Abstract
Background
Untreated sexually transmitted infections (STIs) can lead to serious health complications and may be transmitted to uninfected individuals. Therefore, the early detection and subsequent management of STIs is crucial to control efforts. Time to presentation for STI symptoms and risk of transmission in this period has not been assessed in New Zealand to date.
Methods
All new clients presenting to an urban sexual health clinic (SHC) were invited to complete a questionnaire, which included demographic information, sexual health history, and details about the clinic visit.
Results
Of 331 people approached, 243 (73.4%) agreed to complete the questionnaire. Four incomplete questionnaires were excluded, leaving 239 participants (47.3% female and 52.7% male, 43.8% under the age of 25). The most common reason for seeking healthcare was experiencing symptoms (39.4%) and 41.7% of people with symptoms waited more than seven days to seek healthcare. Around a third (30.6%) of people with symptoms had sex after they first thought they may need to seek healthcare. Infrequent condom use was reported more often by people who had sex with existing partners (84.6%) than by people who had sex with new partners (10.0%).
Conclusions
This is the first study to quantify healthcare-seeking behaviour for STI in New Zealand. Delayed healthcare-seeking (defined as waiting more than seven days) was common and almost a third of people reported engaging in sex while symptomatic. Enabling prompt healthcare-seeking is crucial to minimise transmission risk. Structural barriers such as the financial cost of STI tests must be removed and education around symptom recognition and healthcare system navigation should be provided.
Introduction
Sexually transmitted infections (STIs) can lead to serious health complications including pelvic inflammatory disease, infertility and adverse pregnancy outcomes, as well as increased susceptibility to further STI acquisition including human immunodeficiency virus (HIV) (1–3). Non-diagnosis of any STI also increases the likelihood that an infected individual will transmit the infection to a sexual partner. Therefore, early diagnosis and treatment is a central issue in the control of STIs.
International studies have shown that 20% to 60% of adults visiting health services for STI symptoms had waited longer than seven days before seeking care (4–8). Studies have also reported that many people continue to have sex after noticing symptoms (6, 9–11), potentially transmitting infection to others. While it is assumed that delayed healthcare-seeking is associated with a higher likelihood of sex while symptomatic, few studies have directly assessed the association.
The time to presentation for STI symptoms has not been quantified in New Zealand other than for those with genital warts (12), and there has been no assessment of sexual behaviour while symptomatic. It is therefore not known whether delayed healthcare-seeking for STI symptoms is common in New Zealand and whether it has a potential role in STI transmission. This is important because diagnosis rates of common STIs such as chlamydia and gonorrhoea are high in New Zealand (13).
The objectives of this study were to quantify time to presentation for STI testing in people with STI symptoms, and assess whether delayed healthcare seeking poses a transmission risk.
Methods
Study design
A cross-sectional single-centre observational study design was used to investigate healthcare-seeking behaviour and associated factors.
Setting and sample
The study was conducted at an inner-city public sexual health clinic (SHC) in Wellington, New Zealand, which provides STI testing, treatment and advice, emergency contraception and sexual assault care. Services are free of charge.
Only new clients to the clinic were sampled to avoid including people who were attending for follow-up treatment or for a test of cure, as the study outcome measures were related to healthcare-seeking behaviour for new symptoms.
Recruitment
All new clients attending the SHC between 1st September and 30th November 2015 were given a study pack by the reception staff before their consultation, which included a Participant Information Sheet and a copy of the questionnaire. Those who agreed to take part in the study filled out the questionnaire in the waiting room and returned the completed copy to reception staff. No names were recorded and the clinic patient number was used to link the answers from the questionnaire to STI diagnosis/es. Participants were also given the option to complete the questionnaire online using secure encrypted surveying software, although only one participant chose this route. All refusals to take part were recorded by reception staff on the top of the questionnaire and filed separately for collection by the study coordinator. Participants were offered the option of being entered into a draw to win a grocery voucher of small value (NZ $100) as compensation for their participation. An item at the end of the questionnaire asked for consent to be entered into the draw using a tick-box, and an email address was requested for the purposes of notifying the winner.
