Abstract
Study Objectives
This study tested the hypothesis that sleep fragmentation (SF) delays wound healing in obese B6.BKS(D)-Leprdb/J (db/db) mice with impaired leptin signaling and type 2 diabetes compared with wild-type C57BL/6J (B6) mice.
Methods
Adult male mice (n = 34) were anesthetized and bilateral full-thickness excisional wounds were created on the back of each mouse. Half of the db/db and B6 mice were housed in SF cages equipped with a bar that moved across the cage floor every 2 min, 12 hr/day for 23 days. The other half of each group of mice was housed in the same room and did not experience SF. The dependent measures were number of days required to achieve wound closure, mRNA expression of four inflammatory mediators, blood glucose, insulin, and corticosterone.
Results
SF in the db/db mice caused a significant delay in wound healing relative to db/db mice with no SF. Days to achieve 50 per cent wound healing were 13.3 ± 0.4 with SF compared with 10.3 ± 0.7 without SF. All B6 mice achieved 50 per cent wound healing within 6 days and complete healing after 16 days. SF caused a significant increase in wound levels of TNF-α mRNA only in the db/db mice and an increase in corticosterone only in the B6 mice.
Conclusions
The delayed wound healing in obese, diabetic mice caused by SF is homologous to delayed wound healing in some patients with type 2 diabetes. The results support the interpretation that altered leptinergic signaling and inflammatory proteins contribute to delayed wound healing.
Keywords: sleep fragmentation, wound healing, obesity, diabetes
Statement of Significance
Sleep disruption impairs glucose metabolism. Patients with type 2 diabetes have disrupted glucose metabolism and commonly confront the comorbidity of impaired wound healing. Human skin wounds that do not heal are a significant public health burden. The present data show for the first time that sleep fragmentation significantly delays wound healing in obese, db/db mice with type 2 diabetes, but not in normal weight B6 mice. The results support the interpretation that studies of db/db mice, an animal model of obesity and hyperglycemia, can provide novel insights into the complex relationships among sleep disruption, type 2 diabetes, and wound healing.
Introduction
The goal of this study was to determine whether sleep fragmentation (SF) impairs wound healing in diabetic mice. This goal is framed by evidence outlined below that sleep contributes to the interaction among obesity, type 2 diabetes, and wound healing. Obesity statistics indicate that more than one in three adults in the United States are hyperglycemic and meet diagnostic criteria for prediabetes [1]. World Health Organization data show that worldwide, 39 per cent of adults age 18 years and older are overweight and 13 per cent are obese [2].
Type 2 diabetes, a metabolic disorder estimated to affect more than 370 million people [3], is associated with obesity. Patients with type 2 diabetes also have an increased prevalence of sleep disorders including obstructive sleep apnea (OSA) [4], impaired sleep duration [5], restless leg syndrome [6], and insomnia [7]. The discovery that sleep debt causes metabolic changes similar to those seen in type 2 diabetes [8] has been supported by many studies [9]. Type 2 diabetes in adolescents also is a significant public health burden [10].
Impaired wound healing is a clinically relevant problem [11] and obese, diabetic patients are at increased risk of surgical site infection [12]. SF impairs immune function [13]. Wounds in diabetics are difficult to treat [14] and prolonged inability to promote wound healing is a major cause of amputation [15]. Medical care for nonhealing wounds is estimated to cost more than US$50 billion dollars per year in the United States [11, 16].
The foregoing relationships suggest an association among obesity, SF, and wound healing. The present study used a specific mouse model of obesity, known to exhibit features of human type 2 diabetes, to determine whether SF could causally be associated with impaired wound healing. The control group was comprised of wild type C57BL/6J (B6) mice. B6 mice provide the genetic background for congenic B6.BKS(D)-Leprdb/J (db/db) mice, an accepted model of obesity and type 2 diabetes [17–19]. The obese, diabetic mice are not transgenic animals and arose as a spontaneous mutation in the C57BL/6J strain [20]. These db/db mice synthesize leptin and their obesity arises from the fact that they do not express the long form of the leptin receptor [21, 22]. We are aware of no previous studies determining whether SF differentially alters wound healing in B6 and db/db mice. The foregoing rationale enabled a test of the hypothesis that SF delays wound healing in obese, db/db mice when compared with normal weight B6 mice.
Materials and Methods
Animals
Adult male db/db (Stock# 000697; n = 22) and wild-type B6 (Stock# 000664; n = 12) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). To consider the potential effects of age on wound healing, separate experiments were performed using mature adult mice (25 to 27 weeks of age) and young adult mice (10 weeks of age). Mice were housed in a temperature and humidity-controlled environment with free access to food and water. The light:dark cycle was 12 hr light:12 hr dark. All mice were fed normal Teklad 8640 rodent chow (Envigo, Madison, WI). These studies were reviewed and approved by the University of Tennessee IACUC and adhered to the Guide for the Care and Use of Laboratory Animals (The National Academies Press, 8th ed., Washington, DC, 2011).
Sleep fragmentation
The two treatment conditions included SF and no SF (Figure 1). Mice in each of the two strains were exposed to both treatment conditions, and all mice were housed in SF chambers (model 80391; Lafayette Instruments; Lafayette, IN). During SF, a bar moved across the cage floor every 2 min during the subjective night (0800 to 2000 hr). When the bar reached the mice, they were awakened and ambulated over the bar. Between the intervals of bar movement, the mice remained undisturbed. In the SF condition, the sweeper bar was activated 12 hr/day for 23 days. The control mice were housed in the same room and did not undergo SF. The chambers and the protocol used to cause SF were introduced in 2009 [23]. Subsequent studies show that the SF protocol alters sleep/wake states in mice by increasing wakefulness during the fragmentation period, decreasing latency to sleep onset, and increasing EEG-δ power during slow-wave sleep [23, 24]. Additionally, this SF protocol induces obesity [25], inflammation [26], insulin resistance [27], and cognitive deficits [28]. Thus, the SF protocol satisfies many formal constructs of validity [29], including internal and external validity.
