave propagation distance across the period of sleep (reflecting decreasing connectivity), neuronal connectivity may nonetheless increase in waking—and in case of a positive imbalance within a 24-hour window—may also increase across the 24-hour day in young children. It remains to be tested whether this net increase in connectivity may ultimately add up in anatomical connectivity change resulting in callosal myelin growth across the developmental period [37, 38]. Our novel data indicate that cortical involvement decreased across the night in all age groups, such that slow waves were locally more restricted in the morning compared to evening hours. This finding aligns with adult data which also show more local slow waves in the last hours compared to the first hours of sleep [7]. We conclude from this similarity that cortical involvement dynamics are developmentally stable. Furthermore, because propagation patterns of slow waves survive thalamectomy [39], it was proposed that cortical involvement primarily underlies cortical synaptic connectivity [40]. The observed universal across-night decrease in cortical involvement supports the hypothesis that slow waves are tied to a reduction in neuronal connectivity at the synaptic level [41, 42].
Mean cortical involvement generally increased with older age, which conflicts with data showing that cortical grey matter volume or thickness has been shown to increase during development, reaching a maximum in adolescence and decrease thereafter [43]. However, three aspects need to be considered: First, the timing of maximal grey matter thickness is not uniformly synchronized across the cortex, with the occipital lobe showing an increase until 20 years of age [44]. Second, our oldest age group (ages 9–16 years) included preadolescents and adolescents some of whom are likely to experience continued increases in grey matter volume particularly in occipital and temporal areas [43]. And third, a recent model demonstrates that synaptic refinement and reorganization can account for developmental changes in adolescence, without the requirement of synaptic pruning [45]. It is thus possible that increased connectivity at later developmental periods reflects synaptic optimization rather than pure increase or growth of synapse numbers.
Generally, our findings demonstrate that slow wave speed undergoes no pronounced change across a night and age. Given the small beta values of the main effects as well as the interaction, the dynamics of speed across the night should be considered minor. A focus of future research may extend the applied calculation of slow wave speed to more specified measures of finer resolution (considering more than one propagation streamline, inclusion of propagation direction [46]).
Our results are based on a cross-sectional design based on three cohorts. Longitudinal studies are needed to corroborate these findings. Although no effects in sleep quality were detected between study sites, we cannot rule out the possibility that the inclusion of three cohorts may have affected the results. Due to only minor overlap of ages between the cohorts, controlling for site in the analyses would have masked any age effects.
A caveat of this study is that we targeted stereotyped high-amplitude slow waves by including the 20% of waves with largest amplitudes. Our approach was chosen to assess stereotyped slow waves in particular without the a priori restriction of scalp regions to minimize the potential maturational effects in EEG amplitude and frequency [24], while at the same time allowing for topographical variation within the sample [20]. Future studies may incorporate (1) different scalp locations, (2) unrestricted amplitude, and (3) wider ranges of speed and distance (primary and secondary propagation direction).
Our models include firstly age as linear variable and secondly age as grouping variable. The division into age groups was based on slow wave activity topographical/regional maturational state as well as the maturational state of absolute slow wave activity. This combined integration is critical for the current analyses, which extends existing approaches [35, 47]. The 9- to 16.9-year-old group includes participants in preadolescence/ adolescence, a time that includes a reduction of slow wave activity [48]. This maturational decrease may have interplayed with the generation of variability in the number of detected slow waves in this age group. Of note, one may expect a similar effect creating variability in the youngest group, where slow wave activity increases due to age [24, 29]. However, the slow wave activity increase at preschool-age occurs in a narrower time window (~7–8 years) compared to the decrease during adolescence (~12–14 years) [24]. Further, the standardized detection of waves and calculation of wave propagation metrics minimized the potential influence due to variability in sleep architecture and sleep length. In line with this is the stability of the relationship between age and number of waves detected. This relationship remains when controlling for total sleep time (r(26) = 0.57, p = 0.001). Similarly, age effects in wave propagation distance (r(26) = 0.5, p = 0.007) and cortical involvement (r(26) = 0.44, p = 0.02) remain stable when controlled for total sleep time.
Future work is needed to separate age-effects in propagation metrics from preadolescence to adolescence by considering narrower defined age groups, which should reduce variability within groups. This approach may also yield insights concerning the surprisingly large number of waves detected in one participant of the oldest age group.
In order to capture across-night dynamics from the beginning to the end of the sleep period in a simplified manner, post-hoc analysis focused on changes from the first to the last quintile of the night. We do not exclude the possibility that additional group differences in wave propagation could be detected with percentile-by-percentile post-hoc comparisons in a sufficiently powered sample. No sex differences were observed in our models; however, other studies reported such differences in developmental trajectories [49, 50]. It is possible that a larger sample could reveal such differences. Lastly, we used linear models, which neglect nonlinear associations such as U-shaped relationships. However, quadratic or cubic models did not yield better fits.
In summary, our data support that traveling patterns of sleep slow waves quantify across-night dynamics in neuronal connectivity in childhood and adolescence. The age-specific observations identify specific transitional maturational patterns, which may be associated with heightened, that is, developmental plasticity. It remains speculative whether these age-specific features are related to a neurodevelopmental function of sleep that may differ between children and adults.
Funding
This work was supported by the Clinical Research Priority Program Sleep and Health of the University of Zurich (to S.K.), Swiss National Science Foundation (grant numbers PBZHP3-138801, PBZHP3-147180, to S.K. and P0ZHP1-178697 to S.F.S), National Institutes of Health (grant number R01-MH086566 to M.K.L.), the Jacob’s Foundation (to S.C.D.) and seed funding from the Center for Innovation and Creativity at the University of Colorado Boulder (to M.K.L.).
