Abstract
Acute Graft-versus-host disease (GVHD) is a major immunological complication after allogeneic hematopoietic cell transplantation and a better understanding of the molecular regulation of the disease could help to develop novel targeted therapies. Here we found that a G/C polymorphism within the human microRNA-146a (miR-146a) gene of transplant-recipients, which causes reduced miR-146a levels, was strongly associated with the risk of developing severe acute GVHD (n=289). In mice, deficiency of miR-146a in the hematopoietic system or transfer of recipient-type miR 146a-/- dendritic cells (DCs) enhanced GVHD, while miR-146a mimic-transfected-DCs ameliorated disease. Mechanistically, lack of miR-146a enhanced JAK2 STAT1-pathway activity, which led to higher expression of class II-transactivator (CIITA) and consecutively increased MHCII-levels on DCs. Inhibition of JAK1/2 or CIITA knockdown in DCs prevented miR-146a-/- DC-induced GVHD exacerbation. Consistent with our findings in mice, patients with the miR-146a polymorphism rs2910164 in hematopoietic cells displayed higher MHCII levels on monocytes, which could be targeted by JAK1/2-inhibition.
Our findings indicate that the miR-146a polymorphism rs2910164 identifies patients at high risk for GVHD before allo HCT. Functionally we show that miR-146a acts as a central regulator of recipient-type DC activation during GVHD by dampening the pro-inflammatory JAK-STAT/CIITA/MHCII axis, which provides a scientific rationale for early JAK1/2-inhibition in selected patients.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) represents the only curative therapy option for many hematological malignancies. However, the chance of cure by allo-HCT is limited by acute Graft-versus-host disease (GVHD), an immunological complication caused by allo-reactive donor T cells that leads to a mortality rate of 70 to 90 percent in patients suffering from severe GVHD grade III-IV.
MicroRNAs (miRNAs) are small, double-stranded, non-coding RNA molecules that regulate gene expression at the post-transcriptional level by inducing either mRNA degradation or translational arrest. One miRNA can regulate the expression of multiple target mRNAs 1, allowing them to control the differentiation and function of immune cells at different levels, which has been increasingly recognized in the last decade. Thus, miRNAs are potentially attractive therapeutic targets for the modulation of allogeneic immune responses, since a single miRNA could regulated multiple gene products.
Recently, we have shown in the mouse model and in patient samples that microRNA-146a (miR 146a) is an important negative regulator of donor T cells during acute GVHD, by targeting TRAF6 leading to decreased TNF production 2. In addition to its involvement in adaptive immunity, miR 146a has a central role in regulating innate immune responses 3–5, however its role in JAK-STAT pathway activation and MHCII expression were unclear. The G/C polymorphism in pre-miR-146a (rs2910164) reduces miR-146a expression 6 and has been demonstrated to be associated with Crohn's Disease and autoimmunity 7, 8. We show here that human allo-HCT recipients carrying the rs2910164 CC genotype have a significantly increased risk of developing severe acute GVHD. In a mouse model we found that lack of miR-146a in the host dendritic cells (DCs) exacerbated acute GVHD, via enhanced activity of the JAK-STAT/ class II transactivator (CIITA)/ MHCII axis. Recently we could show the efficacy of JAK1/2 inhibition for patients with acute GVHD refractory to multiple treatments 9. It was so far unclear which patients benefit most of JAK1/2 inhibition. Therefore, these studies were designed to understand if there was a scientific rationale for a novel diagnostic procedure, which is miR-146 rs2910164 genotyping to determine the genetic risk for GVHD which may be used for clinical testing of pre-emptive JAK1/2 inhibition in patients at risk for GVHD.
Materials and Methods
Single Nucleotide Polymorphism (SNP) Analysis - Study Population
For the case-control study, we genotyped 289 allo-HCT recipients for the SNP rs2910164 within the pre-miR-146a sequence. All patients had undergone allo-HCT after myeloablative conditioning at the University Medical Center Freiburg between 2002 and 2014. GVHD grading was performed on the basis of clinical signs, laboratory tests (bilirubin) and when available histopathology for human GVHD. Written informed consent for this study was received from each patient, and the study was approved by the Ethic Committee of the Albert Ludwigs University Freiburg, Germany (Protocol number: 394/13).
All other methods are provided in the Suppl. Methods section.
Results
Association of miR-146a single nucleotide polymorphism (SNP) rs2910164 with GVHD severity
To evaluate the potential role of miR-146a in human GVHD, we determined the miR-146a rs2910164 genotypes of 289 patients that had undergone allo-HCT. The baseline characteristics and GVHD risk factors, including age, CMV reactivation, HLA match status, conditioning regimen and GVHD prophylaxis, were comparably distributed between CC genotype patients and non-CC genotype patients (Suppl. Table 1). The allelic frequencies were comparable to those reported in a large European multicenter study 10 and the genotype frequencies for patients with GVHD grade 0-II and patients with GVHD grade III-IV were in accordance with the Hardy–Weinberg equilibrium (χ2=0.476 and 2.675; p=0.490 and 0.102, respectively).
