Abstract
Lyme disease (LD), caused by bacteria of the Borrelia burgdorferi sensu lato species complex, is the most common vector-borne disease in North America and Europe. A systematic review (SR) was conducted to summarize the global literature on adverse birth outcomes associated with gestational LD in humans. The SR followed an a priori protocol of pretested screening, risk of bias, and data extraction forms. Data were summarized descriptively and random effects meta-analysis (MA) was used where appropriate. The SR identified 45 relevant studies, 29 describing 59 cases reported as gestational LD in the United States, Europe, and Asia (1969–2017). Adverse birth outcomes included spontaneous miscarriage or fetal death (n = 12), newborn death (n = 8), and newborns with an abnormal outcome (e.g. hyperbilirubinemia, respiratory distress and syndactyly) at birth (n = 16). Only one report provided a full case description (clinical manifestations in the mother, negative outcome for the child, and laboratory detection of B. burgdorferi in the child) that provides some evidence for vertical transmission of B. burgdorferi that has negative consequences for the fetus. The results of 17 epidemiological studies are included in this SR. Prevalence of adverse birth outcomes in an exposed population (defined by the authors as: gestational LD, history of LD, tick bites or residence in an endemic area) was compared to that in an unexposed population in eight studies and no difference was reported. A meta-analysis of nine studies showed significantly fewer adverse birth outcomes in women reported to have been treated for gestational LD (11%, 95%CI 7–16) compared to those who were not treated during pregnancy (50%, 95%CI 30–70) providing indirect evidence of an association between gestational LD and adverse birth outcomes. Other risk factors investigated; trimester of exposure, length of LD during pregnancy, acute vs. disseminated LD at diagnosis, and symptomatic LD vs. seropositive women with no LD symptoms during pregnancy were not significantly associated with adverse birth outcomes. This SR summarizes evidence from case studies that provide some limited evidence for transplacental transmission of B. burgdorferi. There was inconsistent evidence for adverse birth outcomes of gestational LD in the epidemiological research, and uncommon adverse outcomes for the fetus may occur as a consequence of gestational LD. The global evidence does not fully characterize the potential impact of gestational LD, and future research that addresses the knowledge gaps may change the findings in this SR. Given the current evidence; prompt diagnosis and treatment of LD during pregnancy is recommended.
Introduction
Lyme disease (LD), the most common tick-borne disease in North America and Europe is caused by spirochetal bacteria of the Borrelia burgdorferi sensu lato species complex (also called Borreliella, but referred to herein as B. burgdorferi) [1]. The most commonly implicated B. burgdorferi species in human infections include B. burgdorferi sensu stricto in both North America and Europe and, B. afzelii and B. garinii in Europe and Asia [2]. Lyme disease was first recognized in North America in 1975 in the area of Lyme, Connecticut, as a result of an investigation into 51 cases (39 of which were children) that presented with a similar form of arthritis [3]. Early symptoms of infection include a characteristic rash (erythema-migrans, EM), fever, headache, and lethargy. If untreated, the disease may affect the heart, nervous system or manifest as arthritis.
Shortly after its discovery in 1975, the possible effects of gestational LD became an area of research interest given that transplacental infections by other species of spirochetes (e.g. Treponema pallidum; relapsing fever Borrelia species and Leptospira interrogans) are known to occur in several animal species (e.g. dogs, mice, cattle) and in humans [4–8]. The literature on transplacental transmission of B. burgdorferi in animals is outside the scope of this systematic review (SR). However, some adverse birth outcomes have been recorded for white-footed mice, dogs, cattle, horses, and a coyote. The most common outcomes were reproductive failure (inability to conceive) and fetal loss during pregnancy [9–16]. Animal model experiments identified B. burgdorferi infection in newborn beagles, indicating that transplacental transmission may occur; however, experiments involving rats, hamsters, and mice have not demonstrated this route of transmission for these species [13,17–19]. Overall, there is some evidence that B. burgdorferi infection in pregnant animals can result in infection of the newborn, fetal death, and fertility issues [10,12,13].
Given the public health importance of LD and our understanding of other spirochetal diseases, a SR was conducted to identify and summarize the global evidence on “What is the evidence that gestational Lyme disease in humans causes adverse birth outcomes including congenital abnormalities?”
Methods
Review protocol, team and expertise
This SR was conducted using an a priori developed protocol that followed standard SR guidelines [20,21] and the review is reported in accordance with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement (S1 Table) [22]. The protocol includes a list of definitions, search algorithms, title/abstract screening form, Risk of Bias tool, and data extraction forms (QA-DE). The protocol (S1 Text), list of relevant included articles (S2 Text) and dataset (S2 Table) are available in the supplementary material.
The review team comprised of individuals with multi-disciplinary expertise in epidemiology, microbiology, entomology, vector-borne diseases, veterinary public health, knowledge synthesis, and information science.
Search strategy
A pretested search algorithm, found below, was implemented in three bibliographic databases on October 16, 2017: Scopus, PubMed/MEDLINE, and Embase. No limits were placed on the search. The search terms were:
((lyme or borrelia or borreliosis) and (pregnancy or pregnant or maternal or fetus or foetus or newborn or congenital))
The capacity of the electronic search to identify all relevant primary research was verified by hand searching reference lists of three book chapters published between 1995 and 2011 [6,23,24] and three randomly chosen review articles from a list of topic relevant reviews identified during title/abstract screening [25–27]. This process netted 13 citations, conference proceedings, and non-indexed papers that were added to the SR. When omitted citations were no longer being identified the process was stopped. Hand searching of the following websites did not yield additional references:
Centers for Disease Control and Prevention (CDC) https://www.cdc.gov/
European Center for Disease Control and Prevention (ECDC) https://ecdc.europa.eu
Public Health Agency of Canada https://www.canada.ca/en/public-health.html
Relevance screening and inclusion criteria
References identified by the search were screened for relevance to the review question using a structured and pre-tested form (S1 Text). Those considered relevant to the review question were procured and relevance was confirmed using another pre-tested form implemented prior to proceeding to the risk of bias evaluation. Primary research on humans with gestational LD and any birth outcome (e.g. healthy infants, pregnancy loss, fetal and newborn abnormalities, adverse outcomes or death) were considered relevant to the research question. Chronic Lyme disease was considered to be outside the scope of this review [28]. Global research in any language was included in this SR to minimise language bias. Primary research was defined as original research where authors generated and reported their own data.
Risk of bias, GRADE and data extraction
Assessment of the risk of bias (RoB) of research relevant to the review question was executed using the whole publication and applying a direct modification of the RoB and Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria endorsed by the Cochrane collaboration [20,29,30]. The RoB assessment evaluates the internal validity of each study using eight criteria from which the reviewers determine an overall RoB (low, unclear, or high) for each outcome. This informs one of the five GRADE criteria [31]. The data extraction form captures pertinent information and results required for summarization and meta-analysis. Two reviewers (LW and JG) independently assessed the RoB and extracted data on each article.
A GRADE assessment was conducted for each relevant outcome where an outcome is a result from a study; the same outcome may be measured in different studies and as part of the systematic review, outcomes that are alike are grouped together and evaluated as follows. In addition to RoB the other criteria include study design, agreement between studies, precision of results, and evidence of a biological gradient for each outcome. This review included research from any study design; however the less controlled the study, the higher the risk of systematic biases that can result in the study findings deviating from the truth. The GRADE framework prescribes a gradually lower GRADE as the risk of bias increases [32]. This means that randomized controlled trials and well-designed cohort studies could be graded moderate/high (*** or ****), whereas case control studies and cross sectional studies are likely to be graded as low (**) and case reports and expert opinion receive a very low grade (*). Across each outcome/study design pair, groups of similar studies would be evaluated for overall RoB, agreement, precision and evidence of a biological gradient, which could result in up-grading (or down-grading) the level of confidence in the evidence for that outcome [32,33].
The final GRADE is assessed considering all five GRADE criteria for each unique outcome to indicate the level of confidence in the evidence [30]. The one to four star grading system indicates: **** high confidence that the effect estimate is close to the true effect; *** moderate confidence in the effect estimate, but future studies may be substantially different; ** limited confidence in the effect estimate, the true effect may be substantially different; * very little confidence in the effect estimate, the true effect is likely to be substantially different [32–34].
Systematic review management and analysis
Search results were imported into reference management software (Endnote X7, Thomson Reuters, USA), duplicates were removed and the list of unique citations was imported into a web-based electronic SR management platform (DistillerSR, Evidence Partners, Ottawa, Canada). All stages of the SR were conducted within this software and collected data were exported into Excel spreadsheets (Microsoft Corporation, Redmond, WA), organised (sub grouped by common exposure and outcome) and summarized (frequencies and percentages).
Post-hoc calculations such as computing unadjusted odds ratios (OR) from contingency table data were done, where necessary, to ensure comparability of the data. Whenever studies reported both unadjusted and adjusted OR measures, the adjusted measure was selected for inclusion in the tables and/or meta-analysis model [20].
Random-effects meta-analysis using the Der Simonian and Laird method was conducted for each unique group of studies if sufficient data were available (ie: if there were ≥2 studies, and the studies were comparable) [35]. For meta-analysis of proportions, the Freeman-Tukey double arcsine transformation was used to stabilize the variances [36]. Heterogeneity was measured using I2, which indicates the proportion of variation in the measures of association across studies due to heterogeneity rather than sampling error [37]. It was not possible to test for publication bias due to a limited number of studies (<10) in any subgroup [38].
Results
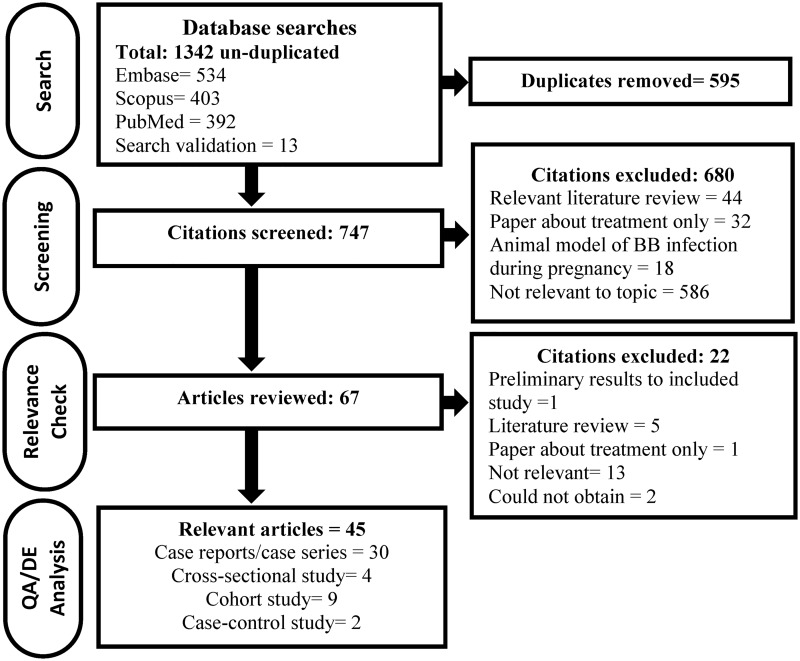
Forty-five relevant primary research studies were identified after screening 746 unique citations and 67 full papers (Fig 1). Thirty-five of these papers were published in English, two in Czech, two in Serbian and one each in French, Italian, Dutch, German, Russian, and Polish. Despite efforts to minimise bias by including all available research, we were unable to procure two potentially relevant articles [39,40].
