Abstract
Introduction
One of the most common distressing conditions experienced by breast cancer survivors is fear of cancer recurrence (FCR). There is, however, no standard intervention for ameliorating FCR. Our clinical experience and previous studies have suggested the potential benefits of problem-solving therapy (PST) and behavioural activation (BA). Given the huge number of cancer survivors and limited number of therapists to competently conduct PST and BA, we have developed PST and BA smartphone applications. This study aimed to evaluate the efficacy of the smartphone-based PST (Kaiketsu-App) and BA (Genki-App) apps in reducing FCR in patients with breast cancer.
Methods and analysis
The SMartphone Intervention to LEssen fear of cancer recurrence project is an open-label, individually randomised, parallel-group trial. Allocation will be managed by a central server using a computer-generated random allocation sequence provided by an independent data centre. Participants will be randomised to smartphone-based intervention plus treatment as usual (TAU) or waitlist control with TAU alone. The primary endpoint of the study is the Japanese version of the Concerns About Recurrence Scale, which will be administered as an electronic patient-reported outcome on the patients’ smartphone after 8 weeks.
Ethics and dissemination
The present study is subject to the ethical guidelines for clinical studies published by Japan’s Ministry of Education, Science and Technology and Ministry of Health, Labour and Welfare and the modified Act on the Protection of Personal Information as well as the ethical principles established for research on humans stipulated in the Declaration of Helsinki and further amendments thereto. The protocol was approved by the Institutional Review Board of Nagoya City University on 15 January 2018 (ID: 60-00-1171).
Trial status
The randomised trial, which commenced on 2 April 2018, currently enrols participants. The estimated end date for this study is in March 2020.
Trial registration number
UMIN000031140; Pre-results.
Keywords: neoplasma, fear of recurrence, cancer survivorship, psychosocial intervention, information and communication technology, quality of life
Strengths and limitations of this study.
This study is the first trial investigating the efficacy of smartphone-based psychological therapy for fear of cancer recurrence (FCR) among breast cancer survivors.
Because many breast cancer survivors return to their households and work, easily accessible therapeutic interventions without hospital visits may offer benefits.
This study focuses on younger breast cancer survivors who are iPhone users; this focus could reduce the external validity of the findings obtained.
The use of the waitlist control conditions may overestimate the efficacy of the smartphone-based psychotherapy.
We will apply two types of psychotherapy and both interventions consist of complex, multifactorial components; thus, we cannot be certain which intervention and components are most beneficial in managing FCR; however, we will adopt a mixed methods design to overcome those issues.
Introduction
Worldwide, breast cancer is one of the most common cancers among women. In Japan, it is also the most common cancer among women, and the incidence is increasing. At present, approximately 90 000 women in Japan are annually diagnosed with breast cancer. Advances in early detection and individualised medical treatment have improved the survival rate of patients with breast cancer. The current 10-year survival rate for patients with breast cancer is over 90%, which contributes to the increasing number of breast cancer survivors.1
Thus, patients with breast cancer have on average a better prognosis than those with many other types of cancer; however, it has been suggested that many breast cancer survivors suffer from uncertainty, anxiety and fear over recurrence.2–4 Our previous study found that the most common unmet needs experienced by patients with ambulatory breast cancer are psychological—especially fear of cancer recurrence (FCR); over half of patients complained of such needs.5 Among patients with breast cancer, FCR is highly prevalent and is associated with poor quality of life.2 5–8
Accordingly, there is an urgent need for an appropriate intervention for FCR. However, there is no standard intervention for ameliorating FCR.8 Recent studies have demonstrated that the following can improve FCR among breast cancer survivors: mindfulness stress reduction (six weekly group sessions); cognitive–behavioural therapy (CBT; five individual face-to-face sessions and three e-consultations over a period of 3 months); and the novel theoretically based intervention of ConquerFear, which includes attention training, metacognition, and acceptance and mindfulness (five individual face-to-face sessions over 10 weeks).9–12 These interventions may be promising; however, one problem with this kind of intervention is the low participation rate owing to time and distance issues (eg, over 60% of potentially eligible subjects have been found to decline participation).9 11–14 In addition, the number of therapists who can provide such specialised care may be severely limited, which is a serious problem in many countries.
Our past experience and some studies indicate the effectiveness of CBT, including problem-solving therapy (PST) and behavioural activation (BA), for FCR among breast cancer survivors (Momino, in press).15–17 We have demonstrated that patients’ problem-solving skills were significantly associated with FCR.15 Our hypotheses of their underlying mechanisms are that PST contributes to patients’ better coping with situations commonly triggering FCR (eg, pain, exposure to news about cancer, regular visit to cancer hospital, and so on) and other stressful situations that increase FOR and that BA also improves FOR through distraction and through increased sense of mastery and pleasure. PST and BA are straightforward interventions that can be administered by less experienced therapists, including nurses.18 However, patients willing to undergo PST or BA are rarely able to do so even in well-resourced countries because a typical course of PST or BA consists of 8–12 face-to-face sessions lasting 1 or 1.5 hours led by a trained therapist.19–21
Though such programs seem promising, they appear to suffer limitations similar to those of the above-mentioned therapeutic interventions.9–12 Given the growing number of women annually diagnosed with breast cancer and the high prevalence among them of unmet needs of FCR, a completely novel approach to therapy provision is required. Recent studies have demonstrated the effectiveness of computerised CBT.22 23 In light of recent developments in information and communication technology (ICT), CBT delivered via smartphones may be a better treatment option for FCR—in terms of accessibility and portability—than a computer-based one.24 We have recently developed a smartphone CBT app, which teaches skills in BA and cognitive restructuring; we demonstrated its efficacy for antidepressant-resistant major depression in a randomised controlled trial.25 We have also developed PST programs as a smartphone app and demonstrated the acceptability and efficacy of the smartphone-based PST in a single-arm pilot study with breast cancer survivors (Imai, submission). The purpose of the present randomised study is to examine the efficacy of smartphone-based PST and BA interventions to reduce FCR in patients with breast cancer in a randomised controlled trial. Since there is no specific standard intervention for ameliorating FCR as mentioned above and our research team’s discussion suggests that setting waitlist control will be more feasible than no intervention, waitlist control is used as a comparator.
