Abstract
Objective
Treatment resistance complicates the management of schizophrenia. Research and clinical translation is limited by inconsistent definitions. To address this we evaluated current approaches and then developed consensus criteria and guidelines.
Method
A systematic review of randomized antipsychotic clinical trials in treatment resistant schizophrenia was performed. Definitions of treatment resistance were extracted. Subsequently, consensus operationalized criteria were developed by a working group of researchers and clinicians through i) a multi-phase, mixed methods approach; ii) identifying key criteria via an online survey; and iii) meetings to achieve consensus.
Results
42 studies met inclusion criteria. Of these, 21 (50%) studies did not provide operationalized criteria, whilst in others, criteria varied considerably, particularly regarding symptom severity, prior treatment duration and antipsychotic dose thresholds. Important for the inability to compare results, only two (5%) studies utilized the same criteria. The consensus group identified minimum and optimal criteria, employing the following principles: 1) current symptoms of a minimum duration and severity determined by a standardized rating scale; 2) ≥moderate functional impairment; 3) prior treatment consisting of ≥2 different antipsychotic trials, each for a minimum duration and dose; 4) adherence systematically assessed and meeting minimum criteria; 5) ideally at least one prospective treatment trial; 6) criteria that clearly separated responsive from treatment resistant patients.
Conclusions
There is considerable variation in current approaches to defining treatment resistance in schizophrenia. We present consensus guidelines that operationalize criteria for determining and reporting treatment resistance, adequate treatment and treatment response in schizophrenia, providing a benchmark for research and clinical translation.
Introduction
Schizophrenia is a severe mental disorder characterized by positive, negative and cognitive symptoms (1). The treatment of schizophrenia was revolutionized by the introduction of chlorpromazine in the 1950s (2). However, it rapidly became clear that some patients showed little if any clinical response despite treatment with multiple different antipsychotic drugs, with the sole exception of clozapine (3). In 1988, clozapine was shown to be effective where other antipsychotic drugs had failed (4), crystallizing the concept that in a proportion of patients schizophrenia is treatment resistant to most antipsychotics.
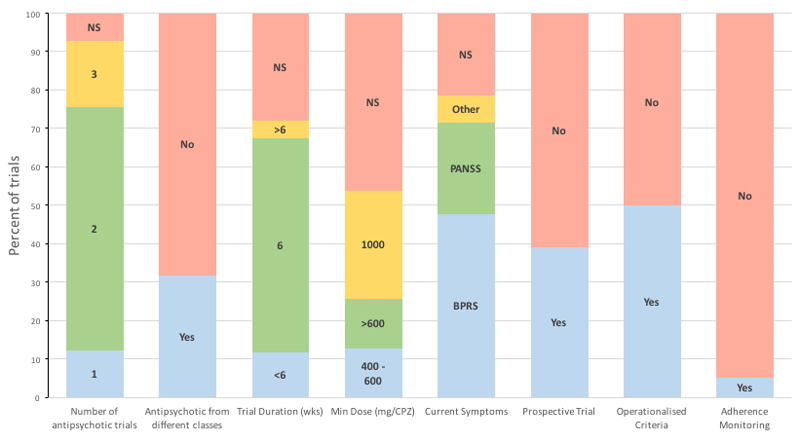
There has been a considerable amount of research into treatment resistance, and its management, which has formed a key component of treatment guidelines around the world (5–8). However, studies have used a variety of different approaches to defining treatment resistance, such that patients included in one study could be excluded from another, as illustrated in figure 1 (9).
Figure 1. Summary of criteria used in clinical trials of treatment resistant schizophrenia.
NS – Not specified. CPZ – Chlorpromazine equivalents
Consequently, comparing studies may be akin to comparing apples to oranges. This is a major hindrance to the field; making the interpretation of meta-analyses difficult, and potentially contributing to failures to replicate findings. For example, a recent network meta-analysis concluded clozapine was no more efficacious than other second-generation antipsychotics for treatment resistant schizophrenia (10), in contrast to an earlier meta-analysis by the same group that excluded studies focused only on treatment resistant patients (11).
Direct comparisons with the same intervention are also affected. For example, Bitter et al (12) found olanzapine to be efficacious; whilst Buchanan et al. (13) found no benefit for it. Heterogeneity of study designs and populations, including less restrictive definitions of resistance (see figure 1), may contribute to these inconsistencies (14).
This lack of uniformity in the definition of treatment resistance also impacts clinical guidelines that seek to distil the evidence from studies. Not surprisingly, given the variation in criteria used in the studies, treatment guidelines use vague definitions that are open to a wide range of interpretations (see table 1), potentially leading to inconsistent clinical management and treatment delays(15; 16).
Table 1. Recommendations for when to consider that a patient’s illness is treatment resistant used in international guidelines.
| Guideline | Requirements of previous treatment | Severity of illness | Other | |||
|---|---|---|---|---|---|---|
| Minimum number of failed APs | Specified AP | Adequate treatment episode duration | Dose | |||
| APA(6) | 2 | “At least one of which is a second-generation AP” | ≥6 weeks | Therapeutic range | “a clinically inadequate response” “and for patients with persistent suicidal ideation or behaviour that has not responded to other treatments” | Nil |
| RANZCP (90) | 2 | Recommends both first and second trial to be of an atypical | 6-8 weeks | Dosages specified for | “Poor response” | “If poor… adherence, or persistent suicide risk, positively offer trial of clozapine.” |
| BAP(91) | 2 | “One of the trials should be of an antipsychotic with an established, favourable, efficacy profile in comparison with other antipsychotics” | ‘Adequate’ | ‘Adequate’ | ‘schizophrenic illness has shown a poor response to, or intolerance of the neurological side effects of [previous treatment]’ | “Poor…adherence and …substance use should be excluded as causes of the …poor response to AP “ |
| IPAP(92) | 2 | “…a typical or, if not available a trial of haloperidol, chlorpromazine or other typical antipsychotic” | 4-6 weeks | ‘Adequate’ | Psychosis or mod-to-severe TD or tardive dystonia after adjusting dose” | Nil |
| Maudsley (79) | 2 | Consider use of either first generation or second generation AP | 2-3 weeks for trial of first AP in FEP. 6 week trial for subsequent 2nd AP before clozapine. | At least minimum effective dose, then titrated to response | Not specified | Nil |
| MOHS(93) | 2 | No | Adequate | Adequate | “illness has not responded adequately to treatment” | 2 trials should be given “sequentially” |
| NICE(5) | 2 | “One of the drugs should be a non-clozapine second-generation AP” | Not specified | Adequate | “illness has not responded adequately to treatment” | 2 trials should be given “sequentially” |
| WFSBP(7) | 2 | “one of which should be an atypical antipsychotic” | 6-8 weeks | Recommended dosage | no improvement at all or only insufficient improvement in the target symptoms | Compliance should be ensured, if necessary by checking drug concentrations |
AP – Antipsychotic; APA – American Psychiatric Assocation; BAP – British Association for Psychopharmacology; FEP – First Episode Psychosis; IPAP - The International Psychopharmacology Algorithm Project; MOHS – Ministry of Health Singapore; NICE – National Institute for Clinical Excellence; RANZCP - Royal Australian and New Zealand College of Psychiatrists; WFSBP - World Federation of Societies of Biological Psychiatry.
In view of this situation, the Treatment Response and Resistance in Psychosis (TRRIP) working group was formed to establish consensus criteria to standardize the definition of treatment resistance. The aim was to develop criteria to aid study design and facilitate comparison of results from different studies. These recommendations are not intended to restrict research using other criteria. However, by providing a consensus benchmark, it will be possible to specify how studies using other criteria differ from the consensus criteria, and to investigate to what degree this might influence results.
General requirements for treatment resistance
Several factors were considered in developing the criteria. First, there is the need for the criteria to encompass a core definition of treatment resistance that captures the worldwide understanding of the concept. Second, the criteria need to be applicable across a range of study designs, extending from longitudinal clinical trials and experimental medicine studies, to cross-sectional mechanistic investigations. Third, the criteria need to identify a group of patients who are clearly distinct from non-resistant patients. Finally, there is the need for the criteria to be practical, so that they can be used in a wide range of settings, but still rigorous.
Three key elements define the concept of treatment resistant schizophrenia. These are: 1) a confirmed diagnosis of schizophrenia based on validated criteria; 2) adequate pharmacological treatment; and 3) persistence of significant symptoms despite this treatment. We recognize that the optimal approach to determining lack of treatment response would be identifying patients at their first psychotic episode and prospectively assessing their response to sequential adequate treatment trials. However, this is unlikely to be practical for the majority of studies, and would be infeasible for identifying the many patients who develop resistance after years of treatment. In view of this fact, criteria need to also allow for cross-sectional identification of treatment resistance.