Fig 1. The flow of citations and research papers through the systematic review process.
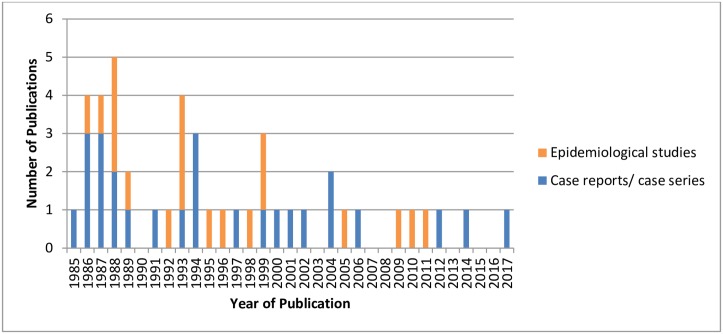
The literature on adverse birth outcomes associated with gestational LD is based on case reports and case series (n = 26), case series with epidemiological data (n = 4), and epidemiological studies (n = 15) (Fig 1). The case report and case series studies reported detailed information on one or more cases of gestational LD and the pregnancy and/or birth outcomes of the case. The case series with epidemiological data provided information on a group of gestational LD cases and associations between possible risk factors (e.g. untreated vs. treated LD) and the risk of an adverse birth outcome. The cohort, cross-sectional, and case control studies investigated possible differences in the frequency of adverse outcomes between an exposed and unexposed control group. Case studies captured in this review were published between 1985 and 2017, whereas the epidemiological studies were published between 1986 and 2011 (Fig 2). Across studies, exposure was defined as: evidence of clinical manifestations of LD during pregnancy, serological evidence of LD during pregnancy or surrogate measures of exposure to LD (e.g. history of tick bites or living in a geographic area considered endemic for LD).
Fig 2. The distribution of publication dates of 45 primary research publications relevant to the impact of gestational Lyme disease included in this systematic review grouped by studies that had epidemiological data or case report data.
Each study was evaluated for its RoB, which evaluates how well the study was conducted. Many criteria for RoB assessment do not apply to case reports, thus case report assessment focused on complete reporting. There were 26 case reports or case series included in this SR and three had an “unclear” RoB designation because the diagnostic tests performed or outcomes were not reported in sufficient detail (Table 1). The RoB for 19 epidemiological studies was 42% low, 42% unclear and 16% high (Table 1). Studies with an “unclear” RoB had one or more criteria that could not be assessed because the required information was not reported including: i) missing information on the blinding of patients and/or outcome assessors, or ii) unexplained loss to follow-up or loss of observations. “High” RoB studies had several flaws in the research process that may bias the results including failure to account for, or examine, important confounders or other biases. Insufficient information to assess RoB criteria is likely a reporting issue in many papers but results in an “unclear” or “high” RoB classification depending on the cumulative deficiencies of the study. The RoB evaluation for each study is available in S2 Table.
Table 1. General characteristics of 45 included primary research publications.
| Category | Count | |
|---|---|---|
| Continent1 | ||
| North America | 19 | |
| Europe | 24 | |
| Asia | 3 | |
| Outcomes Reported1 | ||
| Maternal outcome | 37 | |
| Miscarriage/ pregnancy loss | 13 | |
| Fetal outcome | 8 | |
| Newborn outcome | 35 | |
| Infant/child outcomes | 5 | |
| Study design | Risk of Bias (RoB) Assessment | |
| Case study/ case series | 26 | |
| Low RoB | 23 | |
| Unclear RoB | 3 | |
| Case series 2 | 4 | |
| Low RoB | 1 | |
| Unclear RoB | 2 | |
| High RoB | 1 | |
| Case control | 2 | |
| Low RoB | 1 | |
| Unclear RoB | 1 | |
| Cross sectional | 4 | |
| Low RoB | 2 | |
| Unclear RoB | 2 | |
| Cohort | 9 | |
| Low RoB | 4 | |
| Unclear RoB | 3 | |
| High RoB | 2 | |
1 Total number sums to >45 as studies can fall into more than one category.
2 Case series with epidemiological data.
Case reports of gestational Lyme disease
Details of 59 cases were summarized in 29 publications from the USA, Europe, and Asia describing gestational LD and pregnancy outcomes between 1969 and 2017, Table 2. These case reports and case series received a GRADE of *, indicating that future evidence may be inconsistent with the conclusions of these studies. Across 59 cases, negative outcomes for the fetus or newborn occurred in 36 (61%) pregnancies. Negative outcomes ranged from spontaneous miscarriage (termination of pregnancy prior to when the fetus is considered viable, approximately 28weeks) (n = 10), fetal death and stillbirth after 28 weeks (n = 2) and death shortly following birth (n = 8, four were premature, born before 36 weeks gestation), to a range of congenital abnormalities and health issues (n = 16) including hyperbilirubinemia, respiratory distress, syndactyly, and ureter and heart abnormalities, Table 2. For six infants, a wide range of long term conditions were reported [41–45], Table 2. Healthy children (including one set of twins) were born in 23 pregnancies from mothers who had clinical manifestations (n = 10), serological evidence (n = 3) or both (n = 10) consistent with LD.
Table 2. Summary of pregnancy outcomes from 59 case reports diagnosed with gestational Lyme disease and test results for direct detection of B. burgdorferi or spirochetes or antibodies against the agent of Lyme disease.
| Case Data | Test results by sample taken, test results are noted by +/- and positive results are shaded | Detailed Findings | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mother Clinical and Test Results | Cord Blood | Placenta | Fetus/ Infant Autopsy | Newborn Samples | Child Samples | |||||||||||
| Ref | Country/ State | Year | Age | LD Trimester1 | LD Treated? | Week Pregnancy Ended | Clinical | Serology | Sample Culture | Serology | Tissue | Tissue | Serology | Tissue | serology | |
| North America -USA only | ||||||||||||||||
| [46] | Arkansas (treated in Germany) | 1997 | 34 | 2nd 5 | Yes | term | FP | 2-tier IgM+ 6 | S IgG-, IgM- | PCR - | Healthy twins. 2-tier IgM only: IFA+, IB + Mother tested at approx. 31 weeks pregnant using European protocol. |
|||||
| [47] | California | 1987 | NR | NR | No | term | AR | ELISA- | WS+, culture+ | Newborn died at 8 days with Peripheral cyanosis, systemic hypertension, metabolic acidosis, myocardial dysfunction and abdominal aortic thrombosis. Spirochete appeared similar to the original Long Island tick isolate (B. burgdorferi questionable), cultured from brain; brain and heart positive by staining. Timing of mother’s test is unknown. |
||||||
| [48] | New Jersey | 19912 | NR | 2nd | Yes | term | EM | IB IgM+, IgG+ | Healthy newborn Mother tested at 27 weeks pregnant. |
|||||||
| [49] | New York | 1978 | 25 | unknown | No | 39 | None | IHC+ | Newborn died at 4 hours. Multiple anomalies: large ventricular septal defect, hydrocephalus, omphalocele, clubfoot, spina bifida, and meningomye locele. Spirochetes in fetal tissue. | |||||||
| [49] | New York | 1979 | 33 | unknown | No | 40 | None | IF+ | Newborn died at 30 minutes. Spirochetal fragments in fetal tissue. | |||||||
| [49–51] | New York | 1985 | 24 | 1st 5 | No | term | NR | IFA+, ELISA+3 | DF+, MA+ | DF+, IF+, K+, MA+ | Stillborn. Dark field microscopy showed B. burgdorferi in the liver, adrenal, brain, heart, and placenta. Cultured spirochetes from liver. Lack of tissue inflammation noted. Mother tested postpartum. |
|||||
| [49] | New York | 1985 | 26 | unknown | No | 37 | NR | WS+ | Newborn developed respiratory distress, treated with antibiotics and recovered. | |||||||
| [49] | New York | 1986 | 19 | unknown | No | term | NR | WS+ | Newborn developed respiratory distress, treated with antibiotics and recovered. | |||||||
| [49] | New York | 1986 | 28 | 2nd | Yes | term | EM | IFA-, ELISA- | IFA-, ELISA- | BSK-H+ WS+ |
Healthy newborn Mother appears to have acquired LD 2x during pregnancy and treated 2x. Mother tested at delivery. |
|||||
| [49] | New York | 1986 | 23 | 2nd | Yes | term | EM | S- | WS- | Healthy newborn Mother tested in 2nd and 3rd trimester. |
||||||
| [49] | New York | 1986 | 34 | unknown | No | 17 | None | S- | IF+ | Fetal death. Spirochetes found in brain. Mother tested postpartum. |
||||||
| [49] | New York | 1986 | 25 | unknown | No | 12 | NR | BSK-H+, IHC- | Fetal death. B. burgdorferi isolated from fetal kidney | |||||||
| [49] | New York | 1988 | 21 | unknown | No | 16 | None | S- | IHC+, MA+ | Fetal death. Spirochetes isolated in brain. Mother tested postpartum. |
||||||
| [49,51] | New York | 19892 | 22 | unknown | No | 19 | None | S- | IF+ | Fetal death. Coarctation of the aorta. No inflammation in spirochete positive tissues (not specified) was noted. Mother tested postpartum. |
||||||
| [49,51] | New York | 19892 | 37 | unknown | No | 23 | None | S- | IF+ | Fetal death. Spirochetes found in the kidney. No inflammation in spirochete positive tissues. Mother tested postpartum. |
||||||
| [49,51] | New York | 19892 | 32 | unknown | No | 15 | None | S- | IF+ | IF+ | Fetal death. Spirochetes found in the liver and placenta. Mother tested postpartum. |
|||||
| [49] | New York | 19892 | 27 | unknown | No | 25 | AR | IF+ | Fetal death, large intraventricular septal defect detected. Positive tissue not described. | |||||||
| [27] | New York | 20072 | 42 | 3rd | Yes | 41 | AR | 2-tier 6 IgM+, IgG+ | PCR+ | S IgG+, IgM- | WS-, GM- | Healthy newborn Mother’s synovial fluid PCR+ post-partum (after 1st round of treatment). 2-tier: ELISA+, WB IgM+/IgG+ Mother tested at 34 weeks pregnant. |
||||
| [52] | Wisconsin | 1984 | 28 | 1st | No | 35 | EM, AR | IFA IgG+ | D+ | Newborn died at 39 hours. Congenital hypoplastic left heart complex malformation. Spirochetes, morphologically compatible with B. burgdorferi, found in spleen, renal tubes and bone marrow. No inflammation in spirochete positive tissues. Mother tested postpartum. |
