Abstract
Objective.
To determine feasibility and efficacy of administering docetaxel and carboplatin chemotherapy followed by pelvic radiotherapy and then consolidation chemotherapy in patients with advanced or recurrent endometrial cancer.
Methods.
Patients with surgically staged III–IV (excluding IIIA from positive cytology alone) endometrial cancer or biopsy confirmed recurrent disease were eligible. Treatment consisted of 3 cycles of docetaxel (75 mg/m2) and carboplatin (AUC 6) on a q21 day schedule followed by involved field irradiation (45 Gy)± brachytherapy and three additional cycles of docetaxel and carboplatin. Kaplan–Meier (KM) methods estimated overall survival (OS) and progression free survival (PFS).
Results.
Forty-two patients enrolled, 7 did not complete therapy. 95% (39/41) had primary disease. Median age=58 years (range: 21–81 years). 78% (32/41)=endometrioid histology. Stages=10 IIIA, 21 IIIC, 1 IVA, 7 IVB, (recurrent=1 IC, 1 IIA). There were 23 non-hematologic and 14 grade 3 and 16 grade 4 hematologic toxicities. Seven patients died following treatment with a median follow-up of 28 months (range: 7–70 months). KM estimates and 95% confidence intervals for OS at 1 year were 95% (82–99%), at 3 years 90% (75–96%), and at 5 years 71% (45–86%). Of the 39 with primary disease, 11 progressed or died within 5 years of study enrollment. KM estimates and 95% confidence intervals for PFS at 1 year were 87% (72–94%), at 3 years 71% (51–83%), and at 5 years 64% (42–80%).
Conclusions.
“Sandwiching” radiation between chemotherapy for advanced or recurrent endometrial cancer merits further development based on the reported PFS and OS.
Keywords: Endometrial cancer, Chemotherapy, Radiation, Advanced stage
Introduction
Endometrial cancer is the most common gynecologic malignancy accounting for an estimated 43,470 new cases and 7950 deaths in 2010[1]. Most women present with early stage disease and can be cured with primary therapy, however women with metastatic or relapsed cancer have poor disease specific survival with current adjuvant therapy [2–5].
Patients identified with regional metastatic disease at the time of primary surgery conventionally receive tailored adjuvant radiotherapy. Adjuvant radiotherapy (pelvic or whole abdominal radiotherapy) in advanced stages has been shown to significantly reduce pelvic recurrence, however failures outside the radiation field limit effect on long term survival [3,6,7].
For women with advanced or recurrent disease, single agent chemotherapy regimens exhibit response rates of 20–35% [8–10]. A variety of chemotherapeutic agents have been employed in combination by the Gynecologic Oncology Group (GOG) in attempt to improve response rates, progression-free (PFS) and overall survival (OS) for women with advanced or recurrent endometrial cancer. These studies show that platinum-based combination therapy can improve response rates, [11–13] with the three drug regimen of paclitaxel, adriamycin, and cisplatin (TAP) being the most efficacious but also most toxic in phase III studies[11]. Phase II trials using the better tolerated combination of paclitaxel and carboplatin (TC) demonstrate responses between 45% and 78%. Therefore the GOG conducted a phase III equivalency trial comparing the TAP regimen to TC in women with advanced and recurrent endometrial cancer [14,15].
Given that radiation appears to provide excellent control of targeted tissues, but adds little systemic protection, some authors suggested that combining chemotherapy and radiation therapy may be optimal in patients without overt disease in the upper abdomen. Homesley et al. reported that the GOG experience with RT followed by chemotherapy shows reasonable efficacy despite the fact that 20% of patients were unable to complete all prescribed therapy, largely owing to hematologic toxicity possibly due to marrow-suppressing effects of radiation [16].
The current protocol was undertaken in response to recent retrospective reports suggesting “sandwiching” radiation therapy between two shorter courses of platinum and taxane based chemotherapy may be less toxic with equal or superior efficacy. By delivering adjuvant therapy in a sequential “sandwich” fashion, we hypothesized that response rates will be optimized while limiting toxicities. The primary aim of our study was to determine the efficacy of administering docetaxel and carboplatin with radiotherapy in a “sandwich” fashion in the treatment of advanced stage or recurrent endometrial cancer.