However, the risk of false positives is likely to be greater with the cross-sectional identification of treatment resistance than with prospective determination. This is because cross-sectional identification requires the retrospective determination of response and adequacy of treatment, and is dependent on potentially less reliable sources of information, such as case-notes and patient or informant report data. Whilst recognizing that with any approach there is a risk of false positives, it is important to have criteria that are sufficiently rigorous to capture the construct, yet also practical enough to enable studies to be conducted. In view of this we present two sets of criteria: minimum and optimum criteria. The optimum criteria are to be used where possible; particularly in clinical trials and hypothesis testing where the false positive rate should be low. The minimum criteria might be used for initial studies and hypothesis generation where there are practical limitations on study design and some false positives can be accepted.
Methods
An iterative approach was adopted to develop criteria for treatment resistance in schizophrenia. Initially, a systematic review of definitions of treatment resistant schizophrenia used in clinical trials was conducted. A literature search of PubMed, PsycINFO, and Embase from January 1980 to January 2016 was undertaken using the search string: “(randomized or random or randomly) and (resistant or refractory or clozapine) and (schizophrenia)”. Titles and abstracts were reviewed to initially determine eligibility. The reference lists of each relevant paper were also searched, as were reference lists of relevant review papers, to further identify potential studies. Studies were included if they were randomized controlled trials of a pharmacological intervention in adults with treatment resistant schizophrenia. Studies were excluded if they were naturalistic, or purely of biomarkers such as neuroimaging measures, studies of adjuvant treatments or non-pharmacological interventions, studies of childhood onset or late onset schizophrenia.
The data extracted were: the prerequisites for previous antipsychotic treatment (requirements of different antipsychotics, minimum treatment duration, dose); the specified severity of symptoms; and whether there was a stipulation for resistance to be prospectively demonstrated. Additionally, whether criteria were operationalized or not was recorded. To be considered as operationalized, the study had to report criteria that met the following characteristics: 1) The use of a validated rating scale to determine symptom severity; 2) A specification of minimum symptom duration; and 3) A definition of adequate treatment that specified minimum dose, duration, and number of previous antipsychotics.
Subsequently, a working group - consisting of expert researchers and clinicians, scientists from the pharmaceutical industry and other specialists with experience and expertise in the area of schizophrenia - was identified by the co-chairs of the Treatment Response and Resistance in Psychosis working group (OH, JMK, CUC). This was augmented by attendees at TRRIP meetings held at international conferences in the field. Members of the final working group included researchers who had published recently in the field and researchers who attended the inaugural TRRIP meeting at the Schizophrenia International Research Society Biennial meeting in 2014. The working group mapped out the key criteria and operationalized them.
Second, members of the TRRIP working group were contacted and invited to take part in an on-line survey to identify key areas of agreement and disagreement. The survey was developed by the TRRIP co-chairs and modified with input from TRRIP work group members. In its final version (see Appendix 1), the survey was conducted using SurveyMonkey (www.surveymonkey.com). 48 researchers and clinicians were invited by email to take part in the survey. Over the 30-day collection period, 29 responses (60%), covering 13 countries, were received to the on-line survey; 3 (10%) responses were incomplete. See supplementary information for a summary of the responses to individual items. These responses were synthesized and refined during subsequent discussions amongst the whole group to derive the consensus recommendations for both minimum and optimum criteria.
Third, the working group met to consider and revise criteria for which there was a lack of consensus. The revised criteria were circulated to the TRRIP working group members, and presented as part of an open workshop at an international meeting in the field for further discussion, input and refinement. Finally, consensus was reached regarding this publication through review by all authors.
TRRIP meetings
Criteria were discussed at the Schizophrenia International Research Society biennial meeting (2014 and 2016), the American College of Neuropsychopharmacology Annual Meeting (2014), and the International Congress On Schizophrenia Research (2015), where the open workshop also occurred.
Results
Systematic review
2,808 studies were identified of which 42 met selection criteria and were included in the review (see figure 1). Operationalized criteria were reported in 21 (50%) studies. Only two studies out of 42 used identical criteria to define treatment resistance, and these were from the same research group. In all, 26 studies (62%) required that individuals did not respond to at least two adequate treatment trials; there was no specification regarding class of antipsychotic in 29 (69%) studies; 24 (57%) studies defined an adequate treatment episode as lasting at least 6 weeks; and only 22 (52%) studies specified dosage in terms of chlorpromazine equivalents while the remainder used terms such as “adequate” without providing a dose. 20 (48%) studies rated current symptoms using the Brief Psychiatric Rating Scale (18), while 10 (24%) used the Positive and Negative Syndrome Scale (19). 16 (38%) studies employed a prospective phase of supervised treatment as part of the inclusion process. Two (5%) of the studies described assessment of past adherence, but neither described the methods employed to accomplish this.
Consensus recommendations (Table 2)
Table 2. Consensus criteria for assessment and definition of treatment resistant schizophrenia.
| Domain | Subdomain | Minimum Requirement | Optimum Requirement |
|---|---|---|---|
| Current symptoms | Assessment | Interview using standardised rating scale (e.g., PANSS, BPRS, SANS, SAPS) | Prospective evaluation of treatment using standardised rating scale |
| Severity | At least moderate severity | At least moderate severity and <20% symptom reduction during prospective trial/observation ≥6 weeks | |
| Duration | ≥12 weeks | ≥12 weeks. Specify duration of treatment resistance. | |
| Subjective distress | Not required | Not required | |
| Functioning | At least moderate functional impairment measured using a validated scale (eg z) | At least moderate functional impairment measured using a validated scale (eg SOFAS) | |
| Adequate treatment | Assessment of past response | Information to be gathered from patient/carer reports, staff and case notes, pill counts and dispensing charts. | Information to be gathered from patient/carer reports, staff and case notes, pill counts and dispensing charts. |
| Duration | ≥6 weeks at a therapeutic dose Record minimum and mean (sd) duration for each treatment episode |
≥6 weeks at a therapeutic dose Record minimum and mean (sd) duration for each treatment episode |
|
| Dose | Equivalent to ≥600mg chlorporamzine per day1 Record minimum and mean (sd) dose for each drug |
Equivalent to ≥600mg chlorporamzine per day1 Record minimum and mean (sd) dose for each drug |
|
| Number of anti-psychotics | ≥2 past adequate treatment episodes with different antipsychotic drugs Specify median number of failed antipsychotic trials. |
≥2 past treatment episodes with different antipsychotic drugs and at least one utilizing a long-acting injectable antipsychotic (for at least 4 months). Specify median number of failed antipsychotic trials. | |
| Current Adherence | ≥80% of prescribed doses taken. Adherence should be assessed using ≥ 2 of pill counts, dispensing chart reviews and patient/carer report. Antipsychotic plasma levels monitored on at least one occasion. Specify methods used to establish adherence. |
As for minimum criteria and additionally trough antipsychotic serum levels measured on at least two occasions separated by at least two weeks (without prior notification of patient). | |
| Symptom Domain | Positive/Negative/Cognitive | ||
| Time course | Early-onset (within 1 year of treatment onset)/ Medium-term onset (within >1-5 years of treatment onset)/ Late-onset (after >5 years of treatment onset) | ||
| Ultra-treatment resistant: clozapine | Meets the criteria for treatment resistance above plus failure to respond to adequate clozapine treatment2 | ||
BPRS- Brief Psychiatric Rating Scale; CGI-S-TRS - Clinical Global Impressions-Severity Treatment Resistant Schizophrenia scale; PANSS- Positive and Negative Syndrome Scale; ECT - Electro-convulsive therapy; SANS - Scale for the Assessment of Negative Symptoms; SAPS - Scale for the Assessment of Positive Symptoms; SOFAS- Social and Occupational Functioning Scale
See section 5.5
The consensus criteria are summarized in table 2 and discussed below. See supplementary information for a further discussion of the basis for these recommendations.
1. Terminology
It is recommended that the term “treatment resistant schizophrenia (TRS)” be used to describe cases of schizophrenia meeting the criteria outlined below, and that use of this term is restricted to patients meeting these criteria. The consistent use of this term will facilitate communication and the identification of relevant literature. In the future, if treatments other than antidopaminergic antipsychotics become established for schizophrenia, it may be necessary to add treatment specifiers, such as “dopamine blocking” treatment resistant schizophrenia.
2. Clinical sub-specifiers
The initial trials demonstrating the superiority of clozapine for treatment resistance were undertaken in patients with a high degree of positive symptoms, and in clinical practice this remains the archetypal treatment resistant patient, driven also by the fact that current effective treatments for schizophrenia remain limited to positive symptoms. However, an increasing amount of research has investigated patient groups, that while termed “treatment resistant”, may significantly differ from one another in their symptom profile. As a result there is a need for clarity as to patients’ clinical profile. A patient’s illness may meet criteria based on overall symptoms, or due to specific sub-domains of positive, negative or cognitive symptoms. It may not be appropriate to compare patient groups where the illness is predominantly resistant to treatment in one domain with those in another domain. In view of this, two recommendations are made. First, that the symptom domains used to define resistance are made explicit; and, second, that the domain is specified using the sub-specifiers: positive, negative or cognitive (the latter contingent on developing reliable criteria). Where the patient group is defined as meeting a given threshold of positive symptoms this is specified as “treatment resistant schizophrenia- positive symptom domain”, and similarly “treatment resistant schizophrenia- negative symptom domain”, and “treatment resistant schizophrenia- cognitive symptom domain” for the other categories. Where more than one domain is involved, this may be specified, for example as “treatment resistant schizophrenia- positive and negative symptom domains”.
