Abstract
Objective
The current methods available for determining insulin resistance are complicated; hence, they are only applicable to small-scale studies. Therefore, this study aimed to classify the characteristics of surrogate measures for insulin resistance and establish valid cut-off values for predicting the development of type 2 diabetes mellitus (DM) in Korean populations.
Methods
This prospective study included 7,643 participants aged 40–69 years from the Ansung-Ansan cohort database (2001–2012). Four surrogate measures, namely homeostasis model assessment-insulin resistance (HOMA-IR), visceral adiposity index (VAI), lipid accumulation product (LAP), and triglycerides and glucose (TyG) index, were analyzed. We analyzed each measure using receiver operating characteristic (ROC) curve for the development of type 2 DM. The cut-off value was determined as the value with the highest Youden index score in the specificity dominant area.
Results
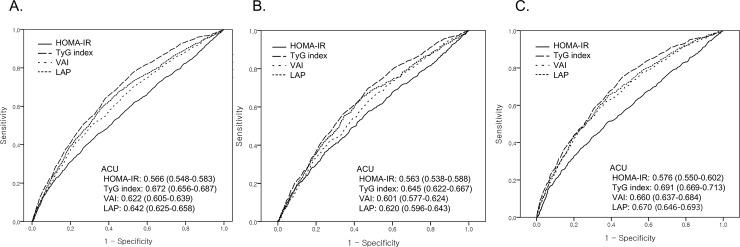
The area under the curve (AUC) was 0.566 (95% confidence interval [CI], 0.548–0.583) for HOMA-IR, 0.622 (95% CI, 0.605–0.639) for VAI, 0.642 (95% CI, 0.625–0.658) for LAP, and 0.672 (95% CI, 0.656–0.687) for TyG index. The AUC of TyG index was significantly higher than that of HOMA-IR, VAI, and LAP (p < 0.001). The cut-off value was 2.54 (sensitivity 36.8%; specificity 73.1%; hazard ratio [HR], 1.41, 95% CI, 1.25–1.59) for HOMA-IR, 2.54 (sensitivity 50.4%; specificity 68.8%; HR, 1.75, 95% CI, 1.55–1.96) for VAI, 36.6 (sensitivity 59.2%; specificity 63.9%; HR, 1.87, 95% CI, 1.64–2.14) for LAP, and 4.69 (sensitivity 62.1%; specificity 63.1%; HR, 2.17, 95% CI, 1.92–2.45) for TyG index.
Conclusions
The TyG index was a better predictor for DM than HOMA-IR. VAI and LAP showed the modest predictability for DM. The TyG index could be a useful supplementary method for identifying individuals at risk for insulin resistance and DM development.
Introduction
Variable degrees of insulin resistance and impaired insulin secretion are major pathophysiological characteristics of type 2 diabetes mellitus (DM) [1]. Insulin resistance is characterized by a reduced physiological response of target tissues to normal levels of insulin and results in decreased glucose utilization in muscle and fat, as well as increased gluconeogenesis in the liver [2–4]. Understanding the contribution of insulin resistance to the pathogenesis of type 2 DM is important for establishing preventive measures and determining optimal therapeutic approaches. Unfortunately, the current methods (e.g., pancreatic suppression test, hyperinsulinemic-euglycemic [HIEG] clamp technique, and minimal model approximation of the metabolism of glucose [MMAMG]) available for determining insulin resistance are complicated, invasive, and expensive; hence, they are only applicable to small-scale studies [5–8]. Instead, indirect indices, such as homeostasis model assessment-insulin resistance (HOMA-IR), visceral adiposity index (VAI), lipid accumulation product (LAP), or triglycerides and glucose (TyG) index, are widely accepted for epidemiological or clinical studies because of their technical simplicity [9, 10, 11]. However, valid cut-off values of the indices used in predicting DM have not been fully evaluated yet. Hence, this study aimed to determine the characteristics of surrogate measures for insulin resistance in Korean populations and establish valid cut-off values for predicting DM.
