Abstract
Purpose
To determine the inter-study reproducibility of magnetic resonance feature tracking (MR-FT) derived left ventricular (LV) torsion and torsion rates for a combined assessment of systolic and diastolic myocardial function.
Materials and Methods
Steady-state free precession (SSFP) cine LV short-axis stacks were acquired at 9:00 (Exam A), 9:30 (Exam B) and 14:00 (Exam C) in 16 healthy volunteers at 3 T. SSFP images were analysed offline using MR-FT to assess rotational displacement in apical and basal slices. Global peak torsion, peak systolic and peak diastolic torsion rates were calculated using different definitions (“twist”, “normalized twist” and “circumferential-longitudinal (CL) shear angle”). Exam A and B were compared to assess the inter-study reproducibility. Morning and afternoon scans were compared to address possible diurnal.
Results
The different methods showed good inter-study reproducibility for global peak torsion (intraclass correlation coefficient (ICC):0.90-0.92;coefficient of variation (CoV):19.0-20.3%) and global peak systolic torsion rate (ICC:0.82-0.84;CoV:25.9-29.0%). Conversely, global peak diastolic torsion rate showed little inter-study reproducibility (ICC:0.34-0.47;CoV:40.8-45.5%). Global peak torsion as determined by the CL shear angle showed the best inter-study reproducibility (ICC:0.90;CoV:19.0%). MR-FT results were not measurably affected by diurnal variation between morning and afternoon scans (CL shear angle: 4.8±1.4°,4.8±1.5° and 4.1±1.6° for Exam A, B and C, respectively; p=0.21).
Conclusion
MR-FT based derivation of myocardial peak torsion and peak systolic torsion rate has high inter-study reproducibility as opposed to peak diastolic torsion rate. The CL shear angle was the most reproducible parameter independently of cardiac anatomy and may develop into a robust tool to quantify cardiac rotational mechanics in longitudinal MR-FT patient studies.
Keywords: inter-study reproducibility, cardiovascular magnetic resonance, feature tracking, torsion, twist, circumferential longitudinal shear angle
Introduction
Left ventricular (LV) torsion describes the systolic twisting motion resulting from basal clockwise rotation and apical counter-clockwise rotation (when viewed from the apex) (1). Previous data attribute up to 40 % of LV stroke volume to ventricular twisting dynamics (1, 2) The phenomenon has been variably described as simple twist (3), normalized twist (often referred to as “Torsion”) (4) and the circumferential-longitudinal (CL) shear angle (5).
The untwisting motion during early diastole – as captured by peak diastolic torsion rate – seems particularly important to early LV filling and therefore represents a potential imaging biomarker for the characterisation of LV diastolic function (6). At the present time, MR myocardial tagging is considered the reference standard for the assessment of LV rotational dynamics. Alternative MR based techniques include tissue phase mapping (7) and displacement encoding with stimulated echoes (DENSE) (8). These techniques require acquisition of additional sequence and are often associated with time-consuming post-processing, limiting their clinical applicability.
MR myocardial feature tracking (MR-FT) is a technique that allows quantification of myocardial deformation from standard steady-state free precession (SSFP) images acquired clinical MR examination (9–12). The feasibility of CMR-FT derived LV torsion has recently been described, demonstrating good intra- and inter-observer reproducibility (13). However, inter-study reproducibility is a key requirement to apply this novel technique in longitudinal studies with repeated measurements. Accordingly, the aim of the present study was to investigate the inter-study reproducibility of CMR-FT derived torsion and recoil rates using the aforementioned definitions and to assess whether this is measurably affected by diurnal physiological alterations.
Materials and Methods
The study complies with the Declaration of Helsinki and was approved by the local ethics committee. 16 healthy volunteers (8 male, 8 female) aged 27.9 ± 5.7 with a body mass index of 26.2 ± 6.8 kg/m2 were recruited from the local university. Exclusion criteria included known cardiac, respiratory, or renal disease or a contraindication to MR. All participants gave written informed consent.
MR Imaging
Participants underwent 3 MR examinations on the same day. All imaging was performed at 3 T (Achieva, Philips Medical Systems, Best, The Netherlands) with participants in the supine position using a 32-channel phased array receiver cardiac coil. On the day of the scans subjects were asked to fast from midnight. The first MR examination was performed at 9:00 (Exam A), followed by a second exam at 9:30 (Exam B). Participants were removed from the scanner and taken off the table between the Exams. Repositioning was performed before Exam B. In order to try and maximise physiological changes, participants then left the department to eat and drink (except of caffeine-containing drinks) as normal. They returned at 14:00 for the third scan (Exam C). Exams A and B were acquired to assess for the inherent variability of MR-FT quantification of LV torsion and torsion rate. Morning scans (Exams A and B) were compared to Exam C to assess for diurnal physiological alterations due to circadian rhythms, different states of hydration or any other reason. Systolic (SBP) and diastolic blood pressures (DBP) as well as heart rate (HR) were measured at the beginning of each Exam A-C.
The MR protocol included an initial survey, coil reference scan and planning to define imaging planes, independently for all three MR scans. Cine images were acquired using a standard ECG-gated balanced steady state free precession (SSFP) sequence. Long-axis views were used to define 11 – 15 equidistant short-axis planes covering the entire LV (in-plane resolution 1.8 x 2 mm, slice thickness 8 mm, gap 8mm, 30 time frames). Identical protocols were used for all three exams (including repositioning prior to every exam) and for all volunteers.
Data Analysis
LV end-diastolic (LVEDV), LV end-systolic volumes (LVESV) and ejection fraction (LVEF) were measured from all Exam A-C using a commercially available software package (CMR 42, Circle, Canada).