3. Symptom thresholds
3.1. Rating scales
As can be seen from our summary of clinical guidelines for treatment resistance (table 1), the current clinical guidelines for symptom response use terms such as “not adequate” that are poorly operationalized. Furthermore, the reliability of these definitions for treatment resistance has not been established. In view of this situation, a clinical or case note diagnosis of treatment resistance based on clinical guidelines cannot be recommended. Instead, it is recommended that a standardized, validated symptom rating scale, such as the Positive and Negative Syndrome Scale (17), the Brief Psychiatric Rating Scale (18), the Scale for the Assessment of Negative Symptoms SANS (19), or the Scale for the Assessment of Positive Symptoms SAPS (20), is used to measure current overall, positive and negative symptom severity.
3.2. Absolute thresholds
There are two components to the symptomatic assessment of treatment resistance. The first is the absolute threshold of current severity. It is conceivable, although in practice unlikely, that a patient never has more than mild symptoms, but has not shown a response to a series of treatments. Whilst the patient’s symptoms are treatment resistant, there are clinical and methodological risks associated with including such a patient in studies. Firstly, mild severity on rating scales is at the borderline with uncertain symptoms. Given that, even when carefully applied, inter-rater reliability for rating scales is 0.85-0.9 (21), the measurement error means that there is the risk of including patients with uncertain symptoms. Secondly, the clinical risk-benefit balance in patients with mild symptoms is very different from that in patients with more severe symptoms, where the severity of the condition provides much stronger support for experimental interventions. In view of this, it is recommended that the minimum threshold for current symptoms should be at least moderate severity, as defined on a standardized rating scale.
By the same token, it is conceivable that a patient could have a rating of moderate severity on just one symptom item and no other ratings. Given measurement error, there is the risk that this patient’s illness is sub-threshold. Thus, it is recommended that the threshold of at least moderate severity is attained for more than one symptom in the given domain or, if there is only one symptom, that it should be at least severe. These criteria are minimum thresholds that are designed to ensure that patients are clearly currently unwell to a degree that would warrant intervention. These severity threshold criteria are intended to apply to each domain. So, for example, a study of resistant positive symptoms would require at least two positive symptoms of moderate or greater severity, or at least one symptom with at least a severe rating, and a study of negative symptoms would require at least two negative symptoms at moderate or greater severity, or at least one symptom with at least a severe rating. A study of both resistant negative and resistant positive symptoms would need to meet these criteria in each domain. Of course, a study may recruit patients who are much more severely unwell. We do not mean to preclude research focusing on patients who are not included in these definitions, but recommend that the criteria used are given relative to these criteria so that their differentiating characteristics are clear and reported. This will facilitate future comparisons across studies.
It should be relatively straightforward to apply the minimum criteria discussed above to positive and negative symptom domains where validated scales exist. However, there is no cognitive symptom domain in the most widely used clinical rating scales (e.g., PANSS, BPRS, SANS, SAPS) and few if any items cover cognitive symptoms in these rating scales. In view of this it is not currently possible to recommend threshold criteria for cognitive symptoms. However, a number of current initiatives, such as the MATRICS and others (22; 23), aim to develop and validate reliable cognitive batteries for the assessment of cognitive symptoms in schizophrenia. These will enable the establishment of criteria for treatment resistance in the cognitive domain in the future. It should also be noted that factor analyses of rating scales have identified other domains, which may be of interest in specific studies. We recommend that where these are used they are specified in the same manner as the domains listed here.
3.3. Symptom change
The second component of symptomatic assessment is the determination of response to treatment relative to a baseline. Ideally this should be performed prospectively for two treatment episodes with different antipsychotic drugs. Whilst this will not always be practical, it is recommended that there is at least one prospective evaluation of treatment efficacy. If this is not possible, then this should be clearly specified and a retrospective assessment of response to treatment obtained as a minimum. A change of 20% is the minimum that can be routinely detected clinically (24). Therefore, a reduction less than 20% will correspond to a clinically insignificant reduction in symptoms. It could be argued that larger reductions may still not be clinically meaningful. However, given that an improvement of ≥20% has been used to identify treatment responders (25), requiring <20% reduction ensures the treatment resistant group does not overlap with treatment responders. Therefore, it is recommended that at the end of the prospective evaluation the absolute symptom severity rating criteria above are still met, and that symptom reduction should be <20% both for the total rating and specific domain of interest before such a patient be included in a prospective treatment trial of treatment-resistant schizophrenia. In the event that a patient shows an improvement of ≥20% during the prospective observation period, then the patient should be re-evaluated and, if he/she still fulfils absolute criteria for treatment-resistance be observed for another prospective evaluation period. Only patients who during the prospective observation improve by <20% and still fulfill absolute severity thresholds for treatment resistance should be called treatment-resistant and included in prospective studies. In contrast, precise quantitative assessment is unlikely to be feasible for retrospective evaluation (which is exactly why we recommend prospective evaluation of treatment resistance). Therefore, for past treatment episodes, we recommend that patients should be rated as less than ‘minimally improved’ on the overall change in the Clinical Global Impression-Schizophrenia Scale (26). It is recommended that multiple sources of information, including patient and caregiver reports, case notes and staff report, are used to evaluate past response. Nevertheless, as measurement error is likely to be larger in the retrospective evaluation of response to past treatment, in order to be conservative, it is recommended that where there is missing information or doubt, investigators err on the side of caution and exclude subjects or prospectively evaluate non-response in at least this subgroup. A further important requirement, is that investigators ensure that rating scales are adjusted to a baseline of zero. For example, a score change from 90 to 60 in the 30-item PANSS, each scored 1-7, represents a 50.0% reduction rather than 33.3%. Using a non-zero score for absent symptoms with the PANSS will lead to underestimation of treatment effects when percentage change in symptoms is calculated (27).
3.4. Functional impact
It is of course conceivable that a subject has symptoms at threshold severity, but that these have little functional impact (28; 29). Thus, in addition to symptom severity it is recommended that functional impairment is measured using a recognized, validated measure and that this is reported. Scales that just index functioning, such as the Role Functioning Scale (30) or the Social and Occupational Functioning Scale (SOFAS) (31), are preferred over scales that include symptom assessment as part of the measure as symptom severity can strongly influence ratings. To be consistent with required symptom thresholds, we propose that there is moderate (eg: score <60 on SOFAS) or more severe functional impairment.
Distress caused by symptoms is also an important factor to consider. However, due to lack of insight associated with schizophrenia (32), some patients may not report distress. Furthermore, distress is de facto subjective and difficult to operationalize. In view of these factors, it is recommended that subjective distress should not be a requirement (although recording or measuring it is desirable to capture patient-centered outcomes).
It should be recognized that symptoms and function may fluctuate as part of the natural history of the disorder and that there is an element of measurement error in the assessment of symptoms (1; 21). Therefore, it is necessary to establish that symptoms have persisted over a reasonable period of time to be clear that a patient is truly treatment resistant. It is recommended that a minimum of 12 weeks duration of symptoms be used, during which symptoms and functional impairment are of at least moderate severity threshold severity and that the minimum duration be clearly identified.
4. Characterizing treatment resistance
4.1. Degree
Treatment resistance is mostly treated as a binary variable as a study entry or treatment decision criterion in research and clinical practice. This is often necessary for research purposes and when making clinical decisions. Clinically, however a continuum is apparent (33). As such, carefully characterizing patients will aide a finer grained assessment of biological mechanisms or treatment effects in well-defined subgroups of patients with treatment resistance. Thus, it is recommended that symptom and functional measures are reported in as much detail as possible. As a minimum, this should include positive and negative symptom ratings using a validated instrument such as the BPRS, PANSS or SAPS and SANS and a measure of functional impairment using a validated measure such as the Role Functioning Scale or SOFAS (17; 19; 20; 31; 34; 35). These measures should also be used to characterize change after an intervention, as treatment may affect certain symptom domains more than others. This characterization will facilitate research into the continuum of treatment resistance, and enable better comparison between studies as well as an estimation of the room for improvement at an individual level.
4.2. Temporal development
A further issue is when treatment resistance begins. Studies show that treatment resistance is present from illness onset in some patients, whilst in others the illness shows an initial response to treatment, but subsequently resistance develops (36–40). From a theoretical perspective, both the mechanisms underlying resistance and the therapeutic implications may be different in these two situations: for example, clozapine does not show clear superiority over other antipsychotic drugs in non-treatment resistant first episode patients (41; 42). Whilst the importance of this is not clear, to facilitate research into these issues, it is recommended that it is specified whether patients have been treatment resistant from within the first year of treatment (early-onset treatment resistance), or have developed it during 1 to five years after onset of treatment (medium-term onset treatment resistance), or later than five years after onset of treatment (late-onset treatment resistance). Ideally, the duration of treatment resistance should also be ascertained and reported. Other factors posited to be relevant to the pathophysiology of resistance, such as development of resistance following relapse and misuse of substances, should be recorded where possible (43). It is important to note that duration of treatment resistance relates to treatment onset and not illness onset, otherwise it could be confounded by duration of untreated psychosis.