Materials and methods
Study populations
The Ansung-Ansan cohort study is an ongoing prospective study that started in 2001 with support from the National Genome Research Institute in Korea’s Center for Disease Control and Prevention. Detailed information on the study design and procedures is available in a previous report [12].
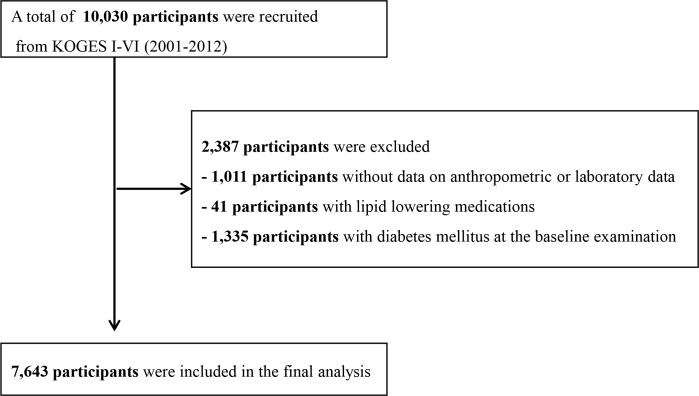
A population-based sample of male and female Koreans aged 40–69 years were enrolled from the following two sites: Ansung, which is a rural community with approximately 190,000 residents and Ansan, which is a rural community with approximately 693,000 residents [13]. A total of 10,038 participants (5,018 from Ansung and 5,020 from Ansan) underwent a baseline health examination at the Ajou University Medical Center and the Korea University Ansan Hospital from June 2001 to January 2003. Follow-up examinations were conducted biennially. Data from the baseline survey and five subsequent surveys (I-VI: 2001–2012) were analyzed in the present study. We excluded the following participants: those with incomplete data, those with lipid lowering medications and those with a clinical history of DM at the baseline examination (Fig 1). In total, 7,643 participants were eligible for this study.
Fig 1. Flowchart showing the final selection.
KOGES, The Korean Genome and Epidemiology study.
Clinical and laboratory measurements
Waist circumference was measured at the end of normal expiration using flexible tape at the narrowest point between the lowest border of the rib cage and the uppermost lateral border of the iliac crest. Height and body weight were measured to the nearest 0.1 cm and 0.2 kg, respectively. Blood pressure was measured in the sitting position after at least 5 minutes of rest. Blood samples were obtained after an overnight fast of at least 8 hours, and biochemical assays, including plasma glucose, total cholesterol, triglycerides, and high-density lipoprotein cholesterol (HDL-C), were measured using the ADVIA 1650 chemistry analyzer (Bayer HealthCare Ltd., Tarrytown, NY, USA). Hemoglobin A1c (HbA1c) level was measured using high-performance liquid chromatography (Variant II; BioRad Laboratories, Hercules, CA, USA).
Definitions
A patient was deemed to have DM if he/she had at least one of the following conditions: the fasting glucose concentration was ≥126 mg/d, glucose concentration was ≥200 mg/dL in an oral 75-g 2-hour glucose tolerance test, HbA1c was ≥6.5%, and the use of glucose-lowering medication. A structured questionnaire was used to investigate regarding the use of glucose-lowering medication.
Insulin resistance was evaluated using HOMA-IR, VAI, LAP, and TyG index. The formulas for HOMA-IR and TyG index were as follows:
Statistical analysis
Summary statistics are presented as mean and standard deviation (SD) or prevalence (%). The values of each surrogate measure for insulin sensitivity were presented by the 10th, 25th, 50th, 75th, and 90th percentile. We analyzed each measure of insulin resistance using receiver operating characteristic (ROC) curve to estimate the predictive ability for the development of DM in 10 years. We performed the de Long’s test to identify which surrogate measures for insulin resistance were significantly superior. The cut-off value of each surrogate measure was determined as the value with the highest Youden index score in the specificity dominant area. Multivariate Cox proportional hazards regression models were constructed to evaluate the hazards ratio (HR) and 95% confidence interval [CI] for DM. Follow-up duration was calculated as the time from the first anthropometric and clinical measures to either the date of development of DM or the end of follow-up (December 31, 2012). In addition, OR for DM according to the continuous value of each measure was analyzed using restricted cubic spline splits with five knots.
