Abstract
Background
Community-college students are at high risk for tobacco use. Because the use of mobile phone text messaging is nearly ubiquitous today, short message service (SMS) may be an effective strategy for tobacco risk communication in this population. Little is known, however, concerning the message structure significantly influencing perceived tobacco risk.
Objective
We aim to outline the rationale and design of Project Debunk, a randomized trial comparing the effects of different SMS text message structures.
Methods
We conducted a 6-month randomized trial comparing 8 arms, based on the combination of the 3 message structures delivered to young adults in a 2×2×2 study design: framing (gain-framed or loss-framed), depth (simple or complex), and appeal (emotional or rational). Participants were invited to participate from 3 community colleges in Houston from September 2016 to July 2017. Participants were randomized to 1 arm and received text messages in 2 separate campaigns. Each campaign consisted of 2 text messages per day for 30 days. Perceived tobacco risk was assessed at baseline, 2 months after the first campaign, and 2 months after the second campaign. We assessed the perceived risk of using conventional products (eg, combustible cigarettes) and new and emerging products (eg, electronic cigarettes). The validity of message structures was assessed weekly for each campaign. A 1-week follow-up assessment was also conducted to understand immediate reactions from participants.
Results
We completed data collection for the baseline survey on a rolling basis during this time and assessed the validity of the message structure after 1 week of SMS text messages. For the entire sample (N=636), the average age was 20.92 years (SD 2.52), about two-thirds were male (430/636, 67.6%), and most were black or African American (259/636, 40.7%) or white (236/636, 37.1%). After 1 week of receiving text messages, the following was noted: (a) loss-framed messages were more likely to be perceived as presenting a loss than gain-framed messages (F7,522=13.13, P<.001), (b) complex messages were perceived to be more complex than simple messages (F7,520=2.04, P=.05), and (c) emotional messages were perceived to be more emotionally involving than rational messages (F7,520=6.46, P<.001).
Conclusions
This study confirms that the recruitment, randomization, and message composition have been successfully implemented. Further analyses will identify specific types of messages that are more effective than others in increasing the perceived risk of tobacco use. If our results suggest that any of the 8 specific message structures are more effective for helping young adults understand tobacco risk, this would provide evidence to include such messages as part of a larger technology-based campaign such as mobile phone apps, entertainment-based campaigns, and social media.
Trial Registration
ClinicalTrials.gov NCT03457480; https://clinicaltrials.gov/ct2/show/NCT03457480 (Archived by WebCite at http://www.webcitation.org/6ykd4IIap)
Registered Report Identifier
RR1-10.2196/10977
Keywords: tobacco use, risk, perception, text messaging, young adult
Introduction
Background
Almost 14% of young adults are currently using cigarettes and 27% have used electronic cigarettes, one of the many new and emerging tobacco products (NETP) [1]. Young adults perceive NETPs such as electronic cigarettes (e-cigarettes) and hookah (ie, waterpipes) as safer ways to enjoy nicotine than conventional products [2-4]. Reduced risk perception has led to uninformed choices among young adults [5], including experimentation with multiple tobacco products, alcohol, and other substances [6-8]. Indicators of socioeconomic disadvantage such as low educational attainment and income status are predictors of tobacco use [9]. In particular, young adults in community college represent an underserved population more susceptible to tobacco use than young adults attending universities or 4-year colleges [10-12].
Following bans on traditional advertising for tobacco, protobacco marketing began to make effective use of modern advertising through social and mobile media channels to reduce the risk perception and promote misinformation about tobacco among young adults [13,14]. Currently, tobacco companies make effective expenditures on product discounts, point-of-sale advertising, direct mail advertising, e-marketing, and social media [15-20]. In addition, with 96% of young adults owning a smartphone, tobacco companies depend on mobile phone strategies for marketing [21]. Tobacco product demonstrations are featured on industry-sponsored websites, and invitations to join Web-based social interactions are encouraged [22-25]. More than 49 protobacco smartphone apps have been identified in app stores under kids and games categories [26]. As a result, there is a clear need for efforts to respond to protobacco marketing by communicating about tobacco risk to young adults, as delineated by the educational mission and research priorities of the United States Food and Drug Administration [27,28].
