Abstract
Introduction
Electroacupuncture at ‘four sacral points’, also known as electrical pudendal nerve stimulation therapy, combines the advantages of pudendal nerve neuromodulation and the technique of deep insertion of long acupuncture needles. It has been used to treat stress urinary incontinence, female urgency-frequency syndrome, idiopathic urgency urinary incontinence and neurological bladders in previous studies. Here, we describe the protocol for a randomised controlled trial for evaluation of the efficacy and safety of electroacupuncture at ‘four sacral points’ for the management of urinary incontinence after stroke.
Methods and analysis
This is an open-label randomised controlled trial with blinded assessments and analyses. A total of 140 eligible patients will be randomly allocated to two groups. The treatment group (n=70) will receive electroacupuncture at ‘four sacral points’ along with routine medical care, while the control group will receive conventional electroacupuncture along with routine medical care. Twenty treatment sessions will occur over a period of 4 weeks. The primary outcome measures will be the self-recorded findings in an incontinent episode diary at baseline and at 4 weeks after baseline. The secondary outcome measures will be the International Consultation on Incontinence Questionnaire Urinary Incontinence—Short Form (ICIQ-UI SF) score and the Barthel Activities of Daily Living Index (Barthel ADL Index) score at baseline and at 4 and 28 weeks after baseline.
Ethics and dissemination
This protocol has been approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University (approval No. 2018-K-059–01). Written informed consent will be obtained from each participant. The results of the study will be published in peer-reviewed journals.
Trial registration number
ChiCTR-IOR-17012847; Pre-result.
Keywords: complementary medicine, urology, stroke medicine, stroke, stroke medicine
Strengths and limitations of this study.
First pilot study to evaluate the efficacy and safety of electroacupuncture at ‘four sacral points’ for the treatment of urinary incontinence after stroke.
Randomised clinical trial with pragmatic design.
A novel acupuncture intervention for the treatment of urinary incontinence after stroke.
Lack of blinding of acupuncturists and participants due to the nature of acupuncture.
Introduction
The International Continence Society has defined urinary incontinence (UI) as the involuntary loss of urine.1 In a systematic review, a random-effects meta-analysis determined the prevalence of UI after stroke to be 23.6%.2 Post-stroke UI may develop because of various reasons, although direct stroke-induced damage to the neuromicturition pathways is considered the most common cause. Typical symptoms include the involuntary leakage of urine accompanied or immediately preceded by urgency. Urodynamic evaluation often reveals uninhibited detrusor contraction.3 Physical consequences include skin dermatitis and urinary tract infections, while psychological consequences include embarrassment and low self-esteem.4 In addition, UI is a powerful prognostic indicator of survival and eventual functional dependence.5 The most recent research on this topic demonstrated a higher mortality rate for stroke patients with UI (56.8%) than for stroke patients without UI (11.9%).6 Therefore, the management of UI after stroke is of great importance.
Evidence-based interventions for post-stroke UI are somewhat limited, but include behavioural and pharmacological interventions, as well as individually tailored structured management plans, or the aid of continence nurse specialists, in order to promote continence.3 Trials of behavioural and pharmacological therapies have provided insufficient evidence to guide the management of post-stroke UI in adults.7 Furthermore, side-effect profiles and anticholinergic burden should be considered before medications are prescribed.8
Neuromodulation therapies, which involve the electrical stimulation of target-specific nerves, are reportedly effective for overactive bladder (OAB) or urgency UI (UUI).9 Neuromodulation includes transvaginal or transanal electrical stimulation (TES), posterial tibial nerve stimulation (PTNS), sacral nerve neuromodulation (SNM) and pudendal nerve (PN) neuromodulation (PNM). 10 Although TES is an easy procedure, it is not tolerated by many patients because of discomfort, mucosal injury and high-intensity stimulation for an acceptable treatment result.11 PTNS is a minimally invasive technique with needle electrodes but it is not direct PN stimulation and requires multiple treatments to maintain initial effect comparing with SNM.12 13 SNM requires surgical procedure with implantation of InterStim device, providing continuous stimulation by close nerve contact. It has a high success rate.14 15 Its common adverse events are pain (15%–42%) and infection (3.4%–6.1%) at the implant site and surgical revision that can mount up to 33%.13 16 PN afferents play a particularly important role in the inhibition of the voiding reflex. PNM as direct PN stimulation may be more effective than SNM because the latter only excites a portion of PN afferents. UUI refractory to SNM can be treated by PNM with the Interstim device or the Bion device (selective PN stimulation);10 17 18 however, the performance of PNM also needs surgery so its disadvantages are similar to those of SNM.10 18
According to the theory of traditional Chinese medicine, UI is primarily caused by kidney and bladder dysfunction in terms of urine control. Accordingly, the principle of acupuncture treatment for UI is to promote the recovery of urine control.19 Acupoints on the lower abdomen, such as CV4 (Guanyuan), CV6 (Qihai) and ST28 (Shuidao), as well as those on the sacral region, such as BL32 (Ciliao), BL33 (Zhongliao) and BL35 (Huiyang), are generally selected for the regulation of the bladder voiding function.20–22 A literature review showed that acupuncture demonstrated more favourable effects than did antimuscarinic drugs for the treatment of OAB and alleviation of symptoms in some comparative trials.23 In addition, acupuncture reportedly improved the quality of life and urodynamic testing parameters in patients with OAB.23 24 In a randomised, double-blind, placebo-controlled study, electroacupuncture significantly increased the maximum cystometric capacity and bladder compliance, decreased the detrusor leak point pressure, alleviated lower urinary tract symptoms and decreased the risk of upper urinary tract damage in patients with post-stroke detrusor overactivity.22 However, high-quality clinical trials with appropriate inclusion criteria, sample size, control design, acupoint selection, depth of needle insertion and efficacy and safety evaluations are necessary to properly evaluate the efficacy of acupuncture for the treatment of post-stroke UI.7 25 26
On the basis of the theory of nerve stimulation, we developed electroacupuncture at ‘four sacral points’,27 28 also known as electroacupuncture neurostimulation therapy or electrical PN stimulation therapy. This approach involves the insertion of long needles at ‘four sacral points’, with electricity to stimulate specific nerves under the sacral region.28 29 When it was first developed, this treatment was used to treat stress UI (SUI)in women, and radiographic evidence with simultaneous records of perineal ultrasonographic pelvic floor muscle contraction, vaginal pressure and pelvic floor surface electromyography have shown that it causes PN excitation.29 In addition, it has been used for the treatment of female urgency-frequency syndrome, idiopathic urgency UI and UI caused by neurological or non-neurological conditions.28 30 31 The mechanism of electroacupuncture at ‘four sacral points’ for the treatment of post-stroke UI is that as this therapy can stimulate PN directly, it is speculated that it is able to inhibit central hyperactivity through the viscerosomatic convergence at S2-S4 common spinal neurons of PNs and bladder nerves to relive the symptoms post-stroke UI.31 32
The effectiveness of post-stroke UI treatment using complementary medicine approaches is worthy of investigation in a well-designed study. To the best of our knowledge, no randomised controlled trials (RCT) comparing the efficacy and safety of electroacupuncture at ‘four sacral points’ with those of conventional electroacupuncture for the treatment of post-stroke UI have been conducted. Here we describe a protocol for an RCT to evaluate the efficacy and safety of electroacupuncture at ‘four sacral points’ for the management of post-stroke UI.