Analyses were carried out using SPSS, version 25.0 (IBM, Armonk, NY, USA) and the statistical package R (version 3.3.2, R Foundation for Statistical Computing). The significance levels were set at 0.05.
Ethics statement
The protocol of the study was approved by the institutional review board of Kangnam Sacred Heart Hospital (IRB No. HKS 2017-07-007), and all participants gave written informed consent. All participants’ records were anonymized before being accessed by the authors, and all methods were carried out in accordance with the approved guidelines and regulations.
Results
Baseline characteristics
Overall, data from 7,643 participants were assessed (3,603 males and 4,040 females). Among them, 17.1% (1,306) had newly diagnosed DM during the 10-year follow-up period. Table 1 summarizes baseline anthropometric, clinical, and biochemical characteristics of the participants.
Table 1. Baseline characteristics of participants according to incident DM.
| Characteristics | Total participants (N = 7,643) |
|---|---|
| Age, years | 51.7 ± 8.8 |
| Male sex, n (%) | 3,603 (47.1) |
| Smoking, n (%) | 3,044 (40.3) |
| BMI, Kg/m2 | 24.4 ± 3.1 |
| Energy intake, Kcal/day | 1,967.7 ± 720.8 |
| Physical activity*, n (%) | 4,649 (62.6) |
| Hypertension, n (%) | 2,271 (29.7%) |
| Systolic BP, mmHg | 120.5 ± 17.9 |
| Diastolic BP, mmHg | 79.9 ± 11.4 |
| HbA1c, % | 5.5 ± 0.3 |
| Fasting glucose, mg/dL | 82.7 ± 8.5 |
| Fasting insulin, μIU/mL | 7.6 ± 4.8 |
| Total cholesterol, mg/dL | 189.6 ± 34.2 |
| HDL Cholesterol, mg/dL | 44.9 ± 10.0 |
| Triglycerides, mg/dL | 154.8 ± 94.0 |
| HOMA-IR | 1.6 ± 1.0 |
| VAI | 2.5 ± 1.9 |
| LAP | 38.5 ± 31.9 |
| TyG index | 4.7 ± 0.2 |
Data were presented as means ± SD or number (%)
* Participants who engaged in physical activity for at least 30 minutes per day
Abbreviations: BMI; body mass index; BP, blood pressure; HbA1c; hemoglobin A1c; HDL; high-density lipoprotein; HOMA-IR: homeostasis model assessment-insulin resistance; TyG index; triglycerides and glucose index
The distribution of surrogate measures for insulin resistance at baseline examination are summarized in Table 2.
Table 2. Distribution of surrogate measures for insulin resistance at baseline examination.
| Indirect index | Percentile | ||||
|---|---|---|---|---|---|
| 10th | 25th | 50th | 75th | 90th | |
| HOMA-IR | 0.64 | 1.03 | 1.40 | 1.92 | 2.48 |
| VAI | 0.97 | 1.34 | 2.00 | 3.05 | 4.67 |
| LAP | 10.7 | 17.9 | 30.2 | 49.7 | 75.7 |
| TyG index | 4.37 | 4.49 | 4.64 | 4.81 | 4.99 |
Abbreviations: HOMA-IR: homeostasis model assessment-insulin resistance; VAI: visceral adiposity index; LAP: lipid accumulation product; TyG index: triglycerides and glucose index
Cut-off values of surrogate measures for insulin resistance
The ROC for newly developed DM in 10 years according to each measure is presented in Fig 2. The AUC was 0.566 (95% CI, 0.548–0.583) for HOMA-IR, 0.622 (95% CI, 0.605–0.639) for VAI, 0.642 (95% CI, 0.625–0.658) for LAP, and 0.672 (95% CI, 0.656–0.687) for TyG index. The AUC of TyG index was significantly higher than that of HOMA-IR, VAI, and LAP (p < 0.001). The AUC of VAI was similar with that of LAP (p = 0.115) while higher than that of HOMA-IR (p value < 0.001). The AUC of each measure was higher in women than in men.