MR-FT was performed using dedicated software (TomTec Imaging Systems, 2D CPA MR, Cardiac Performance Analysis, Version 1.1.2, Unterschleissheim, Germany). MR-FT is based on an algorithm for endo- and epicardial border tracking, which is guided by inhomogeneity of tissue brightness or anatomical features using a maximum likelihood method. The details of the MR-FT algorithm have been published previously (14, 15).
Images were analysed in a random order by a blinded experienced observer (JTK, 3 years experience in MR-FT). From the short-axis stack, the most apical plane still showing LV cavity at systole and the most basal plane still showing a complete circumference of myocardium during the entire cardiac cycle were identified. From these two planes, adjacent planes directed towards the mid-ventricle were chosen to perform MR-FT, since previous data showed inferior performance of MR-FT in the most apical and basal short-axis slices (13).
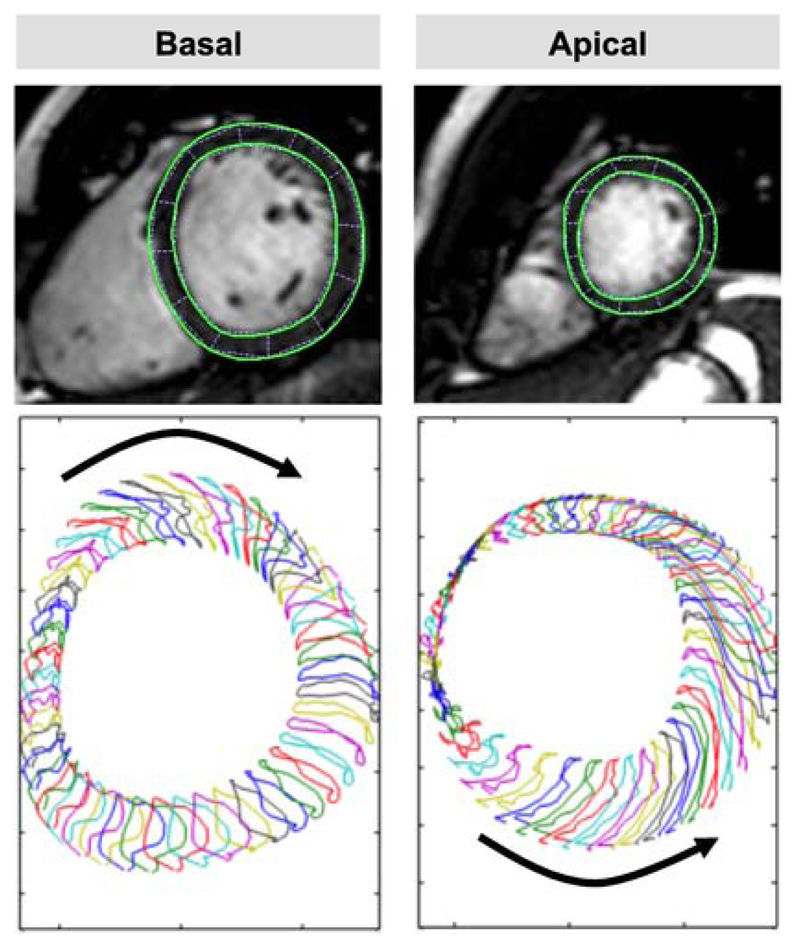
LV endocardial and epicardial borders were manually traced in the apical and basal slice (Fig. 1) using a point-and-click approach with the initial contour set at LV end-diastole. Endocardial and epicardial border surfaces were manually delineated before the automated tracking algorithm was applied. This software automatically tracks 48 subendocardial and subepicardial tissue voxels throughout the cardiac cycle. Tracking performance was visually reviewed to ensure accurate tracking of the ventricular myocardium. In case of insufficient border tracking manual adjustments were made to the initial contour and the algorithm was reapplied. Tracking was repeated for three times including redrawing of the contours in each short-axis view. Results were based on the average of the three repeated measures.
Figure 1.
Cardiovascular Magnetic Feature Tracking (CMR-FT). Rotational dynamics were assessed in basal (left panel) and apical (right panel) short-axis positions. Rotation resulted in an overall apical counter-clockwise and basal clockwise rotation when viewed from the apex. The lower panel shows the tracking of 48 (sub)endocardial features.
Areas within the epicardial and endocardial contours were calculated from the FT result files using MATLAB (The MathWorks, Version R2014b, MA, USA).