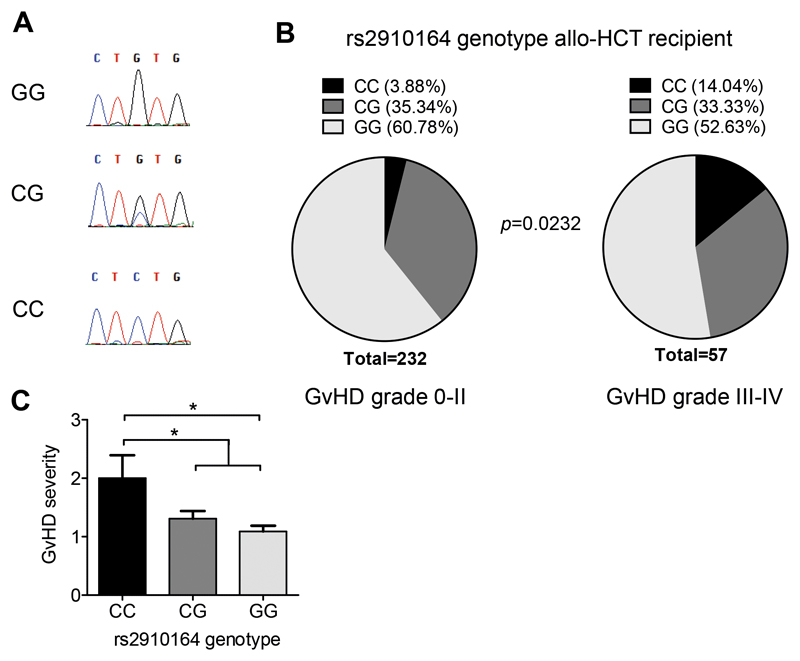
We found that the miR-146a rs2910164 polymorphism was significantly associated with the risk of developing severe acute GVHD grade III-IV (Figure 1A,B; Suppl. Table 2). Individuals carrying the miR 146a rs2910164 CC genotype had a higher risk of severe GVHD compared to those carrying GG (p=0.0083, OR=4.178; 95% CI=1.49-11.71). When comparing patients with the CC genotype to all other patients (CG or GG; "non-CC"), the increased risk for severe GVHD was even more accentuated (p=0.008, OR = 4.045; 95 % CI 1.486-11.01). Moreover, the C allele was identified as a risk factor for the development of severe GVHD (p=0.0476, OR=1.613; 95% CI=1.023-2.543). Similarly, the overall GVHD severity was significantly higher in patients carrying the CC genotype, further confirming the association of rs2910164 with the susceptibility to severe acute GVHD (Figure 1C). These results were confirmed in a second independent validation cohort (n=114), showing that individuals carrying the miR-146a rs2910164 CC genotype had a higher risk of severe GVHD compared to those carrying GG (p=0.0391, OR: 5.889, CI: 1.162-29.840), (Suppl. Figure 1A, B, Suppl. Table 4 and 5). While in miR-146a-/- mice, the lack of miR-146a is complete, in patients with the miR-146a SNP the miR is only reduced compared to the normal population (Suppl. Figure 1C, D). Multivariate analysis showed that the CC genotype was a risk factor for GVHD (p=0.009) independent of T cell depletion (Suppl. Figure 1E).
Figure 1. Host miR-146a SNP rs2910164 is associated with increased risk for severe acute GVHD.
(A) Representative Sanger sequencing chromatograms from individual rs2910164 genotypes that were used to establish the qPCR genotype analysis are shown.
(B) 289 allo-HCT recipients were analyzed for their rs2910164 SNP genotype using Taqman realtime PCR assays. Genotype frequencies for the group of recipients that developed no/mild GVHD (grade 0-II) or severe GVHD (grade III-IV) are depicted. The risk to develop severe GVHD was significantly increased in patients carrying the CC genotype. Fisher's exact test was used to analyze the contingency table.
(C) Mean GVHD severity in the group of patients with CC genotype, CG genotype and GG genotype, respectively, was determined. Patients with a CC genotype developed more severe GVHD. Kruskal-Wallis test followed by Dunn's post hoc test was employed, * p<0.05.
MiR-146a deficiency of the allo-HCT recipient exacerbates murine acute GVHD
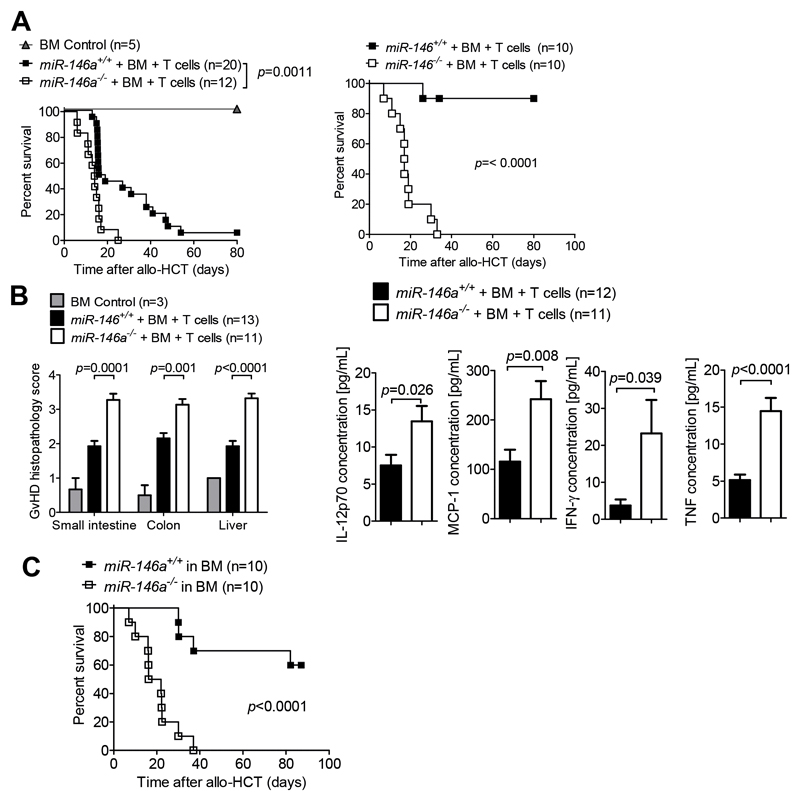
The results from the clinical study strongly implicated an association between the rs2910164 C allele and the CC genotype, which cause reduced mature miR-146a expression10, with severe GVHD. We therefore hypothesized that miR-146a might play a role in regulating recipient cells during GVHD and aimed to investigate this in functional studies using gene-targeted mice deficient for miR 146. T cells and BM isolated from WT BALB/c mice (H-2Kd) were transplanted into lethally irradiated miR-146a+/+ (WT) or miR-146a-/- mice (H-2Kb). We observed a more aggressive course of GVHD with accelerated mortality in miR-146a deficient recipients compared to WT recipients in two different GVHD models (Figure 2A). Additionally, histopathological GVHD scores and the serum levels of IL-12p70, MCP-1, IFN-γ and TNF-α were higher in miR 146a deficient recipients compared to WT mice on d7 after transplantation (Figure 2B). To more precisely determine which recipient tissues require miR 146a for the regulation of GVHD, we next generated BM chimeras with a specific deficiency of miR-146a in either hematopoietic or non-hematopoietic cell types. We observed a marked aggravation of GVHD severity in chimeras lacking miR 146a in the hematopoietic system when compared to mice with a WT hematopoietic system (Figure 2C). In contrast, restriction of miR-146a deficiency to non-hematopoietic recipient cells did not affect the course of GVHD when compared to WT recipients (Suppl. Figure 2A). These observations strongly support the concept that miR 146a plays an important role in regulating recipient cells of hematopoietic origin during acute GVHD.