||||||
| [44] | USA- not specified | 19862 | 34 | 1st | Yes | 20 | EM, AR | S+ | IF-, culture- | IF-, culture - | Fetal death. Mother tested at 8 and 16 weeks pregnant |
|||||
| [44] | USA- not specified | 19862 | 32 | 1st | No | 36 | FP, AR | Newborn with hyperbilirubinemia, recovered. | ||||||||
| [44] | USA- not specified | 19862 | 30 | 2nd | Yes | term | EM, AR | Healthy newborn except for syndactyly | ||||||||
| [44] | USA- not specified | 19862 | 31 | 2nd | Yes | term | EM | S- | Healthy newborn. At 8 months child was diagnosed with cortical blindness and developmental delay. Infant tested at 1 year. |
|||||||
| [44] | USA- not specified | 19862 | 31 | 3rd | No | term | EM, MG | Healthy newborn except for a generalized, petechial, vesicular rash and hyperbilirubinemia | ||||||||
| [53] | USA- not specified | 20172 | 31 | 2nd | Yes | 41 | EM | ELISA+ | Healthy newborn Mother tested at 16 weeks pregnant. |
|||||||
| Europe | ||||||||||||||||
| [42] | Austria | 1969 | NR | Pre 5 | No | NR | EM | IB+ | New born had several minor abnormalities (huge sacral hemangioma, gluteal atrophy). Samples taken 20 years later for serological testing, whole family was positive. | |||||||
| [54] | Czech Republic | 1986 | 26 | 2nd | Yes | 32 | EM | NR- | S- | Premature newborn with respiratory distress syndrome and anemia at birth. Time of newborn test is not reported. |
||||||
| [54] | Czech Republic | 1986 | 24 | 3rd 5 | NR | 41 | EM | S- | Healthy newborn Time of newborn test is not reported. |
|||||||
| [55] | Denmark | 19872 | 29 | 3rd 5 | Yes | 39 | EM | S+ | S- | Healthy newborn Mother tested at 36 weeks pregnant. |
||||||
| [56] | France | 19942 | 27 | 3rd 5 | Yes | term | EM | ELISA IgG+, IgM+ | ELISA IgG-, IgM- | ELISA IgG-, IgM- | Healthy newborn Mother tested at delivery. Newborn tested at 6 and 9 months. |
|||||
| [45] | Germany | 1981 | NR | unknown | No | 37 | none | ELISA IgG+ 4 | ELISA IgG+ 4 | Child had many conditions: intellectually retarded, deaf, enlarged head, fontanelle 4x3 at 4 years, chronic meningitis, protruding eyes, blepharitis, conjunctivitis, strabismus, maculopaplar rash, pruritus, recurrent arthritis of the knees, adenomegaly, hepatosplenomegaly. Serology done retrospectively when child was 4 years old. There is no clinical history of LD before or during pregnancy in the mother. Time of mother’s test is not reported. |
||||||
| [57,58] | Germany | 1984 | 37 | 1st 5 | Yes | term | EM | ELISA IgM+ IgG-, IHA+, IFA- | WS+, MA+ | Newborn died at 23 hours due to prenatal brain damage. Spirochetes identified in the brain and liver. Mother’s 1984 serology samples were negative with 1984 tests and positive with 1986 tests. |
||||||
| [58] | Germany | 19862 | NR | 3rd | NR | term | EM | S- | Healthy newborn Newborn tested more than once between birth and 9 months. |
|||||||
| [59] | Germany | 19942 | 36 | 3rd | Yes- post | term | AR | S IgG-, IgM+ | S IgG-, IgM- | Healthy newborn. Mother had joint pain, IgM+ at day 28 of symptoms, at day 42 IgM- and IgG-. Newborn tested at birth. |
||||||
| [60] | Italy | 1993 | 32 | unknown | No | 39 | None | IFA IgG+ | WS- | WS+, PCR+ | 9 m: ELISA, IFA, WB IgG- & IgM-13m: ELISA-, IFA-WB IgG+, IgM - | Healthy newborn. At 3 weeks: multiple annular erythemas and fever, relapsed at 9, 12, 24 and 36 months. Each episode between 9 and 36 months treated with antibiotics. 13 month IgG western blot was positive for B. garinii 41 kD, 30 kD and 61 kD. 36 months a borrelia-like organism failed to culture on BSK-H. Mother was tested postpartum. |
||||
| [61] | Poland | 2001 | 25 | 3rd | Yes | term | EM | ELISA IgM+, IgG+ | Newborn had hyperbilirubinemia and recovered Mother tested approx. 25 weeks pregnant. |
|||||||
| [62] | Poland | 20122 | 30 | 3rd | Yes | term | EM | 2 -tier IgM+, IgG- | ELISA- | Healthy newborn 2-tier: ELISA IgM+ & WB IgM+ Mother tested postpartum & post treatment. Newborn tested after birth. |
||||||
| [63] | The Netherlands | 20002 | 33 | Pre | Yes | 38 | FP | S IgM+/IgG+ | Healthy newborn. Mother tested at 16, 17 (IgM+ only) and 19 (IgM+ and IgG+) weeks pregnant. |
|||||||
| [64] | The Netherlands | 20022 | 37 | 3rd 5 | Yes | term | EM, FP | 2-tier 6 IgM+, IgG+ | ELISA IgM- | Healthy newborn 2-tier: ELISA IgM+, IgG + & WB IgM+, IgG + Mother tested at 37 weeks pregnant. Newborn tested at birth. |
||||||
| [65] | Serbia | 1991 | 25 | 1st 5 | Yes | term | EM | S IgG-, IgM- | Healthy newborn Mother tested at 10 weeks pregnant. |
|||||||
| [66] | Serbia | 1992 | 29 | 2nd 5 | NR | term | NR | IFA IgM+, IgG+ | Healthy newborn Mother tested at 25 weeks pregnant. |
|||||||
| [66] | Serbia | 1992 | 25 | 1st 5 | NR | term | NS | IFA IgM+, IgG+ | Healthy newborn Mother tested at 18 weeks pregnant. |
|||||||
| [43] | Slovenia | 1986 | 33 | NR | Yes—post | 34 | None | IFA IgG+ | DF+ | Fetal death. Spirochetes seen in lung, liver and brain tissue. Mother tested postpartum: IFA IgG+, 6 months after treatment IgG- |
||||||
| [43] | Slovenia | 19992 | 26 | 1st 5 | Yes | 32 | EM | S+ | DF+ | Died within hours of birth. Hydrocephalus, a fluidothorax, ascites, no malformations. Spirochetes in lung and liver tissue. Mother tested at the end of the second trimester. |
||||||
| [43] | Slovenia | 19992 | 26 | 1st 5 | Yes | 25 | EM | IFA- | WS- | Premature newborn died within minutes of birth. Autopsy: chorioamnionitis and vasculitis of umbilical vessels Mother tested at 6 weeks pregnant. |
||||||
| [43] | Slovenia | 19992 | 25 | 1st 5 | Yes | 25 | EM | IFA- | WS- | Premature newborn died immediately, no relevant findings on autopsy. Mother tested at 11 weeks pregnant. |
||||||
| [43] | Slovenia | 19992 | 26 | 2nd 5 | Yes | 33 | EM | IFA- | Premature newborn had severe hyperbilirubinemia, staphylococcal infection and apnoea. Mother tested at 7 weeks pregnant. |
|||||||
| [43] | Slovenia | 19992 | 23 | 2nd 5 | Yes | 26 | EM | IFA- | Premature newborn with respiratory distress syndrome, bilateral ventricular and periventricular bleeding, intraverebral parieto occipital bleeding. Child recovered. Mother tested at 21 weeks pregnant. |
|||||||
| [43] | Slovenia | 19992 | 31 | 2nd 5 | Yes | 36 | EM | IFA- | Healthy newborn Mother tested at 26 weeks pregnant. |
|||||||
| [43] | Slovenia | 19992 | 27 | 1st 5 | Yes | 36 | EM | IFA- | Newborn had respiratory distress, a wet lung and later pneumothorax and atelectasis. Mother tested at 16 weeks pregnant. |
|||||||
| [43] | Slovenia | 19992 | 28 | 3rd | Yes | 40 | EM | IFA- | Healthy newborn. 7 months old bilateral ureteral stenosis with hydronephrosis identified. Mother tested at 29 weeks pregnant |
|||||||
| [43] | Slovenia | 19992 | 23 | 3rd 5 | Yes | term | EM | IFA- | Healthy newborn, 5 months old vesicoureteral reflux was diagnosed. Mother tested at 33 weeks pregnant. |
|||||||
| [43] | Slovenia | 19992 | 29 | 1st | Yes | term | EM | IFA- | Healthy newborn, 10 months old unilateral ureteral stenosis and hydroureter. Mother tested at 16 weeks pregnant. |
|||||||
| [43] | Slovenia | 19992 | 37 | 1st 5 | Yes | term | EM | IFA- | Healthy newborn with syndactyly Mother tested at 6 weeks pregnant. |
|||||||
| [43] | Slovenia | 19992 | 28 | 1st | Yes | 9 | EM | IFA- | Spontaneous abortion 9 weeks. Mother tested at 7 weeks pregnant. |
|||||||
| [43] | Slovenia | 19992 | 23 | 1st | Yes | 10 | EM | IFA- | Spontaneous abortion 10 weeks. Mother tested at 5 weeks pregnant. |
|||||||
| [41] | Spain | 20142 | 31 | 2nd | Yes | 36 | NS | BSK-H+, PCR+ 6, RLBH+ | Child developed cholelithiasis in utero (diagnosed at 26 weeks), at 2.5 years still suffered from the condition. Positive cell culture for Borrelia ssp. from unknown sample, confirmed LD by PCR and RLBH. Mother tested at 20 weeks pregnant. | |||||||
| Asia | ||||||||||||||||
| [67] | Turkey | 20052 | 18 | unknown | No | 36 | None | S IgM+, WB IgG+ | WB IgM equivocal | Newborn had congenital triventricular hydrocephalus and aquaductus cerebri stenosis, transepandymal cerebrospinal-fluid leakage. Mother WB IgG: 31 kDa & Newborn WB IgM: 41 kDa and 75 kDa Mother tested at 34 weeks pregnant. Newborn tested at birth. |
||||||
| [68] | Russia | 1999 | 21 | 2nd | Yes | term | NS | IFA IgG+ | Healthy newborn Mother tested at 20 weeks pregnant. |
|||||||
1 LD trimester = the trimester that LD was acquired by the case, options "pre" before pregnancy, 1st trimester is pregnancy weeks 1–12, 2nd trimester is pregnancy weeks 13–27 and 3rd trimester is pregnancy weeks 27 to 40+ weeks, "post" after pregnancy.
2 Case date not provided, publication date used.
3 Conflicting postpartum serological results: CDC and New York State Department of Health found strongly reactive results by IFA and ELISA, however Dr. Allen Steere did not detect specific antibodies for B. burgdorferi.
4 ELISA test conducted on mother’s serum over 4 years after the birth of her child. Child’s serum was tested (unknown test) at unreported times between birth and 4 years old.
5 Tick bite reported by mother.
6 Indicates cases in which B. burgdorferi infection in the mother was detected using laboratory methods recommended by current guidelines.
NR = not reported, NS = non-specific,
EM = erythema migrans rash associated with Lyme disease. FP = facial palsy, AR = arthritis, MG = meningitis.
S = serological test not described. ELISA = enzyme-linked immunosorbent assay. IHA = indirect hemagglutination. IFA = indirect immunofluorescence assay. IB = immunoblot, 2-tier = 2-tier testing indicates a positive or equivocal ELISA or IFA followed by a confirmatory western blot. IgG/ IgM = Immunoglobulin G and M are indicated for serology where described.