5. Defining Adequate Treatment
5.1. Duration
It could always be argued that a patient may respond if treatment is given for a little longer, which, taken to the extreme, leads to the requirement that a patient would need to take a given treatment for life to be certain they will not respond. However, few non-responders within the first 6 weeks go on to respond at later time points, and clinical trials for licensing, which form a large basis of the evidence base, generally last 4-6 weeks.(44) Clearly there is the need to balance the risk of false positives with practical considerations. Thus it is recommended that each antipsychotic treatment episode should have lasted at least 6 weeks, at a therapeutic dose (see 5.2), to be deemed ‘adequate’. Thus, given the minimum number of different antipsychotic treatment episodes (see 5.3), the minimum duration of treatment required is 12 weeks. As outlined below (see 5.5), to rule out “pseudo-resistance” due to inadequate treatment adherence, the optimal definition of treatment resistance would include at least one failed trial with a long-acting injectable antipsychotic (LAI), given for at least 6 weeks after it has achieved steady state (generally at least 4 months from commencing treatment) (45; 46).
5.2. Dose
For a treatment episode to be deemed therapeutic, the minimum dose of prescribed oral or injectable antipsychotic should be the target dose (or mid-point of the target dose range) for the acute treatment of schizophrenia given in the manufacturer’s summary of product characteristics. If this is not clear or practical, it is recommended that a total daily dose equivalent to 600mg of chlorpromazine per day (determined using established conversion ratios such as those given in recent papers regarding dose conversion (47–49)) is used as the minimum. It is recommended to err on the side of a higher minimum dose where there is a range of possibilities. If a trial has to be aborted secondary to intolerability prior to reaching criteria for an adequate therapeutic dose maintained for at least 6 weeks, it should not count as a failed adequate treatment trial.
5.3. Number of past treatment episodes
Failure of at least two adequate treatment episodes with different antipsychotic drugs, each meeting the above criteria, is required to establish treatment resistance. In some clinical guidelines it is recommended that these trials include different types of antipsychotic (such as first- and second-generation drugs) (table 1). However, given the overlap in side-effects, efficacy and receptor profiles among currently available non-clozapine antipsychotics, the consensus was that the current data do not provide unequivocal support for therapeutic categories of different antipsychotic drugs (11; 50). There was some disagreement about this conclusion amongst the working group members, as olanzapine, risperidone and amisulpride show consistent, though small, advantages in meta-analyses of efficacy (51). However, consensus was reached that, when considering this from a practical perspective as well, specifying particular drug(s) would limit generalizability, not least because a given drug may not be readily available in some settings (for example, amisulpride in the USA). In view of this, a requirement to use particular categories or drugs (apart from clozapine) is not currently recommended. Of course, particular drugs may be stipulated in a given study where there is a specific reason to focus on patients who have not responded to a certain drug or group of drugs. In practice, many patients will have tried a large number of different drugs (16). In view of this, the total number of failed adequate antipsychotic treatment trials, the drugs and their dose and route, should be ascertained and reported where possible. As mentioned above, a trial with a LAI would be optimal to establish treatment resistance not confounded by treatment non-adherence.
It terms of both duration and number of treatment trials, it is necessary to promptly optimize treatment, yet to also minimize the risk of prematurely discarding potentially effective treatments. Arguments can be made for extending treatment trials, given that a proportion of patients appear to show a delayed response (52), conversely it can also be argued that treatment with a second non-clozapine antipsychotic after initial treatment failure is not warranted, given that response rates seem to be below 20% (36). The proposed criterion of at least two trials lasting a minimum of 6-weeks aims to strike a balance between these two opposing views.
5.4. Clozapine resistant schizophrenia
For clarity and due to the specific role of clozapine in the treatment of resistant schizophrenia (53–57), failure to respond to clozapine is to be used as a subspecifier of treatment resistant illness, i.e., clozapine-resistant schizophrenia. In addition to using the mid-dose range as a minimum requirement for an adequate trial, and the adherence requirements below (5.5), it is recommended that trough serum levels of clozapine are measured on at least two occasions separated by at least a week at a stable dose of clozapine. This is important not only to establish adherence, but also because of the link between serum levels of clozapine and response (58–62). Clozapine levels ≥350 ng/ml (63) constitute an optimum threshold requirement for establishing non-response to clozapine treatment. It is strongly recommended that levels are used, not least because of the major effect of smoking and gender on clozapine’s pharmacokinetics, but where obtaining blood is not possible, a minimum dose of 500mg/day is recommended, unless tolerability issues restrict the dose range. This dose is in the middle of the approved dose range for clozapine, and it was only at doses of over 400mg a day that clozapine proved superior to other antipsychotics in a met-analysis of head-to-head comparisons (64). The duration of an adequate trial of clozapine remains to be definitively determined (65). A number of studies have recommended trial durations of between 4 and 12 months (66–68). Others, however, have suggested that the time course of response is not significantly different to non-clozapine antipsychotics (69–71), and the perception of a delayed response may primarily be due to the time taken to reach a therapeutic level (72). Due to the lack of clarity as to where to proceed following a failed clozapine trial, and the clinical effort required to establish treatment with clozapine, we recommend clozapine therapy should be tried for a duration of at least 3 months following attainment of therapeutic plasma levels.
5.5. Adherence
Due to difficulties with adhering to dosing schedules, lack of illness insight, side effect burden, cognitive impairment and other factors, non-adherence is a significant problem in the treatment of schizophrenia and is often under-recognized (73–76). Non-adherence may be the single largest source of unrecognized error in studies of treatment resistance (73). Consequently, it is important to make strenuous efforts to determine adherence and apply criteria to exclude poorly adherent subjects who can represent false positive “pseudo-resistant” cases. Whilst 100% adherence is rare even in clinical trial settings (77; 78), it is necessary to be close to this figure, otherwise the study will be of non-adherence rather than of treatment resistance.
As a minimum, it is recommended that patients have taken ≥80% of prescribed doses at the prescribed dosage level over the required ≥12-week treatment period during which the criteria for treatment resistance have persisted. This adherence level should be determined by as many sources as feasible, including a minimum of two out of: pill counts, dispensing chart review and patient/caregiver report. Sources should be specified, but patient report alone is unlikely to be sufficient (34). In addition, given that there may still be covert non-adherence, antipsychotic blood levels should be determined in all patients taking oral medication on at least one occasion (and optimally ≥2 occasions each separated by at least two weeks). Because anticipation of blood could encourage an unrepresentative period of increased adherence beforehand, tests need to be conducted without advance notice of when. Where guidelines (such as the Maudsley Prescribing Guidelines(79)) indicate a minimum plasma level associated with response, this should be used as a minimum criterion. However, where there is a lack of consensus as to what is a therapeutic plasma level, a minimum level will need to be set based on what can be expected in people regularly taking the drug at a therapeutic dose (80). Nevertheless, unless blood level monitoring is very frequent, covert non-adherence is still possible. Thus, where possible, or as a pragmatic and likely superior alternative to documenting adequate antipsychotic blood levels on at least one occasion, it is recommended that one of the failed treatment episodes involves a LAI; or alternatively, that adherence has been monitored via direct observation or with technological assistance (81).
6. Defining adequate treatment responders
Cross-sectional and mechanistic studies will often require a comparator group of participants who have shown a good response to treatment. For consistency, the same clinical rating scales need to be used to identify this group as are used to identify the treatment resistant group. In addition, the criteria need to ensure that there is a clear distinction between groups. This precondition requires that the criteria make allowance for measurement error, and have clear separation of thresholds; to avoid the inclusion of participants rated in a borderline zone who are potentially eligible for both groups, dependent on the rater or day that they are rated. As such, it is recommended that as an absolute symptom threshold responders show no more than mild symptom severity across the symptom items in the domain(s) of interest, and have shown this over at least 12 weeks. Where possible it is recommended that response is ascertained prospectively over at least 6 weeks and defined as at least a 20% improvement in symptom scores for the domain of interest as well as meeting the absolute thresholds. Furthermore, there may be circumstances, for example studies in first episode patients, where this threshold may be of insufficient stringency. In these circumstances investigators may choose even more rigorous stability criteria to define adequate treatment response, such as having achieved remission, consisting of no more than mild positive and negative symptoms for ≥6 months (8), or no symptoms at all. In addition to the symptom severity threshold, current functional impairment should not be more than mild (eg >60 on SOFAS) in all circumstances (see table 3 for a summary of criteria).