The use of mobile health (mHealth) SMS (short message service) text messaging may be an effective strategy for tobacco risk communication to young adults. In the United States, 95% of mobile phones are capable of receiving text messages and 96% of the young adults own mobile phones, indicating this is a highly feasible method for transmitting information to this population [21,29]. Although text messaging programs have been implemented for preventive behavioral interventions, including smoking cessation, no published accounts have applied text messaging to communicate about tobacco risk to young adults [30-34]. To the best of our knowledge, this study is the first to examine different styles of mobile phone text messages for tobacco risk communication. Once the most impactful text messages have been identified, they can subsequently be introduced into an advanced digital intervention that can counteract protobacco marketing.
In the United States, a majority of young adults have smartphones, with more advanced text messaging capabilities (eg, WhatsApp). However, it is pertinent to conduct an evaluation of text messages for risk communication, through traditional text messaging. SMS text messaging ensures that all participants are capable of receiving text messages regardless of a smartphone ownership. In addition, traditional text messaging ensures that all participants receive the messages in the same format, thereby allowing a homogeneous exposure to the intervention content and a more reliable evaluation. Such an evaluation will shed light on how the messages perform. If a set of text messages shows effectiveness, then it can readily be implemented among young adult communities outside the United States, where smartphone capabilities may be limited.
Theoretical Framework
We have designed different types of messages based on 3 main structures: framing (gain-framed or loss-framed messages), depth (ie, simple or complex messages), and appeal (ie, emotional or rational messages) [35-38]. For framing, gain-framed messages describe the benefits of quitting or avoiding tobacco use, whereas loss-framed messages emphasize the disadvantages of use [39-41]. In the context of message depth, both complex grammatical structures and longer words have been applied to shape message complexity [42-45]. In terms of appeal, researchers have developed emotional SMS text messages by introducing emotional words (eg, happy and angry) [46-48], paralinguistic cues such as vocal spelling (eg, weeeell and soooo), and emotional icons (eg, “:-)” for a happy face) [49]. Most research has been in gain-framed versus loss-framed text messages [50]. Some literature, predominantly in advertising and promotion, has been dedicated to emotional versus rational appeal [47]. Virtually nothing has been reported on simple versus complex messages in the health risk domain.
The effectiveness of different message characteristics in driving risk communication outcomes stems from the elaboration likelihood model (ELM) [51,52]. The ELM explains motivation of the individual to engage in information processing. Individuals expending more mental or cognitive effort processing messages tend to formulate stronger attitudes toward an issue and deeper understanding—a desirable attribute for conveying tobacco risk information to the public. One of the basic constructs in the model concerns the degree of cognitive efforts expended and involvement that people use to engage with message content. The ELM posits that individuals can engage in either the central or peripheral processing of health information. Central processing involves attention to message content (eg, complex and rational messages; [53]), whereas peripheral processing involves attention to more peripheral cues such as affect or emotions in developing attitudes toward the message [54]. In the context of risk communication, researchers have not yet presented a theoretical framework supporting certain message types over others with respect to increasing perceived risk. For instance, in the context of message-framing, theoretical frameworks (eg, the prospect theory) do not support a specific framing over another with respect to increasing perceived risk [55]. Instead, such frameworks posit that gain and loss framing can have an effect on health behavior depending on whether the individual is risk-aversive or risk-taking. As a result, theoretical frameworks on message framing have not yet examined perceived risk as the end outcome. In addition, results from previous meta-analyses of relevant research have not been able to favor one message style over another, with respect to health outcomes [56-58]. As a result, it is essential to explore the effect of different message characteristics on perceived risk.
Research Objectives
The primary objective of this study was to conduct exploratory analyses to identify the most effective types of text messages that inform about the harms of tobacco use among young adults in community college. This research protocol outlines the rationale and design of Project Debunk, a community-based randomized trial (peer-reviewed and funded; Multimedia Appendix 1). Project Debunk compares the effects of different structures of text messages delivered to young adults in community college, with the overarching goal of setting the stage for a larger mobile phone text messaging campaign in the future. The protocol presents baseline data from the trial and assesses the validity of the message structures after 1 week of SMS text message exposure.