BSK-H = culture Barbour Stoenner Kelly II medium. K = Kelly's medium. PCR = polymerase chain reaction. RLBH = reverse line blot hybridization assay. WS = Warthin starry silver stain, GM = Gomori methenamine stain. D = Dieterle staining method. IF = indirect immunofluorescence DF = Dark field microscopy, not further specified. MA = specific monoclonal antibody H5332. IHC = immunohistochemistry.
Laboratory testing of the newborn or fetus was not reported in 28 cases, Table 3, and for these the possible role of LD was determined by evidence in the mother (clinical manifestations [n = 13], diagnostic test results [n = 3], or both [n = 10]), while for 2 cases the only evidence of infection was identification of spirochetes in the placenta reported to be B. burgdorferi, Table 2. The newborn and fetus cases with laboratory evidence of infection (n = 31), included serological test results (n = 13) of which only two were considered positive and one was borderline, Table 3. Fetal or newborn tissue samples (n = 19) were examined and B. burgdorferi was identified in 17 cases using staining, indirect immunofluorescence (IF), or PCR to confirm the presence of B. burgdorferi. Not all tests used in the studies are considered reliable; those considered reliable include PCR with specific primers, IF using specific antibodies, and culture when it is confirmed by IF or PCR (n = 12) [69–71]. Direct microscopic detection (n = 7) of B. burgdorferi (using bacteria staining and dark field microscopy) is generally considered of limited value in diagnosis due to both the small number of spirochetes seen and the risk of false positive results for a range of reasons reviewed elsewhere [72]. Across the case reports the specificity of the primers used for PCR and methods of culture confirmation were often not mentioned, but we included these reports nevertheless. Evidence of infection in the placenta (presence of spirochetes) and/or cord blood (presence of antibody) was sought in 12/59 cases, Table 3. Of these 5/11 placentas and 1/5 cord blood samples were positive (for IgG antibodies only), two of the placenta positive results were based on the results of indirect immunofluorescence. In the positive cord blood case and one placenta-positive case the child was healthy at birth.
Table 3. An overview of the features of 59 case reports diagnosed with gestational Lyme disease.
| Case characteristic | Positive / total cases | Not reported/ not done |
|---|---|---|
| Pregnant Women | ||
| Pregnant women with clinical manifestations of LD | 41/54 | 5 |
| Pregnant women with laboratory test results | 23/33 | 26 |
| Pregnant women with test results from currently recommended laboratory tests1 | 4/4 | N/A |
| Pregnant women where clinical symptoms were not reported (n = 2) or not specific (n = 8), but laboratory test results were reported2 | 7/10 | N/A |
| Other samples tested | ||
| Spirochetes detected in placenta | 5/11 | N/A |
| Cord blood serology | 1/5 | N/A |
| Fetus, Newborn or Child | ||
| Any test result for a fetus, newborn or child | 18/31 | 28 |
| Spirochete identified in tissue collected at autopsy | 15/18 | 2 |
| Spirochetes identified following autopsy conducted on fetus from pregnant women not diagnosed with gestational LD. | 5/5 | N/A |
| Spirochete identified in tissue sample from a live child | 1/1 | N/A |
| Serology results in the newborn or child | 2/13 | 34 |
| Frequency of Negative Birth Outcomes | ||
| 1st trimester miscarriage | 3/59 | N/A |
| 2nd trimester miscarriage | 7/59 | N/A |
| 3rd trimester fetal death/ stillbirth | 2/59 | N/A |
| Death shortly after birth | 8/59 | N/A |
| Abnormalities/ health issues3 | 16/59 | N/A |
| Long term conditions | 6/16 | N/A |
| Healthy Infants | 23 (1 set of twins)/59 | N/A |
1 A subset of total laboratory tests, evaluated based on laboratory methods recommended by current guidelines [70,71]
2 Spirochetes identified in placenta (n = 3) &/or fetal tissue (n = 3)
3 Examples of adverse outcomes: hyperbilirubinemia, respiratory distress, syndactyly, and ureter and heart abnormalities
Amongst the 59 pregnancies identified in the case studies in this SR, 33 (56%) pregnant women were tested for LD; but diagnostic methods currently considered reliable (direct detection methods as described above or the two-tier EIA followed by the Western Blot) were used in only four cases, Table 3 [27,41,46,64,69,72,73]. In five other cases, the mother was not diagnosed with LD by clinical symptoms or a diagnostic test, but instead was considered retrospectively to have suffered from LD when the cause of fetal or newborn demise was investigated and possible B. burgdorferi spirochetes were identified in the placenta (n = 3) and/or fetal tissue (n = 3) [49,51].
There was treatment information for 19/23 pregnancies that resulted in healthy newborns; 18/19 (95%) were treated for LD during pregnancy and subsequently had no adverse birth outcomes. Healthy newborns were born to mothers that had a wide range of gestational LD symptoms from EM to neuroborreliosis and LD was acquired in all trimesters (Table 2). In contrast, 34/36 pregnancies have treatment information and a negative birth outcome, of these only 14/34 (41%) were treated during pregnancy. Among the 20 cases that were not treated and had negative birth outcomes, 10 occurred in mothers with no clinical history of symptoms consistent with LD according to current guidelines [70,71]. The other 10 untreated cases were mainly diagnosed retrospectively or after parturition, so there was no opportunity for treatment during pregnancy.
Across cases, evidence that transplacental transmission of B. burgdorferi can occur was shown by testing the placenta (n = 11) and deceased fetal/newborn tissue (n = 18), Table 3. Adverse birth outcomes occurred in 4/5 placenta positive cases (2 stillbirths and 2 cases of respiratory distress that recovered), in 2/6 placenta-negative cases (one premature birth and one case reported as relapsing LD beginning at 3 months of age, and spirochetes were identified in one or more fetal tissues in 15/18 autopsies (Table 2). Only one case (in Germany) described the full range of expected observations (clinical manifestations in the mother, negative outcome for the child, and laboratory detection of B. burgdorferi in the child) that would give confidence that vertical transmission of B. burgdorferi, with negative consequences for the fetus, occurs [57,58]. The reports from the autopsies (n = 18) did not provide an explanation for how the presence of B. burgdorferi was associated with the pathology seen in the fetus [49,51,52,57,58]. A common autopsy observation was the lack of inflammation or immune response against B. burgdorferi infection in the fetus. Across all cases there were no consistent clinical outcomes resulting from gestational LD, and a linkage between fetal loss and gestational LD remains unclear from the case reports.
Epidemiological studies
There are 19 epidemiological studies identified in this review, which include nine cohort studies, four cross sectional studies, two case control studies, and four case series. The studies were conducted in the USA (n = 10) and Europe (n = 9) and published between 1986 and 2011. One cohort did not have extractable epidemiological outcomes, but the data from the pregnant LD cases are included in the previous section [66]. A second study is not included in this review because there were no extractable outcomes [74]. This study also received a very high RoB evaluation due to incomplete reporting of methods and outcomes, lack of blinding, failure to account for or examine important confounders, and other biases including the potential of funding bias [74].
Adverse outcomes in LD exposed vs unexposed populations
Eight studies reported differences in prevalence of one or more types of adverse birth outcomes in exposed compared to unexposed populations, Table 4. The definition of ‘exposed’ in these studies included women diagnosed with gestational LD during the study, a history of LD (based on clinical chart review), positive LD serology during pregnancy, or those considered to be at higher risk of LD (measured indirectly by having a history of tick bites or living in a known endemic area for LD risk). Adverse birth outcomes amongst and within the epidemiological studies varied widely and included very common outcomes such as preterm birth and hyperbilirubinemia [75,76], as well as less frequent and more serious major congenital malformations. Some studies reported all adverse outcomes together without additional details by type of outcome (Table 4). Meta-analysis was considered inappropriate for all outcomes due to the variability in study design, definition of LD, and range of adverse outcomes.
Table 4. Measures of association extracted from eight studies on adverse birth outcomes and LD during or before pregnancy, positive LD serology during pregnancy, and surrogate measures of possible exposure to LD (e.g. tick bites or living in an endemic area).
(Significant odds ratios are bolded in the results.).