Measures
The questionnaire was adapted from a published questionnaire used in the ‘Patient Access and the Transmission of Sexually-transmitted Infections’ (PATSI) and ‘Maximising STI Control’ (MSTIC) studies in the UK (14, 15). The draft questionnaire was refined to fit the New Zealand context in consultation with clinic staff. The questionnaire included items on basic demographics, sexual health history including previous STI testing and previous diagnosis, and details about the patient’s visit to the clinic. The primary outcome measures for this study were the participants’ reasons for testing, the number of days between symptom onset and contacting health services (whether walk-in or phoning for an appointment), the sexual behaviour of symptomatic respondents between noticing symptoms and seeking help, and STI diagnosis/es.
Participants were asked their age and responses were grouped into three categories for analysis (<25 years, 25-34 years, and 35+ years). Ethnicity was self-identified and included an option for those who would prefer not to answer. Multiple ethnicities could be selected. If an individual identified with multiple ethnicities a prioritisation approach was used to allocate individuals to a single group using the hierarchy Māori>Pacific peoples>Asian>other groups except New Zealand European>New Zealand European, as is common in New Zealand (16). Participants were asked to indicate the highest qualification or level of school they had completed. Answers were collapsed into a binary variable of lower education (high school qualification or less) and higher education (tertiary qualification e.g. degree, post-graduate diploma).
Participants could select their reason(s) for testing from a list of ten possible answers, or write in their own reason. Multiple answers were allowed (Table 2). Those who indicated they were experiencing or had experienced symptoms were asked how many days the symptoms were present before they contacted any health services. Delay was defined as waiting more than seven days to contact health services after the onset of symptoms, as this is the most common definition of delay found in the literature (7, 8, 10, 11, 14). Participants reporting symptoms were also asked if they had visited another health provider before coming to the clinic, if they had attempted any self-treatment in this time, whether they’d had sex since first thinking they may need to go to a clinic or health services, and with how many total partners and new partners. Lastly, participants were asked to indicate how often they had used condoms in this period using a five-point scale ranging from ‘none of the time’ to ‘all of the time’. This scale was then categorised into two groups; frequent condom use (‘all of the time’, ‘more than half of the time’) and infrequent condom use (‘half of the time’, ‘some of the time’, ‘none of the time’).
Table 2. Reasons for attending for current test, previous testing behaviour and previous diagnoses.
| Females |
Males |
Total |
||||
|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | N | n (%) | |
| Reason for seeking a sexually transmitted infection (STI) test (multiple answers were allowed) | 110 | 121 | 231 | |||
| Developed genital symptoms | 42 (38.2) | 49 (40.5) | 91 (39.4) | |||
| Partner has symptoms or tested positive | 15 (13.6) | 29 (24.0) | 44 (19.0) | |||
| Had unprotected sex | 33 (30.0) | 36 (29.8) | 69 (29.9) | |||
| Intends to have unprotected sex | 10 (9.1) | 23 (19.0) | 33 (14.3) | |||
| No symptoms, just a check-up | 29 (26.4) | 30 (24.8) | 59 (25.5) | |||
| Being referred or called in | 5 (4.5) | 2 (1.7) | 7 (3.0) | |||
| Other | 10 (9.1) | 6 (5.0) | 16 (6.9) | |||
| Had an STI test before | 110 | 74 (67.3) | 124 | 72 (58.1) | 234 | 146 (62.4) |
| Previously diagnosed with an STI (of those who had tested before) | 73 | 27 (37.0) | 69 | 31 (44.9) | 142 | 58 (40.8) |
Data linkage
The questionnaire included an item which asked for consent to access the participants’ STI results for use in the study. When consent was given, a member of the clinical staff accessed the patient records and recorded any positive diagnosis/es. Infections were restricted to bacterial or viral STIs and the protozoal infection trichomoniasis.
Statistical analysis
Chi-square goodness-of-fit tests were used to compare the demographic characteristics of the study sample to the patient population. Binary logistic regression was used to investigate the association between delay behaviour and sex while symptomatic, as well as other selected associations. Where data was missing for an item, the participant was excluded from the particular analysis. The analyses were conducted with Statistical Package for the Social Sciences (SPSS) Statistics version 22 and p-values <0.05 were considered statistically significant.
Ethical approval
Ethical approval was granted by Victoria University of Wellington Human Ethics Committee (ref: 20504).