Materials and methods
Patient eligibility
This phase II study recruited patients at the University of Minnesota Cancer Center and Park Nicollet Methodist Hospitals from 7/2004 to 7/2009. The institutional review board at each site granted approval. Inclusion criteria were: ≥18 years of age with histologically-confirmed International Federation of Gynecology and Obstetrics (FIGO) stage IIIA (excluding positive cytology only)-IVB endometrial cancer of any non-sarcoma histology (1988 staging system). For primary tumors, eligibility required total abdominal hysterectomy and surgical staging. Participants with recurrent endometrial cancer had to have measurable disease as defined by RECIST within four weeks of study entry. Adequate hematologic (WBC≥3000/μl, platelets≥100,000/mm, granulocytes ≥1,500/μl), renal (Creatinine≤1.5 mg/dL), and hepatic (bilirubin≤1.5× institutional normal value and AST≤3× institutional normal value) function and GOG performance status≤1 was required. Patients with previous pelvic radiation, chemotherapy, history of malignancy within the prior 5 years excluding non-metastatic, non-melanomatous skin cancer, or baseline peripheral neuropathy≥grade 2 were ineligible.
Chemotherapy
Chemotherapy consisted of 3 cycles of IV docetaxel at 75 mg/m2 administered over 60 minutes followed by carboplatin AUC=6 administered over 30 minutes every 3 weeks prior to radiation therapy. Chemotherapy doses were calculated based on the patient’s body surface area and creatinine clearance was calculated by the method of Jelliffe each cycle. Oral dexamethasone (8 mg) was administered two times daily for 3 days beginning 1 day prior to docetaxel administration to reduce the incidence of hypersensitivity reactions. Post-radiation chemotherapy was initiated within 6 weeks of completing radiotherapy and included 3 identical cycles of docetaxel and carboplatin.
Toxicity and adverse events were classified according to the NCI’s Common Terminology Criteria for Adverse Events Version 3.0 (CTCAE). Chemotherapy doses were reduced based on pretreatment blood counts if ANC≤500/mm3 forN5 days, febrile neutropenia requiring antibiotics, or grade 4 thrombocytopenia. Dose levels of both agents were reduced by 25% which was a permanent dose reduction; no further reductions were allowed. Treatment interruptions caused by myelosuppressionN3 weeks required discontinuation of protocol therapy. The protocol was amended after accruing eight patients to prescribe the use of growth factors due to two episodes (grade 3 and grade 4) of febrile neutropenia. Dose adjustments for hepatotoxicity were made only for docetaxel.
Radiation therapy
Radiotherapy was initiated within 4 weeks of the 3rd cycle of chemotherapy following adequate hematologic recovery. Radiation was delivered using a 4 field technique to the pelvis using photon beams of 18 or 25 MV. Daily fraction size was 175 cGy per day 5 days per week. The total dose to the pelvic isocenter was 45.5 Gy and to the center of the paraaortic nodal tissue (if nodes were involved) between 43 and 45 Gy. The volume of irradiation was dependent on extent of disease found at the time of hysterectomy or recurrence. All patients received pelvic irradiation. For positive paraaortic nodes, fields were expanded to encompass both the pelvic and paraaortic nodal chains. CT planning was used to design fields. Standard pelvic borders were applied with the superior border being the L5–S1 interspace and laterally fields encompassed the vessels/nodes with a 1 cm margin. The superior border was extended to the T10–T11 interspace for paraaortic irradiation. High dose rate brachytherapy vaginal cuff boost was delivered for vaginal or parametria extension, cervical or lower uterine segment involvement, or at the discretion of the treating radiation oncologist. The vaginal boost consisted of 1–3 HDR applications with the dose prescribed to the vaginal surface for a length of 4 cm. Patients were removed from protocol for treatment interruption >2 weeks.
Patient follow-up
Patients were evaluated weekly during radiation therapy. Before each chemotherapy cycle patients had a physical examination, complete blood count and electrolytes measured. All adverse effects were assessed and reported. Four to 6 weeks after treatment completion, participants underwent disease assessment with imaging. Patients were evaluated for disease progression and recurrence every three months for 2 years and then every 6 months.
Statistical analysis
This study was designed as a two-stage trial with progression-free survival (PFS) of 9 months as the primary endpoint. The first stage enrolled 16 patients; if activity was demonstrated, an additional 26 patients would be enrolled in the second stage of the study. Treatment would be deemed inactive if 24 or fewer had <9 month PFS. PFS was based on GOG 177 where TAP was shown to be the best cytotoxic chemotherapy available in a population of advanced and recurrent endometrial cancers resulting in a median PFS of 8.3 months[11].
Patient baseline demographic and clinical characteristics were summarized. OS was calculated from date of study enrollment to death or was censored at last contact date for surviving patients. PFS was calculated from study enrollment date to date of first known relapse or death or was censored at last contact date for surviving patients without tumor recurrence/progression. Only patients with primary disease were included in the PFS analysis. OS and PFS were censored at 5 years. Kaplan–Meier methods were used to estimate OS and PFS curves. Point estimates and 95% confidence intervals (CI) for OS and PFS are reported at 1, 3 and 5 years.