Table 3. Criteria for establishing a group of patients with adequate treatment response.
| Treatment Response | Symptom severity | Symptoms rated at no more than mild severity |
| Duration | Response sustained for a minimum of 12 weeks | |
| Functioning | Mild or better functioning on a standardised scale (e.g. SOFAS) |
Discussion
Our review of the criteria currently used to define treatment resistance in clinical trials identified significant limitations in published studies. Notably, 50% of studies did not use fully operationalized criteria, rendering it impossible to accurately replicate these studies. Furthermore, there was wide variation in the criteria used, with 95% of studies using different criteria, complicating comparisons across studies. Finally, key aspects of determining treatment resistance were not specified in many studies. For example, assessment of prior antipsychotic adherence was not specified in 95% of studies. These findings indicate a need for criteria that can be used as a benchmark for future studies.
We developed criteria to address this need. Across a wide range of areas, there was a relatively clear consensus in the working group as to how to best define treatment resistant schizophrenia. A summary of the consensus criteria is shown in table 2. The criteria we suggest show agreement in a number of domains with those used in the majority of previous studies in the literature, in particular the requirements for at least two failed treatment trials each of a minimum of six weeks, and the use of standardized rating scales (supplementary table 1). However, our recommendations differ from approaches used by most studies in the literature to date in several key domains. In particular, our recommendations have clear criteria for ensuring adequate adherence, and for the inclusion of functional impairment. Furthermore, our recommendations include specifiers to characterize the sample, and cover reporting standards to aid comparisons across studies. Finally, we recommend a lower minimum antipsychotic dose than many early studies required, reflecting the recognition in the field that very high doses generally increase the risk of side-effects without additional therapeutic benefit.
The universal adoption of these consensus criteria would facilitate literature searches and meta-analyses as well as help to improve the design of studies. The implementation of operationalized criteria should improve the quality and reproducibility of research in the area of treatment resistant schizophrenia, both in the neurobiological and treatment domains, akin to what has been achieved by operationalizing criteria for treatment remission in schizophrenia (8). The next step is to utilize the criteria in different research settings to evaluate their ease of use and reliability, both within and between raters. We encourage interested researchers to help with this effort by forming a Treatment Response and Resistance in Psychosis (TRRIP) Trial Network. It should be noted that these criteria are not intended to govern clinical practice in the sense that clozapine should only be prescribed to patients fulfilling research criteria for treatment resistant schizophrenia. Thus, this is not a treatment guideline and the various clinical scenarios that may prompt clinicians to use different treatments for patients with schizophrenia are not addressed here.
Strengths and Limitations
The recommendations presented here have been developed through an iterative process and in consultation with expert researchers and clinicians from across the world. As such, they extend previous recommendations (e.g. (82; 83)) to reflect a wide body of opinion, and have been refined to be applicable to a variety of settings. Nevertheless, a limitation is that they may not reflect practice or opinion in all locations. We have attempted to consult widely to mitigate this issue, and sought to produce criteria that are sufficiently representative as to be useful to the field. Furthermore, we have attempted to produce practical criteria that can be easily implementable whilst also addressing the limitations of previous approaches.
Although not all invited experts responded to the online survey, they all participated in discussions and the development of the consensus criteria. Moreover, whilst the survey identified some areas where there were small majorities (see supplementary information), subsequent discussions clarified and refined the criteria to enable agreement and all participants subscribe to the final criteria presented here.
Although in clinical care and in treatment guidelines, antipsychotic treatment combined with psychosocial strategies is advocated for the optimal care of people with schizophrenia, we did not specify a minimum level of “adequate” psychosocial interventions as a prerequisite before treatment resistance could be defined. This decision was not based on an underestimate of the importance of psychosocial treatments, but rather based on the current lack of operationalized criteria for determining adequate psychosocial treatment (84). We anticipate revising this aspect once initiatives to develop criteria have reported data that will allow for a standardized approach.
An important conceptual issue is that the recommendations are based on clinical criteria only. The clinical end-point may incorporate multiple pathophysiological pathways, which may have different treatment implications. As such, whilst clinical criteria are the current state-of-the-art, we anticipate that ultimately the classification will be revised and informed by the underlying biology and mechanisms as evidence on these emerges (85–87).
A further potential issue is that there is likely a continuum of treatment response, and that dichotomous categories such as “adequate treatment response” and “treatment resistance” are crude and reductionistic. The endorsement of some (established) rating scales or some “cutoffs” to achieve this, from a list of many other potentially useful options, may be considered as a compromise. Whilst we acknowledge this, clinicians and patients have to make choices about whether to continue with a given treatment, and research studies require patients to be randomized to a given treatment. In this context, the categorisation we propose aims to prioritize specificity over sensitivity and should help facilitate both clinical care and research decisions.
The criteria recommended here reflect a consensus on the balance between practical considerations, the risk of false positives and the potential to translate findings derived from studies into clinical practice. It is acknowledged that alternative cut-offs may be more appropriate in specific studies, but we recommend that these criteria are specified in reference to the benchmarks outlined here, so that it is clear in what way the criteria are different.
Finally, we have codified the concept that treatment resistance may develop at different stages of the illness, or be present from illness onset. Clinically, it is clear that there are some patients who initially experience a good response to antipsychotic treatment and treatment resistance later develops, whilst others have little or no response from illness onset (36–40). This is of considerable potential clinical and mechanistic importance. However, despite this wide-spread clinical observation, there is relatively little research evidence on this issue (36–40). Our categorisation does introduce boundary issues, particularly between early and late treatment resistance, where it may be argued that there is likely to be little difference between a patient who develops treatment resistance after 4 years of treatment, and a patient who develops it after 5 years of treatment.
However, practical considerations required a cut-off that would be easy to apply and that reflected widespread clinical and research definitions of the early course of schizophrenia, which include the first five years following illness onset (88; 89). It is intended that the criteria will stimulate research into whether there are differences between patients who develop treatment resistance early, late or from illness onset, and clarify the reporting of studies.
Conclusions and future directions
Treatment resistant schizophrenia is a major clinical problem, and clinical guidelines throughout the world recommend specific treatments for affected individuals (5–7). A wide variety of criteria have been applied in research studies. As a consequence, clinical guidelines based on these studies use imprecise or inconsistent definitions that are likely to include patients with very different clinical characteristics to the patients included in the clinical trials on which the guidelines are based. Furthermore, the variation in criteria limits comparison of studies, complicates the interpretation of findings, and may contribute to the failure to replicate findings (12; 13).
We have developed operationalized criteria to address this issue based on a process of wide consultation and refinement, involving expert researchers and clinicians, scientists from the pharmaceutical industry and other specialists who are active in the field. It is intended that they provide benchmarks to aid study design and reporting as well as research into the neurobiology of more homogeneously defined subgroups and the development of novel treatment strategies. We acknowledge that some criteria may not be appropriate for certain questions or studies. It is not intended that these criteria prevent studies using alternative criteria, but where researchers use alternative criteria, we strongly recommend that the differences are indicated (and justified) against the benchmark given in table 2.
Supplementary Material
Footnotes
Disclosures:
Ofer Agid Advisory Board / Consultant: Janssen-Ortho (Johnson & Johnson); Sepracor, Sunovion, Roche, Novartis, BMS, Otsuka, Lundbeck, Eli Lilly & Company U.S., Eli Lilly Canada; Sumitomo Dainippon Pharma (DSP). Speaking engagements: Janssen-Ortho (Johnson & Johnson); Novartis; Sepracor Inc.,US.; Sunovion, Lundbeck, Eli Lilly & Company U.S., Eli Lilly Canada; Mylan Pharmaceuticals; Otsuka. Research Contracts: Pfizer, Inc.; Janssen-Ortho (Johnson & Johnson); Otsuka; Boehringer Ingelheim; Neurocrine Biosciences, Sunovion.
Rodrigo A. Bressan has received research grants from FAPESP, CNPq, AstraZeneca, Eli-Lilly, Lundbeck, Roche and Janssen for clinical trials, has participated on Advisory Boards AstraZeneca, Eli-Lilly, Lundbeck, Roche and Janssen and received speaking fees AstraZeneca, Eli-Lilly, Lundbeck, Roche, Janssen and Ache.
Robert W. Buchanan has served on Advisory Boards for AbbVie, Amgen, Boehringer Ingelheim-RCV, and Takeda; and has served on a DSMB for Pfizer.
William T. Carpenter has served on advisory boards for Teva, Genetech and Allergan and has collaborated with HealthAnalytics.
David Castle - David Castle has received grant monies for research from Eli Lilly, Janssen Cilag, Roche, Allergen, Bristol-Myers Squibb, Pfizer, Lundbeck, Astra Zeneca, Hospira; Travel Support and Honoraria for Talks and Consultancy from Eli Lilly, Bristol-Myers Squibb, Astra Zeneca, Lundbeck, Janssen Cilag,Pfizer, Organon, Sanofi-Aventis, Wyeth, Hospira, Servier; and is a current Advisory Board Member for Lu AA21004: Lundbeck; Varenicline: Pfizer; Asenapine: Lundbeck; Aripiprazole LAI: Lundbeck; Lisdexamfetamine: Shire; Lurasidone: Servier. He has no stocks or shares in any pharmaceutical company.