Methods and analysis
This protocol has been written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials guideline.26
Trial design
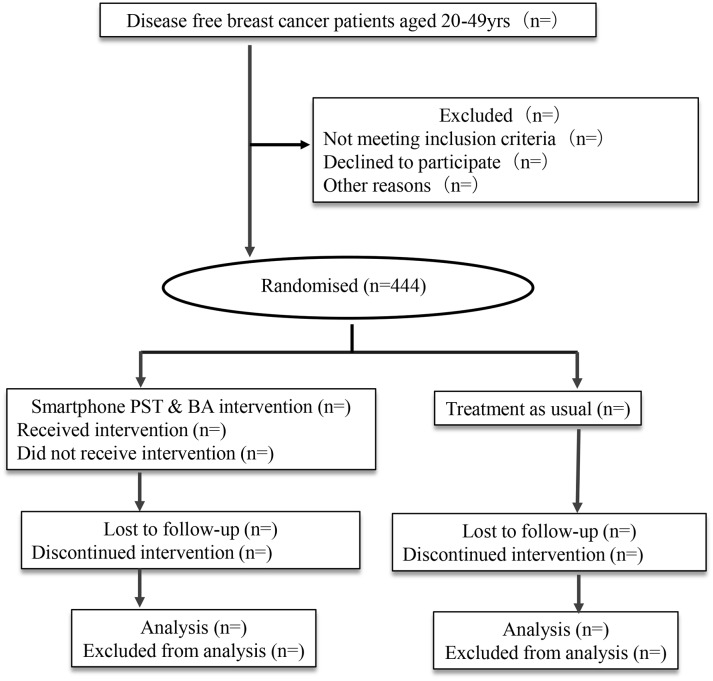
The present study is an individually randomised, parallel-group trial (figure 1). An independent data centre will provide computer-generated random allocation sequences. The allocation sequences are maintained centrally, and the results of the assignment will be sent automatically to the study participants by email. The participants are randomised to smartphone-based intervention plus treatment as usual (TAU) or waitlist control with TAU alone. TAU means general treatment and/or care commonly provided by each patient’s hospital (eg, nurse’s support, and so on).
Figure 1.
Participant flow diagram. BA, behavioural activation; PST, problem-solving therapy.
Interventions: smartphone-based PST (Kaiketsu-App) and BA (Genki-App)
PST provides patients with a structured strategy for solving their problems. PST includes the following five steps27: (1) identification, definition and breakdown of the problem; (2) establishing achievable goals; (3) generating solutions; (4) evaluating and choosing the solution; and (5) implementing the chosen solution and evaluating the outcome after implementation.
The smartphone-based PST program, called Kaiketsu-App (‘Kaiketsu’ means ‘Solution’ in Japanese), for iPhones and iPads (Apple, Cupertino, CA, USA) was developed for this study (figure 2). The development was based on our empirically supported PST manual.17 Kaiketsu-App comprises nine sessions (online supplementary table): three introductory sessions; four sessions about learning the PST in five steps; one session of actual training; and one concluding session. The shortest time necessary to complete Kaiketsu-App is 2 weeks. The main program consists of dialogues between characters, who explain the principles and skills of PST. After the first session, participants have to do homework. The time necessary to complete one session is approximately 30 min.
Figure 2.
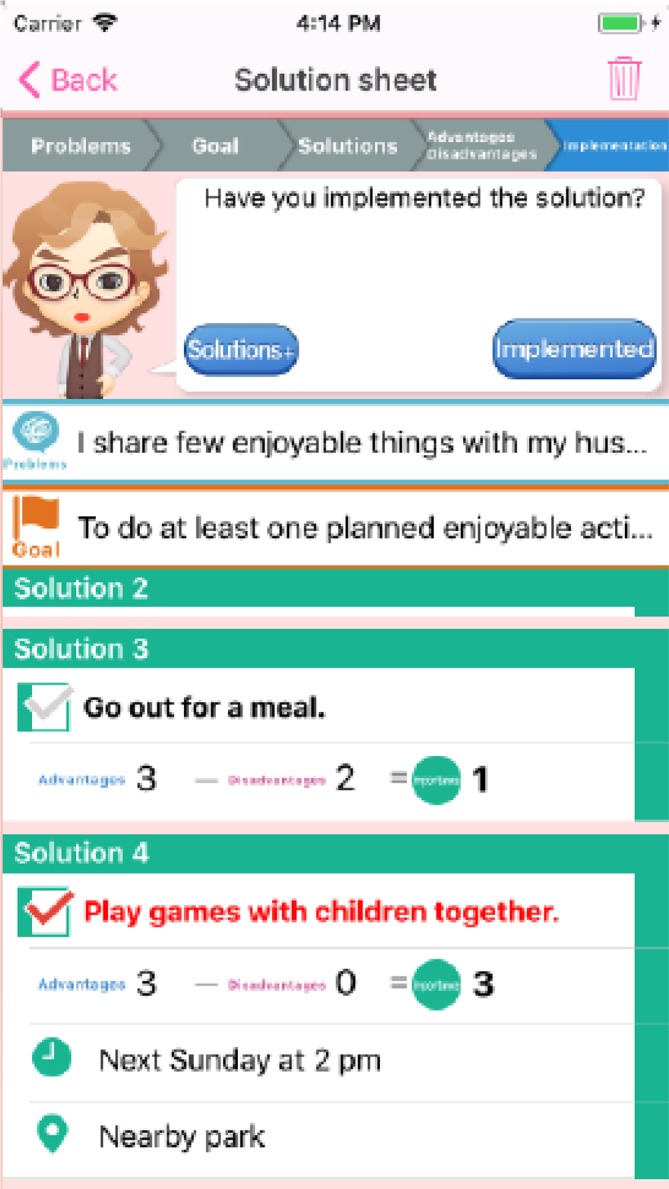
Kaiketsu-App. Application for smartphone-based problem-solving treatment.