Results
Patient characteristics
Between July 2004 and July 2009, 42 patients consented to participate in this trial. One patient withdrew prior to receiving any treatment and per protocol was replaced and excluded from all analyses. Baseline demographic and clinical characteristics of the patients are shown in Table 1. Two patients were treated at disease recurrence. Two participants were taken off protocol due to disease progression, one due to liver toxicity. Three withdrew consent during treatment.
Table 1.
Characteristics of study population.
| N | Mean | SD | |
|---|---|---|---|
| Enrolled in study | 42 | ||
| Withdrew before starting treatment | 1 | ||
| Eligible for analysis | 41 | ||
| Age (years) | 41 | 59 | 9.97 |
| GOG performance status | |||
| 0 | 11 | 26.83 | |
| 1 | 30 | 73.17 | |
| Measurable disease | |||
| Yes | 9 | 21.95 | |
| No | 32 | 78.05 | |
| Race | |||
| White | 35 | 85.37 | |
| Black | 1 | 2.44 | |
| Hispanic | 2 | 4.88 | |
| Other | 2 | 4.88 | |
| Missing | 1 | 2.44 | |
| Disease status | |||
| Primary | 39 | 95.12 | |
| Recurrence | 2 | 4.88 | |
| Stage | |||
| 1C | 1 | 2.44 | |
| IIA | 1 | 2.44 | |
| IIIA | 10 | 24.39 | |
| IIIC | 21 | 51.22 | |
| 1VA | 1 | 2.44 | |
| IVB | 7 | 17.07 | |
| Grade | |||
| 1 | 7 | 17.07 | |
| 2 | 17 | 41.46 | |
| 3 | 17 | 41.46 | |
| Histology | |||
| Endometrioid | 32 | 78.05 | |
| Serous | 4 | 9.76 | |
| Mucinous | 1 | 2.44 | |
| Adenosquamous | 3 | 7.32 | |
| Endometrioid + Serous | 1 | 2.44 | |
| Lymph node dissection | |||
| Yes | 36 | 87.8 | |
| No | 3 | 4.88 |
Chemotherapy
Thirty-five patients (85%) completed all six prescribed cycles of chemotherapy per protocol. In the adjuvant setting, following a surgical staging procedure, there was a mean of 39.1 days (SD=38.2) until chemotherapy was started. Radiation therapy was started a mean of 30.4 days (SD=10.3) following completion of the first three cycles of chemotherapy. There was a mean of 26.9 days (SD=8.3) from radiotherapy completion until the fourth cycle of chemotherapy started. One patient’s pulmonary disease responded to chemotherapy so her physician elected to continue chemotherapy instead of starting radiation and the patient was taken off trial.
Radiation
Two patients did not receive pelvic radiotherapy; one had persistent lung disease and remained on chemotherapy and one declined treatment. One patient received only 21 Gy due to lung metastasis found during radiation. The median dose of external beam pelvic radiation was 45.5 Gy (range=21–60 Gy) and 15 patients received a median of 45.5 Gy to the paraaortic lymph nodes. Twenty-five patients were administered brachytherapy at a dose of 700 cGy in a single fraction at 0.5 cm depth and four patients received 900 cGy in 3 fractions at 0.5 cm depth.
Toxicity
Hematologic toxicities are listed in Table 2. Grade 3/4 neutropenia occurred in 12 patients, with one grade 3 and one grade 4 neutropenic fever observed prior to the addition of growth factor to the protocol. Forty subjects received at least one dose of growth factor. Grade 3/4 anemia occurred in five and one patients, respectively. Six patients received a blood transfusion and six required erythropoietin. There were 23 grade 3 and no grade 4 non-hematological toxicities. The greatest grade 3 non-hematological toxicities were anorexia (three patients) and abdominal pain (three patients). Neuropathy was mild with grade 1 neuropathy in seven patients and grade 2 in two patients (Table 3).
Table 2.
Hematologic toxicities.
| Grade toxicity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adverse events | 0 | 1 | 2 | 3 | 4 | |||||
| N | % | N | % | N | % | N | % | N | % | |
| Total WBC | 17 | 41.4 | 8 | 19.5 | 8 | 19.5 | 4 | 9.7 | 4 | 9.7 |
| ANC/AGC | 20 | 48.7 | 2 | 4.8 | 7 | 17.0 | 2 | 4.8 | 10 | 24.3 |
| Platelets | 21 | 51.2 | 15 | 36.5 | 3 | 7.3 | 2 | 4.8 | 0 | 0 |
| Hemoglobin | 10 | 24.3 | 10 | 24.3 | 15 | 36.5 | 5 | 12.2 | 1 | 2.4 |
| Febrile/Neutropenia | 37 | 90.2 | 0 | 0 | 2 | 4.8 | 1 | 2.4 | 1 | 2.4 |
Table 3.