Leslie Citrome in the past 36 months has engaged in collaborative research with, or received consulting or speaking fees, from: Acadia, Alexza, Alkermes, Allergan, AstraZeneca, Avanir, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Forum, Genentech, Janssen, Jazz, Lundbeck, Merck, Medivation, Mylan, Neurocrine, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, Valeant, Vanda
Christoph U. Correll has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Actavis, Actelion, Alexza; Alkermes, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forum, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Lundbeck, Medavante, Medscape, Merck, Otsuka, Pfizer, ProPhase, Reviva, Roche, Sunovion, Supernus, Takeda, Teva, and Vanda. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Eli Lilly, Janssen, Lundbeck, Pfizer, Takeda and Otsuka. He received grant support from Bristol-Myers Squibb, Otsuka, Lundbeck and Takeda.Dr. Kane has been a consultant for Amgen, Alkermes, Bristol-Meyers Squibb, Eli Lilly, Envivo (Forum) Genentech, H. Lundbeck. Intracellular Therapeutics, Janssen Pharmaceutica, Johnson and Johnson, Merck, Novartis, Otsuka, Pierre Fabre, Proteus, Reviva, Roche,Sunovion and Teva
Zafiris J. Daskalakis has received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc, has also served on the advisory board for Sunovion, Hoffmann-La Roche Limited and Merck and received speaker support from Eli Lilly in the last 5 years.
Michael Davidson has received research grant support and/or travel support and/or speaker fees and/or consultant fees from Lundbeck, Eli Lilly, Servier, Roche, Takeda, Forum, JNJ, Teva, Minerva and holds stocks in CTR
Andrea de Bartolomeis has received research support from Otsuka, Lundbeck, Janssen; lecture fees from Takeda, Sunovion, Lundbeck Chiesi, Roche. Has served as consultant in advisor boards for Jansen, Lundbeck, Otsuka, Roche, Eli Lilly, Takeda.
Richard Drake has received honorarium for advisory board participation for Lundbeck
Bjørn H. Ebdrup has received lecture fees from Bristol-Myers Squibb, Otsuka Pharma Scandinavia AB, and Eli Lilly and Company and is part of the Advisory Board of Eli Lilly Danmark A/S, Janssen-Cilag and Takeda Pharmaceutical Company Ltd.
Helio Elkis has received research grants from FAPESP, Roche and Janssen and received honoraria for travel support, advisory board participation or as speaker from Roche, Janssen and Cristalia
Wolfgang Fleischhacker has received grant or research support, been affiliated with, or received honoraria and travel expenses related to lectures/presentations or consultant activities for AOP Orphan, Acadia, Boehringer Ingelheim, Dainippon Sumitomo, Forest, Gedeon Richter, Janssen, Lundbeck, Otsuka, Reckitt-Benckiser, Roche, Takeda, Targacept, Teva, and Vanda.
Ary Gadelha has participated on Advisory Boards for Janssen-Cilag
Fiona Gaughran has received honoraria for lectures and advisory/consultation work for Lundbeck, Roche, Sunovion and Otsuka and has research funded by a NHS Innovations/Janssen-Cilag award. She has a family member with professional links to GSK and Lilly, and share options in GSK.
Birte Glenthøj is the leader of a Lundbeck Foundation Center of Excellence, CINS, which is partially financed by an independent grant from the Lundbeck Foundation based on international review and partially financed by the Mental Health Services in the Capital Region of Denmark, the University of Copenhagen, the Danish Council for Independent Research, and other foundations. All grants are the property of the Mental Health Services in the Capital Region of Denmark and administrated by them.
William Honer has served on Advisory Boards or consulted for In Silico, Roche, Otsuka/Lundbeck and Eli Lilly. William Honer was supported by the Jack Bell Chair in Schizophrenia
Oliver Howes has received investigator-initiated research funding from and/or participated in advisory/ speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Dr Howes is funded by Medical Research Council-UK (no. MC-A656-5QD30), Maudsley Charity (no. 666), Brain and Behavior Research Foundation, and Wellcome Trust (no. 094849/Z/10/Z) grants to Dr Howes and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Neither Dr Howes or his family have been employed by or have holdings/ a financial stake in any biomedical company.
John Kane has received honoraria for lectures from Bristol-Meyers Squibb, Eli Lilly, Genentech, Janssen and Otsuka;and is a Shareholder in MedAvante, Inc., Vanguard Research Group, LB Pharmaceuticals
James Kennedy has received honoraria from Novartis, Shire, and Purdue Pharma for lectures.
Bruce J. Kinon is an employee of Lundbeck LLC
Stephen M Lawrie has received grant monies for research from Abbvie, Pfizer and Roche, and honoraria for talks and consultancy work from Forum, Janssen-Cilag, Roche and Sunovion.
Jimmy Lee has received travel support and honoraria from Roche and JanssenCilag.
F. Markus Leweke has received honoraria for lectures from AstraZeneca and is a shareholder of curantis UG (ltd.).
Herb Meltzer: Grantee:Dainippon Sumitomo, Sunovion, Boehringer Mannheim, Eli Lilly, Reviva, Janssen, Otsuka, TEVA, MagT Therapeutics. Consultant: ACADIA, Allergan, Neurocrine, Astellas. Stockholder: ACADIA
Tiago Reis Marques reported serving as a speaker for Lundbeck.
Christos Pantelis has participated on Advisory Boards for Janssen-Cilag, Astra-Zeneca, Lundbeck, and Servier. He has received honoraria for talks presented at educational meetings organised by Astra-Zeneca, Janssen-Cilag, Eli-Lilly, Pfizer, Lundbeck and Shire. Christos Pantelis was supported by a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (IDs: 628386 & 1105825)
Gary Remington has received research support from Novartis, and speaker or consultant fees from Neurocrine Biosciences, eNovartis, and Synchroneuron.
Cynthia Siu has received funding and consulting fees from Sunovion and Pfizer that support research and the use of clinical trial databases for analyses and publications. She has also received funding and consulting fees from Hong Kong Health and Medical Research Grant, and the Chinese University of Hong Kong that support research and the use of clinical trial and genetic databases for analyses and data science activities.
Takefumi Suzuki has received manuscript or speaker’s fees from Astellas, Sumitomo Dainippon Pharma, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Meiji Seika Pharma, Novartis, Otsuka Pharmaceutical, and Wiley Japan.
David Taylor: research funding from Sunovion and Lundbeck.
Neil Thomas has received honoraria for talks presented at educational meetings organised by Janssen-Cilag
Andrea de Bartolomeis, Nico van Beveren, Michael L. Birnbaum, Michael A P Bloomfield, Serdar Dursun, Peter Falkai, Ariel Graff-Guerrero, Jaime E. C. Hallak, James H MacCabe, Rob McCutcheon, Carolyn McNabb, Shinchiro Najakima, Susan Rossell, Bruce R Russell, Iris Sommer, Alp Üçok, and James TR Walters have no competing interests.
References
- 1.Howes O, Murray R. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;6736:1–11. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Muñoz F, Alamo C, Cuenca E, Shen WW, Clervoy P, Rubio G. History of the discovery and clinical introduction of chlorpromazine. Ann Clin Psychiatry. 2005;17:113–135. doi: 10.1080/10401230591002002. [DOI] [PubMed] [Google Scholar]
- 3.Claghorn J, Honigfeld G, Abuzzahab FS, Wang R, Steinbook R, Tuason V, Klerman G. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7:377–384. [PubMed] [Google Scholar]
- 4.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 5.National Institute For Clinical Excellence. Schizophrenia: The NICE guideline on core interventions in the treatment and management of schizophrenia in primary and secondary care; National Clinical Practice Guidelines Number CG82. 2014.