| Ref | Study | Adverse outcome definition | Diagnosis of LD or surrogate measure of exposure in mothers | OR* | 95% Conf. Interval | N | |
|---|---|---|---|---|---|---|---|
| Association with adverse birth outcomes due to having a positive LD serological test during pregnancy or history of gestational LD (GRADE ***) | |||||||
| [79] | Carlomagno (1988) | spontaneous miscarriage | Serological screening only (IgG) 2, 3 | 2.14 | 0.50 | 9.09 | 98 |
| [77] | Strobino (1993) | spontaneous miscarriage | Serological screening (IgG or IgM) 2, 4, 9 and clinical history of LD | 0.49 | 0.03 | 8.41 | 1521 |
| [77] | Strobino (1993) | spontaneous miscarriage | Clinical gestational LD 10 | 0.39 | 0.03 | 6.01 | 1746 |
| [77] | Strobino (1993) | spontaneous miscarriage | LD <1 year before conception | 1.73 | 0.69 | 4.36 | 1760 |
| [77] | Strobino (1993) | spontaneous miscarriage | LD >1 year before conception | 1.20 | 0.32 | 4.51 | 1752 |
| [78] | Dlesk (1989) | spontaneous miscarriage | Serological screening only (IgG or IgM)2, 3, 9 | 0.71 | 0.08 | 5.93 | 126 |
| [77] | Strobino (1993) | history of miscarriage | Serological screening (IgG or IgM) 2, 4, 9 and clinical history | 0.86 | 0.19 | 4.00 | 1521 |
| [80] | Bracero (1992) | premature rupture of membranes | Serological screening only (IgG or IgM)2,3, 9 | 1.01 | 0.12 | 8.88 | 134 |
| [80] | Bracero (1992) | premature labour | Serological screening only (IgG or IgM) 2,3, 9 | 1.46 | 0.16 | 13.10 | 134 |
| [80] | Bracero (1992) | low birth weight | Serological screening only (IgG or IgM) 2,3, 9 | 2.27 | 0.41 | 12.58 | 134 |
| [80] | Bracero (1992) | apgar <7 | Serological screening only (IgG or IgM) 2,3, 9 | 3.36 | 0.35 | 32.54 | 134 |
| [80] | Bracero (1992) | small for gestational age | Serological screening only (IgG or IgM) 2,3, 9 | 6.89 | 0.62 | 76.46 | 134 |
| [80] | Bracero (1992) | congenital abnormality, all1 | Serological screening only (IgG or IgM) 2,3, 9 | 5.62 | 0.21 | 150.06 | 134 |
| [81] | Strobino (1999) | congenital cardiac abnormality | History of LD 10 | 0.85+ǂ | 0.39 | 1.89 | 1500 |
| [77] | Strobino (1993) | congenital abnormality, all1 | Clinical gestational LD10 | 0.53ǂ | 0.07 | 4.16 | 1521 |
| [77] | Strobino (1993) | congenital abnormality, all1 | LD <1 year before conception | 1.65ǂ | 0.60 | 4.57 | 1760 |
| [77] | Strobino (1993) | congenital abnormality, all1 | LD > 1 year before conception | 2.94ǂ | 0.98 | 8.86 | 1752 |
| [77] | Strobino (1993) | congenital abnormality, minor1 | Clinical gestational LD10 | 0.80ǂ | 0.10 | 6.28 | 1521 |
| [82] | Williams (1995) | congenital abnormality, major 1 | LD before pregnancy | 3.26 | 0.75 | 14.20 | 2386 |
| [82] | Williams (1995) | congenital abnormality, all1 | LD before pregnancy | 1.13 | 0.26 | 4.85 | 2386 |
| [82] | Williams (1995) | congenital abnormality, major 1 | Clinical gestational LD10 | 6.80 | 0.78 | 59.00 | 2386 |
| [82] | Williams (1995) | congenital abnormality, all1 | Clinical gestational LD10 | 2.37 | 0.28 | 20.42 | 2386 |
| Association with adverse birth outcomes and an IgG or IgM positive cord blood serological test (GRADE **) | |||||||
| [83] | Williams (1988) | adverse birth outcomes | Cord blood serology (IgG) 5 | 0.40 | 0.05 | 3.07 | 255 |
| [82] | Williams (1995) | congenital abnormality, minor1 | Cord blood serology (IgG) 5 | 0.63 | 0.08 | 4.70 | 2386 |
| [84] | Lakos (2010) | adverse birth outcomes | Cord blood serology (IgG)6 | No est. | 74 | ||
| Association with congenital abnormalities and tick bites during pregnancy (GRADE **) | |||||||
| [82] | Williams (1995) | congenital abnormality, all1 | Tick bite during pregnancy | 1.63 | 0.77 | 3.47 | 2386 |
| [77] | Strobino (1993) | congenital abnormality, all1 | Tick bite during pregnancy | 1.35ǂ | 0.72 | 2.53 | 1731 |
| [82] | Williams (1995) | congenital abnormality, major 1 | Tick bite during pregnancy | 1.60 | 0.49 | 5.23 | 2386 |
| [77] | Strobino (1993) | congenital abnormality, major1 | Tick bite during pregnancy | 0.59ǂ | 0.14 | 2.49 | 1731 |
| [82] | Williams (1995) | congenital abnormality, minor1 | Tick bite during pregnancy | 1.62 | 0.64 | 4.11 | 2386 |
| [77] | Strobino (1993) | congenital abnormality, minor1 | Tick bite during pregnancy | 1.76ǂ | 0.90 | 3.46 | 1731 |
| [81] | Strobino (1999) | congenital cardiac abnormality | Tick bite during pregnancy | 0.93+ǂ | 0.56 | 1.56 | 1500 |
| Association with congenital abnormalities and a history (before or during pregnancy) of tick bites, but no LD (GRADE **) | |||||||
| [77] | Strobino (1993) | congenital abnormality, all1 | History of a tick bite with no LD8 | 1.46ǂ | 0.96 | 2.36 | 1731 |
| [77] | Strobino (1993) | congenital abnormality, major1 | History of a tick bite with no LD | 1.52ǂ | 0.75 | 3.07 | 1731 |
| [77] | Strobino (1993) | congenital abnormality, minor1 | History of a tick bite with no LD8 | 1.47ǂ | 0.87 | 2.49 | 1731 |
| Association with congenital abnormalities and mother residing in a LD endemic area compared to a non-endemic LD area (GRADE **) | |||||||
| [83] | Williams (1988) | congenital abnormality, all7 | Residence, endemic LD area | 0.90 | 0.49 | 1.65 | 421 |
| [82] | Williams (1995) | congenital abnormality, all1 | Residence, endemic LD area | 0.87ǂ | 0.70 | 1.06 | 4814 |
| [82] | Williams (1995) | congenital abnormality, major1 | Residence, endemic LD area | 1.08ǂ | 0.77 | 1.53 | 4814 |
| [82] | Williams (1995) | congenital cardiac abnormality | Residence, endemic LD area | 2.4ǂ | 1.25 | 4.59 | 4814 |
| [82] | Williams (1995) | congenital abnormality, minor1 | Residence, endemic LD area | 0.77ǂ | 0.60 | 0.99 | 4814 |
No est = no estimate is available because there were no events in either group.
*Odds Ratios were calculated from the raw data provided in the paper unless otherwise noted.
ǂ = Odds ratio extracted from the paper.
+ = Outcome was adjusted for other variables. [81] is adjusted for maternal age, number of live births, current county of residence, year of birth of study child, occupational x-ray exposure, maternal high blood pressure, and characteristics of residence (wooded area, deer) at the time of birth of the study child. Three studies reported a statistical analysis of the comparability of their exposed and control sampling frames, but did not present adjusted results [77,82,84].
1 Congenital abnormalities were summarized in some studies as all abnormalities together and then subdivided into minor abnormalities and major abnormalities.
2 Lyme disease serology was conducted in the first trimester.
3 Screening for LD positive serology in pregnant women included a single immunoassay.
4 Screening test was an immunoassay confirmed by an immunoblot.
5 Immunoassay used to screen cord blood
6 Immunoblot used to screen cord blood.
7 Study only sampled live births, so the impacts of LD that may lead to fetal demise would have been omitted from these results.
8 Results represent outcomes for women who had tick bites, but no LD. An association with tick bites is also presented for the same sample including women who had LD and for a subset of births where the physician records were available. The associations reported in the paper were conflicting for minor congenital abnormalities [77].
9 The serological test used in this study measured total IgG and IgM.
The results included six studies where no significant association was reported between adverse birth outcomes (e.g. spontaneous miscarriage and congenital abnormalities) and the mother’s LD status, determined by serology or clinical diagnosis [77–82] (Table 4). The RoB was low (n = 3) and unclear (n = 3) across these six studies, study designs and diagnosis of LD varied, but the conclusions were consistent. This gives this group of studies a *** GRADE indicating some confidence the overall conclusions of this research will not change with future research.
Other exposure measures included seropositive cord blood (n = 3 studies), tick bites during pregnancy (n = 3), history of tick bites (n = 1), and residing in an endemic area (n = 2) (Table 4). No association was shown from cord blood serology (IgG or IgM antibodies) results and adverse birth outcomes [82–84]. In addition, congenital abnormalities overall were not associated with surrogate measures of LD exposure including exposure to ticks or expected exposure to ticks by virtue of living in an endemic area (Table 4) [77,81–83]. Among the results from the LD endemic area of Westchester, New York, USA, a significantly higher odds of cardiac abnormalities and lower odds of minor abnormalities was observed compared to a population in a non-endemic area [82](Table 4). In this study, these associations were shown to be unrelated to a clinical history of LD in the mother and are assumed to be independent of LD [82]. Therefore there was no association between gestational LD or surrogate measures of exposure and adverse birth outcomes across the eight studies in Table 4.
Risk factors for adverse outcomes in LD exposed populations
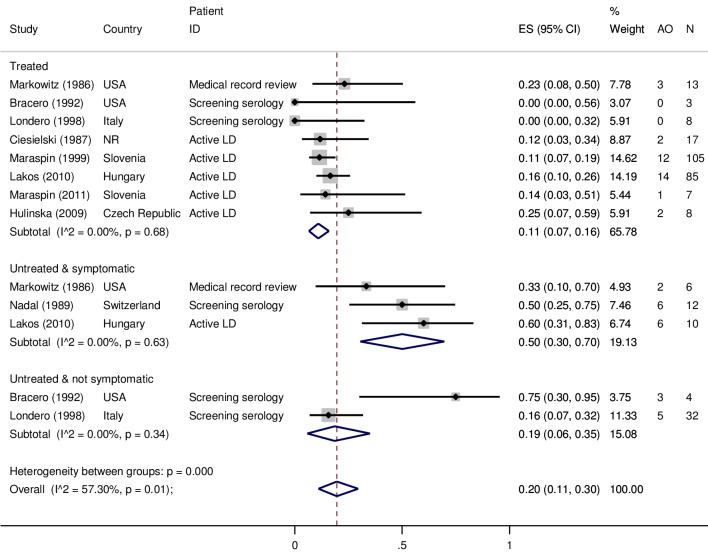
There were ten studies that examined various risk factors for adverse birth outcomes among women diagnosed with gestational LD. In nine of these studies there were data on the proportion of adverse birth outcomes in treated and untreated women with gestational LD (Fig 3). LD status was determined in several ways across these studies; active gestational LD diagnosed by a physician (n = 5) [43,44,84–86], retrospective identification based on medical records (n = 1) [44], or a positive serology result on a screening test (IgG and/or IgM) during pregnancy (n = 3) [87,88]. A random effects meta-analysis was conducted to examine the proportion of adverse outcomes across subgroups based on LD and treatment status: i) diagnosed with gestational LD and treated during pregnancy, ii) diagnosed with gestational LD, but not treated during pregnancy and iii) seropositive on a screening test (single immunoassay [80,87] or two tier test [88]) for LD during pregnancy, but did not have a clinical history of illness and consequently was not treated (Fig 3). There was a significantly higher proportion of adverse birth outcomes in the untreated subgroup (50%, 95%CI 30–70, I2 = 0%) compared to the group that received treatment (11%, 95%CI 7–16, I2 = 0%) (Fig 3). For the subgroup that had a seropositive screening test, but were considered healthy, the results from two studies were quite different from each other and in the meta-analysis this sub-group was not significantly different from the frequency of adverse birth outcomes in the treated and untreated subgroups (19%, 95%CI 6–35, I2 = 0%) (Fig 3).
Fig 3. Random effects meta-analysis of nine studies that reported the proportion of women with gestational Lyme disease that experienced an adverse birth outcome.
Studies were sub-grouped by treatment status: treated active LD, untreated LD that had a clinical history of LD symptoms, and seropositive with no history of LD. LD status was determined by retrospective medical record review, clinical diagnosis with and without serology or culture, or positive IgG and/or IgM serology. (NR = not reported, AO = Adverse outcome).
Among these ten studies, two reported that the country level frequency of adverse birth outcomes was the same as the frequency among women in the study. Specifically, treated patients diagnosed with gestational LD had a lower frequency of spontaneous miscarriages and premature births and similar frequency of congenital malformations compared to the country level frequency [43,84]. This suggests that there was no increased risk of adverse birth outcomes among women with treated gestational LD compared to the country birth statistics. The overall GRADE of the studies in Fig 3 is ** meaning that we have limited confidence the results and estimates presented here will not change with future research. This is mainly due to the limited number of studies and observations in each sub-group despite homogeneity across studies and these limitations prevent exploration of potentially important confounding factors such as geographic region, year study was conducted and methods of diagnosis. Therefore, we caution that the summary results are not generalizable beyond the populations studied.
The odds of an adverse birth outcome in gestational LD cases that were treated compared to those that were untreated were described in four of the studies (Table 5), two involving symptomatic women and two involving asymptomatic women. The largest study was from Hungary and reported significantly increased odds of adverse birth outcomes (OR 7.61, 95% CI 1.90–30.51) [84]. The other studies, two from the USA and one from Italy, found no difference between the treated and untreated groups although the results were in the same direction as the study from Hungary [44,80]. Thus, the data suggest there is some evidence that adverse birth outcomes may occur more frequently if gestational LD is not treated.