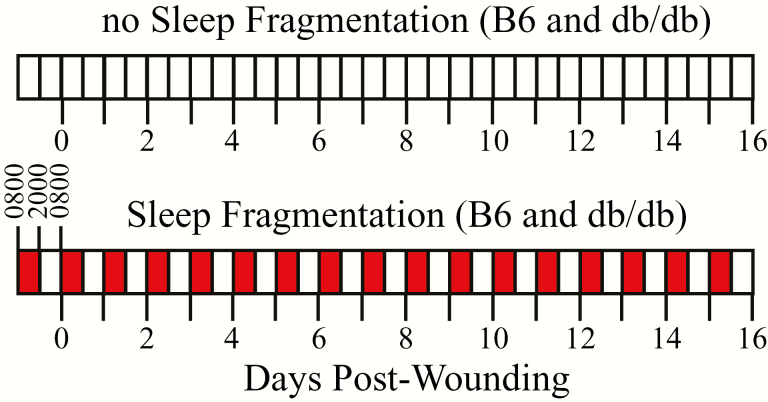
Figure 1.
Schematic of the 16 day experiment for mice that experienced no SF (control) and for mice in which daily fragmentation of sleep occurred from 0800 to 2000 hr (red bars). C57BL/6J (B6) and B6.BKS(D)-Leprdb/J (db/db) mice experienced either SF or no SF. Prior to day zero (abscissa), all mice were acclimated for 7 days to each treatment condition. On day zero, mice were anesthetized and bilateral, dorsal skin biopsy (wounding) was made through the subcutaneous muscle (panniculus carnosus). On alternate days after the skin biopsy, the mice were weighed, blood glucose was measured, and the wounds were photographed (Figure 2). On day 16, mice were euthanized and wound tissue was collected.
Wound healing
After 4 days in either SF or control conditions, mice were anesthetized with isoflurane in 100 per cent oxygen, and the dorsal surface of the mouse was depilated using Nair (Church & Dwight Co., Inc., Ewing, NJ). Three days after depilation, mice were removed from their cages, anesthetized with isoflurane, and a sterile 6-mm biopsy punch was used to create bilateral full-thickness excisional wounds on the dorsal surface area between the shoulders and the pelvis. The foregoing procedures produce a skin wound healing model that has been well-validated and described in detail [30]. Biopsies extended through the superficial musculature (panniculus carnosus). In an effort to limit wound contraction [31], the wounds were protected and kept moist with a transparent semiocclusive dressing (Tegaderm) that was fixed in place with Mastisol (Ferndale Laboratories, Ferndale, MI). Following recovery from anesthesia, the mice were returned to their respective cages until the end of the experiment.
Analysis of wound closure
Quantitative assessment of wound healing was achieved via wound morphometric analyses. Digital images of the bilateral biopsies were obtained immediately after the skin biopsy (Figure 1, day 0) and on days 2, 4, 7, 9, 11, 14, and 16 after the biopsy. On days in which the wounds were photographed, body weights were recorded, and blood glucose levels were measured using a Contour Next EA system (Bayer HealthCare LLC, Mishawaka, IN). Digital images of the biopsy were analyzed using ImageJ [32] to calculate the wound surface area comprising each circular excision. Experimenters performing analyses worked in teams to ensure appropriate blinding to the experimental conditions of the mice. The two bilateral, dorsal wound areas were averaged, and wound closure was expressed as a percent of the original wound area over time.
Serum, gene expression, and wound assays
Sixteen days after creating the bilateral surgical wounds, mice were euthanized via CO2 asphyxiation and cervical dislocation. A cardiac blood sample was obtained for measuring serum corticosterone and insulin. Serum insulin (via Mercodia; Cat No: 10-1247-10) and corticosterone (via Enzo Cat No: ADI-900-097) were measured as described previously [18]. Skin samples (8 mm) from the site of the original wound were obtained from all mice by sharp dissection. Biopsy samples were frozen in liquid nitrogen and stored at −80°C for gene expression analyses of tumor necrosis factor-α (TNF-α), interleukin 1α (IL-1α), chemokine (C-X-C motif) ligand 10 (CXCL10), and cyclooxygenase-2 (COX2). RNA was isolated from frozen tissues using the Qiagen RNeasy kit and quantified by Nano-Drop analysis. cDNA was synthesized from 100 ng of total RNA using iScript (Bio-Rad) and gene expression was measured by real-time PCR using previously published protocols [33].
Statistical analyses
Wound size as a function of SF and time after wounding was analyzed using a two-way analysis of variance (ANOVA) evaluating statistical main effects due to mouse strain (B6 [n = 12] vs db/db [n = 10] in mature adult mice 25 to 27 weeks of age). Post hoc comparisons were made with Bonferroni and Tukey’s multiple comparisons tests. The same analyses were used to evaluate data obtained from the 10-week-old mice. Data are expressed as mean ± standard error of the mean (SEM), and for all comparisons, a probability (p) value of ≤0.05 was considered statistically significant. In a second series of experiments, wound size as a function of SF and time after wounding was evaluated in young adult (10 weeks of age) db/db mice exposed to SF (n = 6) vs no SF (n = 6). The results were evaluated using area under the curve for time to reach 50 per cent wound closure. Relative mRNA abundance of four inflammatory mediators was evaluated with nonparametric Mann–Whitney tests. Body weights were compared using student’s two-tailed, unpaired t-test. All analyses were performed using Prism 7.0a software.