bmjopen-2018-024794supp001.pdf (11.3KB, pdf)
BA intervention was developed based on the hypothesis that anxiety can lead to less pleasurable behaviour and more sedentary life, which then may lead to worsening anxiety.27
The smartphone-based BA program Genki-App (‘Genki’ means ‘Energy or Vitality’ in Japanese) for iPhones and iPads (figure 3) was developed as part of the smartphone-based CBT program which was developed for our previous study.28 Genki-App consists of two sessions (online supplementary table), and approximately 30 min is needed to complete each session: one is outline and introduction of BA therapy including two types of activation (eg, do pleasurable activity again and challenge new activity) and their actual training. (Genki-App includes a self-learning sheet for planning and doing pleasurable and new activity, and for evaluating achievement after conducting its activity.) The other is review of the session, learning knack of BA (start an activity to be able to conduct by yourself; divide big aim into some smaller ones; plan a schedule to conduct an activity; image a situation when you can do it well) and concluding session. The shortest time necessary to complete Genki-App is also 2 weeks. The Genki-App program also mainly consists of dialogues between characters and homework.
Figure 3.

Genki-App. Application for smartphone-based behavioural activation.
Over the 8-week period of the programs, participants are encouraged to complete the sessions and homework through automated email reminders once a week. Although we cannot know the contents of homework for privacy security, treatment adherence (eg, times and length of using each App) can be checked by Google Analytics.
Participants
The inclusion criteria for participants are as follows: (1) diagnosis of breast cancer and awareness of the cancer diagnosis; (2) ages 20–49 years; (3) 1 year following breast surgery; (4) currently disease free; (5) ability to complete an electronic patient-reported outcome (e-PRO) using an iPhone or iPad; and (6) being an iPhone or iPad user and having an apple ID to install applications using App Store. We limit the patients’ age to 20–49 years because one study and our previous investigation demonstrated that individuals of that age are at high risk of FCR and that more than 50% of such people have smartphones.8 29 30
The exclusion criteria for participants are as follows: (1) having active, serious physical disease that affects household and light work and a history or current history of cancer other than breast cancer; (2) inability to understand Japanese; (3) currently undergoing follow-up and treatment in a psychiatry department or by other mental health professionals; (4) patients who have previously undergone structured PST, BA therapy or CBT; and (5) judged inappropriate for participation by the researchers (eg, identity theft, duplicate entry, and so on).
Procedures
Newly developed research management system
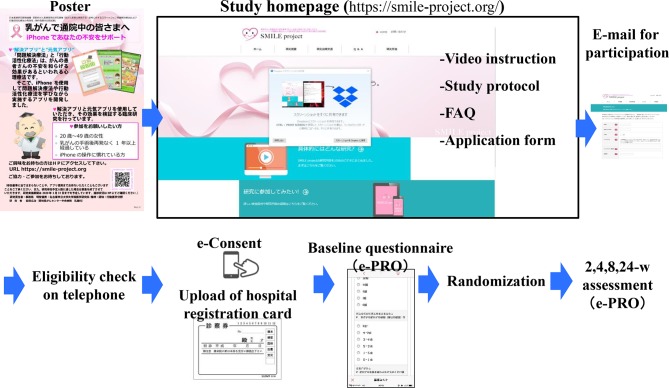
To lessen patient inconvenience in visiting hospital for this study and oncologists’ workload in enrolling study participants, we developed a research management system making full use of ICT technology (figure 4). The study’s website (https://smile-project.org/) provides information about this study. A poster briefly introducing the study and including a QR code for the website has been put up in 10 core cancer hospitals in Japan and study information will be disseminated repeatedly by using several social networking systems (eg, Facebook, patient’s mailing list, and so on). The website explains the purpose of the study, eligibility criteria and methods used; it also features a video briefly introducing the study as well as providing full written information about it. Potential participants who are interested in the study can email the study’s central office, and clinical research coordinators (CRC) at the central office ascertain their eligibility by telephone (table 1).
Figure 4.
Study management system. e-consent, electronic informed consent; e-PRO, electronic patient-reported outcome; FAQ, frequently asked questions.
Table 1.
Schedule for outcome measurement
| Assessment | Time points | ||||
| 0 week | 2 weeks | 4 weeks | 8 weeks | 24 weeks | |
| Understanding of the e-consent | ● | ||||
| Characteristic | ● | ||||
| CARS-J, HADS | ● | ● | ● | ● | ●* |
| FCRI, SCNS-SF34, PTGI-J | ● | ● | ●* | ||
| Satisfaction with interventions | ●* | ||||
| Qualitative assessment of apps | ●* | ||||
*These would be evaluated only for intervention group.