Non-hematologic toxicities.
| Grade toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adverse events | 1 | 2 | 3 | 4 | ||||
| N | % | N | % | N | % | N | % | |
| Allergy | 1 | 2.44 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ear | 0 | 0 | 2 | 4.88 | 0 | 0 | 0 | 0 |
| Cardiac arrhythmia | 1 | 2.44 | 0 | 0 | 0 | 0 | 0 | 0 |
| INR | 1 | 2.44 | 0 | 0 | 0 | 0 | 0 | 0 |
| FIT | 1 | 2.44 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 16 | 39.02 | 19 | 46.34 | 2 | 4.88 | 0 | 0 |
| Fever | 2 | 4.88 | 1 | 2.44 | 0 | 0 | 0 | 0 |
| Weight loss | 2 | 4.88 | 1 | 2.44 | 0 | 0 | 0 | 0 |
| Weight gain | 1 | 2.44 | 1 | 2.44 | 0 | 0 | 0 | 0 |
| Dermatology | 0 | 0 | 22 | 53.66 | 0 | 0 | 0 | 0 |
| Endocrine | 6 | 14.63 | 1 | 2.44 | 0 | 0 | 0 | 0 |
| Constipation | 11 | 26.83 | 8 | 19.51 | 1 | 2.44 | 0 | 0 |
| Anorexia | 3 | 7.32 | 2 | 4.88 | 3 | 7.32 | 0 | 0 |
| Diarrhea | 7 | 17.07 | 7 | 17.07 | 2 | 4.88 | 0 | 0 |
| Dehydration | 0 | 0 | 3 | 7.32 | 1 | 2.44 | 0 | 0 |
| Vomiting | 4 | 9.76 | 2 | 4.88 | 2 | 4.88 | 0 | 0 |
| Mucositis | 1 | 2.44 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemorrhage/bleeding | 2 | 4.88 | 1 | 2.44 | 0 | 0 | 0 | 0 |
| Hepatobiliary/pancreas | 0 | 0 | 0 | 0 | 1 | 2.44 | 0 | 0 |
| Infection (normal ANC) | 2 | 4.88 | 2 | 4.88 | 2 | 4.88 | 0 | 0 |
| Lymphatics | 1 | 2.44 | 3 | 7.32 | 1 | 2.44 | 0 | 0 |
| Metabolic Laboratory | 0 | 0 | 1 | 2.44 | 1 | 2.44 | 0 | 0 |
| Musculoskeletal soft tissue | 2 | 4.88 | 4 | 9.76 | 0 | 0 | 0 | 0 |
| Neuropathy | 7 | 17.07 | 2 | 4.88 | 0 | 0 | 0 | 0 |
| Confusion | 2 | 4.88 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ocular-Visual | 2 | 4.88 | 0 | 0 | 1 | 2.44 | 0 | 0 |
| Pain | 7 | 17.07 | 9 | 21.95 | 3 | 7.32 | 0 | 0 |
| Pulmonary upper respiratory | 4 | 9.76 | 3 | 7.32 | 1 | 2.44 | 0 | 0 |
| Renal/genitourinary | 3 | 7.32 | 2 | 4.88 | 2 | 4.88 | 0 | 0 |
Response
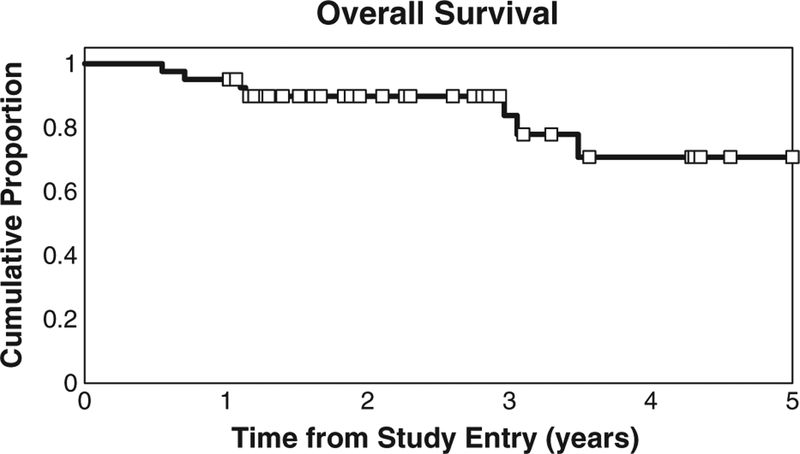
Patients were followed for a median of 28 months (range 7–70 months). Of the 41 patients enrolled, seven patients died during follow-up. The Kaplan–Meier estimate and 95% CI for OS at 1 year is 95% (82–99%), 3 years is 90% (75–96%) and 5 years is 71% (45–86%) (Fig. 1). There are an insufficient number of deaths to estimate median OS.