Results
SF delayed wound healing in obese, db/db mice
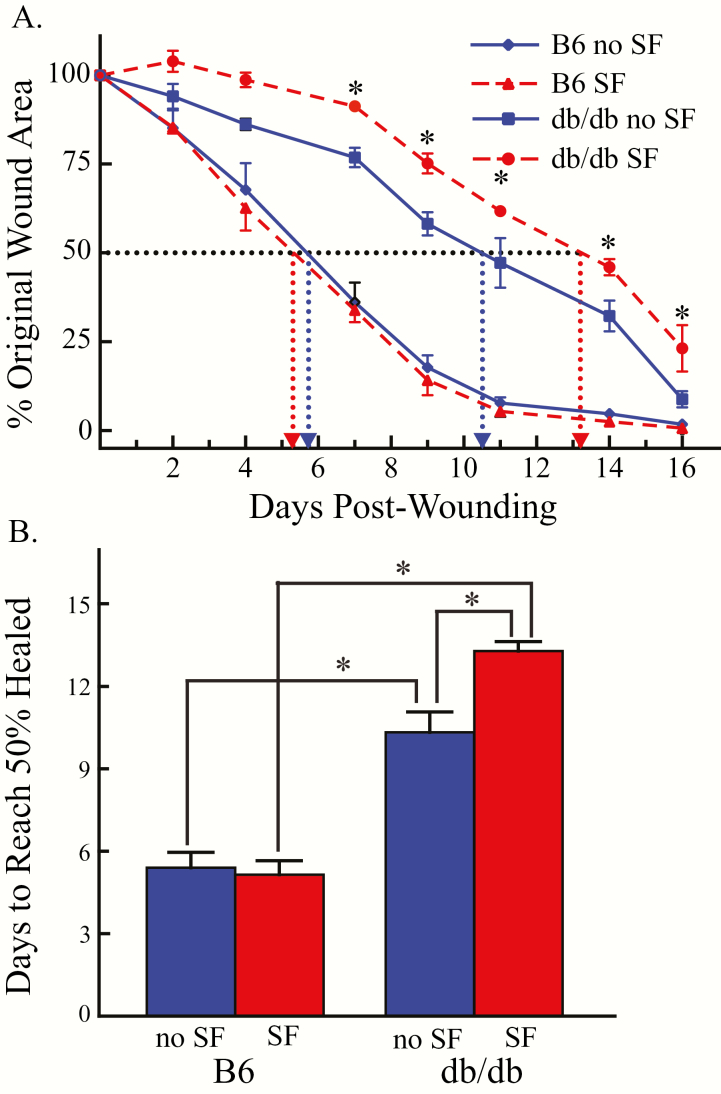
Figure 2 shows representative digital images used to analyze wound closure in B6 mice and in obese, db/db mice. Figure 3A illustrates the effect of SF on the rate of wound closure in B6 (n = 12) and db/db (n = 10) mice. Half of B6 mice and half of the db/db mice experienced SF, in contrast to the other half of the mice that had no SF. For all mice, the percent wound closure was calculated . Two-way ANOVA revealed that wound closure was significantly slowed as a function of SF (p < 0.0001) and mouse strain (p < 0.0001) with a statistically significant interaction between SF and mouse strain (p < 0.0001). In the B6 mice with and without SF, wound closure occurred by day 16 (Figure 3A). On day 16 after the skin biopsy, wound closure was significantly less (p < 0.05) in db/db mice with SF (23.2 ± 6.5%) when compared with db/db mice with no SF (8.8 ± 2.3%), indicating a delay in wound closure. Figure 3B shows that in the wild-type B6 mice, the time to 50 per cent closure was not delayed by SF. By contrast, SF of the obese, diabetic db/db mice significantly delayed the time required to achieve 50 per cent wound closure. Two-way ANOVA revealed that wound closure was significantly slowed as a function of SF (p = 0.03) and mouse line (p < 0.0001). The interaction between SF and mouse line was also statistically significant (p < 0.01). Multiple comparisons test reveal statistically significant (p < 0.05) differences in all pair-wise group comparisons, except between B6 mice with and without SF.
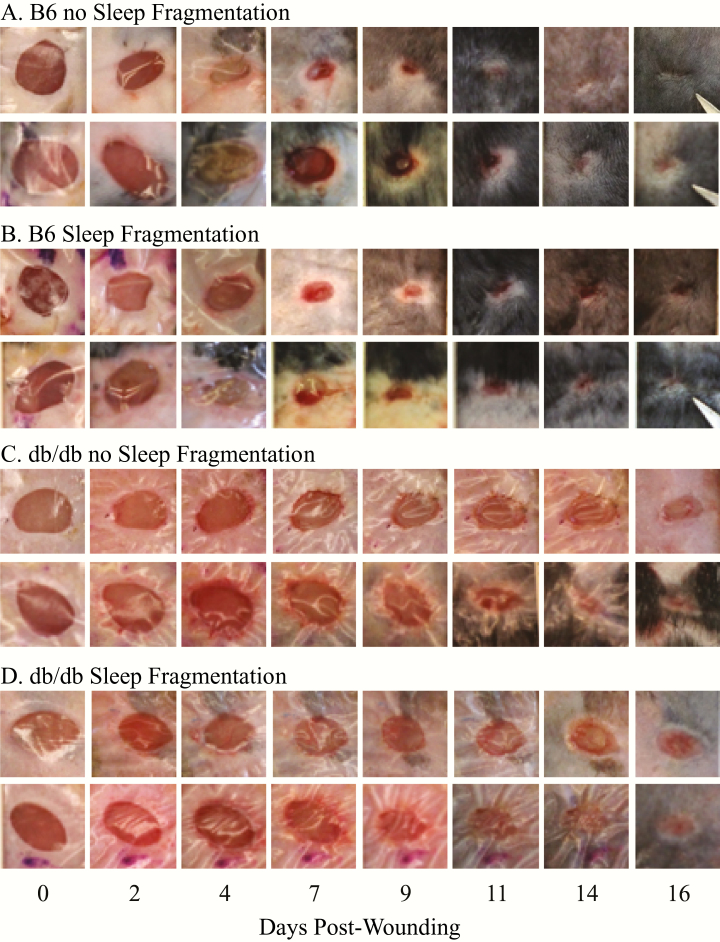
Figure 2.
Representative images of wound closure in C57BL/6J (B6) and B6.BKS(D)-Leprdb/J (db/db) mice without and with SF at 0, 2, 4, 7, 9, 11, 14, and 16 days after the skin biopsy (postwounding). Each row of images shows the same wound from a single mouse. (A) Wound size across time in two B6 mice that experienced no SF. (B) Wound size across time in two B6 mice in the SF group. (C) Wound size in two db/db mice not exposed to SF. (D) Wound size across time of two db/db mice in the SF condition. Photos illustrate the differences in wound closure between B6 and db/db mice as early as 7 days after the skin biopsy. By 16 days after the skin biopsy, the wounds with the least amount of closure were from the db/db mice in the SF condition. These results are expressed quantitatively in Figure 3.
Figure 3.