CARS-J, Japanese version of the Concerns About Recurrence Scale; FCRI, Fear of Cancer Recurrence Inventory; HADS, Hospital Anxiety and Depression Scale; PTGI-J, Post-traumatic Growth Inventory-Japanese version; SCNS-SF34: Short-Form Supportive Care Needs Survey questionnaire.
Electronic informed consent and randomisation at week 0
After screening, the CRCs will seek to obtain the subjects’ electronic informed consent (e-consent) via e-PRO system at week 0. Participants will be requested to upload a picture of identification materials. (Patients will be especially encouraged to attach a photo of the ID card of the hospital where they made regular follow-up visits for breast cancer.) This e-consent procedure is in accordance with the guidance of the US Food and Drug Administration.31 Original informed consent material is shown in the online supplementary appendix.
bmjopen-2018-024794supp002.pdf (732.8KB, pdf)
After providing e-consent and completing the baseline investigation by e-PRO, the participants will be randomly allocated to either the smartphone-based PST and BA group or the waitlist control group in a 1:1 ratio using the electronic data capturing (EDC) web program at the data management centre (figure 1). The random allocation will therefore be concealed.
If a participant is allocated to the intervention group, they will receive a password unique to them for using Kaiketsu-App and Genki-App. On entering the password, participants will be able to proceed to the sections for Kaiketsu-App and Genki-App. If participants are allocated to the control group, they will be informed that use of Kaiketsu-App and Genki-App can be resumed if they wish after week 8.
Data management, central monitoring, data monitoring and auditing
We will collect all data except qualitative interview data through e-PRO; we will obtain qualitative interview data by telephone. (See below for details.) If participants fail to provide their responses regarding FCR, a CRC blinded to the assignment will telephone the subjects to elicit their answers. Data management and central monitoring will be performed using the EDC. The EDC consists of two different and independent parts, one including personal information and the other including trial-related data (eg, assignment, outcomes, and so on) for security. Since the psychological intervention provided by apps will not be invasive and also not produce serious harms, data monitoring committee will not be organised. Similarly, auditing is not also planned for this study.
Data set available
The deidentified anonymised data set will be uploaded to UMIN-ICDR (http://www.umin.ac.jp/icdr/index-j.html) and researchers approved by the steering committee will be able to have access to the data set.
Trial period: weeks 0–8
For participants allocated to the intervention group, an automated email encouraging their adherence to Kaiketsu-App and Genki-App will be sent every week for 8 weeks. The research team can check the patient’s progress (number of times and duration using each application) with Kaiketsu-App and Genki-App using Google Analytics. At weeks 2, 4 and 8, participants will receive an email encouraging them to record their responses on e-PRO.
Follow-up period: weeks 8–24
Participants allocated to the control group can resume the Kaiketsu-App and Genki-App if they wish. At week 24, participants allocated to the intervention group will receive an email encouraging them to provide their responses on e-PRO.
Concomitant treatments
There is no restriction on concomitant treatments.
Stopping rules for participants
Discontinuation of protocol treatment.
If a participant meets any of the following conditions, the research team can discontinue the Kaiketsu-App and Genki-App. However, the participant will not be considered to have dropped out of the trial at that stage and will receive the protocol assessments: (1) the participant wishes to stop the protocol treatment; (2) the research team judges that the risk of the protocol treatment is greater than the benefit for any reason; (3) the research team judges that it is difficult to continue the protocol treatment because of clinical deterioration; and (4) the research team judges that it is inappropriate to continue the protocol treatment for any reason (eg, when identity theft, duplicate entry, and so on, are detected).
Stopping assessment
If a participant withdraws consent for assessment, she will not be followed up. Subjects will be excluded from the intention-to-treat (ITT) cohort of the trial only if their characteristics are found to meet any exclusion criteria at baseline (eg, age over 50 years) after participation.
Assessment measures
Table 1 shows the schedule for outcome measurement.
Primary outcome measure
Fear of recurrence: Japanese version of the Concerns About Recurrence Scale
Originally developed in the USA, the Japanese version of the Concerns About Recurrence Scale (CARS-J) is a 26-item self-report scale to measure fear of recurrence of breast cancer.32 The reliability and validity of CARS-J have been confirmed among Japanese patients with breast cancer.33 CARS-J assesses the overall fear of breast cancer recurrence and four domains of specific fear of recurrence. Overall, fear comprises four items: questions on frequency; potential for upset; consistency; and intensity of fear. The overall fear score with CARS-J is our primary outcome. The range of possible scores for overall fear is 4–24; a higher score indicates greater fear of recurrence.
Secondary outcome measures
Fear of Cancer Recurrence Inventory-Short Form
The Fear of Cancer Recurrence Inventory-Short Form (FCRI-SF) is a 9-item self-report scale originally developed in Canada.34 35 The FCRI evaluates the presence and severity of intrusive thoughts associated with FCR: scores range from 0 to 36; a higher score indicates severe FCR. Unlike with CARS-J, the FCRI-SF can measure fear of recurrence of any type of cancer. The Japanese version of the FCRI-SF was developed after obtaining permission from the original author and using a forward-backward translation process. In this study, the measure will be included as a secondary outcome after its validity and reliability have been ascertained.