Fig. 1.
Kaplan–Meier estimate of OS. One-, 2-, and 3-year OS rates were 95%, 90%, and 71%, respectively.
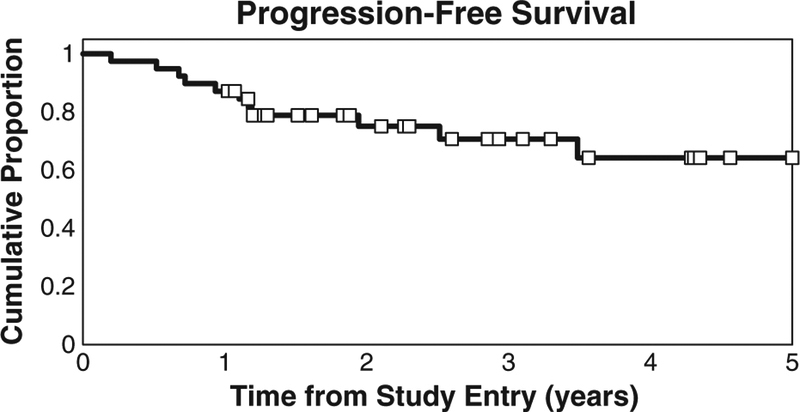
After excluding the two patients enrolled at recurrence, 11 of the 39 patients have progressed or died during follow-up. The Kaplan-Meier estimate and 95% CI for PFS at 1 year is 87% (72–94%), 3 years is 71% (51–83%), and 5 years is 64% (42–80%) (Fig. 2). There are an insufficient number of progressions to estimate median PFS. All patients were followed at least 9 months post-treatment and 35 achievedN9 months PFS. When the 9 patients with measurable disease following initial staging surgery were removed from analysis, the remaining 32 patients had an estimated 1 year OS of 97% (80–100%), 3 year OS of 86% (60–96%), and 5 year OS of 78% (49–92%). The 1 year PFS is 94% (77–98%), 3 year PFS is 85% (63–94%), and 5 year PFS is 77% (50–91%).
Fig. 2.
Kaplan–Meier estimate of PFS. One-, 2-, and 3-year PFS rates were 87%, 71%, and 64%, respectively.
Patterns of initial relapse are shown in Table 4. The majority of relapses occurred distantly primarily in the lung. Two patients had lung metastasis at the start of chemotherapy. One patient had disease progression in the lung during interval radiation despite resolution of her pulmonary nodules following 3 cycles of chemotherapy. There were two local relapses (one was a local and distant failure) that occurred within the radiated field.
Table 4.
Site and frequency of disease persistence/recurrence.
| Site of relapse (N=10) | N (% of total) |
|---|---|
| Distant | 8(80%) |
| Lunga | 3 |
| Breast/Brain | 1 |
| Supraclavicular node | 2 |
| Liver | 1 |
| Paraaortic nodeb | 1 |
| Local | 1(10%) |
| Pelvisc | 1 |
| Local and distant | 1(10%) |
| Carcinomatosis, Supraclavicular node | 1 |
One had disease persistence in lung.
Not in radiation field.
Disease persistent in pelvis at end of therapy.
Conclusion
We demonstrate that “sandwich” therapy using docetaxel and carboplatin is efficacious and well tolerated. Of prospective studies treating patients in this fashion, we report the highest 3-year PFS (71%) and OS (90%) using the combination of carboplatin and docetaxel interposed with involved field radiation for women with advanced stage endometrial cancer.
The rationale of multi-modality therapy is to reduce disease dissemination as well as local-regional pelvic and retroperitoneal recurrences. Recent publications exploring concurrent use of chemo-therapy and radiation in advanced endometrial cancer have shown promise with acceptable toxicity profiles[10,14,17,18]. Secord et al. published a retrospective multicenter analysis of 45 stage III and IV endometrial cancer patients treated using the “sandwich” method. They reported a superior 3-year OS (88%) and PFS (69%) compared to those receiving radiation followed by chemotherapy or chemotherapy followed by radiation. We reported the same 3-year OS and a higher PFS (80%) in our single center review of the “sandwich” method for high risk endometrial cancer [19].