Methods and analysis
Objective
This is a protocol comparing the efficacy and safety of electroacupuncture at ‘four sacral points’ with those of conventional electroacupuncture for the treatment of post-stroke UI. It is designed as a blinded randomised assessment and analysis with two parallel groups over a 4-week treatment period. Randomization will be performed in a random 1:1 allocation sequence.
Recruitment
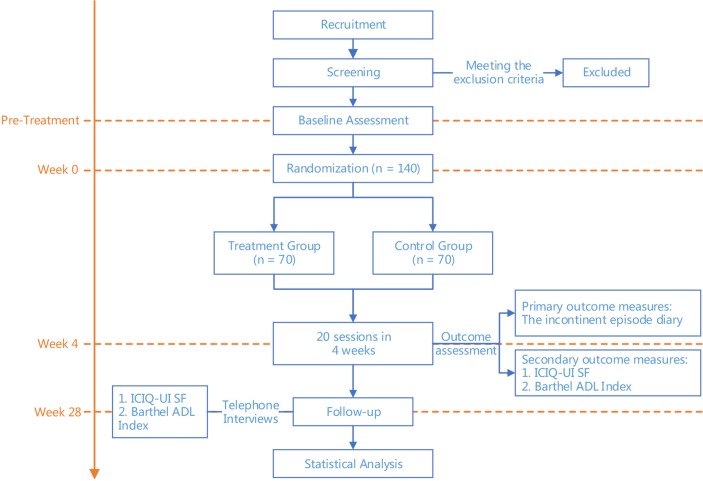
This is a pragmatic RCT comparing electroacupuncture at ‘four sacral points’ with conventional electroacupuncture for the treatment of post-stroke UI. The research structure is shown in figure 1. A total of 140 eligible participants will be recruited from the inpatient and outpatient departments of the First Affiliated Hospital of Zhejiang Chinese Medical University according to the inclusion and exclusion criteria. At the beginning of recruitment, detailed information about the study, including the research objective, study procedure and potential benefits and risks, will be provided to all eligible patients. If the patient agrees to participate, he or she will be asked to sign a written informed consent form. This will be followed by baseline assessment and randomization. A treatment period of 4 weeks and a follow-up period of 24 weeks will follow the recruitment procedure. The neurology department of the First Affiliated Hospital of Zhejiang Chinese Medical University has the major number of patients with post-stroke UI. Our reach team includes neurological physicians and special nurses who will interview potentially eligible patients. Advertisements will be released through health education brochures, posters and videos displayed in the outpatient and inpatient sites of the First Affiliated Hospital of Zhejiang Chinese Medical University. Recruitment information will also be issued through media (eg, newspapers, broadcasts and websites).
Figure 1.
Study flow chart. ADL, Activities of Daily Living; ICIQ UI SF, International Consultation on Incontinence Questionnaire Urinary Incontinence—Short Form.
Design
Randomisation and allocation concealment
The randomisation scheme has been created by the Clinical Evaluation and Analysis Centre of The First Affiliated Hospital of Zhejiang Chinese Medical University, where professionals used SPSS Statistics V.22.0 to generate a random 1:1 allocation sequence using a computer. Professionals involved in allocation will not be recruited in the study. The random allocation is strictly kept in an opaque envelope and is inaccessible to other research staff. After baseline assessment, an envelope with printed randomisation numbers will be opened by an independent staff member in the participant’s presence, in order to determine the group assignment for that participant. All patients who give consent for participation and who fulfil the inclusion criteria will be assigned to a group randomly. Randomisation will be requested by the staff member responsible for recruitment and clinical interviews from the First Affiliated Hospital of Zhejiang Chinese Medical University. The therapists will be informed about the participant’s allocation at the same time. The staff member responsible for recruitment and clinical interviews is not allowed to receive information about the group allocation.
Blinding
Considering the nature of acupuncture, therapists and participants cannot be blinded to the treatment allocation. Data managers and statisticians will be blinded throughout the trial. Telephone interviewers who collect follow-up information will also be blinded. Data managers, statisticians and telephone interviewers are restricted from discussing the treatment allocations with each other. The therapists will not be permitted to communicate with any data managers, statisticians or telephone interviewers. If an unblinding event occurs among data managers, statisticians or telephone interviewers, the relevant work will be transferred to other appropriately blinded data managers, statisticians or telephone interviewers. The Investigator must report all code breaks (with reason) as they occur on the corresponding Case Report Form page.