Psychological distress: Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS), a self-report questionnaire comprising 14 items, was developed for use with patients with medical illness. The HADS consists of an anxiety and a depression subscale (0–21 points each). The total score ranges from 0 to 42; a higher score indicates more severe depression and anxiety.36 The Japanese version of the HADS has been validated for cancer populations.37
Patients’ perceived needs: Short-Form Supportive Care Needs Survey questionnaire
The Short-Form Supportive Care Needs Survey questionnaire (SCNS-SF34) is a self-administered instrument for assessing the perceived needs of patients with cancer.38 The SCNS-SF34 comprises 34 items covering five domains of need: psychological; health system and information; physical and daily living; patient care and support; and sexuality. The total score is obtained by summing all the subscales (range 34–170). A higher score indicates a higher perceived need. The validity and reliability of the Japanese version of SCNS-SF34 have been established.39
Post-traumatic Growth Inventory-Japanese version
The Post-traumatic Growth Inventory (PTGI) is a 21-item self-report scale originally developed in the USA.40 The PTGI includes items that measure positive psychological change experienced as a result of struggle with major life crises or traumatic events. The PTGI-J consists of four subscales: relating to others; new possibilities; personal strength; and spiritual change and appreciation of life. The total score ranges from 0 to 90; a higher score indicates more positive changes.41
Satisfaction with intervention
To assess patients’ perceived satisfaction with the intervention, we ask two additional items. The items are as follows. (1) ‘Please rate your satisfaction with the treatment in meeting your needs during the past 2 months by assigning it a score from 0 to 100; a score of 100 indicates complete satisfaction. A score of 60 is considered the passing level.’ (2) ‘Please rate your overall satisfaction with the treatment during the past 2 months by assigning it a score from 0 to 100; a score of 100 indicates complete satisfaction. A score of 60 is considered the passing level.’ A lower score indicates lower satisfaction. We used this method in our previous study.42
Understanding e-consent
Ten questions will be asked at week 0 related to the participants’ understanding of e-consent: purpose of the study; randomisation; voluntary participation; duration of study; risks and benefits of study participation; free withdrawal anytime from the study; contact method for questions and more detailed information about the study; method of participant identification (uploading a photo of the hospital registration card); which of the video or written documentation in the website was the more helpful in understanding the study contents; and free opinions regarding e-consent.
Qualitative evaluation of intervention
The intervention will consist of multiple complex components; accordingly, simple structured telephone interviews will be conducted at 8 weeks for approximately 30 subjects willing to participate in this additional survey to evaluate the perceived usability and/or merit of the complex intervention. The interview items will be as follows. (1) ‘Please talk freely about the usefulness of the smartphone PST and smartphone behavioral intervention.’ (2) ‘Please talk freely about the usefulness of each of the five steps of the smartphone PST and give your reasons for your opinions.’ (3) ‘Please talk freely about the usefulness of the two parts of the smartphone behavioral intervention and give your reasons for your opinions.’ (4) ‘Please talk freely about the effectiveness and harms of the intervention, for example, the regular encouraging e-mail, and if any other components contributed to improving or deteriorating your fear of recurrence.’ If the participants permit, the answers will be recorded using a voice recorder.
Sociodemographic and biomedical factors e-PRO will also be used to obtain information about the patients’ sociodemographic and biomedical status (marital status, level of education and employment status) and biomedical information (physical function, time since diagnosis, clinical stage and anticancer treatment).
Harms
No specific and serious adverse events are presumed in participants who use the Kaiketsu-App and Genki-App. However, using these apps might lead to psychological distress in some participants depending on their psychological state. We will evaluate these potential adverse events by qualitative evaluation of intervention as mentioned before.
Compensation
Our previous and preliminary trials suggest that few harms occur in this trial. However, if any health hazards occur, these will be covered by the National Health Insurance.
Data analysis
Primary analyses
To examine the treatment effect parameters of all randomly assigned subjects in the primary analysis set according to the ITT principle, we will analyse the primary outcome (CARS-J score at weeks 2, 4 and 8) using a generalised linear model with unstructured covariance and robust SEs. The fixed effects are CARS-J score at baseline, treatment allocation (intervention vs control), time (2, 4 and 8 weeks as categorical variable) and treatment-by-time interaction; the random effects are subjects (as intercepts). The primary outcome of interest is the difference in CARS-J scores between the two groups at week 8. A two-sided p value <0.05 will be used to indicate statistical significance.
Secondary analyses
We will perform secondary analyses to supplement our primary analysis and to obtain a clearer understanding of our clinical questions. The secondary analyses will use models similar to that of the primary analysis and will also examine data for the secondary outcome measures. The secondary analyses will include an assessment of the validity and reliability of FCR-J. These analyses will be conducted for exploratory purposes.
Interim analyses
We do not plan any interim analysis.
Sample size estimation
Our previous phase II study revealed that the mean CARS-J scores were 12.8 at preintervention (baseline), 12.4 at 4 weeks and 11.2 at 8 weeks (Imai, submission). We assumed the following: the mean CARS-J score at 2 weeks would be 12.6; the overall CARS-J scores in the control arms would not change (12.8 at 0, 2, 4 and 8 weeks); the variance of the score would be 30 at all times; and the intraclass correlation would be 0.82 (ie, we assumed a compound symmetry working covariance structure). Thus, for a sample size based on 0.8 power to detect a significant difference at p=0.05 (two sided), 211 participants would be required for each arm. Assuming that 5% of the initial entries would drop out, we would need to recruit 444 participants into the trial.
Publication policy
The protocol paper and study results will be submitted to peer-reviewed journals. The first author of the main paper will be a member of the steering committee (authors of the protocol paper). Another person could be the first author if approved by the steering committee. The list of coauthors will be determined before submitting each paper.