Figure 2. MiR-146a deficiency of the host exacerbates GVHD.
(A) miR-146a+/+ (WT) or miR-146a-/- mice (both C57BL/6 background) were lethally irradiated followed by allo-HCT with BM only (BM Control) or BM + BALB/c T cells.
Left panel: BALB/c into C57BL/6 model, right panel FVB/NRj into C57BL/6 model
(B) On d7 after allo HCT, the serum and the small intestine, colon and liver of miR-146a+/+ and miR-146a-/-recipient mice were isolated. Left panel: Tissues were scored for GVHD severity. Right panel: Inflammatory cytokines were measured in the serum. Data are pooled from 3 independent experiments.
(C) Lethally irradiated WT C57BL/6 mice were transplanted with BM cells from a syngeneic miR 146a+/+ or miR-146a-/- C57BL/6 donor. After >30 days, the chimera were irradiated with 2x2.75 Gy and injected with 5x106 BM cells and 5x105 T cells from an allogeneic BALB/c donor, followed by a survival study. Data are pooled from 3 independent experiments.
MiR 146a functions as a negative regulator of recipient-type DCs during acute GVHD
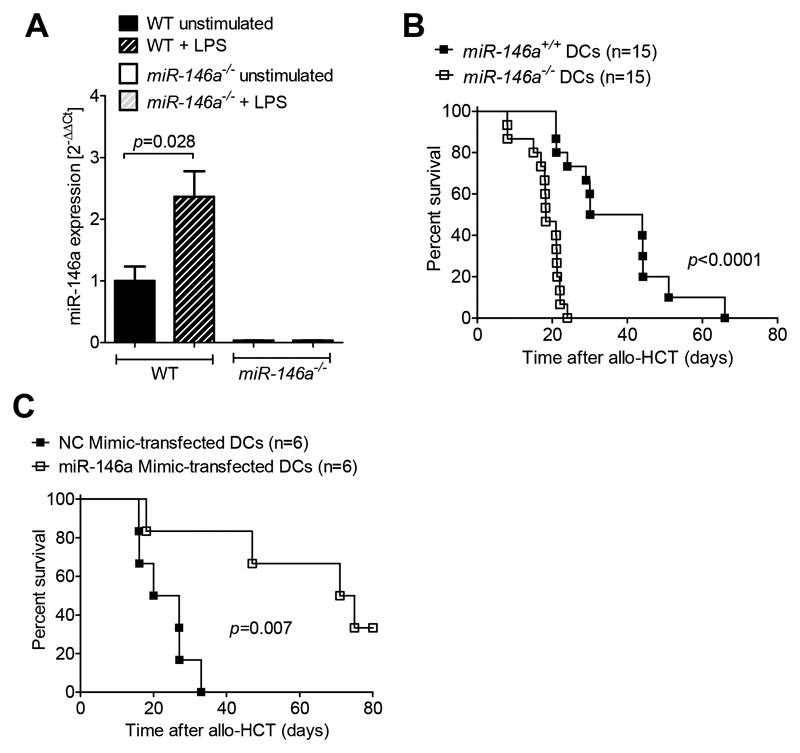
Besides non-hematopoietic antigen presenting cells, recipient-derived DCs that survive the conditioning regimen after allo-HCT11, 12 contribute to the induction of GVHD 13, 14. Therefore, we studied the expression and function of miR-146a in recipient-type DCs. Since lipopolysaccharide (LPS) leakage through the damaged skin or intestinal mucosa has been shown to promote GVHD15 we first examined the impact of LPS stimulation on miR 146 expression. We found that WT DCs upregulated miR-146a expression in response to LPS stimulation (Figure 3A). MiR-146a-/- DCs served as a negative control and showed no detectable miR-146a expression.
Figure 3. LPS stimulation induces miR-146a expression and miR-146a-/- DCs exacerbate GVHD.
(A) RNA was isolated from WT or miR-146a-/- BMDCs that were unstimulated or stimulated with 1 µg/mL LPS for 24h as indicated. MiR-146a expression was analyzed by qRT PCR. Data were pooled from 4 independent experiments.
(B) Allo-HCT was performed as described for the BALB/c into C57BL/6 combination. Groups additionally received 2x106 miR-146a+/+ or miR-146a-/- C57BL/6 BMDCs on the day of transplantation. Survival was monitored for 80 days. Data were pooled from 2 independent experiments.
(C) Allo-HCT was performed as described for the BALB/c into C57BL/6 combination. Additionally, 2x106 WT C57BL/6 BMDCs transfected with a miR-146a mimic or a Negative Control (NC) mimic were i.v. injected on d0.
In order to identify the functional role of miR-146a in recipient-derived DCs, we irradiated WT C57BL/6 mice followed by an allo HCT using donor BALB/c BM cells and BALB/c T cells together with recipient-type (C57BL/6) WT or miR 146a-/- DCs in the donor graft. We observed a more aggressive course of GVHD when miR-146a-/- DCs were transferred compared to WT DCs (Figure 3B). To complement the loss-of-function approach using a gain-of-function method, we overexpressed miR-146a using a specific miRNA mimic before transferring the DCs. Using qRT-PCR we confirmed that transfection with the miR-146a mimic indeed increased miR-146a expression levels by 20-fold in WT C57BL/6 DCs compared to DCs transfected with a negative control (NC) mimic (Suppl. Figure 2B). Co-transplantation of miR-146a mimic-transfected DCs significantly prolonged survival of recipient mice compared to NC mimic-transfected DCs (Figure 3C). To analyze the competitive capacities of miR-146a-/- DCs compared to WT DCs we transferred either miR-146a-/- DCs alone or miR-146a-/- DCs together with WT DCs in mice that had undergone allo-HCT. Mice receiving both WT and miR-146a-/- DCs survived significantly longer than mice receiving miR-146a-/- DCs alone (Suppl. Figure 2C), indicating that WT DCs can partly antagonize the severe GVHD phenotype caused by miR-146a deficient DCs. These findings provide evidence that miR-146a plays a central role for the regulation of recipient-type DCs during allo-responses.