Participants
Sample size
With reference to a similar study 31 with 120 women (efficacy rate, 70.1%:45%), the sample size has been calculated, using PASS V.11 software, as 120 patients for a power (1-beta) of 0.80, an alpha (significance level) of 0.05 and a ratio of 1:1. With consideration of the estimated dropout rate (15%), the total sample size will be 140 (70 in each group).
Inclusion criteria
Male or female patients aged 30–85 years.
Diagnosis of post-stroke UI in accordance with the criteria of the American Stroke Association 33 and the International Continence Society.34
Inpatients or outpatients with a post-stroke interval of 4 weeks to 2 years.
Stable vital signs, normal consciousness and compliance with treatment.
Refractoriness to medications (patients who have taken antimuscarinic agents with no UI improvement).
Provision of written informed consent.
Exclusion criteria
UI caused by other diseases such as Parkinson’s disease, multiple sclerosis, spinal injury or Alzheimer’s disease.
Pre-stroke UI, SUI or mixed UI, or overflow incontinence.
Urinary retention concomitant with UI.
Urethral injury, lower urinary tract obstruction, acute urinary tract infection, refractory urinary tract infection, hydronephrosis, urological calculi or tumours.
Severe cognitive impairment, as defined by a Mini-Mental State Examination score of <22.35
Insufficiency of the heart, lungs, liver, and/or kidneys.
Presence of an implantable electronic device.
Elimination criteria
Inclusion despite non-fulfilment of the inclusion criteria.
Lack of exclusion despite fulfilment of the exclusion criteria.
Eligible participants who receive no interventions.
Dropout criteria
Poor participant compliance (lack of adherence to treatment for personal reasons).
Serious adverse events, complications, or special physiological changes necessitating discontinuation of the intervention.
Voluntary dropout.
Intervention
All participants will receive routine medical care for stroke recovery, including the control of blood pressure, blood sugar and blood lipids and routine rehabilitation training. All of the study-related treatments will be provided by skilled acupuncturists who will strictly follow the detailed procedures for each group. During the treatment course and 24-week follow-up time, the administration of antimuscarinic agents and other drugs for neurogenic detrusor overactivity is prohibited for all the patients. They are also not permitted to receive other acupuncture treatment or physiotherapy for UI. All of the study-related treatments will be provided by certified and skilled acupuncturists who will strictly follow the detailed procedures for each group.
Standard operating procedure
Needle requirements
Disposable sterile acupuncture needles in accordance with national standards within the validity period will be used.
Hand hygiene of the operator
The operator is required to sterilise his or her hands with a sanitizer before the acupuncture procedure.
Sterilisation of the acupuncture points
Within a 5 cm diameter with the acupoint as the centre, sterilise the skin over the acupoints using a cotton swab dipped in 0.45%–0.55% povidone iodine or 75% ethanol.
Procedure
Treatment group: Participants in this group will receive electroacupuncture at ‘four sacral points’.
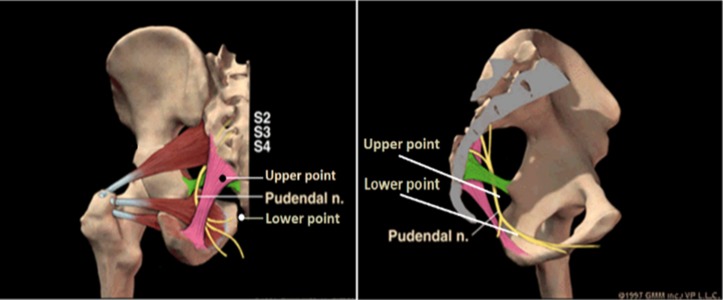
Selection of points: Four sacral points (figure 2 and figure 3) are selected. The two upper points are located on either side of the sacrococcygeal joint, approximately 1 cm from the joint. The two lower points are located on either side of the tip of the coccyx, approximately 1 cm from the coccyx. Acupuncture will be performed at LI15 (Jianyu), LI11 (Quchi), LI10 (Shousanli), SJ5 (Waiguan) and LI4 (Hegu) for participants with upper limb paralysis. For participants with lower limb paralysis, ST31 (Biguan), ST34 (Liangqiu), SP10 (Xuehai), SP9 (Yinlingquan), GB39 (Xuanzhong), GB40 (Qiuxu) and LR3 (Taichong) will also be used. GB20 (Fengchi), SJ17 (Yifeng) and GB12 (Wangu) will be used for participants with dysphagia, with the additional use of ST4 (Dicang), ST6 (Jiache) and LI20 (Yingxiang) for participants with facial paralysis and drooling.
Detailed procedure: At the upper sacral points, a needle (Suzhou Shenlong Medical Apparatus Factory, Suzhou, China) measuring 0.40×100 mm will be inserted perpendicularly to a depth of 80–90 mm to induce a sensation referred to the urethra or anus via stimulation of the main trunk of the PN. At the lower sacral points, a needle measuring 0.40×100 or 0.40×125 mm will be inserted obliquely toward the ischiorectal fossa to a depth of 90–110 mm to induce a sensation referred to the urethra via stimulation of the perineal nerve (figure 4). Once the sensation is induced in the respective regions, two pairs of electrodes from the G6805-A electroacupuncture device (Shantou Medical equipment factory, Shantou, China) will be connected to the two ipsilaterally inserted needles, with the anode connected to the upper needle and the cathode connected to the lower needle. The device will be set to produce electrical stimulation (biphasic 2 ms pulse duration) at a frequency of 2.0 Hz and a moderate intensity of 25–35 mA. Electrostimulation will be performed for 20 min during each treatment. PFM contraction around the urethra (often comfortable) must be maintained during the entire electrostimulation procedure. Conventional acupuncture without electricity will be applied for 20 min at the remaining acupoints.