Time course of wound size in the four groups of mice across 16 days. (A) Results are expressed as mean ± standard error of the mean and show percent of the original wound area as a function of time after the skin biopsy (days postwounding). The number of days required to achieve 50 per cent wound closure was greatest for B6.BKS(D)-Leprdb/J (db/db) mice with SF (filled red circles and dashed red line) and db/db mice with no SF (filled blue squares and solid blue line). The most rapid wound healing occurred in normal weight C57BL/6J (B6) mice without SF (filled blue diamond and solid blue line) and with SF (filled red triangles and red dashed line). The two left-most vertical dotted lines illustrate that the B6 mice achieved wound closure between 5 and 6 days after biopsy, and that there was no significant effect of SF. The two right-most vertical, dotted lines help visualize the differences in number of days (abscissa) to achieve 50 per cent wound closure in the db/db mice with and without SF. The results of Tukey’s multiple comparisons test are summarized by the asterisks. Post hoc comparisons indicated that the db/db mice in the SF condition had significantly larger wounds compared with all of the other three groups, except on days 2 and 4 when the wound size of the two groups of db/db mice did not differ between sleep conditions. (B) Group data summarizing the effect of SF on days required to reach 50 per cent wound closure as a function of mouse strain and SF. Bars summarize mean ± standard error of the mean. Asterisks show significant differences with and without SF. Within mouse strains, SF caused a significant increase in the number of days to reach 50 per cent wound closure only in the diabetic, B6.BKS(D)-Leprdb/J (db/db) mice. C57BL/6J (B6) mice without (blue bar) and with (red bar) SF healed significantly faster than the db/db mice.
SF: gene expression
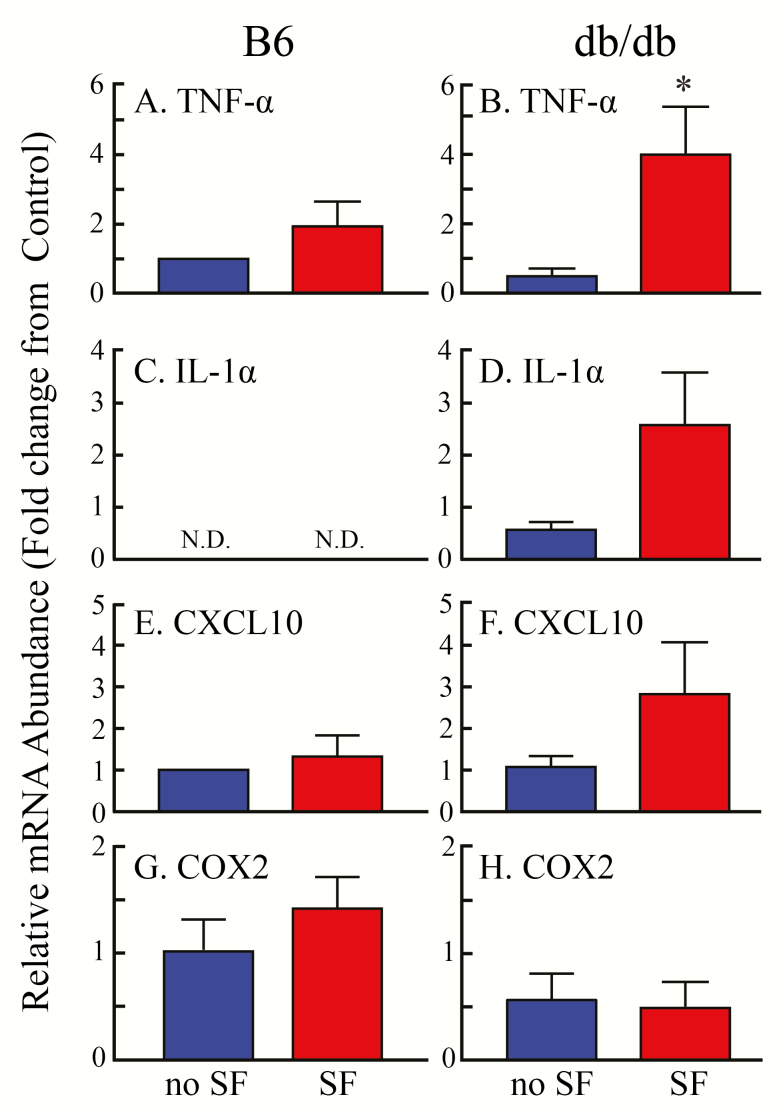
Wound healing occurred more slowly in the db/db mice exposed to SF than in db/db mice with no SF. This result, and the finding that SF did not delay wound healing in wild-type mice, encouraged measurement of inflammatory mediators (e.g., chemokines and cytokines as well as COX2). The chemokines and cytokines chosen for measurement are representative of those elevated in the obese state. In addition, COX2 has previously been shown to be associated with wound healing [34]. Figure 4 compares gene expression obtained from wounds of wild-type B6 mice (left column) with similar measures from obese db/db mice (right column). In B6 mice, TNF-α mRNA level was not altered by SF (Figure 4A, red bar) compared with no SF (blue bar). In contrast, TNF-α expression (Figure 4B) was significantly (p = 0.03) increased during SF in db/db mice relative to db/db mice with no SF. Expression of IL-1α was not detected in B6 mice with or without SF (Figure 4C). In db/db mice, SF caused a nonsignificant (p = 0.06) increase in IL-1α compared with db/db mice with no SF (Figure 4D). There were no significant effects of SF on the inflammatory mediators CXCL10 or COX2 (Figure 4, E–H).
Figure 4.
Fold changes in mRNA expression within the wounds of C57BL/6J (B6) and B6.BKS(D)-Leprdb/J (db/db) mice for the inflammatory mediators: (A and B) tumor necrosis factor-α (TNF-α), (C and D) interleukin 1α (IL-1α), (E and F) chemokine (C-X-C motif) ligand 10 (CXCL10), and (G and H) cyclooxygenase-2 (COX2). Comparison were made between wounds from mice with no SF (blue bars) and mice after SF (red bars). (B) SF caused a significant (p = 0.03) increase in TNF-α in the db/db mice. (C) IL-1α mRNA expression was not detectable (N.D.) in B6 mice or in db/db mice. (D) In the db/db mice, there was a p = 0.06 probability that IL-1α mRNA expression differed as a function of SF. For CXCL10 (E and F) and for COX2 (G and H), there were no significant differences as a function of mouse line or SF.