Definition Of Torsion And Torsion Rate
Torsion and torsion rates were calculated using Microsoft Excel 2011 for Macintosh. Averaged LV rotation profiles were calculated from the rotational displacement of all 48 tissue voxels in apical and basal slices. Three different definitions were used to capture LV torsion:
- Simple twist (further referred to as “twist”), which corresponds to a subtraction of basal clockwise rotation (ϕbase) from apical counter-clockwise rotation (ϕapex) (3):
- Twist normalized to LV length (further referred to as “normalized twist”), which has been commonly described as “Torsion” in the literature and corresponds to Twist divided by the distance (D) between apical and basal imaging planes as calculated from the sum of inter-slice gaps and slice thicknesses (16):
- Circumferential Longitudinal Shear Angle (hereafter referred to as “CL shear angle”), which corresponds to twist normalized to LV length and diameter, where D is the inter-slice distance and ρapex and ρbase the apical and basal radius, respectively, as calculated from the area (endocardial or epicardial) within the contour at each time frame assuming a circle (5):
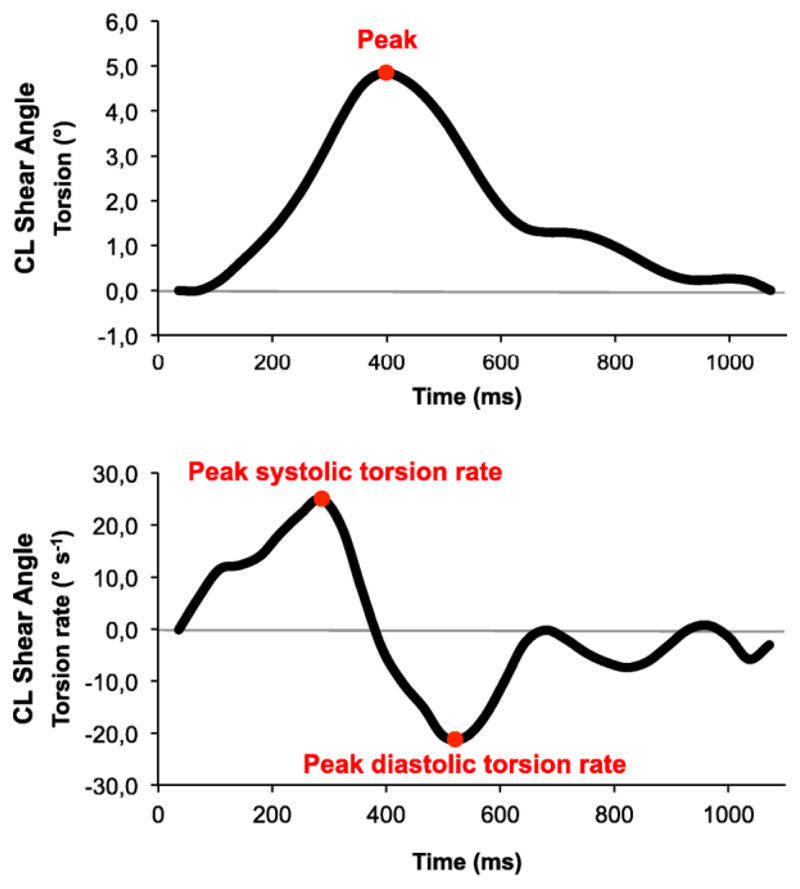
We analysed subepicardial and subendocardial torsion separately and also global LV torsion (averaged subendocardial and subepicardial torsion). Peak systolic (positive) torsion rates and peak diastolic (negative) torsion rates were calculated from the first derivative of torsion (Fig. 2).
Figure 2.
Torsion and torsion rate quantification. Quantification included peak torsion (upper panel) and peak systolic and diastolic torsion rates (lower panel). In this example torsion has been calculating according to the Circumferential-Longitudinal Shear Angle definition.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel and IBM SPSS Statistics version 22 for Macintosh. Data from Exam A-C are expressed as mean (± standard deviation) based on the scans of all 16 volunteers. The Shapiro-Wilk test was applied to ensure normally distributed data. Since systolic and diastolic torsion rates were not normally distributed, a natural logarithmic transformation was performed. The Shapiro-Wilk test was reapplied to check for normal distribution after natural logarithmic transformation. A one-way analysis of variance for repeated measures with Bonferroni post hoc adjustment in case of significance was conducted to evaluate the null hypothesis that there is no change in torsion or torsion rate between the repeated Exams A, B and C. All p values < 0.05 were considered statistically significant.
The inter-study variability (Exam A vs. Exam B) was assessed by intraclass correlation coefficients (ICC) using a model of absolute agreement. Agreement was considered excellent when ICC > 0.74, good when ICC = 0.60–0.74, fair when ICC = 0.40–0.59, and poor when ICC < 0.4 (17). Bland Altman analysis and coefficients of variation (CoV) were calculated. The CoV was defined as the standard deviation of the differences divided by the mean (18). Furthermore, study sample sizes required to detect a relative 10, 15 or 20% change in torsion or torsion rate with a power of 80% and an α error of 0.05 were calculated as follows:
where n is the sample size, α the significance level, P the study power required, and f the value of the factor for different values of α and P (f = 7.85 for α 0.05 and P 0.8), with σ the inter-study standard deviation of differences between Exam A and B, and δ the magnitude of the differences to be detected.
Results
One participant did not attend Exam C, so that a total of 16 measurements were compared for the derivation of inter-study reproducibility (Exam A vs. Exam B) and 15 measurements were compared for the assessment of diurnal variation in torsion and torsion rate (Exam A and B vs. Exam C). All scans were successfully analysed using MR-FT. Results are reported for global parameters. Results for subendocardial and subepicardial torsion and torsion rates are reported in the online supplementary material (Table S1, S2 and S3). Participants LV volumes, LVEF, SBP/DBP and HR did not show any significant differences between the Exams A-C (Table 1).
Table 1.
Comparison of LV dimensions and haemodynamics between the Exams A, B and C. Values are presented as mean ± standard.
| Exam A | Exam B | Exam C | P value* | |
|---|---|---|---|---|
| LVEF (%) | 59 ± 3 | 58 ± 4 | 60 ± 4 | 0.35 |
| LVEDV (ml) | 162 ± 33 | 162 ± 37 | 161 ± 40 | 0.87 |
| LVESV (ml) | 68 ± 17 | 69 ± 20 | 65 ± 18 | 0.15 |
| HR (bpm) | 74 ± 10 | 74 ± 10 | 75 ± 13 | 0.75 |
| SBP (mmHg) | 116 ± 20 | 119 ± 14 | 117 ± 23 | 0.75 |
| DBP (mmHg) | 70 ± 9 | 69 ± 8 | 66 ± 10 | 0.11 |
LVEDV, LV end-diastolic volume; LVESV, LV end-diastolic volume; LVEF, LV ejection fraction; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure.
as derived from one-way ANOVA for repeated measures across the three Exams A, B and C.