Figure 2.
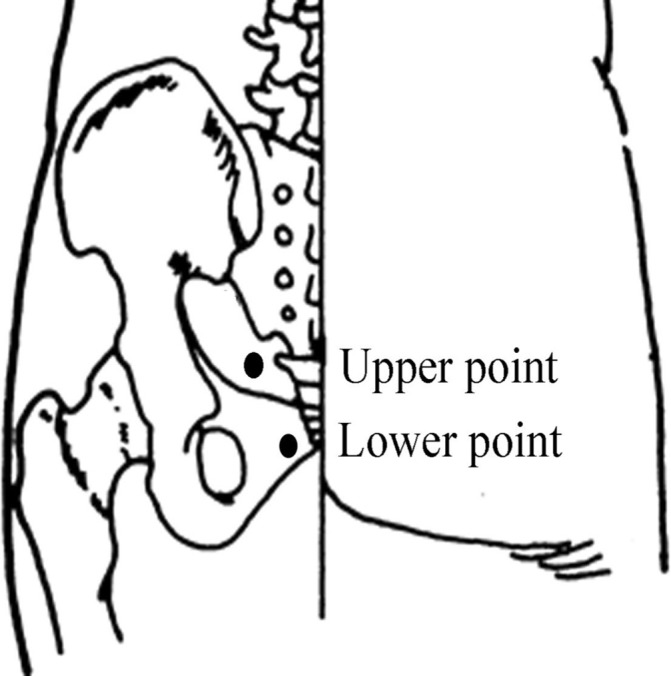
Locations of the ‘four sacral points’ for electroacupuncture.
Figure 3.
Anatomical positions of the ‘four sacral points’ for electroacupuncture.
Figure 4.
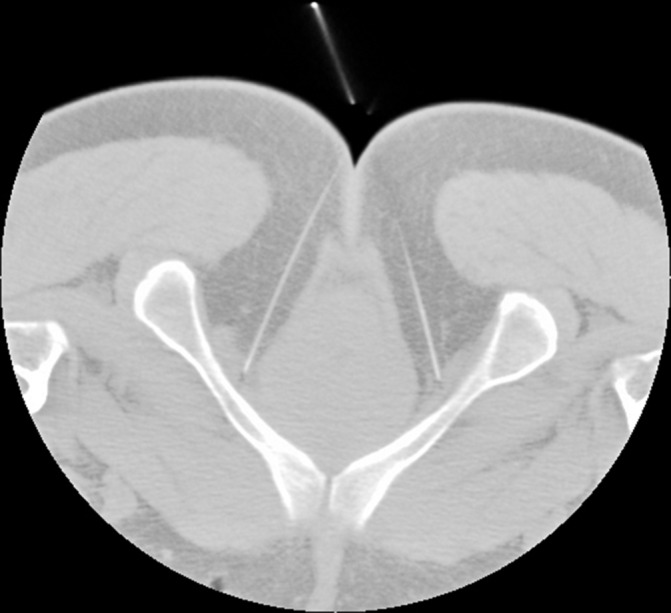
Transverse CT image of the coccygeal apex The tip of the needle inserted at the lower sacral point is visible in the ischiorectal fossa (adjacent to the pudendal nerve in the Alcock’s canal).
Control group: Participants in this group will receive conventional electroacupuncture.
Selection of points: Abdominal points CV6 (Qihai), CV4 (Guanyuan), and ST28 (Shuidao, both sides), corresponding to the conventional electroacupuncture treatment of UI. The point selection procedure for participants with upper or lower limb paralysis, facial paralysis and/or dysphagia will be the same as that used for the treatment group.
Detailed procedure: At CV6 (Qihai), CV4 (Guanyuan) and ST28 (Shuidao), a needle measuring 0.25×40 mm will be inserted perpendicularly to a depth of 25–40 mm to induce a local sensation (distention or sourness). Subsequently, the electrodes from the G6805-A electroacupuncture device will be connected to the needles at these points, with the anode connected to ST28 and the cathode connected to CV6 and CV4. The device will be set to produce electrical stimulation (biphasic 2 ms pulse duration) at a frequency of 2.0 Hz and a moderate intensity that is tolerable by the participants. Electrostimulation will be performed for 20 min during each treatment. Conventional acupuncture without electricity will be applied for 20 min at the remaining acupoints.
Treatment period
All participants will receive treatment every day from Monday to Friday. One course of treatment will comprise 10 sessions. The therapeutic effects will be evaluated after the completion of two treatment courses (4 weeks).
Outcome measures
Primary outcome measures
The incontinent episode diary (table 1) will be used to derive the primary outcome measure. The number of incontinent episodes will be recorded by the participants over a period of 3 days at baseline. A template will be provided for patient use. This data will be recorded again at the end of treatment.
Table 1.
The incontinent episode diary
| Name | Date | ||||
| Record every accidental loss of urine over 3 consecutive days with an X | |||||
| Start at baseline and continue recording for 3 days | |||||
| Day 1 | Day 2 | Day 3 | |||
| eg X | |||||
Secondary outcome measures
1. International Consultation on Incontinence Questionnaire Urinary Incontinence—Short Form (ICIQ-UI SF) 36 (table 2).
Table 2.