SF: body weight, blood glucose, insulin, and corticosterone
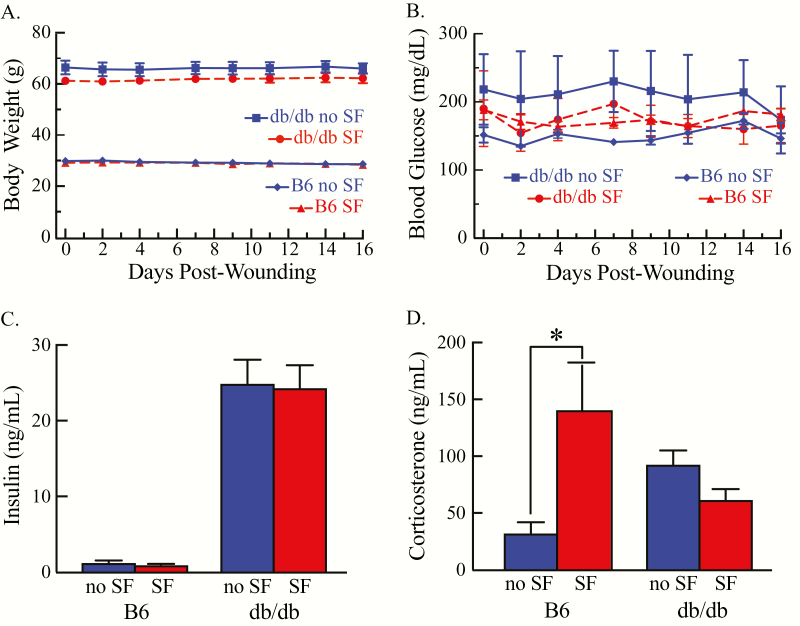
Figure 5A shows that throughout the study, body weight of the db/db mice (mean = 64.40 ± 1.32 g) was significantly greater than body weight of B6 mice (mean = 29.09 ± 0.48 g). The body weight of all mice did not change significantly across the course of the experiments. SF did not significantly alter blood glucose (Figure 5B) or serum insulin levels in either obese or lean mice (Figure 5C). Serum corticosterone levels were measured as an index of potential stress associated with SF. These measures revealed no significant difference in serum corticosterone levels in B6 or db/db mice in the no SF conditions. However, lean mice displayed increases in serum corticosterone in response to SF (Figure 5D).
Figure 5.
Mean ± standard error of the mean of body weight, blood glucose, insulin, and corticosterone for each mouse line. (A) Body weight measurements for B6.BKS(D)-Leprdb/J (db/db) and C57BL/6J (B6) mice taken prior to wounding (day 0) and periodically until the completion of the study (day 16) revealed no significant change within each mouse line. (B) Tail vein blood glucose levels in db/db and B6 mice were stable across the experiment. (C) Insulin levels with SF and without SF did not differ between the B6 and the db/db mice. (D) Only in B6 mice did SF cause a significant (*p < 0.05) increase in corticosterone.
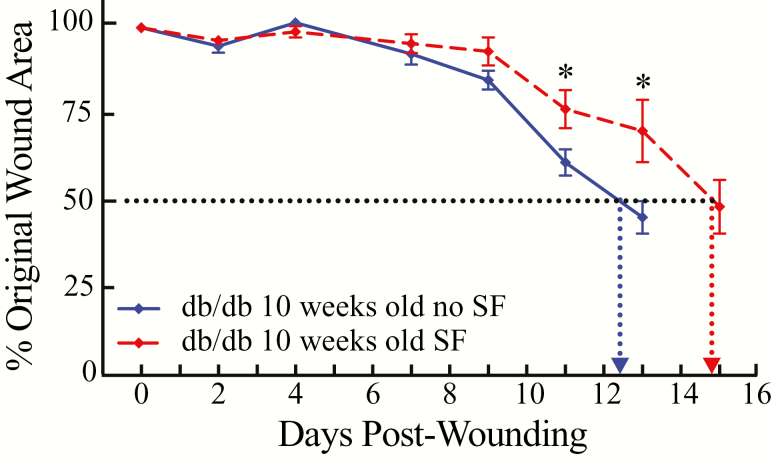
Mice are sexually mature by about 35 days [35] and rapid maturation is one of the advantages of using mice with known genetic backgrounds. Over time, and homologous to humans with type 2 diabetes, db/db mice can develop significant deficits [19]. We conducted a final set of experiments using db/db mice to determine whether the delay in wound healing caused by SF in mature adult mice (25 to 27 weeks of age) would be observed in young adult mice (10 weeks of age). The results of those experiments show that in the SF condition, the 10-week-old db/db mice (n = 6) reached 50 per cent wound healing after 14.9 days (Figure 6). This is in contrast to the no-SF condition in which 10-week-old db/db mice (n = 6) achieved 50 per cent wound healing in 12.4 days. Thus, SF in the 10-week-old obese mice also caused delayed wound healing.
Figure 6.
Time course of wound area to reach 50 per cent closure with and without SF in young, adult B6.BKS(D)-Leprdb/J (db/db) mice (10 weeks of age). Results are expressed as mean ± standard error of the mean and show per cent of the original wound area as a function of time after the skin biopsy (days postwounding). The number of days required to achieve 50 per cent wound closure was greater for db/db mice with SF (filled red symbols and dashed red line) when compared with db/db mice with no SF (filled blue symbols and solid blue line). The two vertical, dotted lines help visualize the differences in number of days (abscissa) to achieve 50 per cent wound closure in the db/db mice with and without SF. The two vertical dotted lines illustrate that the young, adult db/db mice achieved 50 per cent wound closure between 12 and 13 days (12.4 days) without SF (n = 6), and that wound closure was delayed with SF, occurring between 14 and 15 days (14.9 days) (n = 6). The results of a Bonferroni multiple comparisons test are summarized by the asterisks that indicate significant differences with and without SF between the groups on days 11 (p < 0.0369) and 13 (p < 0.0001).
Discussion
The results show that SF significantly delayed wound healing in obese, db/db mice with type 2 diabetes, but not in wild-type B6 mice. SF caused delayed wound healing in mature adult and young adult db/db mice. The process of wound healing is initiated by tissue injury and then progresses through stages of inflammation, proliferation of epithelial cells, revascularization, and scar formation. The forgoing processes are triggered by diverse injuries in a variety of tissue types, indicating that wound healing is an innate cellular process. The present result show that SF disrupts inherent processes involved in wound healing. This is consistent with the discovery that SF significantly alters fundamental cell biology [25, 27, 28, 36]. The present finding that SF delays wound healing in diabetic mice with leptin receptor dysfunction is consistent with evidence that leptin promotes skin wound healing [37].