Global gene expression analysis reveals upregulation of the JAK-STAT signaling pathway in miR 146a-/- DCs
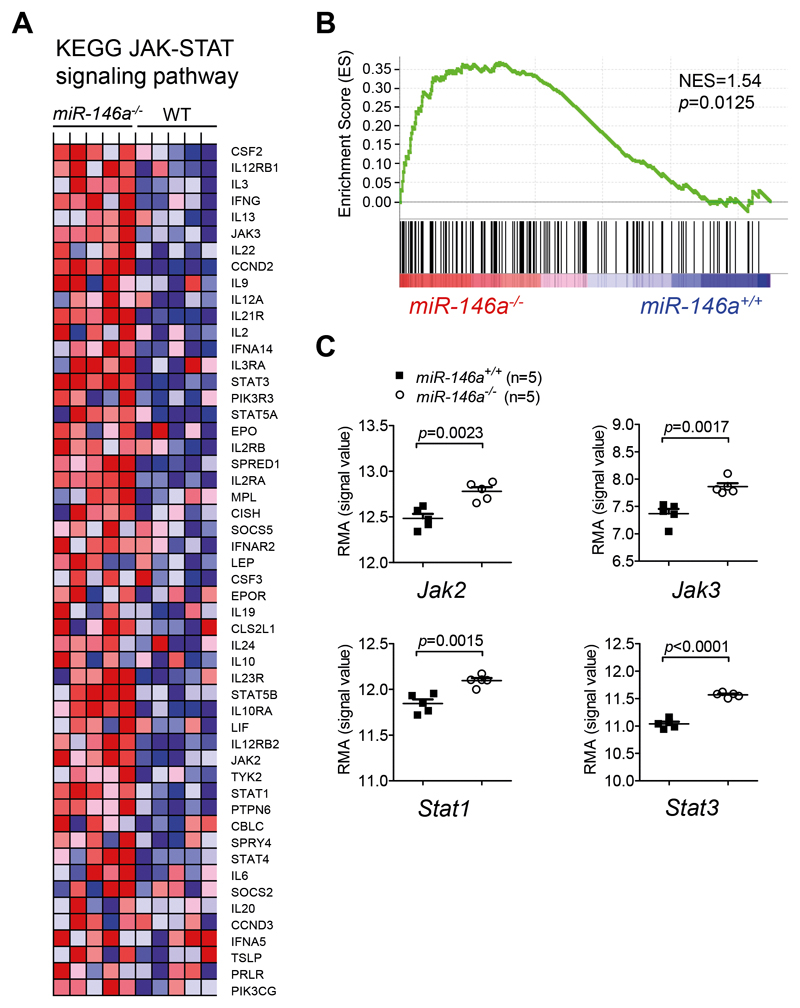
To understand how miR-146a regulates DC activation, we used an unbiased microarray approach to compare gene expression patterns of LPS-stimulated BMDCs derived from miR 146a-/- mice to those from WT mice. To identify groups of genes that share a common biological function and were differentially regulated between miR-146a-/- and WT DCs, we subsequently performed gene set enrichment analysis (GSEA) 16. We found 21 gene sets that were significantly enriched in miR 146a-/- compared to WT DCs (Suppl. Table 3). These included different pro-inflammatory signaling pathways, in particular growth factor and cytokine receptor pathways that signal via the JAK-STAT axis. Compatible with this, the GSEA revealed marked enrichment of the KEGG JAK-STAT signaling pathway (NES=1.54, FDR q=0.025, p=0.0125) in miR 146-/- DCs (Figure 4A-B), including significant upregulation of Jak2, Jak3, Stat1 and Stat3 (Figure 4C).
Figure 4. Microarray and GSEA reveal increased expression of the JAK-STAT signaling pathway in miR-146a-/- DCs.
(A-C) RNA was isolated from miR-146a+/+ or miR-146a-/- BMDCs stimulated with 100 ng/mL LPS for 24h on d8 of culture, and analyzed using GeneChip Mouse Gene 2.0 ST Arrays (Affymetrix). GSEA was used to identify gene sets that exhibited significant overlap with gene expression differences between miR-146a+/+ or miR-146a-/- BMDCs.
(A) Heat map representation of microarray data showing the expression levels of the 52 core enrichment genes for the KEGG JAK-STAT signaling pathway (upregulated in miR-146a-/- DCs as compared to miR-146a+/+ DCs). Rows represent individual genes and columns represent samples. Range of colors (red to blue) shows the range of expression values (high to low).
(B) Enrichment plot, showing enrichment of genes of the KEGG JAK-STAT signaling pathway in miR 146a-/- BMDCs as compared to miR 146a+/+ BMDCs. The enrichment profile is displayed as a green line. The score at the peak of the plot is the enrichment score (ES) for the JAK-STAT signaling gene set. The vertical black lines below the enrichment plot depict individual genes of the gene set. The genes that appear before or at the peak are defined as the core enrichment genes for this gene set. NES=normalized enrichment score.
(C) Expression of key molecules of JAK-STAT signaling, including Jak2, Jak3, Stat1 and Stat3 is significantly higher in miR-146a-/- DCs, as analyzed by microarray.