International Consultation on Incontinence Questionnaire Urinary Incontinence—Short Form (ICIQ-UI SF)
| 1. Please write in your date of birth: | □□ □□ □□ | ||
| date month year | |||
| 2. Are you | Female □ Male □ | ||
| 3. How often do you leak urine? (Tick one box) |
|||
| never | □ | 0 | |
| about once a week or less often | □ | 1 | |
| two or three times a week | □ | 2 | |
| about once a day | □ | 3 | |
| several times a day | □ | 4 | |
| all the time | □ | 5 | |
| 4. We would like to know how much urine you think leaks. How much urine do you usually leak (whether you wear protection or not)? (Tick one box) |
|||
| None | □ | 0 | |
| a small amount | □ | 2 | |
| a moderate amount | □ | 4 | |
| a large amount | □ | 6 | |
| 5. Overall, how much does leaking urine interfere with your everyday life? | |||
| Please ring a number between 0 (not at all) and 10 (a great deal) | |||
| 0 1 2 3 4 5 6 7 8 9 10 | |||
| not at all a great deal | |||
| ICIQ score: sum scores 3+4+5 | □□ | ||
| 6. When does urine leak? (Please tick all that apply to you) | |||
| never—urine does not leak | □ | ||
| leaks before you can get to the toilet | □ | ||
| leaks when you cough or sneeze | □ | ||
| leaks when you are asleep | □ | ||
| leaks when you have finished urinating and are dressed | □ | ||
| leaks for no obvious reason | □ | ||
| leaks all the time | □ |
2. Barthel Activities of Daily Living Index (Barthel ADL Index)37 (table 3).
Table 3.
Barthel Activities of Daily Living Index (Barthel ADL Index)
| The Barthel Index | Patient Name | ||
| Rater Name | |||
| Date: | |||
| Activity | Score | ||
| Feeding | Unable | 0 | |
| Some help required (eg, needs help cutting, spreading butter, etc. or requires a modified diet) | 5 | ||
| Independent | 10 | ||
| Bathing | Dependent | 0 | |
| Independent (or in shower) | 5 | ||
| Grooming | Needs help with personal care | 0 | |
| Independent face/hair/teeth/shaving (implements provided) | 5 | ||
| Dressing | Dependent | 0 | |
| Needs help but can do at least half unaided | 5 | ||
| Independent (including buttons, zips, laces, etc.) | 10 | ||
| Bowels | Incontinent or catheterized and unable to manage alone | 0 | |
| Occasional accident | 5 | ||
| Continent | 10 | ||
| Bladder | Incontinent or catheterized and unable to manage alone | 0 | |
| Occasional accident | 5 | ||
| Continent | 10 | ||
| Toilet use | Dependent | 0 | |
| Needs some help, but can do some things alone | 5 | ||
| Independent (can get on and off, dress and wipe unassisted) | 10 | ||
| Transfer (bed to chair and back) | Unable, no sitting balance | 0 | |
| Major help (one or two people, physical), can sit | 5 | ||
| Minor help (verbal or physical) | 10 | ||
| Independent | 15 | ||
| Mobility (on level surfaces) | Immobile or <50 yards | 0 | |
| Wheelchair independent, including corners; >50 yards | 5 | ||
| Walks with little help from one person (verbal or physical); >50 yards | 10 | ||
| Independent (but may use an aid; for example, walking stick); >50 yards | 15 | ||
| Stairs | Unable | 0 | |
| Needs help (verbal, carrying aid) | 5 | ||
| Independent | 10 | ||
| Total | |||
The ICIQ-UI SF is used in research and clinical practice worldwide. It is a brief and psychometrically robust patient-completed questionnaire for evaluating the frequency and severity of UI in men and women and its impact on quality of life. The ICIQ-UI SF score ranges from 0 to 21.38 The Barthel ADL Index is a 10-item measure of activities of daily living that is frequently used in clinical practice and as a trial outcome measure in stroke medicine.37 It is used to assess baseline abilities to quantify functional changes, including UI, after rehabilitation in stroke patients.
The above outcome measures will be assessed at baseline and at 4 and 28 weeks after baseline.
Adverse events
An adverse event of acupuncture is defined as symptoms or diseases that are against the purpose of the treatment during or following the acupuncture treatment.39 The research staff will record the blood pressure, heart rate, heart rhythm and respiration rate and observe changes in the consciousness of all participants before and after the treatment. The description of AEs, time of occurrence, location of the reaction, level of severity, corresponding management and the necessity for patient withdrawal from the trial will be recorded on the Case Report Forms (CRFs). AEs of acupuncture will be classified according to their location as local and systemic reactions.
Local reactions
Subcutaneous haematoma.
Minor bleeding on withdrawal of the needle.
Subcutaneous bruise.
Pain in the punctured region after treatment.
Skin allergy in the punctured region after treatment.
Local infection.
Systemic reactions
Acupuncture fainting.
Abdominal distention.
Dizziness or vertigo.
Leg weakness.
Muscle spasm.
Systemic allergy.
Systemic infection.
Organ injury.
Severe adverse events (AEs) are defined as symptoms or diseases that result in hospitalisation or prolong hospitalisation, disability, a life-threatening situation or even death.40 Systemic infection and organ injury are two major AEs. Severe AEs will be reported to the Ethics Committee within 24 hours. The Ethics Committee will provide medical suggestions for the research team and decide whether the patient should continue the ongoing treatment.
For patients having common adverse reactions, appropriate medical care will be given to ease local bleeding, irritation, bruising and so on. For those who have severe reactions leading to organ injury, systematic infection, systematic allergy and so on, compensation will be given to cover their medical costs by our research team.
Data management, monitoring and auditing
A research assistant will record the baseline characteristics of all participants. Trained caregivers will record the frequency of UI at baseline and at 4 weeks after baseline. A researcher who remains blinded to treatment group allocation will collect data regarding the frequency of UI from the caregivers. The participants will also complete ICIQ-UI SF and the Barthel ADL Index at baseline and at 4 weeks after baseline. A blinded telephone interviewer will interview the participants and collect data pertaining to ICIQ-UI SF and the Barthel ADL Index at 28 weeks after baseline. All data will be recorded on the CRFs. For participants who discontinue treatment early, we will use last observation carried forward analysis to handle the missing data, meaning that we will input outcome data of patients who are lost to follow-up in our analysis.