Conflict of interest statement. None declared.
Address where work was conducted: Pulmonary Clinic, University Hospital Zürich, Rämistrasse 100, 8091 Zürich, Switzerland.
References
- 1. Shulman EP, et al. The dual systems model: review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nelson EE, et al. Social re-orientation and brain development: an expanded and updated view. Dev Cogn Neurosci. 2016;17:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vyazovskiy VV, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63(6):865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Achermann P, et al. Temporal evolution of coherence and power in the human sleep electroencephalogram. J Sleep Res. 1998;7(Suppl 1):36–41. [DOI] [PubMed] [Google Scholar]
- 5. Tononi G, et al. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kurth S, et al. Traveling slow oscillations during sleep-a marker of brain connectivity in childhood. Sleep. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olini N, et al. Diurnal changes in electrocorticogram sleep slow-wave activity during development in rats. J Sleep Res. 2014;23(3):261–267. [DOI] [PubMed] [Google Scholar]
- 9. Li W, et al. REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20(3):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jha SK, et al. Sleep-dependent plasticity requires cortical activity. J Neurosci. 2005;25(40):9266–9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ringli M, et al. Developmental aspects of sleep slow waves: linking sleep, brain maturation and behavior. Prog Brain Res. 2011;193:63–82. [DOI] [PubMed] [Google Scholar]
- 12. Kurth S, et al. Development of brain EEG connectivity across early childhood: does sleep play a role?Brain Sci. 2013;3(4):1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fattinger S, et al. Overnight changes in the slope of sleep slow waves during infancy. Sleep. 2014;37(2):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tarokh L, et al. Developmental changes in brain connectivity assessed using the sleep EEG. Neuroscience. 2010;171(2):622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurth S, et al. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33(4):475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilhelm I, et al. Sleep slow-wave activity reveals developmental changes in experience-dependent plasticity. J Neurosci. 2014;34(37):12568–12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feinberg I, et al. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72(1):56–65. [DOI] [PubMed] [Google Scholar]
- 18. Kurth S, et al. Mapping the electrophysiological marker of sleep depth reveals skill maturation in children and adolescents. Neuroimage. 2012;63(2):959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurth S, et al. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30(40):13211–13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurth S, et al. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33(4):475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vyazovskiy VV, et al. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30(12):1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huber R, et al. Exposure to pulsed high-frequency electromagnetic field during waking affects human sleep EEG. Neuroreport. 2000;11(15):3321–3325. [DOI] [PubMed] [Google Scholar]
- 23. Iber, C, et al. eds. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24. Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence?J Psychiatr Res. 1982;17(4):319–334. [DOI] [PubMed] [Google Scholar]
- 25. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 26. Rovine MJ, et al. Latent growth curve and repeated measures ANOVA contrasts: what the models are telling you. Multivariate Behav Res. 2018;53(1):90–101. [DOI] [PubMed] [Google Scholar]
- 27. Pinheiro J, et al. R Core Team (2014) nlme: linear and nonlinear mixed effects models. R package version 3. 1–117 2014. http://CRAN.R-project.org/package=nlme. Accessed January 15, 2018.
- 28. Barton K. MuMIn: multi-model inference, R package version 0.12.0. 2009. http://r-forge.r-project.org/projects/mumin/. Accessed September 3, 2018. [Google Scholar]
- 29. Kurth S, et al. Sleep slow oscillations and cortical maturation. In: Marcos G. Frank.ed. Sleep and Brain Activity. 1st ed. Cambridge, MA:Academic Press;2012. [Google Scholar]
- 30. Tarokh L, et al. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33(6):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colrain IM, et al. Changes in sleep as a function of adolescent development. Neuropsychol Rev. 2011;21(1):5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scholle S, et al. Normative values of polysomnographic parameters in childhood and adolescence: quantitative sleep parameters. Sleep Med. 2011;12(6):542–549. [DOI] [PubMed] [Google Scholar]
- 33. Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 1998;27(2):184–188. [DOI] [PubMed] [Google Scholar]
- 34. Johnson MH, et al. Processes of change in brain and cognitive development. Trends Cogn Sci. 2005;9(3):152–158. [DOI] [PubMed] [Google Scholar]
- 35. Campbell IG, et al. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep. 2011;34(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hagenauer MH, et al. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31(4):276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paus T, et al. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54(3):255–266. [DOI] [PubMed] [Google Scholar]
- 38. Dean DC III, et al. Characterizing longitudinal white matter development during early childhood. Brain Struct Funct. 2015;220(4):1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steriade M, et al. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13(8):3284–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Massimini M, et al. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24(31):6862–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tononi G, et al. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. [DOI] [PubMed] [Google Scholar]
- 42. de Vivo L, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355(6324):507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. [DOI] [PubMed] [Google Scholar]
- 44. Sowell ER, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. [DOI] [PubMed] [Google Scholar]
- 45. Hoel EP, et al. Synaptic refinement during development and its effect on slow-wave activity: a computational study. J Neurophysiol. 2016;115(4):2199–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murphy M, et al. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106(5):1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feinberg I, et al. Topographic differences in the adolescent maturation of the slow wave EEG during NREM sleep. Sleep. 2011;34:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feinberg I, et al. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am J Physiol Regul Integr Comp Physiol. 2013;304(4):R296–R303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lenroot RK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruigrok AN, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]