Results
Of 331 new patients who were approached to take part, 243 (73.4%) agreed. Four respondents completed less than 50% of the questionnaire and so were excluded from analysis, leaving 239 (72.2%) responses in the final dataset. The sample was split relatively evenly between females (47.3%) and males (52.7%), with 43.8% under the age of 25 (age range 17-70 years, median 25 years) (Table 1). Almost two-thirds (60.3%) of participants reported a tertiary level qualification.
Table 1. Demographic characteristics.
| Total N | n (%) | |
|---|---|---|
| Gender: | 239 | |
| Female | 113 (47.3) | |
| Male | 126 (52.7) | |
| Age (years): | 235 | |
| <25 | 103 (43.8) | |
| 25-34 | 94 (40.0) | |
| 35+ | 38 (16.2) | |
| Ethnicity*: | 236 | |
| New Zealand European | 110 (46.6) | |
| Māori | 16 (6.8) | |
| Pacific peoples | 3 (1.3) | |
| Asian | 13 (5.5) | |
| Other | 94 (39.8) | |
| Education: | 239 | |
| Lower education (high school qualification or less) | 95 (39.7) | |
| Higher education (tertiary qualification) | 144 (60.3) |
If an individual identified with multiple ethnicities, a prioritisation approach was used to allocate individuals to a single group using the hierarchy Māori>Pacific peoples>Asian>Other>New Zealand European, as has been previously used in New Zealand Ministry of Health publications.
The clinic provided anonymised routinely collected data on all participants who attended the clinic during the study period, which was used to compare the gender, age, and ethnicity of those who completed the questionnaire with those who attended the clinic in the same period but did not participate in the study. Chi-square goodness-of-fit tests revealed there were more females and more under 25 year olds in the study sample than would be expected (X2(1) = 4.69, p = 0.03 and X2(2) = 60.66, p < 0.01, respectively). There was also a significant difference in ethnicity between the study sample and the whole clinic population (X2(4) = 17.80, p < 0.01), where the ‘other ethnicity’ group was overrepresented in the study sample and the New Zealand European group was underrepresented.
The most common survey-reported reason people attended the clinic for an STI test was because they had developed STI symptoms (Table 2). Other common reasons included a partner having symptoms or testing positive, having had unprotected sex with a new partner, or just wanting a check-up.
Many people reported in the survey that they had been tested before (n = 146, 62.4%), and females were more likely to report having had a previous STI test than males after adjustment for age (57.1% of males, 65.5% of females, OR = 1.906, p = 0.026). Of those who reported having previously had a test, 58 (40.8%) said they had been diagnosed with an STI; this corresponds to 24.3% of the sample having been previously diagnosed with an STI (24.6% of men, 23.9% of women, no significant difference). The most common STI that people reported being previously diagnosed with was chlamydia (64.9% of the 37 people who reported which STI(s) they had ever been diagnosed with).
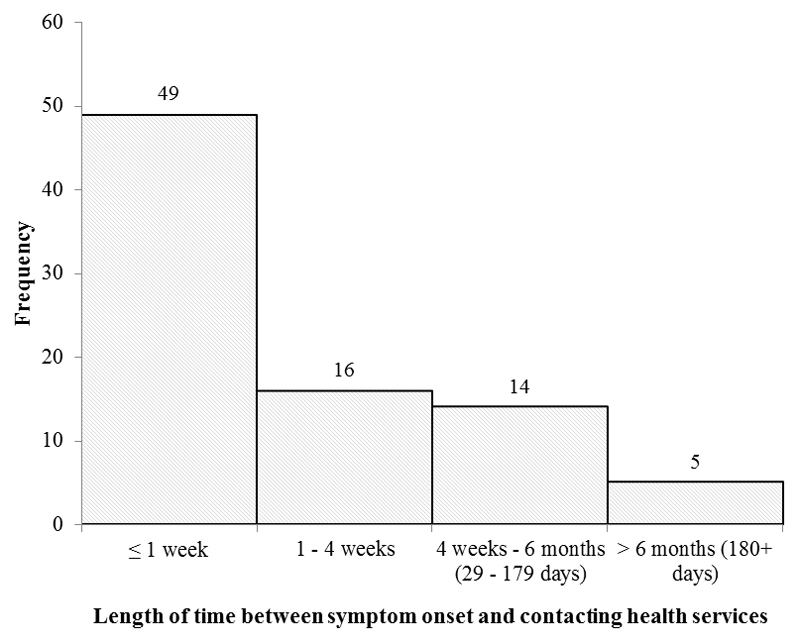
For those who reported symptoms (n = 91), the median length of time reported between symptom onset and contacting health services was 5.5 days (range 0-750 days). The distribution of delay times is shown in Figure 1. Of those experiencing symptoms, 41.7% waited more than seven days from symptom onset to contacting health services according to the participant survey responses (Table 3).