Enhanced JAK-STAT signaling is responsible for the severe GVHD phenotype induced by miR 146a deficient DCs
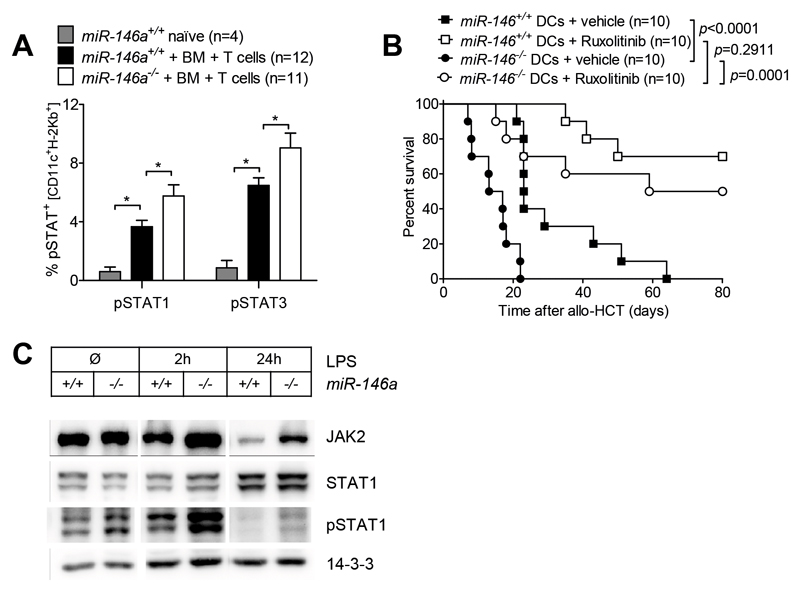
In order to confirm increased JAK-STAT signaling in miR-146a deficient cells in vivo, we performed phospho-flow analysis on the spleens of miR-146a+/+ versus miR-146a-/- allo HCT recipients. Phosphorylation of STAT1 and STAT3 proteins in DCs was enhanced in both WT and miR 146a deficient allo-HCT recipient mice when compared to naïve untreated controls (Figure 5A). We observed that the JAK-STAT pathway was more activated in the WT DC isolated from mice during GVHD compared to naive mice (Figure 5A) which was likely due to inflammatory cytokines released after allo-HCT. Recipient-derived miR-146a-/- DCs displayed significantly increased pSTAT1 and pSTAT3 levels compared to miR-146a+/+ DCs (Figure 5A). To clarify, whether augmented JAK-STAT signaling is responsible for the GVHD exacerbation induced by miRNA-146a-/- DCs after allo-HCT in vivo, we employed the JAK1/2 inhibitor ruxolitinib. Recipient-derived miR-146a-/- or miR-146a+/+ DCs were pre-treated with vehicle (DMSO) or 0.3 µM ruxolitinib for 4h in vitro, before they were transferred into irradiated hosts together with allogeneic BM and T cells. Co-transplantation of DMSO-treated miR-146a-/- DCs induced enhanced mortality compared to co-transplantation of DMSO-treated miR-146a+/+ DCs (Figure 5B). Conversely, the difference between miR-146a-/- and miR-146a+/+ DCs could be completely abrogated by specific JAK1/2 blockade in the DCs before co-transplantation (Figure 5B).
Figure 5. MiR-146a regulates host DCs during GVHD by dampening JAK-STAT signaling.
(A) Allo-HCT was performed as described for the BALB/c into C57BL/6 combination and recipient mice (miR-146a+/+ or miR-146a-/-) were sacrificed on d7. Untreated WT C57BL/6 mice served as naϊve control group. Splenocytes were isolated, stained for CD11c, H-2Kb, pSTAT1 and pSTAT3 and subjected to phospho-flow cytometry. Data were pooled from 2 independent experiments.
(B) BMDCs (miR 146a-/- or miR 146a+/+) were pre-treated with 0.3 µM of JAK1/2 inhibitor (ruxolitinib) or vehicle control (DMSO) for 4h on d7 of culture. After extensive washing, 2x106 pre-treated BMDCs were injected into lethally irradiated recipients (11 Gy) in combination with 5x106 BALB/c BM cells and 5x105 BALB/c T cells. Survival of recipient mice was monitored for 80 days. Data are pooled from 2 independent experiments.
(C) BM derived DCs (miR 146a-/- or miR 146a+/+) were stimulated with 100 ng/mL LPS for different time periods as indicated. After cell lysis, total and phospho-protein levels of JAK2, STAT1 and pSTAT1 were determined by Western Blot.
To validate these findings in an in vitro system we performed Western Blot analysis of miR 146a-/- or miR 146a+/+ DCs stimulated with LPS. We found increased levels of total JAK2 protein in miR-146a-/- compared to miR-146a+/+ DCs 2h and 24h after LPS stimulation (Figure 5C). Importantly, increased JAK2 levels translated into augmented STAT1 phosphorylation (Figure 5C), further confirming de-repressed JAK STAT signaling in miR-146a deficient DCs. Ruxolitinib treatment effectively prevented phosphorylation of STAT1 and STAT3 proteins after LPS stimulation (Suppl. Figure 3A), while it did not affect DC viability (Suppl. Figure 3B). Hence our data indicate that miR-146a negatively regulates JAK-STAT signaling in recipient-type DCs, thereby counteracting GVHD-related inflammation.
MiR-146a deficient DCs have higher levels of the transcription factor CIITA and MHCII
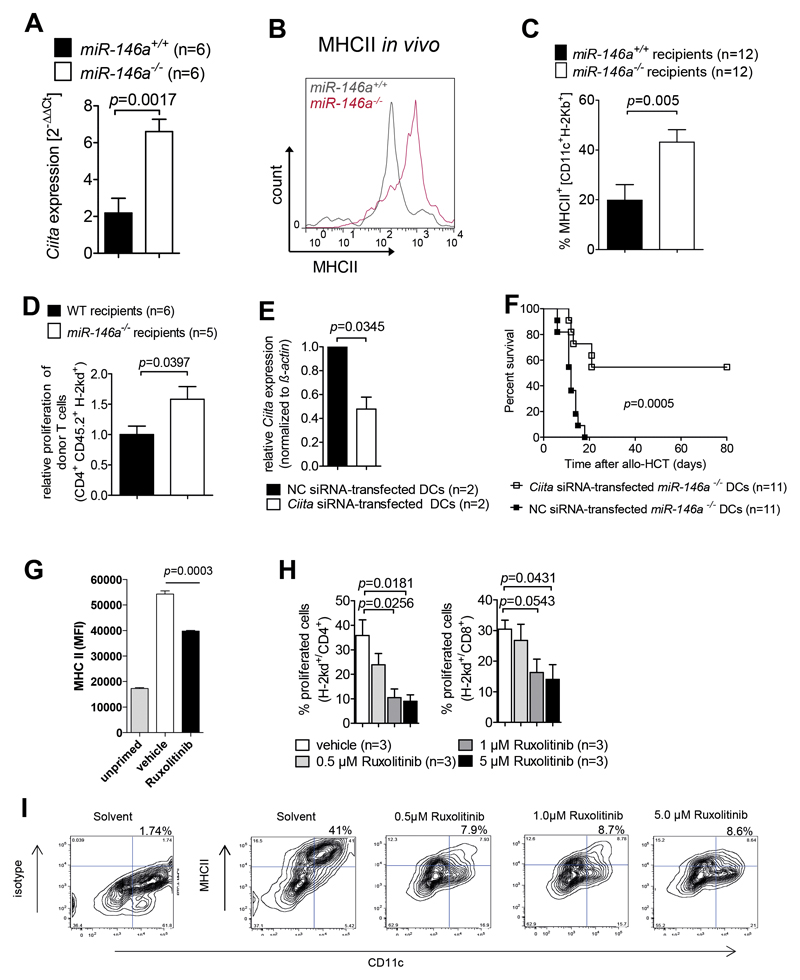
To better define the mechanism how increased JAK-STAT activity translated into a more inflammatory phenotype of miR146a-/- DCs, we searched for transcription factors that are known to be induced by increased JAK/STAT pathway activity. We found that class II transactivator (CIITA), which was shown to positively regulate MHCII expression 17 was significantly increased in miR-146a-/- DCs compared to WT DCs (Figure 6A).