After the completion of the CRFs, two independent researchers blinded to the group allocation will separately input data into an Excel spreadsheet. Another independent supervisor will check the two data sets for consistency. If conflicting data entries are discovered, the supervisor will compare the data sets with the original CRFs and mark the modification on the CRFs. The principal investigator of the research team will have the access to all documents and will protect the electronic documents with a password and create backups of all documents. The First Affiliated Hospital of Zhejiang Chinese Medical University will be responsible for the storage and management of all data.
An independent data monitoring committee (DMC)is made up of members from Clinical Evaluation and Analysis Centre of The First Affiliated Hospital of Zhejiang Chinese Medical University. The DMC, blinded to the treatment allocations, will meet regularly to monitor the study data. The DMC will also perform an interim analysis when 50% of patients have been randomised and have completed the primary outcome measurement.
The First Affiliated Hospital of Zhejiang Chinese Medical University will audit this study mainly for participant enrolment, consent and costs.
Statistical analysis
All statistical analyses will be performed by a statistician from the Clinical Evaluation and Analysis Centre of The First Affiliated Hospital of Zhejiang Chinese Medical University using SPSS Statistics V.22.0. A normality test will be used to determine whether the data are normally distributed. ANCOVA (analysis of covariance) will be used if there is imbalance in the baseline characteristics and outcome measures. Continuous variables with a normal distribution will be expressed as mean ± SDs. Continuous variables with a non-normal distribution or ordinal variables will be expressed as medians (with lower, upper quartiles). Categorical variables will be summarised as counts and proportions. A Student’s t-test will be used if the primary and secondary outcome measures conform to normal distribution. A paired t-test will be used to compare pre-treatment and post-treatment UI occurrence for the primary outcome measure and pre-treatment and post-treatment scores in the secondary outcome measures. The independent sample t-test will be used to compare the difference between two groups for primary and secondary outcome measures. A Wilcoxon signed rank test will be used if the primary and secondary outcome measures conform to abnormal distribution. A Wilcoxon paired test will be used to compare pre-treatment and post-treatment UI occurrence for the primary outcome measure and pre-treatment and post-treatment scores in secondary outcome measures. The inter-group rank sum test will be used to compare the difference between two groups for primary and secondary outcome measures. All reported p-values will be two-sided, and CIs will be at the 95% level. A p-value of <0.05 will be considered statistically significant.
Patient and public involvement
Patients and the public were not directly involved in the design, recruitment or conduct of this pilot study. Since the participants in our study are under chronic conditions, the outcome measures valuated in this study was influenced by patients’ priorities, experience and preferences. As most stroke patients are in great need of acupuncture treatment in China, we did not view the intervention as burdensome and the burden of the intervention was not assessed by the patients themselves. The results of this study will be disseminated in peer-reviewed journals and at academic conferences. A summary of the study report will be written for patients through online website (https://sandychenshan.haodf.com/) and WeChat (a free messaging and calling application) account or group.
Ethics and dissemination
This study will adhere to the principles of the Declaration of Helsinki. This study will be conducted at the First Affiliated Hospital of Zhejiang Chinese Medical University.
Modification of the protocol
Any modifications to the protocol, including changes of study objectives, study design, patient population, sample sizes, study procedures or significant administrative aspects, will require a formal application to the Zhejiang Provincial Administration of Traditional Chinese Medicine as well as the Chinese clinical trial registry.
Confidentiality
All study participants will be given an identification number throughout the trial to assure confidentiality. All participants’ information will be stored in locked cabinets with limited access.
Dissemination
The initial data will be accessible via Research Manager (ResMan). The results of this study will be published in open-access and peer-reviewed journals and presented at relevant conferences.
Discussion
According to the present study protocol, in addition to electroacupuncture at ‘four sacral points’ in the treatment group and conventional electroacupuncture in the control group, the same acupoints for other stroke symptoms will also be selected for both groups. Other stroke symptoms include unilateral limb weakness, facial paresis, dysphasia and dysphagia.41 In China, acupuncture has been a primary medical intervention for stroke.42 In fact, not providing acupuncture therapy to a stroke patient is considered impractical.
Antimuscarinic agents are considered first-line drugs for neurogenic detrusor overactivity.43 However, because of their moderate efficacy and troublesome side effects, quite a few patients exhibit refractory disease.44 According to the eligibility criteria, participants who are refractory to medication will be included in this RCT.
With regard to the outcome measures, the incontinent episode diary will be used as objective measure to record the frequency of incontinence. Patients will record their findings in the incontinent diary for only 3 days, because a longer recording period can lead to decreased patient compliance.45 46 In order to improve adherence, health education about the importance of UI management will be conducted by a special nurse and brochures on post-stroke UI will be provided to patients. The ICIQ-UI SF and Barthel ADL Index will be used as subjective measures to investigate UI symptoms and QoL. The ICIQ-UI SF is a useful tool to assess UI with regard to the severity of symptoms and impact on QoL at baseline and at follow-up.47 The Barthel ADL Index (feeding, bathing, grooming, dressing, bowels, bladder, toilet use, transfer, mobility and stairs) is an important predictor of stroke outcomes. Studies involving stroke survivors should include ADL assessments for better management of stroke patients.37
In summary, we have described a protocol for a pilot RCT to evaluate the efficacy and safety of electroacupuncture at ‘four sacral points’ for the treatment of post-stroke UI. The results of this trial should lead to a greater understanding of promising alternative options for post-stroke UI.