Figure 1. Frequency of delay times reported by survey participants.
Table 3. Healthcare-seeking behaviour of symptomatic respondents.
| Females |
Males |
Total |
||||
|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | N | n (%) | |
| Previously visited a health provider for this set of symptoms | 41 | 11 (26.8) | 46 | 11 (23.9) | 87 | 22 (25.3) |
| Attempted self-treatment | 39 | 13 (33.3) | 46 | 9 (19.6) | 85 | 22 (25.9) |
| Waited longer than 7 days to visit a health provider | 38 | 17 (44.7) | 46 | 18 (39.1) | 84 | 35 (41.7) |
| Days between symptom onset and contacting health services (Median (IQR)) | 38 | 6.5 (3.0-30.0) | 46 | 5.0 (3.0 – 14.0) | 84 | 5.5 (3.0-18.0) |
Of those with symptoms who answered the section on sexual behaviour (n = 85), 26 (30.6%) reported they had continued to have sex after symptom onset (26.1% of men and 35.9% of women) (Table 4). Of those who reported sex while symptomatic, 13 had had sex with their existing partner and 10 people had had sex with at least one new partner (3 did not report whether the partner(s) were new or existing) (Table 4). Among the 26 people who reported sex while symptomatic, infrequent condom use was reported more by those who had sex with existing partners (84.6%, n = 11) than by those who had sex with new partners (10.0%, n = 1). Those who waited more than seven days to contact health services were more likely to have sex with any partner (existing or new) than those who waited less than seven days (OR = 3.25, 95% CI 1.23 – 8.62, p = 0.018).
Table 4. Sexual behaviour since symptom onset and contacting healthcare services.
| Females |
Males |
Total |
||||
|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | N | n (%) | |
| Sex while symptomatic | 39 | 14 (35.9) | 46 | 12 (26.1) | 85 | 26 (30.6) |
| Sex while symptomatic with existing partner only | 36 | 8 (22.2) | 46 | 5 (10.9) | 82 | 13 (15.9) |
| Sex while symptomatic with at least one new partner | 36 | 3 (8.3) | 46 | 7 (15.2) | 82 | 10 (12.2) |
In total, 146 (61.1%) participants gave permission for their STI test results to be extracted from the clinic records for use in the study. There were no demographic differences between participants who consented to linkage and those that did not. Of the 63 people who reported seeking an STI test due to experiencing STI symptoms and gave consent to access their results, 25 (39.7%) were positive for at least one STI. There was no statistically significant difference in the likelihood of delaying seeking help for symptoms for those who tested positive compared to those who tested negative (OR = 0.56, 95% CI 0.19 – 1.66, p = 0.30). Among symptomatic participants with a positive STI result, clinic records showed 32% had a C. trachomatis infection, 24% had HPV (genital warts), 16% had HSV and 16% were cases of non-specific urethritis. Among asymptomatic patients who were diagnosed with an STI (n = 8), the majority (75%) were due to C. trachomatis.
Discussion
The most common reason for seeking healthcare at the SHC among this cohort was the presence of STI symptoms. The length of delay was highly variable, with some people seeking healthcare immediately and others waiting for several months. The data showed that 41.7% had symptoms present for more than seven days before contacting health services. One third of people with symptoms reported they had had sex since they first thought they needed to go to a clinic or health services, and, as expected, the people that waited longer than seven days were more likely to have sex while symptomatic.
To our knowledge, this is the first study examining healthcare-seeking delay for STI symptoms and sexual behaviour while symptomatic in New Zealand. A previous study of 66 clients assessed time to presentation of patients with genital warts at the Auckland Sexual Health Service, but the study did not include data from patients with other symptoms suggestive of an STI and did not assess whether patients continued to have sex while symptomatic (12).