Methods
Study Design
Project Debunk has gathered data in the following 2 phases: (1) qualitative research for text message development and (2) a randomized trial. This research protocol briefly describes the methods used for the message-development phase and outlines the detailed information about the trial phase at baseline and 1 week after message exposure (ie, the intervention).
In design, the trial is being conducted as a 6-month-long randomized trial comparing 8 arms, based on the combination of the 3 message structures: framing, depth, and appeal (Figure 1). Participants are randomly assigned to one of the 8 arms. They are receiving text messages in 2 separate waves or campaigns. Each campaign consists of 2 text messages per day for 30 days (ie, 60 text messages). The 2 campaigns are 2 months and 1 week apart.
Figure 1.
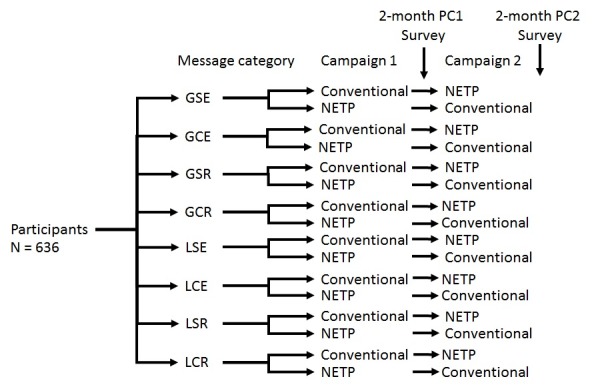
Study randomization flowchart. Conventional indicates conventional tobacco products including cigarettes, cigars, smokeless; NETP indicates new and emerging tobacco products, including snus, hookah, and e-cigarettes. In this study design, there is a break of one week between post-campaign 1 survey and campaign 2. GSE: gain-framed, simple emotional; GCE: gain-framed, complex, emotional; GSR: gain-framed, simple, rational; GCR: gain-framed, complex, rational; LSE: loss-framed, simple, emotional; LCE: loss-framed, complex, emotional; LSR, loss-framed, simple, rational; LCR: loss-framed, complex, rational; PC1: post-campaign 1; PC2: post-campaign 2.
Allowing for a crossover design, participants within each of the 8 arms are randomly divided into 2 groups: group 1 is receiving text messages about conventional tobacco products during campaign 1 and then about NETP during campaign 2. Group 2 is receiving text messages about NETP during campaign 1 and then about conventional tobacco during campaign 2. This crossover design was advised by the Tobacco Center of Regulatory Science on Youth and Young Adults (TX TCORS) Scientific Steering Committee, as it will allow us to explore potential differences between the 2 categories of products within and between participants, with respect to their perceived risk of tobacco use. Data collection for the trial is being conducted at baseline, 2 months post campaign 1 (PC1), 2 months post campaign 2 (PC2), weekly throughout each campaign (a weekly manipulation check assessment), and 7 days after each campaign.
Population
Eligibility criteria for the trial included the following: aged 18 to 25 years, enrolled in community college, using mobile phone text-messaging features on a regular basis, willing to provide their phone number, capable of receiving text messages from our text messaging system, able to read and speak English, and accept to provide a signature on a written informed consent form. The age range of 18 to 25 years was chosen to define emerging young adulthood, as recommended by the National Research Council of the Institute of Medicine in the United States [59]. Three community college campuses from the Houston Community College (HCC) system were targeted for recruitment. Students attending the HCC system are 58% female and have a mean age of 25.6 years. Their racial or ethnic profile is as follows: 30.2% African American, 14.6% Asian American, 14.2% white, 36.9% Hispanic, and 4.2% other [60]. The 3 community colleges were selected based on their ethnically diverse student population and their proximity to our research institution. In addition, we have an existing research relationship with such institutions. All methods and procedures used in the project have been approved by the Institutional Review Board of Ethics of the University of Texas MD Anderson Cancer Center (2014-0474), as well as the HCC System Institutional Review Board.