Supplementary Material
Footnotes
Contributors: SC conceived and wrote the protocol; SW and LX contributed to the study design; HL contributed to the sample size calculation and wrote the statistical analysis plan; ZH drew the flow charts; CZ and HZ prepared the figures and tables. All authors have read and approved the final manuscript.
Funding: This work was supported by Zhejiang Provincial Administration of Traditional Chinese Medicine (grant number 2016ZA076; 2018ZA045).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Ethics Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University (approval No. 2018-K-059–01).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Abrams P, Andersson KE, Birder L, et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn 2010;29:213–40. 10.1002/nau.20870 [DOI] [PubMed] [Google Scholar]
- 2. Ruffion A, Castro-Diaz D, Patel H, et al. Systematic review of the epidemiology of urinary incontinence and detrusor overactivity among patients with neurogenic overactive bladder. Neuroepidemiology 2013;41:146–55. 10.1159/000353274 [DOI] [PubMed] [Google Scholar]
- 3. Mehdi Z, Birns J, Bhalla A, et al. Post-stroke urinary incontinence. Int J Clin Pract 2013;67:1128–37. 10.1111/ijcp.12183 [DOI] [PubMed] [Google Scholar]
- 4. Cai W, Wang J, Wang L, et al. Prevalence and risk factors of urinary incontinence for post-stroke inpatients in Southern China. Neurourol Urodyn 2015;34:231–5. 10.1002/nau.22551 [DOI] [PubMed] [Google Scholar]
- 5. Pizzi A, Falsini C, Martini M, et al. Urinary incontinence after ischemic stroke: clinical and urodynamic studies. Neurourol Urodyn 2014;33:420–5. 10.1002/nau.22420 [DOI] [PubMed] [Google Scholar]
- 6. John G, Primmaz S, Crichton S, et al. Urinary incontinence and indwelling urinary catheters as predictors of death after new-onset stroke: a report of the south london stroke register. J Stroke Cerebrovasc Dis 2018;27:118–24. 10.1016/j.jstrokecerebrovasdis.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 7. Thomas LH, Cross S, Barrett J, et al. Treatment of urinary incontinence after stroke in adults. Cochrane Database Syst Rev 2008;23:CD004462 10.1002/14651858.CD004462.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panicker JN. Urogenital symptoms in neurologic patients. Continuum 2017;23:533–52. 10.1212/CON.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 9. Amundsen CL, Komesu YM, Chermansky C, et al. Two-year outcomes of sacral neuromodulation versus onabotulinumtoxina for refractory urgency urinary incontinence: a randomized trial. Eur Urol 2018;74:66–73. 10.1016/j.eururo.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bosch JL. Electrical neuromodulatory therapy in female voiding dysfunction. BJU Int 2006;98 Suppl 1:43–8. 10.1111/j.1464-410X.2006.06316.x [DOI] [PubMed] [Google Scholar]
- 11. van Balken MR, Vergunst H, Bemelmans BL. The use of electrical devices for the treatment of bladder dysfunction: a review of methods. J Urol 2004;172:846–51. 10.1097/01.ju.0000134418.21959.98 [DOI] [PubMed] [Google Scholar]
- 12. Eftekhar T, Teimoory N, Miri E, et al. Posterior tibial nerve stimulation for treating neurologic bladder in women: a randomized clinical trial. Acta Med Iran 2014;52:817–21. [PubMed] [Google Scholar]
- 13. Tutolo M, Ammirati E, Van der Aa F. What is new in neuromodulation for overactive bladder? Eur Urol Focus 2018;4:49–53. 10.1016/j.euf.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 14. Smits MA, Oerlemans D, Marcelissen TA, et al. Sacral neuromodulation in patients with idiopathic overactive bladder after initial botulinum toxin therapy. J Urol 2013;190:2148–52. 10.1016/j.juro.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 15. Noblett K, Siegel S, Mangel J, et al. Results of a prospective, multicenter study evaluating quality of life, safety, and efficacy of sacral neuromodulation at twelve months in subjects with symptoms of overactive bladder. Neurourol Urodyn 2016;35:246–51. 10.1002/nau.22707 [DOI] [PubMed] [Google Scholar]
- 16. Lee C, Pizarro-Berdichevsky J, Clifton MM, et al. Sacral neuromodulation implant infection: risk factors and prevention. Curr Urol Rep 2017;18:16 10.1007/s11934-017-0663-1 [DOI] [PubMed] [Google Scholar]
- 17. Peters KM. Alternative approaches to sacral nerve stimulation. Int Urogynecol J 2010;21:1559–63. 10.1007/s00192-010-1282-2 [DOI] [PubMed] [Google Scholar]
- 18. Groen J, Amiel C, Bosch JL. Chronic pudendal nerve neuromodulation in women with idiopathic refractory detrusor overactivity incontinence: results of a pilot study with a novel minimally invasive implantable mini-stimulator. Neurourol Urodyn 2005;24:226–30. 10.1002/nau.20131 [DOI] [PubMed] [Google Scholar]
- 19. Sun Z, Yu N, Yue J, et al. Acupuncture for urinary incontinence after stroke: a protocol for systematic review. BMJ Open 2016;6:e008062 10.1136/bmjopen-2015-008062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Song FJ, Jiang SH, Zheng SL, et al. [Electroacupuncture for post-stroke urinary incontinence: a multi-center randomized controlled study]. Zhongguo Zhen Jiu 2013;33:769–73. [PubMed] [Google Scholar]