Fields et al. and Lupe et al. prospectively evaluated the use of the “sandwich” method in advanced staged endometrial cancer and reported similar 3 year PFS (54%/53%) and OS’s (52%/68%) [20,21]. Fields’ study had only patients with UPSC while N50% of Lupe’s population had high risk histology which is in contrast to our population with only 12% (5/41) having serous histology. Our lower risk histology may account for the improved PFS and OS.
There are currently no prospective randomized trials to support the concept that combination chemotherapy and radiation is superior to the standard of care single modality treatment established in GOG 177 [11]. That trial compared TAP to doxorubicin and cisplatin (AP) in chemotherapy-naïve women with measurable stage III, IV, or recurrent endometrial cancer. TAP significantly improved PFS and OS compared with AP however improvement was at the expense of neurotoxicity. Peripheral sensory neuropathy was reported as 12% grade 3 and 27% grade 2 (TAP) versus <1% grade 3 and 4% grade 2 (AP). Overall, 40% of patients receiving TAP vs. 5% receiving AP experienced grade 2 or 3 neuropathy which may contribute substantially to a negative quality of life.
Though many oncologists have adopted TC to treat advanced endometrial cancer, presumably to combat neurotoxicity, only data from phase II and retrospective studies support use in combination for advanced endometrial cancer[12,14,22]. The GOG however, will soon answer the question of the two vs. three drug comparability in the recently completed GOG 209 trial comparing the standard TAP to the less toxic regimen of TC. In this trial, radiation was allowed provided it was completed at least 4 weeks prior to starting chemotherapy. These results will answer an important question regarding selection of chemotherapy to use in advanced endometrial cancer.
In the presented trial we chose to use docetaxel based on the increased neurotoxicity associated with paclitaxel in a population that often has baseline neuropathy. The activity of docetaxel in endome-trial cancer has been previously reported. Katsumata et al. used docetaxel at 70 mg/m2 every 3 weeks in stage III, IV or recurrent endometrial cancer with an overall response rate of 31% [23]. Günthert et al. reported response rates of 21% in a similar previously untreated population [24]. Recently the GOG conducted a phase II study in previously treated recurrent endometrial cancer patients studying docetaxel 36 mg/m2 administered weekly every 28 days. They reported modest activity with two (7.7%) partial responses and eight (30.8%) with stable disease [25].
In our study, only two patients reported grade 2 neuropathy; none experienced grade 3 or 4 neuropathy. Lupe et al. reported that 31% of their patients receiving TC interposed by radiation experienced a grade 3 or 4 toxicity with peripheral neuropathy and neutropenia being the most commonly cited [21]. The decision to use docetaxel as opposed to paclitaxel and carboplatin over cisplatin is largely due to the grades 3 and 4 neuropathy observed in approximately 40% of patients receiving cisplatin and paclitaxel. Endometrial cancer patients often are older and many have previously-diagnosed diabetes, therefore neuropathy tends to be a significant issue in this population. We believe that administration of docetaxel instead of paclitaxel decreased neurotoxicity allowing for completion of six total prescribed courses of chemotherapy.
With the lower incidence of neurotoxicity as well as shorter infusion times when compared to paclitaxel, docetaxel offers both a side effect advantage and substantial clinical benefit. Additionally, we show with this particular regimen, patients have minimal delay between treatment modalities with on average about a month between the third chemotherapy cycle to the start of radiotherapy and with less than a month before the fourth chemotherapy cycle is started following radiation completion.
The low rate of recurrence in the radiation field is notable in our study. One patient had disease persistence in field following therapy completion and one recurred with carcinomatosis. These findings are similar to that of Lupe et al. who reported only two pelvic recurrences and one patient with a local and distant failure in patients treated in the “sandwich” fashion [21]. With chemotherapy alone in the treatment of advanced endometrial cancer, pelvic recurrences have been reported to be as high as 46% [26]. Our findings with that of others suggest there may be improved pelvic control using multi-modality therapy compared to chemotherapy alone.
Although prospective, our study is limited by its heterogeneous population with regard to disease stage and cytoreductive status. Additionally, further follow up time is necessary to report any late grade 3 or higher treatment-related gastrointestinal adverse events that may accompany radiation therapy. However, the cumulative probability of these events appeared to be acceptable at 5% following the sequencing of radiation followed by chemotherapy as reported in GOG 184 [16]. Another limitation could be that the majority of patients had endometrioid histology and 24% had stage IIIa disease, both historically good prognostic factors. The most common type of endometrial cancer is endometrioid and it is not surprising that this study reflects that histological distribution. Unlike GOG 184, we excluded patients with stage IIIa disease based on positive cytology alone. However, the impact of positive peritoneal cytology on survival remains controversial [27–29]. Despite what may be commonly thought, stage IIIa is not without risk for recurrence and outcomes have been shown to vary greatly depending on amount of pelvic disease present [30].