Torsion And Torsion Rates
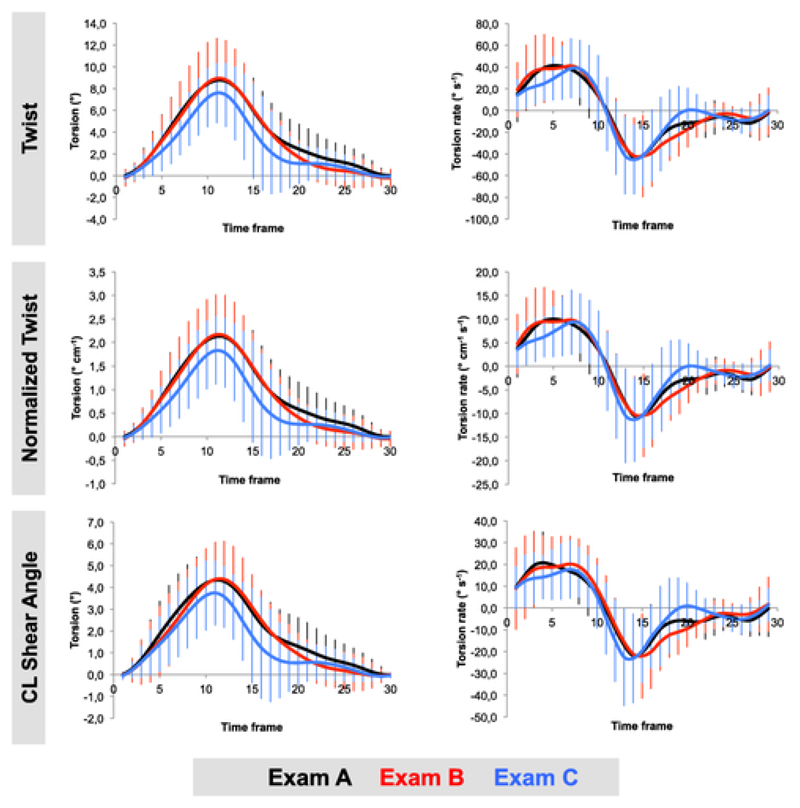
Global torsion and torsion rate parameters as derived from the three different definitions and for all three Exams are summarised in Table 2 and displayed in Fig. 3. Subepicardial peak systolic torsion rate as determined by “twist” and “normalized twist” was significantly different between Exam B and C (both p = 0.02, see Table S1 in the supplementary material). There were no significant differences in any of the other torsion and torsion rate parameters between all three repeated exams as determined by either definition. There was no measurable diurnal variation between morning and afternoon scans.
Table 2.
Comparison of repeated global torsion (mean (standard deviation)) and torsion rates (median (25th;75th percentiles)) as determined by the three different definitions.
| Exam A | Exam B | Exam C | P value* | ||
|---|---|---|---|---|---|
| Twist | Peak Torsion [°] | 9.6 (1.3) | 9.7 (3.5) | 8.3 (3.3) | 0.16 |
| Peak Systolic Torsion Rate [° s-1] | 66.4 (43.3;72.3) | 51.1 (45.7;80.1) | 50.2 (40.4;59.9) | 0.35 | |
| Peak Diastolic Torsion Rate [° s-1] | -57.4 (-82.3;-46.8) | -61.3 (-85.8;-44.6) | -65.9 (-79.8;-52.3) | 0.98 | |
| Normalized Twist | Peak Torsion [° cm-1] | 2.3 (0.8) | 2.3 (0.8) | 2.0 (0.8) | 0.21 |
| Peak Systolic Torsion Rate [° cm-1 s-1] | 15.4 (10.7;19.9) | 14.8 (10.5;18.5) | 12.5 (9.5;16.8) | 0.44 | |
| Peak Diastolic Torsion Rate [° cm-1 s-1] | -13.6 (-19.5;-11.5) | -15.9 (-19.2;-11.2) | -14.5 (-21.4;-11.6) | 0.96 | |
| CL Shear Angle | Peak Torsion [°] | 4.8 (1.4) | 4.8 (1.5) | 4.1 (1.6) | 0.21 |
| Peak Systolic Torsion Rate [° s-1] | 27.0 (21.1;33.5) | 26.9 (19.7;36.0) | 24.6 (19.1;29.0) | 0.24 | |
| Peak Diastolic Torsion Rate [° s-1] | -21.9 (-37.0;-19.0) | -25.3 (-33.0;-17.0) | -28.5 (-32.1;-19.2) | 0.87 | |
CL Shear Angle, circumferential-longitudinal Shear Angle
as derived from one-way ANOVA for repeated measures across the three Exams A, B and C.
Figure 3.
Global torsion (left panel) and torsion rate (right panel) profiles averaged across the study group. There was good agreement between all three Exams (A-C). Torsion was slightly lower during Exam C, however this difference was not statistically significant.
Inter-study Reproducibility
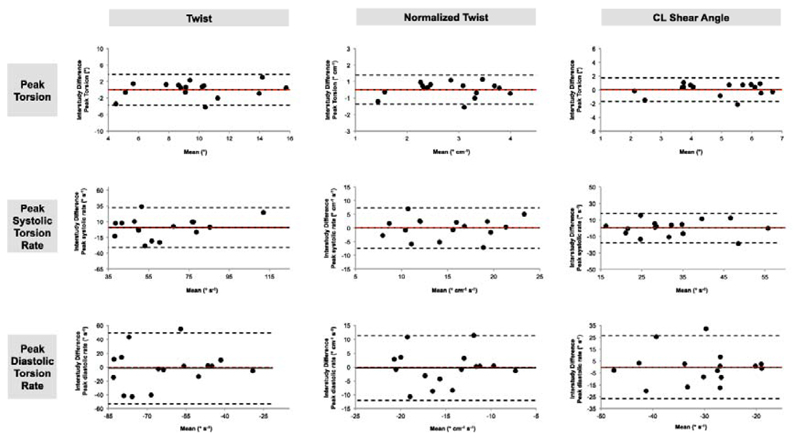
Mean differences ± 2 SD (Bland-Altman analysis), CoV and ICC for global torsion and torsion rates are summarised in Table 3 for the different definitions (Exam A vs. Exam B). Reproducibility was comparable between all three definitions. There was good reproducibility for global peak torsion (ICC ≥ 0.90) and global peak systolic torsion rate (ICC ≥ 0.82) using any definition. The lowest variability was found for global peak torsion as determined by the CL shear angle (CoV 19.0%). Peak global torsion as determined by normalized twist showed the highest ICC (0.92). In contrast, reproducibility for global peak diastolic torsion rate was fair to poor using any definition. Bland-Altman plots for global torsion and torsion rates are displayed in Fig. 4.