- 21. Paik SH, Han SR, Kwon OJ, et al. Acupuncture for the treatment of urinary incontinence: A review of randomized controlled trials. Exp Ther Med 2013;6:773–80. 10.3892/etm.2013.1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Liu L, Wang X. Electroacupuncture at points Baliao and Huiyang (BL35) for post-stroke detrusor overactivity. Neural Regen Res 2013;8:1663–72. 10.3969/j.issn.1673-5374.2013.18.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forde JC, Jaffe E, Stone BV, et al. The role of acupuncture in managing overactive bladder; a review of the literature. Int Urogynecol J 2016;27:1645–51. 10.1007/s00192-015-2935-y [DOI] [PubMed] [Google Scholar]
- 24. Olivera CK, Meriwether K, El-Nashar S, et al. Nonantimuscarinic treatment for overactive bladder: a systematic review. Am J Obstet Gynecol 2016;215:34–57. 10.1016/j.ajog.2016.01.156 [DOI] [PubMed] [Google Scholar]
- 25. Solberg M, Alræk T, Mdala I, et al. A pilot study on the use of acupuncture or pelvic floor muscle training for mixed urinary incontinence. Acupunct Med 2016;34:7–13. 10.1136/acupmed-2015-010828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Z, Wang Y, Xu H, et al. [Observation on therapeutic effect of electroacupuncture for post-stroke urge incontinence]. Xin Zhong Yi 2010;42:73–5. [Google Scholar]
- 27. Wang S, Chen G, Li L. [“Four sacral needles” therapy for female stress incontinence]. Shanghai J Acu-Mox 2006;25:15–17. [Google Scholar]
- 28. Lu J. [Observation on therapeutic effect of electroacupuncture neurostimulation therapy for urge urinary incontinence]. Zhongguo Zhen Jiu 2012;32:691–5. [PubMed] [Google Scholar]
- 29. Wang S, Zhang S. Simultaneous perineal ultrasound and vaginal pressure measurement prove the action of electrical pudendal nerve stimulation in treating female stress incontinence. BJU Int 2012;110:1338–43. 10.1111/j.1464-410X.2012.11029.x [DOI] [PubMed] [Google Scholar]
- 30. Wang S, Zhang S, Zhao L. Long-term efficacy of electrical pudendal nerve stimulation for urgency-frequency syndrome in women. Int Urogynecol J 2014;25:397–402. 10.1007/s00192-013-2223-7 [DOI] [PubMed] [Google Scholar]
- 31. Wang S, Lv J, Feng X, et al. Efficacy of electrical pudendal nerve stimulation versus transvaginal electrical stimulation in treating female idiopathic urgency urinary incontinence. J Urol 2017;197:1496–501. 10.1016/j.juro.2017.01.065 [DOI] [PubMed] [Google Scholar]
- 32. Wang S. Electroacupuncture pudendal nerve stimulation and its application. J Acupunct Sci 2013;11:117–21. 10.1007/s11726-013-0671-8 [DOI] [Google Scholar]
- 33. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20. 10.1002/nau.20798 [DOI] [PubMed] [Google Scholar]
- 35. Grut M, Fratiglioni L, Viitanen M, et al. Accuracy of the Mini-Mental Status Examination as a screening test for dementia in a Swedish elderly population. Acta Neurol Scand 1993;87:312–7. 10.1111/j.1600-0404.1993.tb05514.x [DOI] [PubMed] [Google Scholar]
- 36. Timmermans L, Falez F, Mélot C, et al. Validation of use of the International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form (ICIQ-UI-SF) for impairment rating: a transversal retrospective study of 120 patients. Neurourol Urodyn 2013;32:974–9. 10.1002/nau.22363 [DOI] [PubMed] [Google Scholar]
- 37. Duffy L, Gajree S, Langhorne P, et al. Reliability (inter-rater agreement) of the Barthel Index for assessment of stroke survivors: systematic review and meta-analysis. Stroke 2013;44:462–8. 10.1161/STROKEAHA.112.678615 [DOI] [PubMed] [Google Scholar]
- 38. Hajebrahimi S, Nourizadeh D, Hamedani R, et al. Validity and reliability of the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form and its correlation with urodynamic findings. Urol J 2012;9:685–90. [PubMed] [Google Scholar]
- 39. Li YQ, Lu YY, Zhang J, et al. [Analysis on the situation of adverse reaction to acupuncture and acupuncture risk]. Zhongguo Zhen Jiu 2011;31:764–8. [PubMed] [Google Scholar]
- 40. Zhao L, Zhang FW, Li Y, et al. Adverse events associated with acupuncture: three multicentre randomized controlled trials of 1968 cases in China. Trials 2011;12:87 10.1186/1745-6215-12-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician 2015;91:528–36. [PubMed] [Google Scholar]
- 42. Zhang JH, Wang D, Liu M. Overview of systematic reviews and meta-analyses of acupuncture for stroke. Neuroepidemiology 2014;42:50–8. 10.1159/000355435 [DOI] [PubMed] [Google Scholar]
- 43. Mehnert U, Kessler TM. The management of urinary incontinence in the male neurological patient. Curr Opin Urol 2014;24:586–92. 10.1097/MOU.0000000000000109 [DOI] [PubMed] [Google Scholar]
- 44. Smith AL, Wein AJ. Urinary incontinence: pharmacotherapy options. Ann Med 2011;43:461–76. 10.3109/07853890.2011.564203 [DOI] [PubMed] [Google Scholar]
- 45. Robinson D, McClish DK, Wyman JF, et al. Comparison between urinary diaries completed with and without intensive patient instructions. Neurourol Urodyn 1996;15:143–8. [DOI] [PubMed] [Google Scholar]
- 46. Gordon D, Groutz A. Evaluation of female lower urinary tract symptoms: overview and update. Curr Opin Obstet Gynecol 2001;13:521–7. 10.1097/00001703-200110000-00012 [DOI] [PubMed] [Google Scholar]
- 47. Nyström E, Sjöström M, Stenlund H, et al. ICIQ symptom and quality of life instruments measure clinically relevant improvements in women with stress urinary incontinence. Neurourol Urodyn 2015;34:747–51. 10.1002/nau.22657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.