Fig 2. ROC curves of incident diabetes mellitus in 10 years based on each surrogate measure for insulin resistance.
A. Total; B. Men; C. Women.
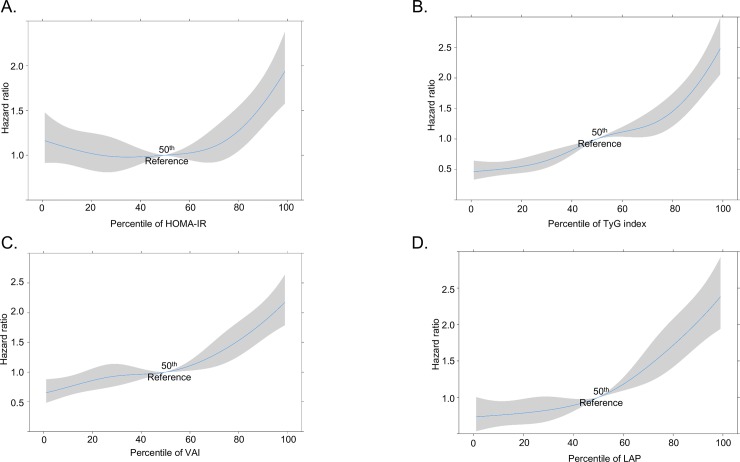
The cut-off values with their corresponding sensitivity, specificity, and HR are summarized in Table 3. In the restricted cubic spline regression, each surrogate measure showed a dose-dependent relationship with the risk of DM (Fig 3).
Table 3. Cut-off values with their corresponding sensitivity, specificity, and hazard ratio (HR).
| Surrogate measures | Cut-off value | Sensitivity | Specificity | HR (95% CI)* |
|---|---|---|---|---|
| HOMA-IR | 1.83 | 38.6 | 73.1 | 1.41 (1.25–1.59) |
| VAI | 2.54 | 50.4 | 68.8 | 1.75 (1.55–1.96) |
| LAP | 36.6 | 59.2 | 63.9 | 1.87 (1.64–2.14) |
| TyG index | 4.69 | 62.1 | 63.1 | 2.17 (1.92–2.45) |
Abbreviations: HOMA-IR: homeostasis model assessment-insulin resistance; TyG index; triglycerides and glucose index; HR: hazard ratio
*Adjusted for age, sex, BMI, smoking and hypertension, physical activity, energy intake
Fig 3. The odds ratio for DM according to the percentile of each surrogated measure.
A. HOMA-IR; B. VAI; C. LAP; D. TyG index Abbreviations: HOMA-IR: homeostasis model assessment-insulin resistance; VAI: visceral adiposity index; LAP: lipid accumulation product; TyG index: triglycerides and glucose index.
Discussion
In this community-based prospective cohort, we confirmed that TyG index was a better predictor for DM compared with VAI, LAP and HOMA-IR. VAI and LAP showed modest predictability for DM while HOMA-IR scarcely predicted DM.
HOMA-IR is a well-known robust tool for the assessment of insulin resistance and is associated with the development of DM [14–16]. The present study shows dose dependent association between HOMA-IR and the risk of DM. However, the AUC value was much lower than those in previous studies. According to studies conducted in Iran, China, and Korea, the AUC value of HOMA-IR was approximately 0.7–0.8 [17–19]. Interestingly, a significant decline of the diagnostic performance of HOMA-IR for type 2 DM was observed with aging [19]. Several previous studies showed a decline in the AUC of HOMA-IR for metabolic syndrome in elderly individuals as well [20, 21]. Considering that the participants of our study were older than those of previous studies, such differences might arise from age-related effects.