Table 3.
Inter-study reproducibility (comparison of Exam A and Exam B) for global torsion and torsion rates as determined by three different definitions.
| Mean difference ± 2SD | CoV[%] | ICC (95% CI) | ||
|---|---|---|---|---|
| Twist | Peak Torsion [°] | 0.0 ± 3.9 | 20.3 | 0.91 (0.74-0.97) |
| Peak Systolic Torsion Rate [° s-1] | 0.7 ± 33.1 | 26.9 | 0.84 (0.53-0.94) | |
| Peak Diastolic Torsion Rate [° s-1] | 1.7 ± 53.9 | 43.1 | 0.36 (0.00-0.78) | |
| Normalized Twist | Peak Torsion [° cm-1] | 0.0 ± 0.9 | 19.9 | 0.92 (0.76-0.97) |
| Peak Systolic Torsion Rate [° cm-1 s-1] | 0.0 ± 7.7 | 25.9 | 0.84 (0.54-0.95) | |
| Peak Diastolic Torsion Rate [° cm-1 s-1] | 0.3 ± 12.3 | 40.8 | 0.47 (0.00-0.82) | |
| CL Shear Angle | Peak Torsion [°] | 0.0 ± 1.7 | 19.0 | 0.90 (0.72-0.97) |
| Peak Systolic Torsion Rate [° s-1] | 0.3 ± 18.7 | 29.0 | 0.82 (0.48-0.94) | |
| Peak Diastolic Torsion Rate [° s-1] | 0.1 ± 27.9 | 45.5 | 0.34 (0.00-0.78) | |
SD, standard deviation; CoV, coefficient of variation; ICC, intraclass correlation coefficient; CI, confidence interval; CL Shear Angle, circumferential-longitudinal shear angle
Figure 4.
Bland-Altman Plots for inter-study reproducibility (comparison of Exam A and Exam B) obtained for global peak torsion, global peak systolic torsion rate and global peak diastolic torsion rate, as calculated according to the three different definitions: twist, normalized twist and circumferential-longitudinal shear angle. Dashed red line: zero mean difference. Dashed black line: limits of agreement (1.96 standard deviations).
Sample Size Calculations
The differences in reproducibility between peak torsion and peak systolic torsion rate on the one hand, and peak diastolic torsion rate on the other hand are reflected in the sample size calculations. Sample sizes required to detect a relative 10%, 15% or 20% change in global torsion and torsion rates are summarised in Table 4. Sample sizes range from n=15 to detect a 20% change in peak torsion as determined by CL shear angle (corresponds to a magnitude of 1.0 ° in the present study) and n=322 to detect a 10% change in peak diastolic torsion rate as determined by CL shear angle (corresponds to a magnitude of 3.1 ° s-1 in the present study).
Table 4.
Sample size calculations for global torsion as determined by the three different definitions to detect a relative change in 10, 15 or 20 % of mean (Exam A and B) torsion and torsion rate, respectively.
| Sample size [n] | ||||
|---|---|---|---|---|
| 10 % | 15 % | 20 % | ||
| Twist | Peak Torsion | 65 | 29 | 17 |
| Peak Systolic Torsion Rate | 114 | 51 | 29 | |
| Peak Diastolic Torsion Rate | 292 | 130 | 73 | |
| Normalized Twist | Peak Torsion | 63 | 28 | 16 |
| Peak Systolic Torsion Rate | 106 | 47 | 27 | |
| Peak Diastolic Torsion Rate | 262 | 117 | 66 | |
| CL Shear Angle | Peak Torsion | 59 | 26 | 15 |
| Peak Systolic Torsion Rate | 141 | 63 | 36 | |
| Peak Diastolic Torsion Rate | 322 | 143 | 81 | |
CL Shear Angle, circumferential-longitudinal shear angle
Discussion
The current study aimed to assess the inter-study reproducibility of MR-FT for the analysis of LV torsion and torsion rates. Firstly, it demonstrates that there is good inter-study reproducibility of peak torsion and peak systolic torsion rate whilst peak diastolic torsion rate quantification is highly variable between repeated measurements. Secondly, we compared a number of different MR-FT measures of torsion and found comparable inter-study reproducibility of the different methods. Finally, there was no measurable diurnal variation of global torsion and torsion rates.