Figure 6. MiR-146a deficient DCs display increased expression of CIITA which leads to enhanced MHCII expression on the cell surface.
(A) RNA was isolated from miR-146a+/+ or miR-146a-/- BMDCs stimulated with 100 ng/mL LPS for 24h on d8 of culture. Ciita expression levels in RNA were analyzed by qRT-PCR. mRNA for CIITA was increased in miR-146a-/- BMDCs compared to miR-146a+/+ DCs. Data are pooled from 2 independent experiments.
(B, C) Allo-HCT was performed as described for the BALB/c into C57BL/6 combination and recipient mice (miR-146a+/+ or miR-146a-/-) were sacrificed on d7. The amount of surface MHCII expression on miR-146a-/- or miR-146a+/+ CD11c+ DCs isolated from the spleens of allo-HCT recipients on d7 is shown. (B) Representative flow cytometry plot gated on CD11c+H 2Kb+ DCs. (C) The bar diagram shows data pooled from 2 independent experiments.
(D) miR-146a+/+ (WT) or miR-146a-/- mice (both C57BL/6 background) were lethally irradiated followed by allo-HCT with BALB/c BM + BALB/c T cells. Donor T cell proliferation was determined by the extent of CellTrace™ Violet dye dilution.
(E) Ciita levels were determined by qRT-PCR in miR-146a-/- C57BL/6 BMDCs transfected with Ciita siRNA or a negative control (NC) siRNA. Data are pooled from 2 independent experiments.
(F) Allo-HCT was performed as described for the BALB/c into C57BL/6 combination. Groups additionally received either 2x106 miR-146a-/- C57BL/6 BMDCs transfected with Ciita siRNA or 2x106 miR-146a-/- C57BL/6 BMDCs transfected with a negative control (NC) siRNA on the day of transplantation. Survival was monitored for 80 days. Data were pooled from 2 independent experiments.
(G) MHCII levels are shown on splenic DC isolated from mice that were fed twice via oral gavage either ruxolitinib or vehicle prior to (white and black column) or w/o (grey column) OVA/CpG priming for 20 hours.
(H) The bar diagram shows the percentage of proliferated CD4 or CD8 T cells cocultured for 72h with BMDCs that were treated with ruxolitinib at the indicated concentrations for 24h, then washed and activated with LPS (during the activation phase no ruxolitinib was present). Data are pooled from 3 independent experiments.
(I) The flow cytometry plots show the expression of MHCII on BMDCs that were treated 24h with ruxolitinib, then washed and activated with OVA+LPS (during the activation phase no ruxolitinib was present). The percentages of cells in the right upper quadrant are indicated. A representative staining of 3 independent experiments for each concentration of ruxolitinib is shown.
Consistent with our findings, a functional connection between the JAK/STAT pathway and CIITA expression has been previously reported in the context of cytomegalovirus mediated immune escape 18. In line with the enhanced transcription of CIITA we found that miR-146a-/- DCs showed higher surface expression levels of MHCII compared to miR 146a+/+ DCs both after in vitro stimulation with LPS (Suppl. Figure 4A) and after allo-HCT in vivo (Figure 6B, C).
To investigate whether the higher level of MHCII resulted in an increased ability to stimulate allogeneic T cell responses, we analyzed in vivo donor T cell proliferation and found a stronger T cell expansion in miR-146a-/- recipients compared to WT recipients (Figure 6D). To determine whether higher CIITA levels and subsequent MHCII expression contributed to the more severe GVHD phenotype caused by miR-146a-/- DCs we performed a knock-down of Ciita in miR-146a-/- DCs using siRNA. Ciita expression was significantly decreased by the knockdown (Figure 6E). Transfer of miR-146a-/- DCs transfected with Ciita siRNA along with the allogeneic graft led to prolonged survival of mice compared to mice receiving miR-146a-/- DCs transfected with a negative control siRNA (Figure 6F). JAK1/2 inhibition reduced MHCII levels on DCs in vivo (Figure 6G) indicating that JAK1/2 activity was critical for MHCII upregulation in vivo. In vitro treatment of DCs with ruxolitinib led to a decreased MHCII expression and reduced their ability to stimulate CD4 and CD8 T cells (Figure 6H, I).
In addition to higher expression levels of MHC class II, expression of CD80 and CD86 was increased in LPS-stimulated miR-146a-/- DCs compared to WT DCs (Suppl. Figure 4B,C), while miR-146a-/- DCs expressed reduced levels of the co-inhibitory molecule PD L1 (Suppl. Figure 4D). Moreover, LPS-stimulated miR-146a-/- DCs produced increased amounts of the pro-inflammatory cytokines IL-12p70 and MCP-1 compared to WT DCs (Suppl. Figure 5A,B) and allogeneic T cells stimulated with miR-146a-/- DCs produced greater amounts of the pro-inflammatory cytokines IFN-γ and IL-17A (Suppl. Figure 5C,D). Taken together, miR-146a-/- DCs show a more activated phenotype, increased allo-stimulatory capacity and enhanced pro-inflammatory cytokine secretion compared to WT DCs after LPS stimulation in vitro or during allo-responses in vivo.