In our phase II study, we found the “sandwich” protocol both feasible and well tolerated. Neutropenia was the most frequent toxicity. Furthermore, with findings of a 3 year PFS of 71% and OS of 90% we believe this therapy is an active and promising regimen for the treatment of patients with advanced stage endometrial cancer. Future randomized trials are necessary to compare chemotherapy and radiation given in the “sandwich” fashion to other means of sequencing these treatment modalities.
Acknowledgments
This study was supported financially by Sanofi-Aventis and in part by NIH P30 CA77598 utilizing the Masonic Cancer Center, University of Minnesota Biostatistics and Bioinformatics Core. We would like to thank Justin Chura, MD for his assistance in data collection as well as Elaine Bell RN, BSN, OCN Oncology Research Manager for her excellence in study coordination and patient care during this trial.
Footnotes
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- [1].Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- [2].Sovak MA, Hensley ML, Dupont J, Ishill N, Alektiar KM, Abu-Rustum N, Barakat R, Chi DS, Sabbatini P, Spriggs DR, Aghajanian C. Paclitaxel and carboplatin in the adjuvant treatment of patients with high-risk stage III and IV endometrial cancer: a retrospective study. Gynecol Oncol 2006;103:451–7. [DOI] [PubMed] [Google Scholar]
- [3].Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley HD, Malfetano JH, Mychalczak BR, King ME. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a gynecologic oncology group study. Gynecol Oncol 2005;97:755–63. [DOI] [PubMed] [Google Scholar]
- [4].Bruzzone M, Miglietta L, Franzone P, Gadducci A, Boccardo F. Combined treatment with chemotherapy and radiotherapy in high-risk FIGO stage III–IV endometrial cancer patients. Gynecol Oncol 2004;93:345–52. [DOI] [PubMed] [Google Scholar]
- [5].Greven KM, Lanciano RM, Corn B, Case D, Randall ME. Pathologic stage III endometrial carcinoma. Prognostic factors and patterns of recurrence. Cancer 1993;71:3697–702. [DOI] [PubMed] [Google Scholar]
- [6].Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley H, Lee RB, Gehrig PA, Zaino R. Adjuvant whole abdominal irradiation in clinical stages I and II papillary serous or clear cell carcinoma of the endometrium: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol 2006;100:349–54. [DOI] [PubMed] [Google Scholar]
- [7].Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, Thigpen JT, Benda JA. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:36–44. [DOI] [PubMed] [Google Scholar]
- [8].Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, Liao S. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol 2004;22:3902–8. [DOI] [PubMed] [Google Scholar]
- [9].Thigpen JT, Blessing JA, Homesley H, Creasman WT, Sutton G. Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol 1989;33:68–70. [DOI] [PubMed] [Google Scholar]
- [10].Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol 2006;103:155–9. [DOI] [PubMed] [Google Scholar]
- [11].Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, Kline R, Burger RA, Goodman A, Burks RT. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 2004;22:2159–66. [DOI] [PubMed] [Google Scholar]
- [12].Price FV, Edwards RP, Kelley JL, Kunschner AJ, Hart LA. A trial of outpatient paclitaxel and carboplatin for advanced, recurrent, and histologic high-risk endometrial carcinoma: preliminary report. Semin Oncol 1997;24 S15–78- S15–82. [PubMed] [Google Scholar]
- [13].Akram T, Maseelall P, Fanning J. Carboplatin and paclitaxel for the treatment of advanced or recurrent endometrial cancer. Am J Obstet Gynecol 2005;192: 1365–7. [DOI] [PubMed] [Google Scholar]
- [14].Hoskins PJ, Swenerton KD, Pike JA, Wong F, Lim P, Acquino-Parsons C, Lee N. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: a phase II study. J Clin Oncol 2001;19:4048–53. [DOI] [PubMed] [Google Scholar]
- [15].Nakamura T, Onishi Y, Yamamoto F, Kouno S, Maeda Y, Hatae M. Evaluation of paclitaxel and carboplatin in patients with endometrial cancer. Gan To Kagaku Ryoho 2000;27:257–62. [PubMed] [Google Scholar]