It is important to estimate a valid cut-off value for the clinical use of HOMA-IR. Although a number of studies suggested the cut-off values, there is great variability. In several population-based studies, the cut-off values of HOMA-IR were made based on the percentile criterion (75 -90th percentile according to studies) of values in the general population [22–24]. However, considering the distribution of HOMA-IR varied according to participants’ demographic characteristics such as age, sex and race, it is difficult to estimate the optimal cut-off value with the percentile criterion. For example, the 75th percentile of HOMA-IR was 2.53 in healthy Koreans, while 1.6 in healthy Iranians, 2.0 in healthy Swedish men and 3.8 in French men [20, 22–24]. In addition, it is unclear whether the proposed cutoff values of HOMA-IR based on the percentile criterion could predict clinically relevant outcomes [15, 20, 21].
In order to resolve such doubts, several studies were conducted to determine the valid cut-off value of HOMA-IR for predicting the development of DM. Despite the fact that DM develops over a long period of time, most of the studies were cross-sectional and may have contained various confounding factors [25–27]. Limited data regarding HOMA-IR cut-off values were obtained from longitudinal studies. Ghasemi et al. suggested that the cut-off value of HOMA-IR was 2.17 (sensitivity 50%, specificity 76.7%) for males and 1.85 (sensitivity 75.9%, specificity 58.3%) for females [17]. According to Lee et al., the cut-off value of HOMA-IR was 1.97 (sensitivity 65.5%, specificity 82.9%) [18]. The results obtained in our study are consistent with those obtained in these studies. However, considering the low sensitivity of HOMA-IR for DM in the present study, this cut-off value has limitations for the application to clinical settings.
One significant drawback of HOMA-IR is that a standard assay for measurement of fasting insulin is absent. To overcome this, insulin-free equations for estimating insulin resistance have been developed. One well-known useful insulin-free surrogate measure is the triglycerides and glucose (TyG) index [10]. It is a more simple and inexpensive method compared with insulin-based surrogate measures. TyG is well correlated with the gold standard methods for insulin resistance such as HIEG clamp or MMAMG [10, 28]. Moreover, there was a modest correlation between the TyG index and insulin stimulated glucose uptake during insulin suppression testing [29]. Several population-based studies demonstrated that high TyG index was associated with DM, hypertension, nonalcoholic fatty liver disease, and atherosclerosis [30–35]. However, there have been few studies to estimate the cut-off value of TyG index for predicting development of DM. Guerrero-Romero et al. suggested that the best value of the TyG index for diagnosis of insulin resistance was 4.68 using the HIEG clamp test with a small sample size; this cut-off value is similar to our findings [10]. We suggest our results could support the cut-off value from the study of Guerrero-Romero et al. To the best of our knowledge, this is the first study to estimate a valid cut-off value of TyG index using data from a population-based longitudinal study.
Notably, we found that TyG index had better predictive power for development of DM, compared with HOMA-IR. The correlation between HIEG clamp test and TyG index is known to be comparable with the correlation between HIEG clamp test and HOMA-IR [28, 36]. However, a direct comparison between TyG index and HOMA-IR regarding their ability to predict DM development has not performed yet. Therefore, another significance of the present study is providing evidence of clinical usefulness of TyG index for identifying individuals at risk for DM. Regarding low cost and universal use of blood glucose and triglycerides tests, the TyG index can be a good supplementary test measure for insulin resistance.
Interestingly, the AUC of the surrogate measures differed by sex. This result is consistent with those of a previous study [17, 19]. Considering that estrogens promote peripheral fat storage, whereas androgens promote the accumulation of visceral abdominal fat, the alteration of sex hormone might affect the insulin resistance and the diagnostic performance of each measure for type 2 DM [37].
The main strength of this study was the data source, the Ansung-Ansan Cohort, which is a long-term (10-year follow-up), community-based cohort. Despite this strength, the present study has some limitations. First, because this study was performed among Korean adults, the result might not be applicable to other ethnicities. Second, the study lacks in directly comparing the surrogate measure and gold standard methods for insulin resistance such as HIEG clamp or MMAMG. Further studies are necessary for this issue.