The proportion of people waiting longer than seven days to seek healthcare for STI symptoms (41.7%) is consistent with that reported in recent studies from other developed countries. For example, a recent study in the USA found that 38% of men and 39% of women with symptoms delayed seeking care for more than seven days (17). In a study by Mercer and colleagues in the UK, 45.7% of genitourinary medicine (GUM) clinic patients had been symptomatic for more than seven days before seeking care, although a subsequent study five years later indicated the median length of delay had decreased from seven to three days (14, 18).
It is not known from this data what factors influenced the time taken to contact health services. However, previous research by the authors involving qualitative interviews with university students in New Zealand identified several barriers to STI testing (19). These were: underestimating the risk of acquiring an STI; perceiving STIs as not serious; fear of invasive procedure; self-consciousness in genital examination; being too busy; the financial cost of an STI test; clinician attributes (e.g. gender) and attitude; and concern of being stigmatised. It is likely that these factors will have also played a role in the health-seeking behaviour for the participants in the current study. In addition, while a person may notice some symptoms, they may not initially ascribe the symptoms to a possible STI, which is likely to affect the time to presentation. This may be especially relevant among women, who may be more likely to ascribe their symptoms to natural bodily functions, such as regular discharge (9).
The participants were asked how many days their symptoms were present before contacting health services, but provider delay was not queried in this study. It may be that the time between symptom onset and actually seeing a health professional was longer than recorded, due to appointment availability. Provider delay is an important aspect of timeliness to STI testing and may be influenced by a range of factors including staffing and triage processes. It would be useful to assess the extent and impact of provider delay in NZ, perhaps using clinical audit. It is also possible that the time to contact health services was affected by other health provider factors such as opening hours and distance to services. Further research should also be undertaken to assess these aspects of delay to healthcare.
In total, 26.1% of men and 35.9% of women reported having sex after the onset of symptoms. This is very similar to figures recently reported from the UK (25.2% of men 38.3% of women) (18). That females are more likely to engage in sex while symptomatic than males is a consistent finding across many studies (9–11, 20); however, the number of participants in this study was too small to reliably test this association.
Engaging in sexual activity after the onset of symptoms poses a serious risk for transmission of infection. This study showed that those who delayed healthcare seeking were more likely to have sex while symptomatic. Although this association has been generally assumed, only a few studies have directly assessed the association (10, 11, 21). Of these, two studies reported that delay behaviour was associated with sex while symptomatic (11, 21). Conversely, one study found that sexual activity while symptomatic was associated with attending healthcare quicker (10).
As waiting longer than seven days to contact health services was associated with having sex after symptom onset, enabling prompt healthcare seeking may mitigate some transmission risk. Public health messages should emphasize early action for STI symptoms including the need to abstain from sex with all partners until healthcare has been sought and appropriate treatment and/or advice given. Additional interventions could involve providing education to improve STI symptom recognition, correcting myths about what an STI test involves (19), or providing support and guidance to navigate the healthcare system. Health funders and health providers in particular have a crucial role in minimising the structural barriers that may delay or prevent testing. Structural barriers include the location of health services, availability of appointments, the financial cost of getting an STI test, and the cultural responsiveness of the health provider (19, 22).
The main limitations of this research are the small sample size and the generalisability of the data. The sample size was a result of the short time frame available for recruitment (three months). Repeating this study in a larger sample would allow for comparison of demographic and sexual behaviour characteristics between people who delay seeking healthcare for symptoms and those who do not. This would enable identification of specific groups of people that could benefit from additional information and resources to aid them in seeking timely healthcare.
Attendees at SHCs are not representative of the general population, so these data should not be extrapolated to other groups (23). In addition, the sample was younger than the overall clinic population, and had a higher proportion of women and people reporting their ethnicity as ‘other’; therefore results may not be representative of the overall clinic population. However, the study response rate was high at 73.4%. A large proportion of the group also had a tertiary level qualification, which is likely due to educated people experiencing better health and more healthcare, thus being more likely to attend the clinic and take part in research.