- 6.Lehman A, Lieberman J. Practice guideline for the Treatment of Patients With Schizophrenia. Am J Psychiatry. 2004;161:1–56. [PubMed] [Google Scholar]
- 7.Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Möller H-J. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 1: Acute treatment of schizophrenia. World J Biol Psychiatry. 2005;6:132–191. doi: 10.1080/15622970510030090. [DOI] [PubMed] [Google Scholar]
- 8.Correll CU, Kishimoto T, Nielsen J, Kane JM. Quantifying Clinical Relevance in the Treatment of Schizophrenia. Clin Ther. 2011;33:B16–B39. doi: 10.1016/j.clinthera.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki T, Remington G, Mulsant BH, Rajji TK, Uchida H, Graff-Guerrero A, Mamo DC. Treatment resistant schizophrenia and response to antipsychotics: a review. Schizophr Res. 2011;133:54–62. doi: 10.1016/j.schres.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Samara MT, Dold M, Gianatsi M, Nikolakopoulou A, Helfer B, Salanti G, Leucht S. Efficacy, Acceptability, and Tolerability of Antipsychotics in Treatment-Resistant Schizophrenia. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2015.2955. [DOI] [PubMed] [Google Scholar]
- 11.Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;6736:1–12. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 12.Bitter I, Dossenbach MRK, Brook S, Feldman PD, Metcalfe S, Gagiano Ca, Füredi J, Bartko G, Janka Z, Banki CM, Kovacs G, et al. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuro-Psychopharmacology Biol Psychiatry. 2004;28:173–180. doi: 10.1016/j.pnpbp.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan RW, Ball MP, Weiner E, Kirkpatrick B, Gold JM, McMahon RP, Carpenter WT. Olanzapine treatment of residual positive and negative symptoms. Am J Psychiatry. 2005;162:124–129. doi: 10.1176/appi.ajp.162.1.124. [DOI] [PubMed] [Google Scholar]
- 14.Kane JM, Correll CU. The Role of Clozapine in Treatment-Resistant Schizophrenia. JAMA psychiatry. 2016;73:187–8. doi: 10.1001/jamapsychiatry.2015.2966. [DOI] [PubMed] [Google Scholar]
- 15.Purcell H, Lewis S. Postcode prescribing in psychiatry: Clozapine in an English county. Psychiatr Bull. 2000;24:420–422. [Google Scholar]
- 16.Howes OD, Vergunst F, Gee S, McGuire P, Kapur S, Taylor D. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J psychiatry J Ment Sci. 2012;201:481–485. doi: 10.1192/bjp.bp.111.105833. [DOI] [PubMed] [Google Scholar]
- 17.Kay SR, Flszbein A, Opler LA. The Positive and Negative Syndrome Scale for Schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 18.Overall JE, Gorham DoR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 19.Andreasen NC. Scale for the assessment of negative symptoms. Iowa City: University of Iowa; 1984. [Google Scholar]
- 20.Andreasen NC. Scale for the assessment of positive symptoms. Iowa City: University of Iowa; 1984. [Google Scholar]
- 21.Bell M, Milstein R, Beam-Goulet J, Lysaker P, Cicchetti D. The Positive and Negative Syndrome Scale: Reliability, Comparability and Predictive Value. J Nerv Ment Dis. 1992;180:723–728. doi: 10.1097/00005053-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Levaux MN, Potvin S, Sepehry AA, Sablier J, Mendrek A, Stip E. Computerized assessment of cognition in schizophrenia: Promises and pitfalls of CANTAB. Eur Psychiatry. 2007;22:104–115. doi: 10.1016/j.eurpsy.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Keefe RSE, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31:2318–25. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
- 25.Leucht S. Measurements of response, remission, and recovery in schizophrenia and examples for their clinical application. J Clin Psychiatry. 2014;75(Suppl 1):8–14. doi: 10.4088/JCP.13049su1c.02. [DOI] [PubMed] [Google Scholar]
- 26.Haro JM, Kamath Sa, Ochoa S, Novick D, Rele K, Fargas a, Rodríguez MJ, Rele R, Orta J, Kharbeng a, Araya S, et al. The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand Suppl. 2003;107:16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- 27.Obermeier M, Schennach-Wolff R, Meyer S, Möller H-J, Riedel M, Krause D, Seemüller F. Is the PANSS used correctly? a systematic review. BMC Psychiatry. 2011;11:113. doi: 10.1186/1471-244X-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howes OD, Shotbolt P, Bloomfield M, Daalman K, Demjaha A, Diederen KMJ, Ibrahim K, Kim E, McGuire P, Kahn RS, Sommer IE. Dopaminergic function in the psychosis spectrum: an [18F]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophr Bull. 2013;39:807–14. doi: 10.1093/schbul/sbr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sommer IEC, Daalman K, Rietkerk T, Diederen KM, Bakker S, Wijkstra J, Boks MPM. Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr Bull. 2010;36:633–41. doi: 10.1093/schbul/sbn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman SH, Sewell DR, Cooley EL, Leavitt N. Assessing levels of adaptive functioning: the Role Functioning Scale. Community Ment Health J. 1993;29:119–31. doi: 10.1007/BF00756338. [DOI] [PubMed] [Google Scholar]
- 31.Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101:323–329. [PubMed] [Google Scholar]
- 32.Baier M. Insight in schizophrenia: A review. Curr Psychiatry Rep. 2010;12:356–361. doi: 10.1007/s11920-010-0125-7. [DOI] [PubMed] [Google Scholar]
- 33.Brenner HD, Dencker S, Goldstein M, Hubbard J, Keegan D, Kruger G, Kulhanek F, Liberman RP, Malm U, Midha K. Defining treatment refractoriness in schizophrenia. Schizophr Bull. 1990;16:563–565. doi: 10.1093/schbul/16.4.551. [DOI] [PubMed] [Google Scholar]
- 34.Guy W. ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 35.Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF) Br J Psychiatry. 1995;166:654–659. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- 36.Agid O, Arenovich T, Sajeev G, Zipursky RB, Kapur S, Foussias G, Remington G. An algorithm-based approach to first-episode schizophrenia: Response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72:1439–1444. doi: 10.4088/JCP.09m05785yel. [DOI] [PubMed] [Google Scholar]
- 37.Wiersma D, Nienhuis FJ, Slooff CJ, Giel R. Natural course of schizophrenic disorders: a 15-year followup of a Dutch incidence cohort. Schizophr Bull. 1998;24:75–85. doi: 10.1093/oxfordjournals.schbul.a033315. [DOI] [PubMed] [Google Scholar]
- 38.Kolakowska T, Williams A, Ardern M, Reveley M, Jambor K, Gelder M, Mandelbrote B. Schizophrenia with good and poor outcome. I: early clinical features, response to neuroleptics and signs of organic dysfunction. Br J psychiatry. 1985;146:229–246. doi: 10.1192/bjp.146.3.229. [DOI] [PubMed] [Google Scholar]
- 39.Emsley R, Nuamah I, Hough D, Gopal S. Treatment response after relapse in a placebo-controlled maintenance trial in schizophrenia. Schizophr Res. 2012;138:29–34. doi: 10.1016/j.schres.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 40.Emsley R, Oosthuizen P, Koen L, Niehaus D, Martinez L. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33:80–3. doi: 10.1097/JCP.0b013e31827bfcc1. [DOI] [PubMed] [Google Scholar]
- 41.Lieberman Ja, Phillips M, Gu H, Stroup S, Zhang P, Kong L, Ji Z, Koch G, Hamer RM. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28:995–1003. doi: 10.1038/sj.npp.1300157. [DOI] [PubMed] [Google Scholar]
- 42.Girgis RR, Phillips MR, Li X, Li K, Jiang H, Wu C, Duan N, Niu Y, Lieberman JA. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J psychiatry J Ment Sci. 2011;199:281–288. doi: 10.1192/bjp.bp.110.081471. [DOI] [PubMed] [Google Scholar]
- 43.Sheitman BB, Lieberman Ja. The natural history and pathophysiology of treatment resistant schizophrenia. J Psychiatr Res. 1998;32:143–50. doi: 10.1016/s0022-3956(97)00052-6. [DOI] [PubMed] [Google Scholar]
- 44.Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-Onset Hypothesis of Antipsychotic Action: A Hypothesis Tested and Rejected. Arch Gen Psychiatry. 2003;60:1228–1235. doi: 10.1001/archpsyc.60.12.1228. [DOI] [PubMed] [Google Scholar]
- 45.Brissos S, Veguilla MR, Taylor D, Balanzá-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4:198–219. doi: 10.1177/2045125314540297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Citrome L. New second-generation long-acting injectable antipsychotics for the treatment of schizophrenia. Expert Rev Neurother. 2013;13:767–783. doi: 10.1586/14737175.2013.811984. [DOI] [PubMed] [Google Scholar]
- 47.Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: The minimum effective dose method. Schizophr Bull. 2014;40:314–326. doi: 10.1093/schbul/sbu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leucht S, Samara M, Heres S, Patel MX, Furukawa T, Cipriani A, Geddes J, Davis JM. Dose Equivalents for Second-Generation Antipsychotic Drugs: The Classical Mean Dose Method. Schizophr Bull. 2015;41:1397–1402. doi: 10.1093/schbul/sbv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–93. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 50.Howes O, Egerton A, Allan V. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15:2550–2559. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J-P, Gallego Ja, Robinson DG, Malhotra AK, Kane JM, Correll CU. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2013;16:1205–18. doi: 10.1017/S1461145712001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163:743–745. doi: 10.1176/ajp.2006.163.4.743. [DOI] [PubMed] [Google Scholar]