Inter-study reproducibility is crucial when repeated examinations are required. As shown previously, LV volumetric assessment derived from MR has high inter-study reproducibility and reduces sample sizes by up to 90% when compared to echocardiography (18). Higher reproducibility means that smaller changes can be detected with increased reliability. This is particularly important when subtle changes need to be quantified in serial examinations e.g. effects induced by pharmacological or life-style interventions. MR-FT represents a relatively novel approach to quantify LV rotational dynamics (13, 19). Previous MR-FT studies primarily focused on ventricular strain measurements. The reproducibility and repeatability varies between studies with most studies reporting reasonable reproducibility of global strain values (20, 21). As demonstrated by Morton et al. the highest inter-study reproducibility for LV MR-FT has been reported for LV global circumferential strain (20). In contrast, segmental strain parameters – which would be particularly relevant for assessing patients with coronary artery disease – were less reproducible. This study demonstrates that the reproducibility of global LV torsion is as good as global LV circumferential strain (20). Higher reproducibility of global measurements is most likely a result of averaging rotation from all tracked features in one imaging plane. In order to further maximise reproducibility, all measurements were repeated three times and corresponding averaged rotational profiles were included in the calculation of torsion. This strategy has improved the reproducibility in previous MR-FT investigations (10, 13) and is based on speckle tracking echocardiography, which typically includes averaged deformation indexes from three to five consecutive cardiac cycles (3, 22). Furthermore, we did not include the very basal and apical short-axis planes in our analyses, since previous studies confirmed improved intra- and inter-observer reproducibility, as well as a more robust evaluation of physiological alterations during inotropic stimulation, measuring rotation of slices slightly closer to the mid-ventricle (13). This is probably because through-plane motion is particularly pronounced in the very basal and apical short-axis imaging planes and can cause loss of tracked features from the imaging plane.
The sample size calculations in the current study demonstrate that relatively small samples are required to detect a 20% change in peak torsion and peak systolic torsion rate. It is important to note that the image quality of our healthy volunteers was good in all cases. Reproducibility and sample sizes may therefore be different in patients or when image quality is reduced. However, although dobutamine stimulation may reduce image quality, a previous MR-FT study in a patient group with coronary artery disease even demonstrated improved MR-FT reproducibility during dobutamine exposure as compared to measurements at rest (23).
The degree of variability of a given clinical parameter that may be considered as acceptable is also dependent on the magnitude of change to be expected in follow-up examinations. Longitudinal studies on patients undergoing cardiac resynchronization therapy (CRT) revealed increased LV torsion after CRT implantation in responders by on average +300% (24) whilst a decrease by on average -40% was reported in non-responders (25). Therefore, our reported reproducibility of MR-FT derived rotational dynamics appears as if it may be sufficient to detect clinically relevant changes. However, the high variability of peak diastolic torsion rate (CL shear angle: ICC = 0.34; CoV = 45.5%) means that this parameter does not yet appear applicable to clinical trials with repeated measurements. The high variability of peak diastolic torsion rates is most likely explained by through-plane motion, which occurs particularly in healthy individuals during the fast early LV relaxation, causing loss of tracked features from the imaging plane in a short period of time. LV peak diastolic torsion rate (often referred to as “diastolic untwisting” or “diastolic recoil”) has shown to be a sensitive imaging biomarker of LV diastolic function in a variety of cardiovascular disorders (5). Accordingly, robust quantification of this parameter is highly desirable. Future studies will need to investigate whether two-dimensional (2D) feature tracking can be refined enough to overcome this limitation. If 2D MR-FT has reached its performance limit, correction of through-plane motion by the development of three dimensional (3D) feature tracking might improve the quantification of diastolic recoil.
In the present study we have investigated the inter-study reproducibility of LV torsion and torsion rate as determined by the different definitions: twist, normalized twist and CL shear angle. The simple twist definition is generally not recommended when using MR because the results are not comparable between patients with different anatomy (26). In fact, the CL shear angle definition seems most promising to make LV torsion comparable between different sized hearts since it is takes both LV length and LV diameter into consideration (5). Our results demonstrate that the inter-study reproducibility between all considered definitions – twist, normalized twist and CL shear angle – is in fact comparable. Therefore, this supports the use of CL shear angle in future MR-FT studies because it is independent of cardiac anatomy.
Our study aimed to assess diurnal variation of LV rotational dynamics by comparing morning and afternoon measurements. It is important to note, that based on our sample size calculations, our study was underpowered to detect changes in torsion rate parameters below 20%. However, we found a statistically significant difference in subepicardial peak systolic torsion rate for twist and normalized twist (Exam B vs. C). This finding seems to be paralleled by a trend towards lower LV torsion in Exam C as compared to Exam A and B. In contrast, no differences or trends were observed for conventional LV functional parameters. These findings might be in accordance with previous data by Hansen et al. who demonstrated that changes in sympathomimetic stimulation – which should also occur during the course of one day – influence LV torsion even more than conventional indexes such as LV stroke volume and LVEF (27).
The fact that previous studies have been performed using different definitions e.g. twist, normalized twist or CL shear angle definition of LV torsion, makes it difficult to compare the reported reproducibility. There are only limited inter-study reproducibility data on CMR based quantification of torsion. In a substudy of the Multi-Ethnic-Study of Atherosclerosis, Donekal et al. reported inter-study reproducibility of CMR myocardial tagging with an ICC of 0.73 for peak torsion, ICC of 0.4 for peak systolic torsion rate and ICC of 0.37 for peak diastolic torsion rate as determined by the normalized twist definition (28). Swobodo et al. showed good inter-study reproducibility for twist measurements using complementary spatial modulation of magnetization (29). Furthermore, Reyhan et al. (30) described moderate inter-study reproducibility for twist and CL shear angle using Fourier analysis of stimulated echoes (FAST) – a novel MR tagging method. Speckle tracking echocardiography (STE) represents another technique that has been used for the study of LV rotational dynamics in a variety of cardiovascular disorders. STE is widely available and benefits from high temporal resolution. However, there are legitimate concerns that STE might not be robust enough for LV torsion quantification in longitudinal studies (31). STE suffers from limited image quality especially occasionally poor acoustic windows, making it impossible to perform STE in significant numbers of study samples (32, 33). Kim et al. reported feasibility in 56/160 (35%) participants (32), Park et al. in only 274/1408 (20%) participants enrolled for STE derived quantification of LV twist (33). In general 2D echocardiography does not allow a reliable and uniform selection of apical and basal slices in individual subjects, which has proved to be crucial for robust quantification of myocardial twisting motion (3, 13). Whether or not 3D echocardiography may overcome this limitation remains to be demonstrated. The reported inter-study reproducibility of 2D STE varies between excellent in a sample of 5 healthy volunteers (3) and poor to moderate in a study of 20 subjects (33). 2D STE is limited to the simple twist definition, since an accurate assessment of the inter-slice distance between apical and basal slices is generally not possible. Therefore, a comparison of torsion between subjects with different sized hearts cannot be achieved as discussed above.