Models of diabetes, SF, and wound healing
A wide range of occupational, situational, or disease states cause sleep to be fragmented into short episodes that are disrupted by arousals. The heterogeneous causes of SF are a potential confound for efforts to understand the health impact of fragmented sleep. This confound has been diminished by development of devices and procedures that standardize experimentally imposed SF [23, 38–40]. Sleep varies significantly among mouse strains and sleep is a heritable phenotype in mice and humans. The B6 mouse strain provides the genomic background for many genetically altered mice. B6 mice and obese db/db mice produce the cytokine leptin but, as noted earlier, due to a spontaneous mutation [20], db/db mice do not express the long form of the leptin receptor. This allelic mutation causes db/db mice to develop excessive adipose tissue, peripheral nerve damage with sensory loss, hyperglycemia, and dyslipidemia homologous to human type 2 diabetes. These phenotypes have encouraged four decades of research using db/db mice as a model of type 2 diabetes [19, 41].
The present results are consistent with evidence that human wound healing is impaired by obesity [42]. Diabetic, obese mice were shown many years ago to be of translational relevance for studies of impaired wound healing [43]. The present results demonstrate for the first time in db/db mice that SF further delays wound healing. The finding of normal wound healing in the B6 mice compared with the db/db mice is consistent with the earlier finding of no difference in wound healing between homozygous db/db mice and heterozygous db/+ control mice (Table 1 of [43]).
Pioneering experiments using rats reported that 72 hr of total sleep deprivation caused no alterations in wound healing [44]. There are three significant differences between the present study and the study of sleep deprived rats [44].
First, sleep deprivation and SF are different yet can share similar traits. Sleep deprivation is a physiological stress [45] as evidenced by decreases in the stress hormone cortisol during sleep and increase in cortisol during sleep deprivation [46]. Human SF also has been associated with increases in cortisol [47]. There are differing results from SF studies in mice that used corticosterone as a measure of stress. SF produced by the same type of instrumentation as used in the present study has been reported to cause both no increase in corticosterone [23] and a significant increase in corticosterone [48]. The present measures of corticosterone do not resolve these previous equivocal findings and indicate further that the effects of SF on corticosterone levels can vary between mouse strains. SF increased corticosterone in B6 mice but not in db/db mice (Figure 5).
A second major difference between the sleep deprivation study in rat and the present study concerns the nature of the skin wound. The present study used a standard protocol to create an excisional wound [30, 43, 49, 50]. The skin wound in the rat study was created via bilateral, subcutaneous implant of 5 cm by 1 mm polytetrafluoroethylene tubing.
Third, in the present study, the conclusion that SF delayed wound healing was based on direct measures of wound size. In the rat study, the conclusion that 72 hr of sleep deprivation did not alter wound healing was inferred from biochemical measures of DNA, protein, and hydroxyproline derived from postmortem tissue. Additionally, the present results obtained from mature db/db mice (25 to 27 weeks of age) were replicated by a second study of young adult db/db mice (10 weeks of age), with and without SF.
Complex comorbidities: SF, diabetes, and impaired healing
In patients with type 2 diabetes, a meta-analysis of nearly 4000 studies indicated that disruption of sleep duration and quality alters glycemic control [9]. Type 2 diabetes is associated with a wide range of sleep disorders including restless leg syndrome [6], OSA [51], and insomnia [7]. OSA severity is associated with poor glucose control and evidence suggests a causal link between OSA and development of diabetes [52].
Type 2 diabetes is characterized by a state of chronic, low grade inflammation, hyperglycemia, and elevated proinflammatory mediators [53]. Cytokines have multiple functions including modulation of immunity, inflammation, and sleep [13, 54]. The proinflammatory cytokine TNF-α is increased in type 2 diabetes and obesity [55]. TNF-α has been studied extensively in relation to wound healing [56] and sleep [13]. There is also evidence that increases in TNF-α can contribute to cognitive and behavioral deficits [25, 28, 57]. The inflammatory process is crucial for wound healing. Systemic inflammation has been hypothesized to disrupt the normal apoptotic machinery in activated immunocytes [56, 58]. This disruption potentially perpetuates the inflammatory response to injury and infection. The data in Figure 4 show that SF significantly increased wound levels of TNF-α mRNA expression only in the db/db mice that experienced SF. This result is consistent with previous studies showing that SF increases TNF-α [24]. The present result in Figure 4 may reflect the fact that wound cytokines samples were obtained on day 16 when all mice, except for the sleep fragmented db/db mice, had healed. The data in Figure 3 are the first to document the time course to achieve 50 per cent wound healing in B6 and db/db mice, with and without SF. Thus, in the absence of prior data to guide this study, cytokine samples were taken on day 16. Chemokines and cytokines are elevated in obesity and, as shown here, appear to be further altered by SF. Levels of COX2 are known to vary with wound healing [34].
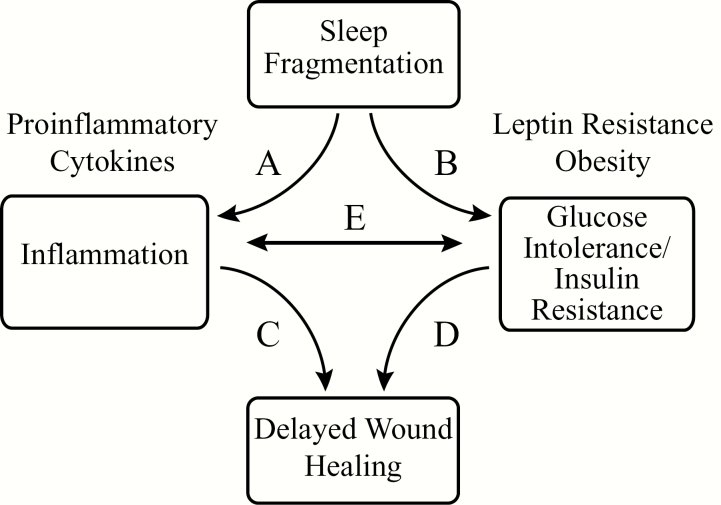
Previous reviews have described the impact of sleep, hormonal status, and body weight on type 2 diabetes [59]. Figure 7 summarizes the complex interactions between inflammation, SF, glucose intolerance/insulin resistance, and obesity, all of which affect wound healing. This schematic, however, should not obscure the fact that wound healing in diabetes is humbling in complexity and associated with more than 100 physiological factors [60]. The present results encourage application of multivariate analyses to quantify the direct and indirect impact of SF on delayed wound healing.