- 53.Frogley C, Taylor D, Dickens G, Picchioni M. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15:1351–71. doi: 10.1017/S146114571100201X. [DOI] [PubMed] [Google Scholar]
- 54.Fakra E, Azorin J-M. Clozapine for the treatment of schizophrenia. Expert Opin Pharmacother. 2012;13:1923–1935. doi: 10.1517/14656566.2012.709235. [DOI] [PubMed] [Google Scholar]
- 55.Van Sant SP, Buckley PF. Pharmacotherapy for treatment- refractory schizophrenia. Expert Opin Pharmacother. 2011;12:411–434. doi: 10.1517/14656566.2011.528200. [DOI] [PubMed] [Google Scholar]
- 56.Essali A, Al-Haj Haasan N, Li C, Rathbone J. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD000059.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71:1115–1124. doi: 10.4088/JCP.10r06264yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mauri MC, Volonteri LS, Dell’Osso B, Regispani F, Papa P, Baldi M, Bareggi SR. Predictors of clinical outcome in schizophrenic patients responding to clozapine. J Clin Psychopharmacol. 2003;23:660–4. doi: 10.1097/01.jcp.0000095351.32154.3a. [DOI] [PubMed] [Google Scholar]
- 59.Schulte PFJ. What is an adequate trial with clozapine? Clin Pharmacokinet. 2003;42:607–618. doi: 10.2165/00003088-200342070-00001. [DOI] [PubMed] [Google Scholar]
- 60.Bell R, McLaren A, Gaianos J, Copolov D. The clinical use of plasma clozapine levels. Australas Psychiatry. 1998;32:567–574. doi: 10.3109/00048679809068332. [DOI] [PubMed] [Google Scholar]
- 61.Cooper TB. Clozapine plasma level monitoring: current status. Psychiatr Q. 1996;67:297–311. doi: 10.1007/BF02326373. [DOI] [PubMed] [Google Scholar]
- 62.Jann MW, Grimsley SR, Gray EC, Chang W-H. Pharmacokinetics and pharmacodynamics of clozapine. Clin Pharmacokinet. 1993;24:161–176. doi: 10.2165/00003088-199324020-00005. [DOI] [PubMed] [Google Scholar]
- 63.Remington G, Agid O, Foussias G, Ferguson L, McDonald K, Powell V. Clozapine and therapeutic drug monitoring: Is there sufficient evidence for an upper threshold? Psychopharmacology (Berl) 2013;225:505–518. doi: 10.1007/s00213-012-2922-7. [DOI] [PubMed] [Google Scholar]
- 64.Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Lobos CA, Schwarz S, Davis JM. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166:152–163. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- 65.Beck K, Howes O. Optimising treatment of refractory schizophrenia. Psychopharmacology (Berl) 2013;227:373–374. doi: 10.1007/s00213-013-3076-y. [DOI] [PubMed] [Google Scholar]
- 66.Breier a, Buchanan RW, Irish D, Carpenter WT. Clozapine treatment of outpatients with schizophrenia: outcome and long-term response patterns. 1993. Psychiatr Serv. 2000;51:1249–53. doi: 10.1176/appi.ps.51.10.1249. [DOI] [PubMed] [Google Scholar]
- 67.Meltzer HY, Bastani B, Kwon KY, Ramirez LF, Burnett S, Sharpe J. A prospective study of clozapine in treatment-resistant schizophrenic patients. I. Preliminary report. Psychopharmacology (Berl) 1989;99(Suppl):S68–72. doi: 10.1007/BF00442563. [DOI] [PubMed] [Google Scholar]
- 68.Lieberman J, Safferman A. Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry. 1994:1744–1752. doi: 10.1176/ajp.151.12.1744. [DOI] [PubMed] [Google Scholar]
- 69.Sherwood M, Thornton AE, Honer WG. A quantitative review of the profile and time course of symptom change in schizophrenia treated with clozapine. J Psychopharmacol. 2012;26:1175–84. doi: 10.1177/0269881112440513. [DOI] [PubMed] [Google Scholar]
- 70.Rosenheck R, Evans D, Herz L, Cramer J, Xu W, Thomas J, Henderson W, Charney D. How long to wait for a response to clozapine: a comparison of time course of response to clozapine and conventional antipsychotic medication in refractory schizophrenia. Schizophr Bull. 1999;25:709–19. doi: 10.1093/oxfordjournals.schbul.a033412. [DOI] [PubMed] [Google Scholar]
- 71.Conley RR, Carpenter WT, Tamminga Ca. Time to clozapine response in a standardized trial. Am Psychiatry. 1997;154:1243–7. doi: 10.1176/ajp.154.9.1243. [DOI] [PubMed] [Google Scholar]
- 72.Fabrazzo M, La Pia S, Monteleone P, Esposito G, Pinto A, De Simone L, Bencivenga R, Maj M. Is the time course of clozapine response correlated to the time course of clozapine plasma levels? A one-year prospective study in drug-resistant patients with schizophrenia. Neuropsychopharmacology. 2002;27:1050–5. doi: 10.1016/S0893-133X(02)00319-6. [DOI] [PubMed] [Google Scholar]
- 73.McCutcheon R, Beck K, Bloomfield MAP, Marques R, Rogdaki M, Howes OD. Treatment resistant or resistant to treatment? Antipsychotic plasma levels in patients with poorly controlled psychotic symptoms. J Psychopharmacol. 2015;29:892–897. doi: 10.1177/0269881115576688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: Epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12:216–226. doi: 10.1002/wps.20060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Velligan D, Wang M, Diamond P, Glahn DC, Castillo D, Bendle S, Francis Lam YW, Ereshefsky La, Miller AL. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58:1187–1192. doi: 10.1176/ps.2007.58.9.1187. [DOI] [PubMed] [Google Scholar]
- 76.Jónsdóttir H, Opjordsmoen S, Birkenaes AB, Engh Ja, Ringen PA, Vaskinn A, Aamo TO, Friis S, Andreassen Oa. Medication adherence in outpatients with severe mental disorders: relation between self-reports and serum level. J Clin Psychopharmacol. 2010;30:169–75. doi: 10.1097/JCP.0b013e3181d2191e. [DOI] [PubMed] [Google Scholar]
- 77.Besch C. Compliance in clinical trials. Aids. 1995;9:1–10. doi: 10.1097/00002030-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 78.McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, Germano D, Bravin J, McDonald T, Blair A, Adlard S, et al. Randomized Controlled Trial of Interventions Designed to Reduce the Risk of Progression to First-Episode Psychosis in a Clinical Sample With Subthreshold Symptoms. Arch Gen Psychiatry. 2002;59:921. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 79.Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. maudsle. John Wiley & Sons; 2015. [Google Scholar]
- 80.Hiemke C, Baumann P, Bergemann N, Conca a, Dietmaier O, Egberts K, Fric M, Gerlach M, Greiner C, Gründer G, Haen E, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44:195–235. doi: 10.1055/s-0031-1286287. [DOI] [PubMed] [Google Scholar]
- 81.Mullard A. Do you want chips with that? Nat Rev Drug Discov. 2015;14:735–737. doi: 10.1038/nrd4769. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki T, Remington G, Mulsant BH, Uchida H, Rajji TK, Graff-Guerrero A, Mimura M, Mamo DC. Defining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendation. Psychiatry Res. 2012;197:1–6. doi: 10.1016/j.psychres.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Lee J, Psych M, Takeuchi H, Fervaha G, Sin GL, Psych M, Foussias G, Agid O, Farooq S, Psych M, Psych F, et al. Subtyping Schizophrenia by Treatment Response : Antipsychotic Development and the Central Role of Positive Symptoms. Can J Psychiatry. 2015;60:515–522. doi: 10.1177/070674371506001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huhn M, Tardy M, Spineli LM, Kissling W, Förstl H, Pitschel-Walz G, Leucht C, Samara M, Dold M, Davis JM, Leucht S. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta-analyses. JAMA psychiatry. 2014;71:706–15. doi: 10.1001/jamapsychiatry.2014.112. [DOI] [PubMed] [Google Scholar]
- 85.Howes OD, Kapur S. A neurobiological hypothesis for the classification of schizophrenia: Type a (hyperdopaminergic) and type b (normodopaminergic) Br J Psychiatry. 2014;205:1–3. doi: 10.1192/bjp.bp.113.138578. [DOI] [PubMed] [Google Scholar]
- 86.Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–10. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- 87.Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, McGuire PK. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75:e11–3. doi: 10.1016/j.biopsych.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 88.Lenior ME, Dingemans PMaJ, Linszen DH, De Haan L, Schene aH. Social functioning and the course of early-onset schizophrenia: Five-year follow-up of a psychosocial intervention. Br J Psychiatry. 2001;178:53–58. doi: 10.1192/bjp.179.1.53. [DOI] [PubMed] [Google Scholar]
- 89.Hafner H, Maurer K, Loffler W, an der Heiden W, Hambrecht M, Schultze-Lutter F. Modeling the Early Course of Schizophrenia. Schizophr bull. 2003;29:325–340. doi: 10.1093/oxfordjournals.schbul.a007008. [DOI] [PubMed] [Google Scholar]
- 90.McGorry PD. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of schizophrenia and related disorders. Aust N Z J Psychiatry. 2005;39:1–30. doi: 10.1080/j.1440-1614.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 91.Barnes TRE. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25:567–620. doi: 10.1177/0269881110391123. [DOI] [PubMed] [Google Scholar]
- 92.The International Psychopharmacology Algorithm Project. The International Psychopharmacology Algorithm Project. 2006.
- 93.Verma S, Chan LL, Chee KS, Chen H, Chin SA, Chong SA, Chua W, Fones C, Fung D, Khoo CL, Kwek SKD, et al. Ministry of Health Clinical Practice Guidelines : Schizophrenia. 2011;52:521–526. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.