Our study is limited due to the inclusion of healthy volunteers rather than patients. Reproducibility might vary between healthy volunteers and patients with different cardiovascular disorders. However, previously published data suggest that volunteer and patient reproducibility may well be similar as discussed above. The average BMI of our population was high (27 ± 7), which could be related to changes in LV rotational dynamics (34). The sample size of this study was relatively small. As discussed above, although there was a trend towards lower LV torsion in Exam C, the study was underpowered to detect significant differences of global LV torsion and torsion rate parameters. Ideally, a direct comparison of the reproducibility between myocardial tagging, representing the reference standard for such acquisitions, and MR-FT would have been performed. A general limitation of MR-FT is the restriction to 2D acquisitions as discussed above. Therefore, we recommend that future developments focus on the development of 3D models to overcome some of the fundamental limitations of 2D MR-FT.
In conclusion, the inter-study reproducibility of MR-FT derived LV peak torsion and peak systolic torsion rate is good whilst the reproducibility of peak diastolic torsion rate is only fair to poor irrespective of the definition of torsion. MR-FT quantification of peak torsion and systolic torsion rates could therefore be a useful tool to accurately quantify LV systolic function in future longitudinal patient studies. Since the CL shear angle definition of torsion normalises for cardiac geometry, and thus allows LV torsion to be compared between differently sized hearts, we recommend using this definition in future patient studies.
Supplementary Material
Grant support
We are grateful for the financial support by the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research) and the Research program of the Faculty of Medicine of the Georg-August-University Göttingen, Germany. JTK received a travel grant by the RWG Foundation. PL holds a Sir Henry Dale Fellowship funded jointly by the Wellcome Trust and the Royal Society (grant no. 099973/Z/12/Z).
References
- 1.Esch BT, Warburton DE. Left ventricular torsion and recoil: implications for exercise performance and cardiovascular disease. J Appl Physiol (1985) 2009;106(2):362–9. doi: 10.1152/japplphysiol.00144.2008. [DOI] [PubMed] [Google Scholar]
- 2.Carlsson M, Ugander M, Mosen H, Buhre T, Arheden H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;292(3):H1452–9. doi: 10.1152/ajpheart.01148.2006. [DOI] [PubMed] [Google Scholar]
- 3.Goffinet C, Chenot F, Robert A, Pouleur AC, le Polain de Waroux JB, Vancrayenest D, et al. Assessment of subendocardial vs. subepicardial left ventricular rotation and twist using two-dimensional speckle tracking echocardiography: comparison with tagged cardiac magnetic resonance. Eur Heart J. 2009;30(5):608–17. doi: 10.1093/eurheartj/ehn511. [DOI] [PubMed] [Google Scholar]
- 4.Stuber M, Scheidegger MB, Fischer SE, Nagel E, Steinemann F, Hess OM, et al. Alterations in the local myocardial motion pattern in patients suffering from pressure overload due to aortic stenosis. Circulation. 1999;100(4):361–8. doi: 10.1161/01.cir.100.4.361. [DOI] [PubMed] [Google Scholar]
- 5.Russel IK, Gotte MJ, Bronzwaer JG, Knaapen P, Paulus WJ, van Rossum AC. Left ventricular torsion: an expanding role in the analysis of myocardial dysfunction. JACC Cardiovasc Imaging. 2009;2(5):648–55. doi: 10.1016/j.jcmg.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Opdahl A, Remme EW, Helle-Valle T, Edvardsen T, Smiseth OA. Myocardial relaxation, restoring forces, and early-diastolic load are independent determinants of left ventricular untwisting rate. Circulation. 2012;126(12):1441–51. doi: 10.1161/CIRCULATIONAHA.111.080861. [DOI] [PubMed] [Google Scholar]
- 7.Codreanu I, Robson MD, Golding SJ, Jung BA, Clarke K, Holloway CJ. Longitudinally and circumferentially directed movements of the left ventricle studied by cardiovascular magnetic resonance phase contrast velocity mapping. J Cardiovasc Magn Reson. 2010;12:48. doi: 10.1186/1532-429X-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong J, Yu X. Strain and torsion quantification in mouse hearts under dobutamine stimulation using 2D multiphase MR DENSE. Magn Reson Med. 2010;64(5):1315–22. doi: 10.1002/mrm.22530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowallick JT, Edelmann F, Lotz J, Lamata P, Schuster A. Imaging Diastolic Dysfunction with Cardiovascular Magnetic Resonance. Journal of Cardiol Ther. 2014;1(4):58–64. [Google Scholar]
- 10.Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, et al. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson. 2014;16(1):60. doi: 10.1186/s12968-014-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster A, Kutty S, Padiyath A, Parish V, Gribben P, Danford DA, et al. Cardiovascular magnetic resonance myocardial feature tracking detects quantitative wall motion during dobutamine stress. J Cardiovasc Magn Reson. 2011;13:58. doi: 10.1186/1532-429X-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster A, Paul M, Bettencourt N, Morton G, Chiribiri A, Ishida M, et al. Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol. 2013;166(2):413–20. doi: 10.1016/j.ijcard.2011.10.137. [DOI] [PubMed] [Google Scholar]