The miR-146a SNP rs2910164 causing reduced activity of the miR in hematopoietic cells is associated with higher MHCII expression in humans
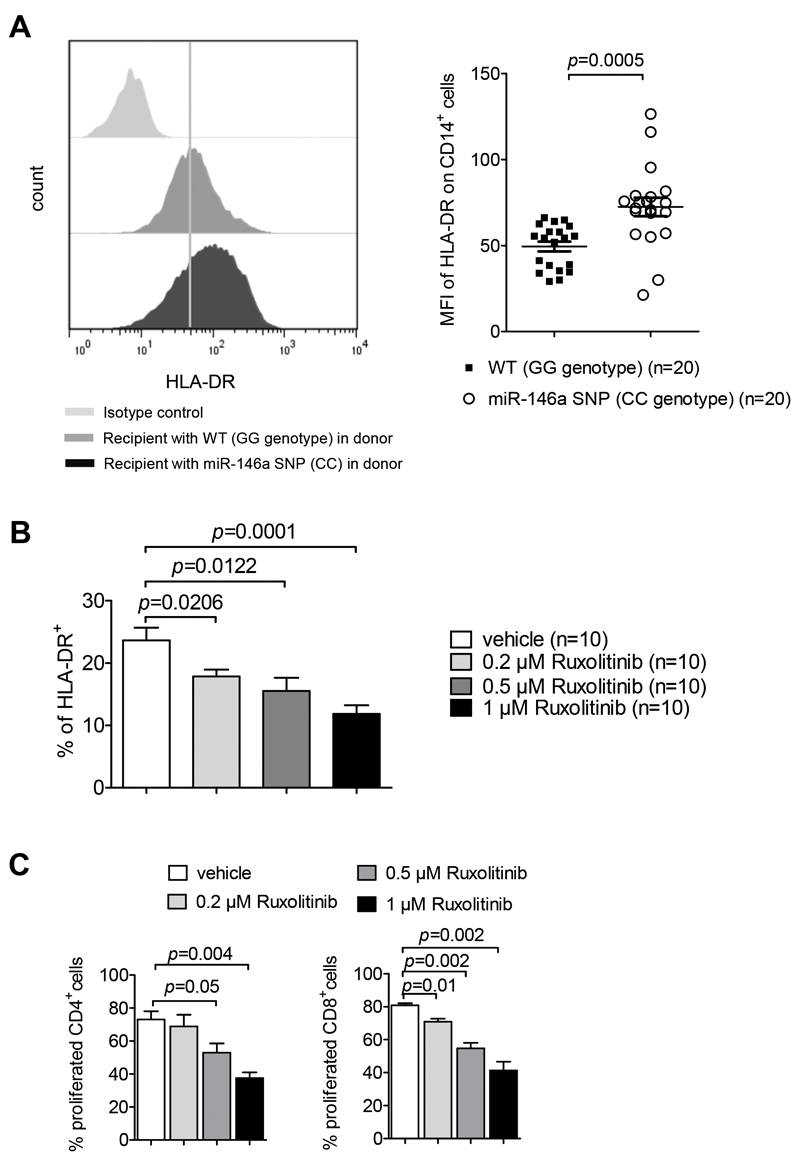
To clarify whether the findings we had made in mice on the functional connection between miR146a deficiency and higher MHCII expression were relevant for patients, we analyzed MHCII levels on monocytes of allo-HCT patients that had a defect in hematopoietic cells (donors carrying the SNP rs2910164 (CC genotype) and proven 100% donor cell engraftment). We observed that CD14+ monocytes in the peripheral blood of patients with the SNP rs2910164 had higher surface expression levels of MHCII (Figure 7A), supporting our hypothesis that miR-146a regulates MHCII expression in patients. Chimerism analysis of enriched monocytes (>90% purity) in five patients with CC genotype and five patients with a GG genotype showed that in patients the donor chimerism in the CD14+ monocyte compartment was 100%. The patients received no immunosuppressive therapy at the time of sample collection. Consistent with a role of the JAK/STAT pathway in MHCII regulation, pharmacological JAK1/2 inhibition also reduced MHCII expression in human monocyte-derived DCs (moDCs) and moDC-dependent T cell proliferation (Figure 7B, C). The same reduction of MHCII expression by ruxolitinib was seen when monocytes instead of GM-CSF stimulated DCs were studied (Suppl. Figure 6A).
Figure 7. SNP rs2910164 leads to increased MHCII expression on CD14+ cells.
(A) PBMCs of recipients transplanted with PBSC from donors carrying the CC genotype of SNP rs2910164 or the wildtype (GG) of rs2910164 were isolated by a Ficoll gradient and the amount of surface expression of HLA-DR on CD14+ cells was analyzed by flow cytometry. Representative histograms of HLA-DR expression and the median fluorescence intensity (MFI) of the HLA-DR expression on CD14+ cells is shown, data were pooled from 5 independent experiments.
(B) Human moDCs were exposed to increasing concentrations of ruxolitinib and then activated with LPS. MHCII levels were determined by flow-cytometry, data were pooled from 3 independent experiments.
(C) The bar diagram shows the percentage of proliferated CD4 or CD8 human T cells that were cocultured for 72h with moDCs that were treated with ruxolitinib at the indicated concentrations, then washed and activated with LPS. Data are pooled from 3 independent experiments, the same moDC-T cell donor combination was used in all experiments.
Discussion
Patients with acute steroid-refractory GVHD have a dismal prognosis19. Recently we reported the efficacy of JAK1/2 inhibition for patients with acute GVHD that were refractory to multiple previous therapies9. We observed complete responses in 46.3% of the patients9 but it was so far unclear which patients benefit most of JAK1/2 inhibition. In this report we provide the first experimental evidence that the miR-146a variant rs2910164 in human allo-HCT recipients significantly increases the risk for acute severe GVHD. In addition to this clinical analysis, by using gain- and loss-of-function approaches we show that miR-146a regulates the activation status of recipient-type DCs and thereby controls the severity of acute GVHD after allo-HCT. Mechanistically, we demonstrate that miR 146a acts as a negative regulator of JAK-STAT signaling in recipient-type DCs during allogeneic responses in mice. To date, there have only been few studies investigating the role of miR 146a in DCs and none of them had deciphered a functional link to JAK-STAT signaling. Consistent with our findings, it was shown that miR-146a regulates human DC activation by interfering with Toll-like receptor 2 (TLR2) downstream signaling events 20 and consecutively limits pro-inflammatory cytokine production21. In addition, STAT1, which is critically involved in DC maturation,22 has been identified as a direct target of miR-146a 23, 24. In agreement with these data, our findings support the concept that miR 146a acts as a negative feedback regulator of the JAK-STAT signaling pathway in DCs. Increased JAK-STAT signaling was connected to upregulation of MHCII on miR-146a-/- DCs, increasing their potential to activate (allogeneic) T cells. JAK-STAT signaling downstream of the IFN-γ receptor induces the transcription factor CIITA, which drives MHCII expression25. These data are compatible with our finding that CIITA is upregulated in miR-146a-/- DCs which is connected to higher MHCII expression levels. Finally, our previous reports on the efficacy JAK1/2 signaling in murine26 and human GVHD9 also support this concept. While our data suggest that patients carrying the CC genotype of miR-146a may benefit particularly of JAK2 inhibition, it is likely that not only those patients will benefit from JAK inhibition but also others.