Study period
The study period of this trial will be from April 2017 to March 2020; the participant entry period will be April 2018 to September 2019.
Patient and public involvement statement
The study protocol was designed with a patient (breast cancer survivor) and she participated in this study as a researcher. She appropriately discussed with other patients when a patient’s preferences and/or opinions should be considered. She will play a same role on implementing the study. Thus, patients were and will always be involved in the study. Results of the study will be shown in the study homepage.
Ethics and dissemination
The present study is subject to ethical guidelines for clinical studies published by Japan’s Ministry of Education, Science and Technology and Ministry of Health, Labour and Welfare and the modified Act on the Protection of Personal Information as well as the ethical principles established for research on humans stipulated in the Declaration of Helsinki and further amendments thereto.
If important protocol modifications are needed, the investigators will discuss them and report to the review board for approval.
With regard to dissemination, the results obtained will be submitted for publication in peer-reviewed journals. The main and relevant findings will be presented at conferences.
Discussion
To our knowledge, the present study is the first trial investigating the efficacy of smartphone-based psychological therapy for fear of recurrence among breast cancer survivors. Considering the huge number of breast cancer survivors and low participant rate with other types of therapeutic interventions, smartphone-based psychological therapy may offer a more accessible option. As many cancer survivors return to their households and to work, easily accessible therapeutic interventions may offer additional benefits in managing fear of recurrence. The present study focuses on younger patients with breast cancer who are iPhone users. However, many other patients suffering from fear of recurrence use other types of smartphones (eg, Android); in addition, including patients aged 50 years and above would constitute a broader targeted population. If the efficacy of the smartphone-based intervention program among our participants is confirmed, the program will have promising applicability in real clinical settings.
The present study has some methodological limitations. First, not all patients who are interested in and willing to use Kaiketsu-App and Genki-App possess a smartphone. This may weaken the applicability of the results from this trial to all patients with breast cancer with fear of recurrence. Especially, the results may not be applicable to patients in low/middle-income countries and to those with poor ICT literacy.
Second, we will use a waitlist control as the comparator owing to feasibility and ethical considerations. The odds of response was found to be statistically significantly greater for no treatment than the waitlist.43 The waitlist may therefore lead to some overestimation of the efficacy of smartphone-based psychotherapy.
Third, we will apply two types of psychotherapy—BA and PST—as interventions. Both psychotherapeutic interventions consist of complex, multifactorial components. Thus, if the interventions prove superior to the waitlist controls, we cannot determine which intervention and components are most efficacious or beneficial in managing fear of recurrence. However, to overcome this limitation, we will adopt a mixed methods design and can check adherence with each intervention (detailed in ‘Methods and analysis’) so that we can identify the most useful components of the interventions.
Fourth, we will request that participants upload images for identification (they will be especially encouraged to attach a photo of the hospital registration card) to avoid individuals masquerading as patients with breast cancer. However, possible deception cannot be completely prevented in our recruitment system.
Finally, lack of the third group, which is the in-person PST and BA treatment arm, would lessen the impact of this study. If we set the in-person PST and BA treatment arm to compare effect sizes in this group with the other two groups, we would be able to dissect the specific mechanisms of change.
Supplementary Material
Acknowledgments
We thank Ms T Mashiko for her data management support. We also thank Ms Y Yanase, Ms A Nomura, Ms K Tojima, Ms I Sakakima and Ms K Kobori for their support for the study. We thank the Edanz Group (www.edanzediting.com) for editing a draft of this manuscript.
Footnotes
Contributors: TA, TAF and MH developed the smartphone applications and MU and FI also contributed to modification of these applications. TA, TY, KM, FK, NS, TM, TAF, HI and YU participated in the design of the study. TY played a chief role in the statistical parts. TA drafted the manuscript. All authors participated in, read and approved the final manuscript.
Funding: This study is supported by a Grant-in-Aid for Japan Agency for Medical Research and Development (JP17ck0106324h). This study is supported in part by a Grant-in-Aid for Scientific Research (25285194) and Young Scientists (17k13942) from the Japanese Ministry of Education, Culture, Science and Technology, grant from Nagoya City University, Foundation for Promotion of Cancer Research in Japan and the National Cancer Center Research and Development Fund (27-A-3 and 30-A-11).
Competing interests: TA has received lecture fees from AstraZeneca, Daiichi-Sankyo, Dainippon-Sumitomo, Eizai, Hisamitsu, Lilly, MSD, Meiji-seika Pharma, Mochida, Pfizer, Novartis, Otsuka, Shionogi, Takeda, Tanabe-Mitsubishi, Terumo and Yoshitomi. TA has received research funds from Daiichi-Sankyo, Eizai, MSD, Pfizer, Novartis and Tanabe-Mitsubishi. TY received research funds from AC MEDICAL, A2 Healthcare, CAC Croit, FMD K&L Japan, Japan Tobacco, Japan Media, Luminary Medical, Medidata Solutions, ONO PHARMACEUTICAL, Kyowa Hakko Kirin and DAIICHI SANKYO. TY received consulting fees from ONO PHARMACEUTICAL, Kowa, Japan Tobacco, CHUGAI PHARMACEUTICAL, TSUMURA & CO, CAC Croit, ASAHI INTECC, Asahi Kasei Pharma and Clinical Trial. FK has received lecture fees from MSD. TAF has received lecture fees from Janssen, Meiji, Mitsubishi-Tanabe, MSD and Pfizer. He has received research support from Mitsubishi-Tanabe. HI has received lecture fees from Daiichi Sankyo, Chugai, AstraZeneca, Pfizer and Eisai. He has received research support from Daiichi Sankyo, Chugai, AstraZeneca, Pfizer, MSD, Kyowahakou Kirin, GSK, Lilly, Novartis and Bayer. YU has received lectures fees from Asteras, Daiichi-Sankyo, Dainippon-Sumitomo, Eizai, Jannsen, Kyowahakko-Kirin, Ono, Meiji-seika Pharma, Mochida, Pfizer, Novartis, Otsuka, Sawai, Shionogi, Taiho, Tanabe-Mitsubishi and Tsumura Pharma.