Figure 7.
Schematic summarizing interacting factors currently known to affect wound healing. (A) SF promotes inflammation, in part, by increasing proinflammatory cytokines [48, 57, 71, 72]. (B) SF increases glucose intolerance/insulin resistance, obesity, and leptin resistance [25, 36, 55]. (C) Inflammation delays wound healing via macrophage dysfunction which in turn leads to impaired angiogenesis, disorganized apoptosis, and impaired cell migration [73, 74]. (D) Impaired glucose metabolism delays wound healing through the sequela caused by diabetes such as neuropathy, vasculopathy, and immunodeficiency [75, 76].
Limitations and future directions
There are many reasons to be cautious about the extent to which data from mice faithfully emulate human disease [61]. This potential limitation makes it even more remarkable that disrupting sleep promotes obesity in humans [5] and in mice [25]. Evidence confirming a relationship between SF and energy metabolism also emphasizes the good agreement between studies of mice [62] and humans [63].
The present results are limited to male mice. Human studies document sex differences in wound healing [64] and in secretion of inflammatory cytokines caused by sleep restriction [65]. It follows that there are male and female differences in the prevalence of obesity and type 2 diabetes [66]. Sex differences in leptin levels also vary by mouse strain. For example, in an outbred mouse strain (Swiss CD-1 mice), plasma leptin levels are significantly greater in males than in females [67]. The foregoing relationships, recent discoveries of sex differences between B6 and db/db strains [68], and the present results all encourage future studies to determine whether delays in wound healing vary by sex for db/db and B6 mice.
The present study was designed to determine whether the well-validated SF protocol causes delayed wound healing in mice with type 2 diabetes, not to quantify the role of obesity versus diabetes as a potential causal contributor to delayed wound healing. The results justify future studies specifically aiming to quantify the extent to which SF alters wound healing in obese mice compared with obese mice with type 2 diabetes. As demonstrated elsewhere [68, 69], such adequately powered studies will require the use of male and female B6 mice with diet-induced obesity (DIO), normal weight B6 mice, leptin-deficient ob/ob mice, and obese, diabetic db/db mice. Statistical evaluations of such studies require a three-way ANOVA model to evaluate potential main effects and interactions attributed to mouse line (four levels), with and without SF (two levels), and as a function of sex (two levels). Complex experimental designs of the type outlined above must also contend with the fact that DIO mice are prediabetic (https://www.jax.org/strain/380050) and may develop type 2 diabetes as they age.
In summary, the results support the interpretation that experimentally induced SF delays wound healing in male db/db mice that are obese and diabetic but not in wild-type B6 mice. These findings, and the biological parallels between sleep and metabolism in mice and humans, suggest an association between sleep and the clinically significant problem of impaired wound healing [11, 16].
Funding
Support was provided by grants from the National Institutes of Health, Bethesda, MD, HL65272 (R.L. and H.A.B.); Office of Research Engagement, University of Tennessee, and by the Physicians’ Medical Education and Research Foundation, Knoxville, TN and also supported by the Beaman Endowed Professorship (H.A.B.) and Robert H. Cole Endowed Professorship (R.L.).
Conflict of interest statement. All authors have completed the conflict of interest statement provided by http://www.icmje.org/conflicts-of-interest/.
Acknowledgments
Portions of these results have been presented as an abstract [70]. For expert assistance, the authors thank Aaron Baer, B.A., Emily Miller, B.S., and Josiah Brandt, B.S.
References
- 1. CDC. Diabetes home. https://www.cdc.gov/diabetes/basics/prediabetes.html. Accessed July 4, 2018.
- 2. WHO. Obesity and overweight http://wwwwhoint/mediacentre/factsheets/fs311/en/. Accessed April 1, 2017.
- 3. Kahn SE, et al. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shechter A, et al. ;. Sleep AHEAD Research Group of the Look AHEAD Research Group. Sleep architecture following a weight loss intervention in overweight and obese patients with obstructive sleep apnea and type 2 diabetes: relationship to apnea-hypopnea index. J Clin Sleep Med. 2014;10(11):1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh M, et al. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med. 2005;1(4):357–363. [PubMed] [Google Scholar]
- 6. Merlino G, et al. Association of restless legs syndrome in type 2 diabetes: a case-control study. Sleep. 2007;30(7):866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vgontzas AN, et al. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32(11):1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spiegel K, et al. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. [DOI] [PubMed] [Google Scholar]
- 9. Lee SWH, et al. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91–101. [DOI] [PubMed] [Google Scholar]
- 10. Viner R, et al. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet. 2017;389(10085):2252–2260. [DOI] [PubMed] [Google Scholar]
- 11. Sen CK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Latham R, et al. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22(10):607–612. [DOI] [PubMed] [Google Scholar]
- 13. Imeri L, et al. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10(3):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armstrong DG, et al. Classifying diabetic foot surgery: toward a rational definition. Diabet Med. 2003;20(4):329–331. [DOI] [PubMed] [Google Scholar]
- 15. Berlanga-Acosta J. Diabetic lower extremity wounds: the rationale for growth factors-based infiltration treatment. Int Wound J. 2011;8(6):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fife CE, et al. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry. Wounds. 2012;24(1):10–17. [PubMed] [Google Scholar]
- 17. Sullivan KA, et al. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28(3):276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burke SJ, et al. db/db mice exhibit features of human type 2 diabetes that are not present in weight-matched C57BL/6J mice fed a Western diet. J Diabetes Res. 2017;2017:8503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Brien PD, et al. Mouse models of diabetic neuropathy. ILAR J. 2014;54(3):259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hummel KP, et al. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. [DOI] [PubMed] [Google Scholar]
- 21. Banks AS, et al. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275(19):14563–14572. [DOI] [PubMed] [Google Scholar]
- 22. Bjørbaek C, et al. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272(51):32686–32695. [DOI] [PubMed] [Google Scholar]
- 23. Ramesh V, et al. Sleep fragmentation differentially modifies EEG delta power during slow wave sleep in socially isolated and paired mice. Sleep Science. 2009;2:64–75. [Google Scholar]
- 24. Kaushal N, et al. TNF-α and temporal changes in sleep architecture in mice exposed to sleep fragmentation. PLoS One. 2012;7(9):e45610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity (Silver Spring). 2014;22(3):758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang SX, et al. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes (Lond). 2014;38(4):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gozal D, et al. Protein-tyrosine phosphatase-1B mediates sleep fragmentation-induced insulin resistance and visceral adipose tissue inflammation in mice. Sleep. 2017;40:1–10. [DOI] [PubMed] [Google Scholar]