Collectively, our clinical and pre-clinical observations emphasize a role for miR-146a as key negative regulator of JAK-STAT signaling in DCs, thereby modulating acute GVHD. Our study sets the stage for future designing of rational GVHD prevention/therapy concepts implementing the analysis of the GVHD susceptibility genotype miR-146a rs2910164 before transplantation in order to identify high-risk patients, who may particularly benefit from JAK1/2 inhibitors in a pre-emptive or a therapeutic setting upon GVHD development.
Supplementary Material
Acknowledgments
This study was supported by the ERC Consolidator grant (681012 GVHDCure to RZ), DFG (SFB1160 P14 to RZ), the German Cancer Consortium (DKTK Program Molecular Targeted Therapy) to RZ and NvB and the Excellence Initiative of the German Research Foundation (GSC-4, BIOSS) II, projects B4 and B13 to TB and RZ, respectively).
Footnotes
Author contributions
Contribution: N.S. designed the experiments, performed the experiments, evaluated the data and wrote the manuscript; K.H designed and performed the experiments and evaluated the data. D.M. contributed to experimental design, performed the experiments and analyzed the data. G.P., W.M., A.H., D.W., P.B. and M.K. helped with experiments and data analysis; D.P. performed microarray analysis; A.S.-G. performed histopathological scoring; A.H., P.B., D.W. performed experiments T.B., N.v.B. and J.D. contributed to experimental design and data interpretation. J. Finke helped to analyze the patient samples; J. Ferrara helped to design experiments and to analyze data. U.S. helped to perform the SNP genotype analysis; and R.Z. developed the overall concept, analyzed the data, and helped to write the manuscript.
Conflict of interest statement: The authors have declared that no conflict of interest exists.
References
- 1.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005 Feb 17;433(7027):769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 2.Stickel N, Prinz G, Pfeifer D, Hasselblatt P, Schmitt-Graeff A, Follo M, et al. MiR-146a regulates the TRAF6/TNF-axis in donor T cells during GVHD. Blood. 2014 Oct 16;124(16):2586–2595. doi: 10.1182/blood-2014-04-569046. [DOI] [PubMed] [Google Scholar]
- 3.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006 Aug 15;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahid MA, Pauley KM, Satoh M, Chan EK. miR-146a is critical for endotoxin-induced tolerance: Implication in innate immunity. The Journal of biological chemistry. 2009 Dec 11;284(50):34590–34599. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labbaye C, Testa U. The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. Journal of hematology & oncology. 2012;5:13. doi: 10.1186/1756-8722-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jazdzewski K, Murray EL, Franssila K, Jarzab B, Schoenberg DR, de la Chapelle A. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2008 May 20;105(20):7269–7274. doi: 10.1073/pnas.0802682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazouli M, Papaconstantinou I, Stamatis K, Vaiopoulou A, Zeglinas C, Vassiliou I, et al. Association study of genetic variants in miRNAs in patients with inflammatory bowel disease: preliminary results. Digestive diseases and sciences. 2013 Aug;58(8):2324–2328. doi: 10.1007/s10620-013-2640-y. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Zhang K, Zhou R. Meta-analysis of pre-miRNA polymorphisms association with susceptibility to autoimmune diseases. Immunological investigations. 2014;43(1):13–27. doi: 10.3109/08820139.2013.822389. [DOI] [PubMed] [Google Scholar]
- 9.Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multi-center survey. Leukemia. 2015;29:2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lofgren SE, Frostegard J, Truedsson L, Pons-Estel BA, D'Alfonso S, Witte T, et al. Genetic association of miRNA-146a with systemic lupus erythematosus in Europeans through decreased expression of the gene. Genes and immunity. 2012 Apr;13(3):268–274. doi: 10.1038/gene.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. The Journal of experimental medicine. 2006 Nov 27;203(12):2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young JW, Merad M, Hart DN. Dendritic cells in transplantation and immune-based therapies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007 Jan;13(1 Suppl 1):23–32. doi: 10.1016/j.bbmt.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999 Jul 16;285(5426):412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 14.Koyama M, Kuns RD, Olver SD, Raffelt NC, Wilson YA, Don AL, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nature medicine. 2012;18:135–142. doi: 10.1038/nm.2597. [DOI] [PubMed] [Google Scholar]
- 15.Cooke KR, Gerbitz A, Crawford JM, Teshima T, Hill GR, Tesolin A, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. The Journal of clinical investigation. 2001 Jun;107(12):1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005 Oct 25;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 18.Miller DM, Rahill BM, Boss JM, Lairmore MD, Durbin JE, Waldman JW, Sedmak DD. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med. 1998;187:675–683. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin P, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–1163. doi: 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurkin J, Schichl YM, Koeffel R, Bauer T, Richter S, Konradi S, et al. miR-146a is differentially expressed by myeloid dendritic cell subsets and desensitizes cells to TLR2-dependent activation. Journal of immunology. 2010 May 1;184(9):4955–4965. doi: 10.4049/jimmunol.0903021. [DOI] [PubMed] [Google Scholar]
- 21.Park H, Huang X, Lu C, Cairo MS, Zhou X. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. The Journal of biological chemistry. 2015 Jan 30;290(5):2831–2841. doi: 10.1074/jbc.M114.591420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson SH, Yu CR, Mahdi RM, Ebong S, Egwuagu CE. Dendritic cell maturation requires STAT1 and is under feedback regulation by suppressors of cytokine signaling. Journal of immunology. 2004 Feb 15;172(4):2307–2315. doi: 10.4049/jimmunol.172.4.2307. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis and rheumatism. 2009 Apr;60(4):1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 24.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010 Sep 17;142(6):914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nature reviews Microbiology. 2010 Apr;8(4):296–307. doi: 10.1038/nrmicro2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–3842. doi: 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.