Patient consent: Not required.
Ethics approval: The protocol was approved by the Institutional Review Board of Nagoya City University on 15 January 2018 (ID: 60-00-1171).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ito Y, Miyashiro I, Ito H, et al. . Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci 2014;105:1480–6. 10.1111/cas.12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodgkinson K, Butow P, Hunt GE, et al. . Breast cancer survivors’ supportive care needs 2-10 years after diagnosis. Support Care Cancer 2007;15:515–23. 10.1007/s00520-006-0170-2 [DOI] [PubMed] [Google Scholar]
- 3. Härtl K, Schennach R, Müller M, et al. . Quality of life, anxiety, and oncological factors: a follow-up study of breast cancer patients. Psychosomatics 2010;51:112–23. 10.1016/S0033-3182(10)70671-X [DOI] [PubMed] [Google Scholar]
- 4. Gordon NH, Siminoff LA. Measuring quality of life of long-term breast cancer survivors: the Long Term Quality of Life-Breast Cancer (LTQOL-BC) Scale. J Psychosoc Oncol 2010;28:589–609. 10.1080/07347332.2010.516806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akechi T, Okuyama T, Endo C, et al. . Patient’s perceived need and psychological distress and/or quality of life in ambulatory breast cancer patients in Japan. Psychooncology 2011;20:497–505. 10.1002/pon.1757 [DOI] [PubMed] [Google Scholar]
- 6. Harrison JD, Young JM, Price MA, et al. . What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer 2009;17:1117–28. 10.1007/s00520-009-0615-5 [DOI] [PubMed] [Google Scholar]
- 7. Koch L, Jansen L, Brenner H, et al. . Fear of recurrence and disease progression in long-term (≥ 5 years) cancer survivors–a systematic review of quantitative studies. Psychooncology 2013;22:1–11. 10.1002/pon.3022 [DOI] [PubMed] [Google Scholar]
- 8. Simard S, Thewes B, Humphris G, et al. . Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv 2013;7:300–22. 10.1007/s11764-013-0272-z [DOI] [PubMed] [Google Scholar]
- 9. Lengacher CA, Reich RR, Paterson CL, et al. . Examination of Broad Symptom Improvement Resulting From Mindfulness-Based Stress Reduction in Breast Cancer Survivors: A Randomized Controlled Trial. J Clin Oncol 2016;34:2827–34. 10.1200/JCO.2015.65.7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lengacher CA, Johnson-Mallard V, Post-White J, et al. . Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology 2009;18:1261–72. 10.1002/pon.1529 [DOI] [PubMed] [Google Scholar]
- 11. Butow PN, Turner J, Gilchrist J, et al. . Randomized Trial of ConquerFear: A Novel, Theoretically Based Psychosocial Intervention for Fear of Cancer Recurrence. J Clin Oncol 2017;35:4066–77. 10.1200/JCO.2017.73.1257 [DOI] [PubMed] [Google Scholar]
- 12. van de Wal M, Thewes B, Gielissen M, et al. . Efficacy of Blended Cognitive Behavior Therapy for High Fear of Recurrence in Breast, Prostate, and Colorectal Cancer Survivors: The SWORD Study, a Randomized Controlled Trial. J Clin Oncol 2017;35:2173–83. 10.1200/JCO.2016.70.5301 [DOI] [PubMed] [Google Scholar]
- 13. Fukui S, Kugaya A, Okamura H, et al. . A psychosocial group intervention for Japanese women with primary breast carcinoma. Cancer 2000;89:1026–36. [DOI] [PubMed] [Google Scholar]
- 14. Fukui S, Kugaya A, Kamiya M, et al. . Participation in psychosocial group intervention among Japanese women with primary breast cancer and its associated factors. Psychooncology 2001;10:419–27. 10.1002/pon.534 [DOI] [PubMed] [Google Scholar]
- 15. Akechi T, Momino K, Yamashita T, et al. . Contribution of problem-solving skills to fear of recurrence in breast cancer survivors. Breast Cancer Res Treat 2014;145:205–10. 10.1007/s10549-014-2929-3 [DOI] [PubMed] [Google Scholar]
- 16. Akechi T, Hirai K, Motooka H, et al. . Problem-solving therapy for psychological distress in Japanese cancer patients: preliminary clinical experience from psychiatric consultations. Jpn J Clin Oncol 2008;38:867–70. 10.1093/jjco/hyn115 [DOI] [PubMed] [Google Scholar]
- 17. Hirai K, Motooka H, Ito N, et al. . Problem-solving therapy for psychological distress in Japanese early-stage breast cancer patients. Jpn J Clin Oncol 2012;42:1168–74. 10.1093/jjco/hys158 [DOI] [PubMed] [Google Scholar]
- 18. Patel V, Weobong B, Weiss HA, et al. . The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial. Lancet 2017;389:176–85. 10.1016/S0140-6736(16)31589-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nezu AM, Nezu CM, Felgoise SH, et al. . Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients. J Consult Clin Psychol 2003;71:1036–48. 10.1037/0022-006X.71.6.1036 [DOI] [PubMed] [Google Scholar]
- 20. Dimidjian S, Goodman SH, Sherwood NE, et al. . A pragmatic randomized clinical trial of behavioral activation for depressed pregnant women. J Consult Clin Psychol 2017;85:26–36. 10.1037/ccp0000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hopko DR, Armento ME, Robertson SM, et al. . Brief behavioral activation and problem-solving therapy for depressed breast cancer patients: randomized trial. J Consult Clin Psychol 2011;79:834–49. 10.1037/a0025450 [DOI] [PubMed] [Google Scholar]