- [16].Homesley HD, Filiaci V, Gibbons SK, Long HJ, Cella D, Spirtos NM, Morris RT, DeGeest K, Lee R, Montag A. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecol Oncol 2009;112:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Onda T, Yoshikawa H, Mizutani K, Mishima M, Yokota H, Nagano H, Ozaki Y, Murakami A, Ueda K, Taketani Y. Treatment of node-positive endometrial cancer with complete node dissection, chemotherapy and radiation therapy. Br J Cancer 1997;75:1836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alvarez Secord A, Havrilesky LJ, Bae-Jump V, Chin J, Calingaert B, Bland A, Rutledge TL, Berchuck A, Clarke-Pearson DL, Gehrig PA. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol 2007;107:285–91. [DOI] [PubMed] [Google Scholar]
- [19].Geller MA, Ivy J, Dusenbery KE, Ghebre R, Isaksson Vogel R, Argenta PA. A single institution experience using sequential multi-modality adjuvant chemotherapy and radiation in the “sandwich” method for high risk endometrial carcinoma. Gynecol Oncol 2010;118:19–23. [DOI] [PubMed] [Google Scholar]
- [20].Fields AL, Einstein MH, Novetsky AP, Gebb J, Goldberg GL. Pilot phase II trial of radiation “sandwiched” between combination paclitaxel/platinum chemotherapy in patients with uterine papillary serous carcinoma (UPSC). Gynecol Oncol 2008;108:201–6. [DOI] [PubMed] [Google Scholar]
- [21].Lupe K, D’Souza DP, Kwon JS, Radwan JS, Harle IA, Hammond JA, Carey MS. Adjuvant carboplatin and paclitaxel chemotherapy interposed with involved field radiation for advanced endometrial cancer. Gynecol Oncol 2009;114:94–8. [DOI] [PubMed] [Google Scholar]
- [22].Michener CM, Peterson G, Kulp B, Webster KD, Markman M. Carboplatin plus paclitaxel in the treatment of advanced or recurrent endometrial carcinoma. J Cancer Res Clin Oncol 2005;131:581–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Katsumata N, Noda K, Nozawa S, Kitagawa R, Nishimura R, Yamaguchi S, Aoki D, Susumu N, Kuramoto H, Jobo T, Ueki K, Ueki M, Kohno I, Fujiwara K, Sohda Y, Eguchi F. Phase II trial of docetaxel in advanced or metastatic endometrial cancer: a Japanese Cooperative Study. Br J Cancer 2005;93:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gunthert AR, Ackermann S, Beckmann MW, Camara O, Kiesel L, Rensing K, Schroder W, Steiner E, Emons G. Phase II study of weekly docetaxel in patients with recurrent or metastatic endometrial cancer: AGO Uterus-4. Gynecol Oncol 2007;104:86–90. [DOI] [PubMed] [Google Scholar]
- [25].Garcia AA, Blessing JA, Nolte S, Mannel RS. A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: a study by the Gynecologic Oncology Group. Gynecol Oncol 2008;111:22–6. [DOI] [PubMed] [Google Scholar]
- [26].Mundt AJ, McBride R, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Significant pelvic recurrence in high-risk pathologic stage I–IV endometrial carcinoma patients after adjuvant chemotherapy alone: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys 2001;50:1145–53. [DOI] [PubMed] [Google Scholar]
- [27].Dede M, Yenen MC, Goktolga U, Duru NK, Guden M, Dilek S, Pabuccu R. Is adjuvant therapy necessary for peritoneal cytology-positive surgical-pathologic Stage I endometrial cancer? Preliminary results. Eur J Gynaecol Oncol 2004;25: 591–3. [PubMed] [Google Scholar]
- [28].Tebeu PM, Popowski GY, Verkooijen HM, Casals J, Ludicke F, Zeciri G, Usel M, Bouchardy C, Major AL. Impact of peritoneal cytology on survival of endometrial cancer patients treated with surgery and radiotherapy. Br J Cancer 2003;89: 2023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wethington SL, Barrena Medel NI, Wright JD, Herzog TJ. Prognostic significance and treatment implications of positive peritoneal cytology in endometrial adenocarcinoma: Unraveling a mystery. Gynecol Oncol 2009;115:18–25. [DOI] [PubMed] [Google Scholar]
- [30].Jobsen JJ, ten Cate LN, Lybeert ML, van der Steen-Banasik EM, Scholten A, van der Palen J, Slot A, Kroese MC, Schutter EM, Siesling S. The number of metastatic sites for stage IIIA endometrial carcinoma, endometrioid cell type, is a strong negative prognostic factor. Gynecol Oncol 2010;117:32–6. [DOI] [PubMed] [Google Scholar]