In conclusion, TyG index was better predictor for DM compared with HOMA-IR. VAI and LAP showed modest predictability for DM. TyG index could be used as a simple and supplementary method to identify individuals at risk for insulin resistance and DM development.
Acknowledgments
Data in this study were from the Korean Genome and Epidemiology Study (KoGES; 4851–302). National Research Institute of Health, Centers for Disease Control and Prevention, Ministry for Health and Welfare, Republic of Korea. This research was supported by Hallym University Research Fund 2017 (HURF-2017-48).
Data Availability
The datasets used and/or analyzed during the current study are owned by a third-party organization (The Korean Genome and Epidemiology Study-Ansan and Ansung study, KoGES; 4851-302). This data is available by online sharing service under the permission of the division of epidemiology and health index in the Korea Centers for Disease Control and Prevention (KCDC). More details in English are available on the following website: URL: http://www.nih.go.kr/NIH/eng/contents/NihEngContentView.jsp?cid=65203&menuIds=HOME004-MNU2261-MNU2262-MNU2263-MNU2267. In addition, we did not have any special access to this data that other researchers would not have. The data underlying the results presented in the study are available from the KCDC (URL: https://is.cdc.go.kr/koges/distributeRequest/user/agreeView.do).
Funding Statement
This research was supported by Hallym University Research Fund 2017 (HURF-2017-48).
References
- 1.Kwak SH, Park KS. Pathophysiology of Type 2 Diabetes in Koreans. Endocrinol Metab (Seoul). 2018;33:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–71. 10.1016/j.cell.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–5. [DOI] [PubMed] [Google Scholar]
- 4.Hanefeld M, The metabolic syndrome: roots, myths, and facts In: Hanefeld M, Leonhardt W, editors, The Metabolic Syndrome, Jena: Gustav Fischer;1997,p.13–24. [Google Scholar]
- 5.Defronzo R, Tobin J, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J physiol. 1979;237:214–23. [DOI] [PubMed] [Google Scholar]
- 6.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987;79:790–800. 10.1172/JCI112886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with insulin suppression test and euglycemic clamp. Diabetes. 1981;30:387–92. [DOI] [PubMed] [Google Scholar]
- 8.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and b cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 9.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. 10.1210/jcem.85.7.6661 [DOI] [PubMed] [Google Scholar]
- 10.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51. 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 11.Mazidi M, Kengne AP, Katsiki N, Mikhailidis DP, Banach M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J Diabetes Complications. 2018;32:266–70 10.1016/j.jdiacomp.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Han BG; KoGES group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int J Epidemiol. 2017;46:e20 10.1093/ije/dyv316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korean Statistical Information Service. Available from: http://kosis.kr. Accessed 5 May 2018.
- 14.Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91:1063–77. 10.1016/j.mcna.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 15.Antuna-Puente B, Disse E, Rabasa-Lhoret R, Laville M, Capeau J, Bastard JP. How can we measure insulin sensitivity/resistance? Diabetes Metab. 2011;37:179–88. 10.1016/j.diabet.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 16.Song YS, Hwang YC, Ahn HY, Park CY. Comparison of the Usefulness of the Updated Homeostasis Model Assessment (HOMA2) with the Original HOMA1 in the Prediction of Type 2 Diabetes Mellitus in Koreans. Diabetes Metab J. 2016;40:318–25. 10.4093/dmj.2016.40.4.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghasemi A, Tohidi M, Derakhshan A, Hasheminia M, Azizi F, Hadaegh F. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol. 2015;52:905–15 10.1007/s00592-015-0730-3 [DOI] [PubMed] [Google Scholar]