- 13.Kowallick JT, Lamata P, Hussain ST, Kutty S, Steinmetz M, Sohns JM, et al. Quantification of left ventricular torsion and diastolic recoil using cardiovascular magnetic resonance myocardial feature tracking. PLoS One. 2014;9(10):e109164. doi: 10.1371/journal.pone.0109164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hor KN, Baumann R, Pedrizzetti G, Tonti G, Gottliebson WM, Taylor M, et al. Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp. 2011;(48) doi: 10.3791/2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010;3(2):144–51. doi: 10.1016/j.jcmg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Yoneyama K, Gjesdal O, Choi EY, Wu CO, Hundley WG, Gomes AS, et al. Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2012;126(21):2481–90. doi: 10.1161/CIRCULATIONAHA.112.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oppo K, Leen E, Angerson WJ, Cooke TG, McArdle CS. Doppler perfusion index: an interobserver and intraobserver reproducibility study. Radiology. 1998;208(2):453–7. doi: 10.1148/radiology.208.2.9680575. [DOI] [PubMed] [Google Scholar]
- 18.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90(1):29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 19.Nucifora G, Muser D, Morocutti G, Piccoli G, Zanuttini D, Gianfagna P, et al. Disease-specific differences of left ventricular rotational mechanics between cardiac amyloidosis and hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2014;307(5):H680–8. doi: 10.1152/ajpheart.00251.2014. [DOI] [PubMed] [Google Scholar]
- 20.Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson. 2012;14:43. doi: 10.1186/1532-429X-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster A, Morton G, Hussain ST, Jogiya R, Kutty S, Asrress KN, et al. The intra-observer reproducibility of cardiovascular magnetic resonance myocardial feature tracking strain assessment is independent of field strength. Eur J Radiol. 2013;82(2):296–301. doi: 10.1016/j.ejrad.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TS, Appleton CP. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr. 2009;22(3):299–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Schneeweis C, Qiu J, Schnackenburg B, Berger A, Kelle S, Fleck E, et al. Value of additional strain analysis with feature tracking in dobutamine stress cardiovascular magnetic resonance for detecting coronary artery disease. J Cardiovasc Magn Reson. 2014;16(1):72. doi: 10.1186/s12968-014-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sade LE, Demir O, Atar I, Muderrisoglu H, Ozin B. Effect of mechanical dyssynchrony and cardiac resynchronization therapy on left ventricular rotational mechanics. Am J Cardiol. 2008;101(8):1163–9. doi: 10.1016/j.amjcard.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Fung JW, Yip GW, Chan JY, Lee AP, Lam YY, et al. Improvement of left ventricular myocardial short-axis, but not long-axis function or torsion after cardiac resynchronisation therapy: an assessment by two-dimensional speckle tracking. Heart. 2008;94(11):1464–71. doi: 10.1136/hrt.2007.127498. [DOI] [PubMed] [Google Scholar]
- 26.Young AA, Cowan BR. Evaluation of left ventricular torsion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:49. doi: 10.1186/1532-429X-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen DE, Daughters GT, 2nd, Alderman EL, Ingels NB, Stinson EB, Miller DC. Effect of volume loading, pressure loading, and inotropic stimulation on left ventricular torsion in humans. Circulation. 1991;83(4):1315–26. doi: 10.1161/01.cir.83.4.1315. [DOI] [PubMed] [Google Scholar]
- 28.Donekal S, Ambale-Venkatesh B, Berkowitz S, Wu CO, Choi EY, Fernandes V, et al. Inter-study reproducibility of cardiovascular magnetic resonance tagging. J Cardiovasc Magn Reson. 2013;15(1):37. doi: 10.1186/1532-429X-15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swoboda PP, Larghat A, Zaman A, Fairbairn TA, Motwani M, Greenwood JP, et al. Reproducibility of myocardial strain and left ventricular twist measured using complementary spatial modulation of magnetization. J Magn Reson Imaging. 2014;39(4):887–94. doi: 10.1002/jmri.24223. [DOI] [PubMed] [Google Scholar]
- 30.Reyhan M, Kim HJ, Brown MS, Ennis DB. Intra- and interscan reproducibility using Fourier Analysis of STimulated Echoes (FAST) for the rapid and robust quantification of left ventricular twist. J Magn Reson Imaging. 2014;39(2):463–8. doi: 10.1002/jmri.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parisi V, Losi MA, Contaldi C, Chiacchio E, Pastore F, Scatteia A, et al. Speckle-tracking analysis based on 2D echocardiography does not reliably measure left ventricular torsion. Clin Physiol Funct Imaging. 2013;33(2):117–21. doi: 10.1111/cpf.12002. [DOI] [PubMed] [Google Scholar]
- 32.Kim HK, Sohn DW, Lee SE, Choi SY, Park JS, Kim YJ, et al. Assessment of left ventricular rotation and torsion with two-dimensional speckle tracking echocardiography. J Am Soc Echocardiogr. 2007;20(1):45–53. doi: 10.1016/j.echo.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Park CM, March K, Williams S, Kukadia S, Ghosh AK, Jones S, et al. Feasibility and reproducibility of left ventricular rotation by speckle tracking echocardiography in elderly individuals and the impact of different software. PLoS One. 2013;8(9):e75098. doi: 10.1371/journal.pone.0075098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Y, Alharthi MS, Thota VR, Yin L, Li C, Emani UR, et al. Evaluation of left ventricular rotation in obese subjects by velocity vector imaging. Eur J Echocardiogr. 2010;11(5):424–8. doi: 10.1093/ejechocard/jep230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.