Table 5. Measures of association extracted from six studies examining the odds of an adverse birth outcome in patients with gestational LD by treatment status, timing of exposure, severity and progression of LD.
(Significant odds ratios are bolded in the results.).
| Ref | Study | Adverse birth outcome definition | Mother’s risk factor | OR* | 95% Conf. Interval | N | |
|---|---|---|---|---|---|---|---|
| Association with adverse birth outcomes and untreated compared to treated gestational LD (GRADE **) | |||||||
| [84] | Lakos (2010)1 | adverse outcome | Untreated symptomatic LD 2 | 7.61 | 1.90 | 30.51 | 95 |
| [44] | Markowitz (1986) | adverse outcome | Untreated symptomatic l LD 2 | 1.67 | 0.20 | 14.05 | 19 |
| [80] | Bracero (1992) | adverse outcome | Untreated asymptomatic LD | 16.33 | 0.48 | 555.63 | 7 |
| [88] | Londero (1998) | adverse outcome | Untreated asymptomatic LD | 3.00 | 0.18 | 49.32 | 40 |
| Association with adverse birth outcomes and postnatal treatment of gestational LD compared to treatment during pregnancy (GRADE **) | |||||||
| [84] | Lakos (2010) | adverse outcome | Postnatal treatment of gestational LD2 | 2.57 | 0.86 | 7.69 | 95 |
| Association with adverse birth outcomes and the length of gestational LD infection (GRADE **) | |||||||
| [84] | Lakos (2010) | adverse outcome | Clinical gestational LD 2 | 1.00ǂ | NR | NR | 95 |
| Association with adverse birth outcomes and acquiring LD during the first trimester of pregnancy compared to later in pregnancy (GRADE **) | |||||||
| [84] | Lakos (2010) | congenital abnormality, all | Clinical gestational LD 2 | 0.17 | 0.02 | 1.37 | 80 |
| [84] | Lakos (2010) | adverse birth outcomes | Clinical gestational LD 2 | 0.92 | 0.31 | 2.75 | 86 |
| Association with adverse birth outcomes and disseminated LD compared to early LD (EM) at diagnosis (GRADE **) | |||||||
| [44] | Markowitz (1986) | adverse birth outcomes | Disseminated vs. early LD 2 | 3.75 | 0.45 | 31.62 | 19 |
| Association with adverse birth outcomes among EM positive women (early LD) with and without additional LD symptoms (GRADE **) | |||||||
| [89] | Hercogova (1993) | adverse birth outcomes | Symptomatic gestational LD 2 | No est. | 15 | ||
No est = no estimate is available because there were no events in either group.
*Odds Ratios were calculated from the raw data provided in the paper unless otherwise noted.
ǂ = Odds ratio extracted from the paper. One study reported a statistical analysis of the comparability of their exposed and control sampling frames, but did not present adjusted results [77,82,84].
1 Results are available for mode (e.g. oral) of antibiotic treatment; all modes are in agreement with the overall result.
Possible risk factors other than LD treatment status were investigated in three studies including trimester of B. burgdorferi exposure, length of LD during pregnancy, early vs. disseminated gestational LD, and women presenting with an EM only compared to those with an EM and other symptoms of LD [44,84,89](Table 5). None of these studies found a significant association between adverse birth outcomes and these possible risk factors, although most of these studies were small and may have had limited power to detect a difference. For example, in one study with only 19 observations the association was not significant, but there was a higher proportion of adverse birth outcomes in women with disseminated LD (cardiac manifestations and neuroborreliosis; 43% had adverse birth outcomes) compared to early LD (EM only; 17% had adverse birth outcomes) [44]. There were no significant associations between trimester of LD infection and adverse birth outcomes [84] Table 5, or with the frequency of spontaneous miscarriages [77,79]. Birth weight, a surrogate measurement for newborn health, was unrelated to gestational LD in two studies [77,82]. Spirochetes found in the placenta were not associated with adverse birth outcomes in a case series where 3/60 placentas were spirochete positive and all infants were healthy [85]. Coinfection with Anaplasma phagocytophilum based on PCR of the blood and/or placenta was reported in one study for 37.5% (3/8) LD positive women that had adverse birth outcomes (n = 2) and healthy twins (n = 1). The small sample size prohibited investigation of the association between coinfection and adverse birth outcomes [90,91].
Discussion
The literature included in this SR was published between 1985 and 2017, 58% of which are case studies. As evidence, case studies are helpful to generate hypotheses for future research, but cannot be used to further our understanding of a causal relationship, if one exists, between gestational LD and adverse birth outcomes. There were a number of reporting issues in the case studies included in this SR, mostly related to missing or limited information on the mother’s clinical symptoms and the use of diagnostic methods and laboratory tests currently considered unreliable [69–72]. The latter issue also applies to many of the epidemiology studies. This is not to indicate that the results from these studies are false, but that they are questionable, which is a feature of the age of the majority of studies identified for inclusion in this SR.
Diagnosis of LD relies on clinical evaluation, plausible exposure history to infected ticks, and if needed, supplemental diagnostic laboratory tests [70,71]. Reliable test methods would include direct demonstration of B. burgdorferi in tissues or in culture by IF or PCR using, respectively, specific antibodies or primers, or results of a two tier serological test interpreted by current guidelines, the latter being the most common type of testing for diagnosis of LD [92–94]. Typically, two tier serological testing includes an enzyme immunoassay (EIA) to detect IgM or IgG serum antibodies to B. burdorferi; positive or equivocal tests are followed by an immunoblot assay (IB, e.g. Western blot) to confirm the positive screening test result [92–94]. Although these guidelines improved the performance of LD testing, all currently available testing options are imperfect [73,95]. Thus, the inadequate sensitivity of serological tests in early LD necessitates physician awareness of LD and careful clinical assessment supported by laboratory testing results when appropriate. Pregnant women who have acquired LD should be treated according to current guidelines [92–94] as the meta-analysis in this SR suggested that treatment of LD during pregnancy was associated with a decrease in the risk of adverse birth outcomes.
The issue of misdiagnosis or misclassification of LD across studies in this SR is important to carefully consider. The use of unreliable tests or test protocols for LD could contribute to misclassification of cases, since 58% of the studies in this SR were conducted prior to the current serological testing guidelines and LD serological tests were used to screen healthy pregnant women in some of the epidemiology studies [77–80,82–84,87,88,92]; there is a measurable risk of false positives and thus misclassification of observations in the sample population [96]. Among several case reports there is little information on the diagnosis of LD and given the age of these articles there appears to be a reasonable risk that some cases were misclassified as gestational LD. Unfortunately, both false positives and false negatives could have occurred in these studies and had an impact on the results in an unknown direction and magnitude possibly resulting in the distortion of detected associations or failure to detect associations, which undermines our confidence in the research results. Considering the potential for misclassification of LD in the included studies, additional research using currently accepted methods of LD diagnosis, an improved understanding of LD, and larger sample sizes (e.g. via large multi-center observational studies) is needed to more adequately explore possible effects of gestational LD and further investigate potential risk factors suggested in this SR.
It is biologically plausible that transplacental transmission of B. burgdorferi occurs given our understanding of transplacental spirochete transmission for other species of spirochetes (T. pallidum) in humans [6,7]. There are examples among the 59 case reports included in this SR that suggested transplacental transmission occurs including 4 cases of infection in the fetus or newborn determined using relatively reliable laboratory diagnostic methods. Of these only one case reported clinical LD in the mother, an adverse birth outcome and potential demonstration of B. burgdorferi in the child; that would provide some confidence that vertical transmission of B. burgdorferi occurred and may have resulted in a negative outcome for the fetus [57,58]. Examination of the pathological findings from case studies where B. burgdorferi was identified in various fetal tissues does not provide evidence that the presence of B. burgdorferi was linked to the pathological findings and there was a lack of inflammatory response noted in several cases [43,44,47,49–52,57,87,89,97]. These findings are in alignment with literature reviews by medical practitioners on this topic [6,98]. Therefore, it is possible that vertical transmission with negative outcomes can occur, but there are knowledge gaps in terms of the pathology and frequency of occurrence.
Common adverse birth outcomes reported across studies in this SR included preterm birth and hyperbilirubinemia, which are also common outcomes in the general population [75,76]. The potential for increased risk of an adverse outcome in women with gestational LD that is not treated was shown in this SR; however an explanation for this was not addressed in the available research and it is very possible that this was due to many factors such as sub-optimal maternal health as opposed to a single specific pathology caused by B. burgdorferi infection. Congenital malformations of the cardiac or genitourinary system are also among the most common malformations reported [75,99] and were frequently reported in case reports and epidemiological studies in this SR. Hypotheses that there may be higher rates of cardiac malformations as a result of gestational LD were investigated in the early epidemiology studies and case reports included in this SR, but these studies were unable to clarify a relationship with gestational LD [51,52,81]. Given recent research characterising the impact of B. burgdorferi on the cardiac system, additional work on the teratogenic potential of B. burgdorferi particularly on the cardiac system may be warranted [100]. However, the evidence in this SR on congenital malformations does not provide sufficient evidence to exclude or confirm a role for B. burgdorferi in congenital malformations. Future research is needed to address knowledge gaps such as the pathogenesis of B. burgdorferi infection in the developing fetus and its relationship to adverse birth outcomes.
Several risk factors were investigated based on the pathology observed in the early case reports and our biological understanding of LD. These studies failed to find an association between the mother’s LD status and cardiac, minor or major malformation, spontaneous miscarriages, and fetal death [77,80–82]. Adverse birth outcomes were also not associated with the severity of gestational LD (early vs. disseminated), length of LD during pregnancy, or trimester of infection [44,84,89]. However, there was some evidence of increased risk of adverse outcomes in symptomatic women who were not treated with antibiotics, and it is possible that associations with rare or infrequent outcomes were not detected because the sample sizes (or number of LD cases) in most of the epidemiological studies was small and the range of reported outcomes was quite large.
There are several limitations to the evidence included in this SR. This includes limited generalisability of the results to populations other than those studied as there is not enough research to determine whether population differences exist and how they could have impacted the findings. Country level or regional rates for adverse birth outcomes are influenced by many factors related to socio economic factors, healthcare, and genetic predispositions that should be considered when weighing the generalizability of the data [77,82]. Other possible sources of variation in the frequency and type of outcomes include differences among genospecies of B. burgdorferi (e.g. B. burgdorferi sensu stricto, B. afzelii, and B. garinii) that can cause different manifestations of LD. The data on Borrelia species was scarce among the studies in this SR and should be considered in the design of future research to clarify if there are different outcomes or impacts of gestational LD depending on the pathogen [6].
Conclusion
This SR summarizes the research and anecdotal evidence on the potential impact of gestational LD on adverse birth outcomes. Overall there is a limited amount of evidence; with 29 case report articles and 17 epidemiological studies on this topic, and the results highlight a number of knowledge gaps and significant uncertainty about the impact of LD during pregnancy. Due to the variability in the study size and study design, the lack of evidence in the epidemiological research does not rule out uncommon consequences of LD during pregnancy. There is some evidence to suggest that it is biologically plausible for B. burgdorferi to be vertically transmitted to the fetus, however these studies have been unable to define a characteristic pathological effect of B. burgdorferi infection in the fetus, thus there are significant knowledge gaps about the relationship of B. burgdorferi infection and adverse birth outcomes [32]. Given the uncertainty around the impact of B. burgdorferi on the fetus and the consistent evidence suggesting fewer adverse birth outcomes if LD is promptly treated, it is recommended that physicians continue to remain thorough in their diagnosis and treatment of LD in pregnant women and that new research address the knowledge gaps identified in this review.