- 22. Andrews G, Cuijpers P, Craske MG, et al. . Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLoS One 2010;5:e13196 10.1371/journal.pone.0013196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. So M, Yamaguchi S, Hashimoto S, et al. . Is computerised CBT really helpful for adult depression?-A meta-analytic re-evaluation of CCBT for adult depression in terms of clinical implementation and methodological validity. BMC Psychiatry 2013;13:113 10.1186/1471-244X-13-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watts S, Mackenzie A, Thomas C, et al. . CBT for depression: a pilot RCT comparing mobile phone vs. computer. BMC Psychiatry 2013;13:49 10.1186/1471-244X-13-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mantani A, Kato T, Furukawa TA, et al. . Smartphone Cognitive Behavioral Therapy as an Adjunct to Pharmacotherapy for Refractory Depression: Randomized Controlled Trial. J Med Internet Res 2017;19:e373 10.2196/jmir.8602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. . SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mynors-Wallis L. Problem-solving treatment for anxiety and depression: A practical guide. New York: Oxford University Press, 2005. [Google Scholar]
- 28. Watanabe N, Horikoshi M, Yamada M, et al. . Adding smartphone-based cognitive-behavior therapy to pharmacotherapy for major depression (FLATT project): study protocol for a randomized controlled trial. Trials 2015;16:293 10.1186/s13063-015-0805-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Momino K, Akechi T, Yamashita T, et al. . Psychometric properties of the Japanese version of the Concerns About Recurrence Scale (CARS-J). Jpn J Clin Oncol 2014;44:456–62. 10.1093/jjco/hyu032 [DOI] [PubMed] [Google Scholar]
- 30. Crist JV, Grunfeld EA. Factors reported to influence fear of recurrence in cancer patients: a systematic review. Psychooncology 2013;22:978–86. 10.1002/pon.3114 [DOI] [PubMed] [Google Scholar]
- 31. Services USDoHaH, OfHRP, Administration FaD, et al. . Use of Electronic Informed Consent Questions and Answers. 2016. https://www.fda.gov/downloads/drugs/guidances/ucm436811.pdf
- 32. Vickberg SM. The Concerns About Recurrence Scale (CARS): a systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann Behav Med 2003;25:16–24. 10.1207/S15324796ABM2501_03 [DOI] [PubMed] [Google Scholar]
- 33. Momino K, Akechi T, Yamashita T, et al. . Psychometric properties of the Japanese version of the Concerns about Recurrence Scale (CARS-J). Jpn J Clin Oncol. In Press. [DOI] [PubMed] [Google Scholar]
- 34. Simard S, Savard J. Fear of cancer recurrence inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer 2009;17:241–51. 10.1007/s00520-008-0444-y [DOI] [PubMed] [Google Scholar]
- 35. Simard S, Savard J. Screening and comorbidity of clinical levels of fear of cancer recurrence. J Cancer Surviv 2015;9:481–91. 10.1007/s11764-015-0424-4 [DOI] [PubMed] [Google Scholar]
- 36. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 37. Kugaya A, Akechi T, Okuyama T, et al. . Screening for psychological distress in Japanese cancer patients. Jpn J Clin Oncol 1998;28:333–8. 10.1093/jjco/28.5.333 [DOI] [PubMed] [Google Scholar]
- 38. Boyes A, Girgis A, Lecathelinais C. Brief assessment of adult cancer patients’ perceived needs: development and validation of the 34-item Supportive Care Needs Survey (SCNS-SF34). J Eval Clin Pract 2009;15:602–6. 10.1111/j.1365-2753.2008.01057.x [DOI] [PubMed] [Google Scholar]
- 39. Okuyama T, Akechi T, Yamashita H, et al. . Reliability and validity of the Japanese version of the Short-form Supportive Care Needs Survey questionnaire (SCNS-SF34-J). Psychooncology 2009;18:1003–10. 10.1002/pon.1482 [DOI] [PubMed] [Google Scholar]
- 40. Tedeschi RG, Calhoun LG. The posttraumatic growth inventory: measuring the positive legacy of trauma. J Trauma Stress 1996;9:455–71. 10.1002/jts.2490090305 [DOI] [PubMed] [Google Scholar]
- 41. Taku K, Calhoun LG, Tedeschi RG, et al. . Examining posttraumatic growth among Japanese university students. Anxiety Stress Coping 2007;20:353–67. 10.1080/10615800701295007 [DOI] [PubMed] [Google Scholar]
- 42. Akizuki N, Akechi T, Nakanishi T, et al. . Development of a brief screening interview for adjustment disorders and major depression in patients with cancer. Cancer 2003;97:2605–13. 10.1002/cncr.11358 [DOI] [PubMed] [Google Scholar]
- 43. Furukawa TA, Noma H, Caldwell DM, et al. . Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta-analysis. Acta Psychiatr Scand 2014;130:181–92. 10.1111/acps.12275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-024794supp001.pdf (11.3KB, pdf)
bmjopen-2018-024794supp002.pdf (732.8KB, pdf)