- 28. Nair D, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184(11):1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cronbach LJ, et al. Construct validity in psychological tests. Psychol Bull. 1955;52(4):281–302. [DOI] [PubMed] [Google Scholar]
- 30. Park SA, et al. Full-thickness splinted skin wound healing models in db/db and heterozygous mice: implications for wound healing impairment. Wound Repair Regen. 2014;22(3):368–380. [DOI] [PubMed] [Google Scholar]
- 31. Davidson JM, et al. Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv Wound Care (New Rochelle). 2013;2(4):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schneider CA, et al. NIH image to imagej: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burke SJ, et al. Oral corticosterone administration reduces insulitis but promotes insulin resistance and hyperglycemia in male nonobese diabetic mice. Am J Pathol. 2017;187(3):614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roumana-Souza B, et al. Selective inhibition of COX-2 improves cutaneous wound healing of pressure ulcers in mice through reduction of iNOS expression. Life Sci. 2016;153:82–92. [DOI] [PubMed] [Google Scholar]
- 35. Fox JG, et al. , eds. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models. 2nd ed Burlington, MA: Academic Press; 2007; No. III. [Google Scholar]
- 36. Hakim F, et al. Chronic sleep fragmentation during the sleep period induces hypothalamic endoplasmic reticulum stress and PTP1b-mediated leptin resistance in male mice. Sleep. 2015;38(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takokoro S, et al. Leptin promotes wound healing in the skin. PLoS ONE. 2015;10(3):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKenna JT, et al. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146(4):1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sinton CM, et al. Validation of a novel method to interrupt sleep in the mouse. J Neurosci Methods. 2009;184(1):71–78. [DOI] [PubMed] [Google Scholar]
- 40. Sutton BC, et al. Sleep fragmentation exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior in a mouse model of musculoskeletal sensitization. Sleep. 2014;37(3):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kobayashi K, et al. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000;49(1):22–31. [DOI] [PubMed] [Google Scholar]
- 42. Pierpont YN, et al. Obesity and surgical wound healing: a current review. ISRN Obes. 2014;2014:638936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsuboi R, et al. A wound healing model using healing-impaired diabetic mice. J Dermatol. 1992;19(11):673–675. [DOI] [PubMed] [Google Scholar]
- 44. Landis CA, et al. Effects of 72 hours sleep deprivation on wound healing in the rat. Res Nurs Health. 1997;20(3):259–267. [DOI] [PubMed] [Google Scholar]
- 45. Wright KP Jr, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van Cauter E, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67 (Suppl 1):2–9. [DOI] [PubMed] [Google Scholar]
- 47. Stamatakis KA, et al. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dumaine JE, et al. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am J Physiol Regul Integr Comp Physiol. 2015;308(12):R1062–R1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goren I, et al. Severely impaired insulin signaling in chronic wounds of diabetic ob/ob mice: a potential role of tumor necrosis factor-alpha. Am J Pathol. 2006;168(3):765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao G, et al. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen. 2010;18(5):467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grimaldi D, et al. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care. 2014;37(2):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pamidi S, et al. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010;24(5):703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tellechea A, et al. Inflammatory and angiogenic abnormalities in diabetic wound healing: role of neuropeptides and therapeutic perspectivew. Open Circu Vasc J. 2010;3:43–55. [Google Scholar]
- 54. Zielinski MR, et al. Sleep and innate immunity. Front Biosci (Schol Ed). 2011;3:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Esser N, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150. [DOI] [PubMed] [Google Scholar]
- 56. Sinno H, et al. Complements and the wound healing cascade: an updated review. Plast Surg Int. 2013;2013:146764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ramesh V, et al. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J Neuroinflammation. 2012;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Olmos G, et al. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014;2014:861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barone MT, et al. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91(2):129–137. [DOI] [PubMed] [Google Scholar]
- 60. Brem H, et al. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perlman RL. Mouse models of human disease: an evolutionary perspective. Evol Med Public Health. 2016;2016(1):170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang S, et al. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol. 2007;581(Pt 2):649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Spaeth AM, et al. Objective measurements of energy balance are associated with sleep architecture in healthy adults. Sleep. 2017;40(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ashcroft GS. Sex differences in wound healing. Adv Mol Cell Biol. 2004;34:321–328. [Google Scholar]
- 65. Vgontzas AN, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. [DOI] [PubMed] [Google Scholar]
- 66. Kautzky-Willer A, et al. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gui Y, et al. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes Res. 2004;12(9):1481–1491. [DOI] [PubMed] [Google Scholar]
- 68. Angel C, et al. Buprenorphine depresses respiratory variability in obese mice with altered leptin signaling. Anesthesiology. 2018;128(5):984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Glovak Z, et al. Leptin status alters buprenorphine-induced antinociception in obese mice with dysfunctional leptin receptors. Neurosci Lett. 2017;660:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McLain JM, et al. Sleep fragmentation delays wound healing in a diabetic mouse model. Shock. 2017;47:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kapsimalis F, et al. Cytokines and normal sleep. Curr Opin Pulm Med. 2005;11(6):481–484. [DOI] [PubMed] [Google Scholar]
- 72. Minoguchi K, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126(5):1473–1479. [DOI] [PubMed] [Google Scholar]
- 73. Guo S, et al. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brockmann L, et al. Regulation of TH17 cells and associated cytokines in wound healing, tissue regeneration, and carcinogenesis. Int J Mol Sci. 2017;18:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lecube A, et al. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One. 2011;6(8):e23366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ackermann PW, et al. Influence of comorbidities: neuropathy, vasculopathy, and diabetes on healing response quality. Adv Wound Care (New Rochelle). 2013;2(8):410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]