While the proportions of Māori, Pacific, and Asian people who took part in the study were similar to general attendance at the clinic, the actual numbers were small due to the overall study sample size being small. Laboratory data indicate that Māori and Pacific Peoples experience a higher prevalence of STIs (24). The reasons for this are unclear and likely complex, although accessing healthcare may be a potential contributing factor (Ward et al., 2013). Recent research involving young Māori in the Waikato identified discrimination and stigma as key barriers to accessing STI testing for Māori (22). More research into indigenous sexual health in New Zealand is needed.
A further limitation is that the questionnaire did not ask which symptoms the participants had experienced, or about symptom severity. It is probable that the type and/or severity of symptoms influences the speed with which an individual seeks healthcare. A previous study of people attending public clinics in Kenya found that women with genital ulcers or lower abdominal pain presented earlier than women with other complaints and men with genital ulcers presented later (11), although another study from the Netherlands found no relationship between type of symptoms experienced and delay behaviour (7).
This is the first study to quantify delay behaviour for STI testing in New Zealand, and therefore provides original data for service planners and providers to work with. These data suggest that healthcare-seeking behaviour for STI symptoms could be improved in New Zealand, and that this could have a beneficial effect on the transmission of STIs.
Acknowledgments
We thank the participants of this study for taking part in this research. We are grateful to the staff at the sexual health clinic in Wellington for their support of this work and their contribution to data collection. We also thank Hansa Patel for assisting with data entry for the study.
Footnotes
Competing interests:
The authors have no conflicts of interest to declare
Contributor Information
Hayley J Denison, Centre for Public Health Research, Massey University, Wellington, New Zealand. Doctoral candidate, School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand.
Lisa Woods, School of Mathematics and Statistics, Victoria University of Wellington, Wellington, New Zealand.
Collette Bromhead, College of Health, Massey University, Wellington, New Zealand.
Jane Kennedy, Wellington Sexual Health Service, Wellington, New Zealand.
Rebecca Grainger, Department of Medicine, University of Otago Wellington, New Zealand.
Annemarie Jutel, Graduate School of Nursing, Midwifery and Health, Faculty of Health, Victoria University of Wellington, Wellington, New Zealand.
Elaine M Dennison, School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand. Professor, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, United Kingdom.
References
- 1.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201(Suppl 2):S134–55. doi: 10.1086/652395. [DOI] [PubMed] [Google Scholar]
- 2.Idahl A, Boman J, Kumlin U, Olofsson JI. Demonstration of Chlamydia trachomatis IgG antibodies in the male partner of the infertile couple is correlated with a reduced likelihood of achieving pregnancy. Hum Reprod. 2004;19(5):1121–6. doi: 10.1093/humrep/deh155. [DOI] [PubMed] [Google Scholar]
- 3.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):305–10. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akinnawo EO, Oguntimehin F. Health-seeking behaviour of STD patients in an urban area of southwest Nigeria: an exploratory study. Health Transit Rev. 1997;7(Suppl):307–13. [PubMed] [Google Scholar]
- 5.Hook EW, 3rd, Richey CM, Leone P, Bolan G, Spalding C, Henry K, et al. Delayed presentation to clinics for sexually transmitted diseases by symptomatic patients. A potential contributor to continuing STD morbidity. Sex Transm Dis. 1997;24(8):443–8. doi: 10.1097/00007435-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Fortenberry JD. Health care seeking behaviors related to sexually transmitted diseases among adolescents. Am J Public Health. 1997;87(3):417–20. doi: 10.2105/ajph.87.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leenaars P, Rombouts R, Kok G. Seeking medical care for a Sexually Transmitted Disease: Determinants of delay-behavior. Psychol Health. 1993;8(1):17–32. [Google Scholar]