- 18.Lee CH, Shih AZ, Woo YC, Fong CH, Leung OY, Janus E, et al. Optimal Cut-Offs of Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) to Identify Dysglycemia and Type 2 Diabetes Mellitus: A 15-Year Prospective Study in Chinese. PLoS One. 2016;11:e0163424 10.1371/journal.pone.0163424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek JH, Kim H, Kim KY, Jung J. Insulin Resistance and the Risk of Diabetes and Dysglycemia in Korean General Adult Population. Diabetes Metab J. 2018;42:296–307 10.4093/dmj.2017.0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon S, Park JH, Jang EJ, Park YK, Yu JM, Park JS, et al. The Cut-off Values of Surrogate Measures for Insulin Sensitivity in a Healthy Population in Korea according to the Korean National Health and Nutrition Examination Survey (KNHANES) 2007–2010. J Korean Med Sci 2018;33:e197 10.3346/jkms.2018.33.e197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, De Francisco A, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 2013;13:47 10.1186/1472-6823-13-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet Med. 2000;17: 299–307. [DOI] [PubMed] [Google Scholar]
- 23.Esteghamati A, Ashraf H, Esteghamati AR, Meysamie A, Khalizadeh O, Nakhjavani M, et al. Optimal threshold of homeostasis model assessment for insulin resistance in an Iranian population: the implication of metabolic syndrome to detect insulin resistance. Diabetes Res Clin Pract. 2009;84:279–87. 10.1016/j.diabres.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 24.Marques-Vidal P, Mazoyer E, Bongard V, Gourdy P, Ruidavets JB, Drouet L, et al. Prevalence of insulin resistance syndrome in Southwestern France and its relationship with inflammatory and haemostatic markers. Diabetes Care. 2002;25:1371–7. [DOI] [PubMed] [Google Scholar]
- 25.Ryu S, Sung KC, Chang Y, Lee WY, Rhee EJ. Spectrum of insulin sensitivity in the Korean population. Metabolism. 2005;54:1644–51. 10.1016/j.metabol.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Choi S, Kim HJ, Chung YS, Lee KW, Lee HC, et al. Cutoff values of surrogate measures of insulin resistance for metabolic syndrome in Korean non-diabetic adults. J Korean Med Sci. 2006;21:695–700. 10.3346/jkms.2006.21.4.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomé MA, Botana MA, Cadarso-Suarez C, Rego-Irateta A, Fernandez-Mariño A, Mato JA, et al. Prevalence of metabolic syndrome in Galicia (NW Spain) on four alternative definitions and association with insulina resistance. J Endocrinol Invest. 2009;32:505–11. 10.3275/6102 [DOI] [PubMed] [Google Scholar]
- 28.Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–e100. 10.1016/j.diabres.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 29.Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011;60:1673–6. 10.1016/j.metabol.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 30.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 31.Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One. 2014;9:e90430 10.1371/journal.pone.0090430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16:108 10.1186/s12933-017-0589-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17:41 10.1186/s12933-018-0692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S, Du T, Zhang J, Lu H, Lin X, Xie J, et al. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:15 10.1186/s12944-017-0409-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16:175 10.1186/s12944-017-0562-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerrero-Romero F, Villalobos-Molina R, Jiménez-Flores JR, Simental-Mendia LE, Méndez-Cruz R, Murguía-Romero M, et al. Fasting Triglycerides and Glucose Index as a Diagnostic Test for Insulin Resistance in Young Adults. Arch Med Res. 2016;47:382–7. 10.1016/j.arcmed.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 37.Stefanska A, Bergmann K, Sypniewska G. Metabolic syndrome and menopause: pathophysiology, clinical and diagnostic significance. Adv Clin Chem 2015;72:1–75. 10.1016/bs.acc.2015.07.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are owned by a third-party organization (The Korean Genome and Epidemiology Study-Ansan and Ansung study, KoGES; 4851-302). This data is available by online sharing service under the permission of the division of epidemiology and health index in the Korea Centers for Disease Control and Prevention (KCDC). More details in English are available on the following website: URL: http://www.nih.go.kr/NIH/eng/contents/NihEngContentView.jsp?cid=65203&menuIds=HOME004-MNU2261-MNU2262-MNU2263-MNU2267. In addition, we did not have any special access to this data that other researchers would not have. The data underlying the results presented in the study are available from the KCDC (URL: https://is.cdc.go.kr/koges/distributeRequest/user/agreeView.do).