Supporting information
(PDF)
(PDF)
(DOC)
(XLSX)
Acknowledgments
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors thank the Public Health Agency of Canada’s library staff for their assistance in the procurement of articles.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Steere AC (2006) Lyme borreliosis in 2005, 30 years after initial observations in Lyme Connecticut. Wiener Klinische Wochenschrift 118: 625–633. 10.1007/s00508-006-0687-x [DOI] [PubMed] [Google Scholar]
- 2.van den Wijngaard CC, Hofhuis A, Harms MG, Haagsma JA, Wong A, de Wit GA, et al. (2015) The burden of Lyme borreliosis expressed in disability-adjusted life years. European journal of public health 25: 1071–1078. 10.1093/eurpub/ckv091 [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, et al. (1977) Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum 20: 7–17. [DOI] [PubMed] [Google Scholar]
- 4.Larsson C, Andersson M, Guo BP, Nordstrand A, Hagerstrand I, Carlsson S, et al. (2006) Complications of pregnancy and transplacental transmission of relapsing-fever borreliosis. Journal of Infectious Diseases 194: 1367–1374. 10.1086/508425 [DOI] [PubMed] [Google Scholar]
- 5.Yagupsky P, Moses S (1985) Neonatal Borrelia species infection (relapsing fever). American Journal of Diseases of Children 139: 74–76. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro ED, Gerber MA (2011) Chapter 17: Borrelia Infections: Lyme Disease and Relapsing Fever In: Britt W, editor. Infectious Diseases of the Fetus and Newborn, 7th ed: Elsevier. [Google Scholar]
- 7.Kollmann D, Dobson S (2011) Treponema pallidum In: Remington JK, J., editor. Infectious Diseases of the Fetus and Newborn Infant, seventh ed,. Philadelphia: Saunders. [Google Scholar]
- 8.Madjunkov M, Chaudhry S, Ito S (2017) Listeriosis during pregnancy. Arch Gynecol Obstet 296: 143–152. 10.1007/s00404-017-4401-1 [DOI] [PubMed] [Google Scholar]
- 9.Anderson JF, Johnson RC, Magnarelli LA (1987) Seasonal prevalence of Borrelia burgdorferi in natural populations of white-footed mice, Peromyscus leucopus. Journal of Clinical Microbiology 25: 1564–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess EC (1988) Borrelia burgdorferi infection in Wisconsin horses and cows. Ann N Y Acad Sci 539: 235–243. [DOI] [PubMed] [Google Scholar]
- 11.Burgess EC, Wachal MD, Cleven TD (1993) Borrelia burgdorferi infection in dairy cows, rodents, and birds from four Wisconsin dairy farms. Veterinary Microbiology 35: 61–77. [DOI] [PubMed] [Google Scholar]
- 12.Burgess EC, Windberg LA (1989) Borrelia sp. infection in coyotes, black-tailed jack rabbits and desert cottontails in southern Texas. Journal of Wildlife Diseases 25: 47–51. 10.7589/0090-3558-25.1.47 [DOI] [PubMed] [Google Scholar]
- 13.Gustafson JM, Burgess EC, Wachal MD, Steinberg H (1993) Intrauterine transmission of Borrelia burgdorferi in dogs. American Journal of Veterinary Research 54: 882–890. [PubMed] [Google Scholar]
- 14.Silver RM, Yang L, Daynes RA, Branch DW, Salafia CM, Weis JJ (1995) Fetal outcome in murine Lyme disease. Infection & Immunity 63: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorensen K (1990) Lyme disease antibodies in thoroughbred broodmares. correlation to early pregnancy failure. Journal of Equine Veterinary Science 10: 166–168. [Google Scholar]
- 16.Eisner RJ, Meirs DA, Meirs DA, III, Ralston SL (1994) Lack of correlation between exposure to lyme disease (Borrelia burgdorferi) and pregnancy loss in mares. Journal of Equine Veterinary Science 14: 102–105. [Google Scholar]
- 17.Mather TN, Telford SR, Adler GH (1991) Absence of transplacental transmission of Lyme disease spirochetes from reservoir mice (Peromyscus leucopus) to their offspring. Journal of Infectious Diseases 164: 564–567. [DOI] [PubMed] [Google Scholar]
- 18.Moody KD, Barthold SW (1991) Relative infectivity of Borrelia burgdorferi in Lewis rats by various routes of inoculation. American Journal of Tropical Medicine & Hygiene 44: 135–139. [DOI] [PubMed] [Google Scholar]
- 19.Woodrum JE, Oliver JH (1999) Investigation of venereal, transplacental, and contact transmission of the Lyme disease spirochete, Borrelia burgdorferi, in Syrian hamsters. Journal of Parasitology 85: 426–430. [PubMed] [Google Scholar]
- 20.Higgins J, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions. Cochrane collaboration. [Google Scholar]
- 21.Young I, Waddell L, Sanchez J, Wilhelm B, McEwen SA, Rajic A (2014) The application of knowledge synthesis methods in agri-food public health: recent advancements, challenges and opportunities. Prev Vet Med 113: 339–355. 10.1016/j.prevetmed.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Altman DG, Liberati A, Tetzlaff J (2011) PRISMA statement. Epidemiology 22: 128; author reply 128. 10.1097/EDE.0b013e3181fe7825 [DOI] [PubMed] [Google Scholar]
- 23.Gardner T (1995) Lyme Disease In: Remington JK, J., editor. Infectious Diseases of the fetus and newborn infant. 4th ed: W. B. Saunders company; pp. 447–529. [Google Scholar]
- 24.Gardner T (2001) Chapter 11, Lyme Disease In: Remington JK, J., editor. Infectious Diseases of the Fetus and Newborn, 5th ed: Saunders. [Google Scholar]
- 25.McClure EM, Goldenberg RL (2009) Infection and stillbirth. Seminars In Fetal & Neonatal Medicine 14: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mylonas I (2011) Borreliosis during pregnancy: a risk for the unborn child? Vector Borne & Zoonotic Diseases 11: 891–898. [DOI] [PubMed] [Google Scholar]
- 27.Walsh CA, Mayer EW, Baxi LV (2007) Lyme disease in pregnancy: case report and review of the literature. Obstetrical & Gynecological Survey 62: 41–50. [DOI] [PubMed] [Google Scholar]
- 28.Lantos PM (2015) Chronic Lyme disease. Infect Dis Clin North Am 29: 325–340. 10.1016/j.idc.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64: 383–394. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 30.Group GW (2013) GRADE guidelines—best practices using the GRADE framework.
- 31.Higgins JPT, Altman DG (2008) Chapter 8: Assessing risk of bias in included studies In: In: Higgins Jpt G Se, editor. Cochrane Handbook for Systematic Reviews of Interventions Version 501 (updated September 2008): The Cochrane Collaboration. [Google Scholar]
- 32.Schunemann H, Hill S, Guyatt G, Akl EA, Ahmed F (2011) The GRADE approach and Bradford Hill's criteria for causation. J Epidemiol Community Health 65: 392–395. 10.1136/jech.2010.119933 [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. (2011) GRADE guidelines: 9. Rating up the quality of evidence. Journal of clinical epidemiology 64: 1311–1316. 10.1016/j.jclinepi.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 34.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64: 401–406. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 36.Freeman MF, Tukey JW Transformations Related to the Angular and the Square Root. [Google Scholar]
- 37.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to Meta-Analysis. Chichester; Hoboken: John Wiley & Sons. [Google Scholar]
- 38.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj 343: d4002 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 39.Hercogova J, Moidlova M, Zirny J (1994) Could borrelia found in the placenta influence the fetus? Study of 19 women with erythema migrans during pregnancy. In Program and Abstracts of the 6th Interantioanl Conference on Lyme Borreliosis Bologna, Italy: Societa Editrice Esculapio. pp. p76.
- 40.Sigal LH (2005) Pregnancy complicated by Lyme disease.
- 41.Troyano-Luque J, Padilla-Perez A, Martinez-Wallin I, Alvarez de la Rosa M, Mastrolia SA, Trujillo JL, et al. (2014) Short and long term outcomes associated with fetal cholelithiasis: a report of two cases with antenatal diagnosis and postnatal follow-up. Case Reports in Obstetrics and Gynecology 2014: 714271 10.1155/2014/714271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gasser R, Dusleag J, Reisinger E, Stauber R, Grisold M, Pongratz S, et al. (1994) A most unusual case of a whole family suffering from late Lyme borreliosis for over 20 years. Angiology 45: 85–86. 10.1177/000331979404500114 [DOI] [PubMed] [Google Scholar]
- 43.Maraspin V, Cimperman J, Lotric-Furlan S, Pleterski-Rigler D, Strle F (1999) Erythema migrans in pregnancy. Wiener Klinische Wochenschrift 111: 933–940. [PubMed] [Google Scholar]
- 44.Markowitz LE, Steere AC, Benach JL, Slade JD, Broome CV (1986) Lyme disease during pregnancy. JAMA 255: 3394–3396. [PubMed] [Google Scholar]
- 45.Lampert F (1986) Infantile multisyslem inflammatory disease: another case of a new syndrome European Journal of Pediatrics 144: 593–596. [DOI] [PubMed] [Google Scholar]
- 46.Schaumann R, Fingerle V, Buchholz K, Spencker FB, Rodloff AC (1999) Facial palsy caused by Borrelia infection in a twin pregnancy in an area of nonendemicity. Clinical Infectious Diseases 29: 955–956. 10.1086/520481 [DOI] [PubMed] [Google Scholar]
- 47.Lavoie PE, Lattner BP, Duray PH, Malawista SE, Barbour AG, Johnson RC (1987) Culture positive, seronegative transplacental Lyme borreliosis infant mortality. Arthritis Rheum. pp. S50.