- 8.Khan A, Fortenberry JD, Temkit MH, Tu W, Orr DP, Batteiger BE. Gender differences in sexual behaviours in response to genitourinary symptoms. Sex Transm Infect. 2005;81(3):262–6. doi: 10.1136/sti.2004.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voeten HA, O'Hara HB, Kusimba J, Otido JM, Ndinya-Achola JO, Bwayo JJ, et al. Gender differences in health care-seeking behavior for sexually transmitted diseases: a population-based study in Nairobi, Kenya. Sex Transm Dis. 2004;31(5):265–72. doi: 10.1097/01.olq.0000124610.65396.52. [DOI] [PubMed] [Google Scholar]
- 10.Irwin DE, Thomas JC, Spitters CE, Leone PA, Stratton JD, Martin DH, et al. Self-reported sexual activity and condom use among symptomatic clients attending STD clinics. Sex Transm Dis. 1999;26(5):286–90. doi: 10.1097/00007435-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Moses S, Ngugi EN, Bradley JE, Njeru EK, Eldridge G, Muia E, et al. Health care-seeking behavior related to the transmission of sexually transmitted diseases in Kenya. Am J Public Health. 1994;84(12):1947–51. doi: 10.2105/ajph.84.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland JA, Reid M, Powell R, Petrie KJ. The role of illness perceptions: psychological distress and treatment-seeking delay in patients with genital warts. Int J STD AIDS. 2005;16(10):667–70. doi: 10.1258/095646205774357334. [DOI] [PubMed] [Google Scholar]
- 13.ESR Annual Surveillance Report - Sexually Transmitted Infections in New Zealand, 2013. Porirua, New Zealand: The Institute of Environmental Science and Research Ltd; 2014. [Google Scholar]
- 14.Mercer CH, Sutcliffe L, Johnson AM, White PJ, Brook G, Ross JD, et al. How much do delayed healthcare seeking, delayed care provision, and diversion from primary care contribute to the transmission of STIs? Sex Transm Infect. 2007;83(5):400–5. doi: 10.1136/sti.2006.024554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aicken CR, Cassell JA, Estcourt CS, Keane F, Brook G, Rait G, et al. Rationale and development of a survey tool for describing and auditing the composition of, and flows between, specialist and community clinical services for sexually transmitted infections. BMC Health Serv Res. 2011;11:30. doi: 10.1186/1472-6963-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cormack D, Robson C. Classification and output of multiple ethnicities: considerations for monitoring Māori Health. Wellington: Te Rōpū Rangahau Hauora a Eru Pōmare; 2010. [Google Scholar]
- 17.Malek AM, Chang CC, Clark DB, Cook RL. Delay in Seeking Care for Sexually Transmitted Diseases in Young Men and Women Attending a Public STD Clinic. Open AIDS J. 2013;7:7–13. doi: 10.2174/1874613620130614002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercer CH, Aicken CR, Estcourt CS, Keane F, Brook G, Rait G, et al. Building the bypass-implications of improved access to sexual healthcare: evidence from surveys of patients attending contrasting genitourinary medicine clinics across England in 2004/2005 and 2009. Sex Transm Infect. 2012;88(1):9–15. doi: 10.1136/sextrans-2011-050257. [DOI] [PubMed] [Google Scholar]
- 19.Denison HJ, Bromhead C, Grainger R, Dennison EM, Jutel A. Barriers to sexually transmitted infection testing in New Zealand: a qualitative study. Aust N Z J Public Health. 2017;41(4):432–7. doi: 10.1111/1753-6405.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonck K, Mwai C, Ndinya-Achola J, Bwayo J, Temmerman M. Health-seeking and sexual behaviors among primary healthcare patients in Nairobi, Kenya. Sex Transm Dis. 2002;29(2):106–11. doi: 10.1097/00007435-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Thi Thu H, Ziersch A, Hart G. Healthcare-seeking behaviours for sexually transmitted infections among women attending the National Institute of Dermatology and Venereology in Vietnam. Sex Transm Infect. 2007;83(5):406–10. doi: 10.1136/sti.2006.022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tipene J, Green A. He Pūkenga Kōrero. Rangatahi and sexually transmitted infections in the Waikato. A report submitted to the Health Research Council of New Zealand. 2017 Aug 31; [Google Scholar]
- 23.Catchpole M, Connor N, Brady A, Kinghorn G, Mercey D, Band B, et al. Behavioural and demographic characteristics of attenders at two genitourinary medicine clinics in England. Genitourin Med. 1997;73(6):457–61. doi: 10.1136/sti.73.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ESR Annual Surveillance Report - Sexually Transmitted Infections in New Zealand, 2014. Porirua, New Zealand: The Institute of Environmental Science and Research Ltd; 2015. [Google Scholar]