- 48.Schutzer SE, Janniger CK, Schwartz RA (1991) Lyme disease during pregnancy. Cutis 47: 267–268. [PubMed] [Google Scholar]
- 49.MacDonald AB (1989) Gestational Lyme borreliosis. Implications for the fetus. Rheumatic Diseases Clinics of North America 15: 657–677. [PubMed] [Google Scholar]
- 50.MacDonald AB, Benach JL, Burgdorfer W (1987) Stillbirth following maternal Lyme disease. New York State Journal of Medicine 87: 615–616. [PubMed] [Google Scholar]
- 51.MacDonald AB (1986) Human fetal borreliosis, toxemia of pregnancy, and fetal death. Zentralblatt fur Bakteriologie, Mikrobiologie, und Hygiene—Series A, Medical Microbiology, Infectious Diseases, Virology, Parasitology 263: 189–200. [DOI] [PubMed] [Google Scholar]
- 52.Schlesinger PA, Duray PH, Burke BA, Steere AC, Stillman MT (1985) Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Annals of Internal Medicine 103: 67–68. [DOI] [PubMed] [Google Scholar]
- 53.O’Brien JM, Baum JD (2017) Low-grade fever, erythematous rash in pregnant woman • Dx? Journal of Family Practice 66: E9–E10. [PubMed] [Google Scholar]
- 54.Andrasova V, Svarovsky J, Matousek B (1988) [Lyme disease in pregnancy]. Ceskoslovenska Gynekologie 53: 39–41. [PubMed] [Google Scholar]
- 55.Mikkelsen AL, Palle C (1987) Lyme disease during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 66: 477–478. [DOI] [PubMed] [Google Scholar]
- 56.Remy JM, Chevrant-Breton O, Logeais B, Patoux-Pibouin M, Chevrier S, Chevrant-Breton J (1994) Traitement de la maladie de lyme pendant la grossesse, a propos d'un cas Nouvelles Dermatologiques 13: 682. [Google Scholar]
- 57.Weber K, Bratzke HJ, Neubert U, Wilske B, Duray PH (1988) Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. Pediatric Infectious Disease Journal 7: 286–289. [DOI] [PubMed] [Google Scholar]
- 58.Weber K, Neubert U (1986) Clinical features of early erythema migrans disease and related disorders. Zentralblatt fur Bakteriologie, Mikrobiologie, und Hygiene—Series A, Medical Microbiology, Infectious Diseases, Virology, Parasitology 263: 209–228. [DOI] [PubMed] [Google Scholar]
- 59.Bussen S, Steck T (1994) [Manifestation of Lyme arthritis in the puerperal period]. Zeitschrift fur Geburtshilfe und Perinatologie 198: 150–152. [PubMed] [Google Scholar]
- 60.Trevisan G, Stinco G, Cinco M (1997) Neonatal skin lesions due to a spirochetal infection: a case of congenital Lyme borreliosis? International Journal of Dermatology 36: 677–680. [DOI] [PubMed] [Google Scholar]
- 61.Brzostek T (2004) Ludzka erlichioza granulocytarna współistniejaca z borelioza z Lyme u kobiety ciezarnej Przeglad epidemiologiczny 58: 289–294. [PubMed] [Google Scholar]
- 62.Moniuszko A, Czupryna P, Pancewicz S, Kondrusik M, Penza P, Zajkowska J (2012) Borrelial lymphocytoma-A case report of a pregnant woman. Ticks and Tick-borne Diseases 3: 257–258. 10.1016/j.ttbdis.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 63.Grandsaerd MJ, Meulenbroeks AA (2000) Lyme borreliosis as a cause of facial palsy during pregnancy. European Journal of Obstetrics, Gynecology, & Reproductive Biology 91: 99–101. [DOI] [PubMed] [Google Scholar]
- 64.Hemels MAC, Gerards LJ, Kwee A, Wolfs TFW (2002) Lyme-borreliose in de zwangerschap: Gevolgen voor de pasgeborene?. Tijdschrift voor Kindergeneeskunde 70: 65–67. [Google Scholar]
- 65.Isailović G, Veljković M, Soć N, Krstić B, Bjekić M (1993) Erythema migrans posle uvoda krpelja kod trudnice. Glas Srpska akademija nauka i umetnosti Odeljenje medicinskih nauka: 173–175. [PubMed] [Google Scholar]
- 66.Jovanovic R, Hajric A, Cirkovic A, Mikovic Z, Dmitrovic R (1993) Lajmska bolest i trudnoca. Glas—Srpska Akademija Nauka i Umetnosti, Odeljenje Medicinskih Nauka: 169–172. [PubMed] [Google Scholar]
- 67.Önk G, Acun C, Kalayci M, Çaǧavi F, Açikgöz B, Tanriverdi HA (2005) Gestational lyme disease as a rare cause of congenital hydrocephalus. Journal of the Turkish German Gynecology Association 6: 156–157. [Google Scholar]
- 68.Nafeev AA (2001) Sluchai bolezni Laima pri beremennosti. Zhurnal Mikrobiologii Epidemiologii i Immunobiologii: 121–122. [PubMed] [Google Scholar]
- 69.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP (2005) Diagnosis of lyme borreliosis. Clin Microbiol Rev 18: 484–509. 10.1128/CMR.18.3.484-509.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. (2006) The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43: 1089–1134. 10.1086/508667 [DOI] [PubMed] [Google Scholar]
- 71.Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, et al. (2011) Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect 17: 69–79. 10.1111/j.1469-0691.2010.03175.x [DOI] [PubMed] [Google Scholar]
- 72.Wang G (2002) Direct detection methods for Lyme Borrelia, including the use of quantitative assays. Vector Borne Zoonotic Dis 2: 223–231. 10.1089/153036602321653806 [DOI] [PubMed] [Google Scholar]
- 73.Waddell LA, Greig J, Mascarenhas M, Harding S, Lindsay R, Ogden N (2016) The Accuracy of Diagnostic Tests for Lyme Disease in Humans, A Systematic Review and Meta-Analysis of North American Research. PLoS One 11: e0168613 10.1371/journal.pone.0168613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones CR, Smith H, Gibb E, Johnson L (2005) Gestational Lyme Disease Case Studies of 102 Live Births. Lyme Times: 36–38. [Google Scholar]
- 75.(2017) Perinatal health indicators for Canada 2017, a report from the Canadian perinatal surveillance system. Ottawa.
- 76.Agency EP (2013) America's Children and the Environment, 3rd Ed United States: EPA; 264 p. [Google Scholar]
- 77.Strobino BA, Williams CL, Abid S, Chalson R, Spierling P (1993) Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. American Journal of Obstetrics & Gynecology 169: 367–374. [DOI] [PubMed] [Google Scholar]
- 78.Dlesk A, Broste SK, Harkins PG, McCarty PA, Mitchell PD (1989) Lyme seropositivity and pregnancy outcome in the absence of symptoms of Lyme disease. Arthritis Rheum pp. S46.
- 79.Carlomagno G, Luksa V, Candussi G, Magaton Rizzi G, Trevisan G (1988) Lyme Borrelia positive serology associated with spontaneous abortion in an endemic Italian area. Acta Europaea Fertilitatis 19: 279–281. [PubMed] [Google Scholar]
- 80.Bracero LA, Wormser GP, Leikin E, Tejani N (1992) Prevalence of seropositivity to the Lyme disease spirochete during pregnancy in an epidemic area. A preliminary report. journal of maternal and fetal investigations 2: 265–268. [Google Scholar]
- 81.Strobino B, Abid S, Gewitz M (1999) Maternal Lyme disease and congenital heart disease: A case-control study in an endemic area. American Journal of Obstetrics & Gynecology 180: 711–716. [DOI] [PubMed] [Google Scholar]
- 82.Williams CL, Strobino B, Weinstein A, Spierling P, Medici F (1995) Maternal Lyme disease and congenital malformations: a cord blood serosurvey in endemic and control areas. Paediatric and Perinatal Epidemiology 9: 320–330. [DOI] [PubMed] [Google Scholar]
- 83.Williams CL, Benach JL, Curran AS, Spierling P, Medici F (1988) Lyme Disease during Pregnancy A Cord Blood Serosurvey. Annals of the New York Academy of Sciences 539: 504–506. [Google Scholar]
- 84.Lakos A, Solymosi N (2010) Maternal Lyme borreliosis and pregnancy outcome. International Journal of Infectious Diseases 14: e494–498. 10.1016/j.ijid.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 85.Figueroa R, Bracero LA, Aguero-Rosenfeld M, Beneck D, Coleman J, Schwartz I (1996) Confirmation of Borrelia burgdorferi spirochetes by polymerase chain reaction in placentas of women with reactive serology for Lyme antibodies. Gynecologic & Obstetric Investigation 41: 240–243. [DOI] [PubMed] [Google Scholar]
- 86.Maraspin V, Ruzic-Sabljic E, Pleterski-Rigler D, Strle F (2011) Pregnant women with erythema migrans and isolation of borreliae from blood: course and outcome after treatment with ceftriaxone. Diagnostic Microbiology & Infectious Disease 71: 446–448. [DOI] [PubMed] [Google Scholar]
- 87.Nadal D, Hunziker UA, Bucher HU, Hitzig WH, Duc G (1989) Infants born to mothers with antibodies against Borrelia burgdorferi at delivery. European Journal of Pediatrics 148: 426–427. [DOI] [PubMed] [Google Scholar]
- 88.Londero F, San Marco L, Silvestri D, Ruscio M, Bassini D (1998) Enfermedad de Lyme y embarazo. Progresos en Obstetricia y Ginecologia 41: 146–148. [Google Scholar]
- 89.Hercogova J, Tomankova M, Frosslova D, Janovska D (1993) Early stage of Lyme borreliosis during pregnancy: Treatment of 15 women with erythema migrans. Cesko-Slovenska Gynekologie 58: 229–232. [PubMed] [Google Scholar]
- 90.Hulinska D, Votypka J, Vanousova D, Hercogova J, Hulinsky V, Drevova H, et al. (2009) Identification of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in patients with erythema migrans. Folia Microbiologica 54: 246–256. 10.1007/s12223-009-0039-0 [DOI] [PubMed] [Google Scholar]
- 91.Knox KK, Thomm AM, Harrington YA, Ketter E, Patitucci JM, Carrigan DR (2017) Powassan/Deer Tick Virus and Borrelia Burgdorferi Infection in Wisconsin Tick Populations. Vector Borne Zoonotic Dis 17: 463–466. 10.1089/vbz.2016.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.CDC (1995) Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 44: 590–591. [PubMed] [Google Scholar]
- 93.CDC (2017) Lyme Disease Diagnosis and Testing.
- 94.Lindsay LR, Bernat K, Dibernardo A (2014) Laboratory diagnostics for Lyme disease. Canada Communicable Disease Report 40. [DOI] [PMC free article] [PubMed]
- 95.Leeflang MM, Ang CW, Berkhout J, Bijlmer HA, Van Bortel W, Brandenburg AH, et al. (2016) The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis 16: 140 10.1186/s12879-016-1468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Canadian Public Health Laboratory N (2007) The laboratory diagnosis of Lyme borreliosis: Guidelines from the Canadian Public Health Laboratory Network. Can J Infect Dis Med Microbiol 18: 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ciesielski CAR, H.; Johnson, S.; (1987) Prospective study of pregnancy outcome in women with Lyme disease (abstract). 27th ICAAC.
- 98.Elliott DJ, Eppes SC, Klein JD (2001) Teratogen update: Lyme disease. Teratology 64: 276–281. 10.1002/tera.1074 [DOI] [PubMed] [Google Scholar]
- 99.Egbe A, Uppu S, Lee S, Stroustrup A, Ho D, Srivastava S (2015) Congenital malformations in the newborn population: a population study and analysis of the effect of sex and prematurity. Pediatr Neonatol 56: 25–30. 10.1016/j.pedneo.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 100.Muehlenbachs A, Bollweg BC, Schulz TJ, Forrester JD, DeLeon Carnes M, Molins C, et al. (2016) Cardiac Tropism of Borrelia burgdorferi: An Autopsy Study of Sudden Cardiac Death Associated with Lyme Carditis. The American journal of pathology 186: 1195–1205. 10.1016/j.ajpath.2015.12.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(DOC)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.