Recruitment and Enrollment
Recruitment took place at each of the participating HCC campuses from September 2016 to July 2017. We set up recruitment stations or booths equipped with a highly visible logo of the research institution. Printed materials (eg, posters and fliers) announcing the study were displayed in common areas such as student lounges. During participant recruitment at each campus, the research staff explained the purpose of the study to students and answered their questions. Students interested in the trial were screened for eligibility. Subsequently, eligible students provided informed consent to participate in the trial.
Following consent, participants completed a 20-min self-administered baseline survey on their personal mobile phones. This method of enrollment has yielded relatively high recruitment rates (80.1%) during our previous research activities with community college students [61]. Recruitment continued until a sample size of 645 participants was reached. In total, 9 participants were not eligible for the study (over the age of 25 years), so they were dropped, reaching a sample of 636 participants. Up to 6 follow-up reminders were sent via phone and email to remind participants to complete follow-up surveys to progress through the study.
Text Message Interventions for Each Group
From January 2014 to August 2015, our research team from the TX TCORS developed a library of text messages, considering previous scientific literature, developments in social media related to tobacco use, and common terminology. Collectively, the research team has extensive experience in tobacco cessation and prevention, public health, health communication, psychology, and creative writing. Text message design also involved focus group discussions conducted among community college students [62].
Ultimately, our team generated 976 text messages that communicate the risks of tobacco use to college students, both users and nonusers. The messages were developed according to a combination of the 3 structures described above (framing, depth, and appeal), resulting in the following 8 categories:
Complex, gain-framed, emotional (CGE)
Complex, gain-framed, rational (CGR)
Complex, loss-framed, emotional (CLE)
Complex, loss-framed, rational (CLR)
Simple, gain-framed, emotional (SGE)
Simple, gain-framed, rational (SGR)
Simple, loss-framed, emotional (SLE)
Simple, loss-framed, rational (SLR)
In addition, for each category, messages were developed to communicate about the harm of conventional tobacco products and NETPs. Messages describing conventional products included information about combustible cigarettes, variants of cigars, cigarillos, and pipes. Messages about NETP included information about e-cigarettes (including other vaping devices), snus, and hookah. Examples of text messages are presented in Multimedia Appendix 2. Experts and students reviewed and rated each message. For validation of message categories, agreement needed to be ≥70% between experts and students for all the 3 message structures. Further validation of message categories was conducted using a linguistic inquiry and word-counting library designed to count words under specific themes (eg, emotional words) [63].
Randomization and Blinding
This is a double-blind study. Following screening and consent, members of our research staff provided participants with a study identification number and a link to the baseline survey to the mobile phone of each participant. This procedure confirmed that the participant’s device fully met the needs of the study. Following the baseline survey, participants were assigned to one of the 8 arms following a computer-generated randomization list using a resource called assessment, intervention, and measurement (AIM). AIM is a centralized repository at the MD Anderson Cancer Center, managed by a team of experts in the science of collecting and managing participant-reported outcomes. The allocation sequence was generated by the AIM system and automatically sent text messages based on allocation, ensuring that our research team is blind to the allocation of each participant. The allocation sequence is password protected and accessible only to nonresearch staff responsible for the AIM system.
Data Collection
Figure 2 depicts how data are collected for the study. Data collection took place at baseline and will continue at the end of each week throughout campaign 1 and campaign 2 of text message dissemination, as well as 7 days PC1, 7 days PC2, 2 months PC1, and 2 months PC2. Participants will provide data through Web-based surveys received through mobile phones.
Figure 2.
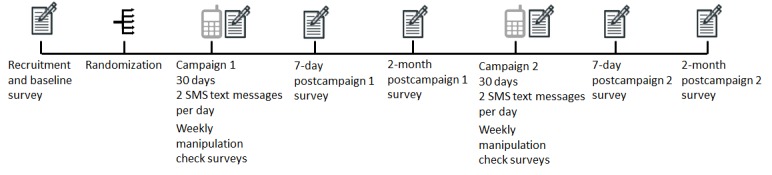
Data collection procedure for the study. SMS: short message service.
We developed the surveys with skip patterns to minimize the burden on participants. Using mobile phones from different brands and data carriers, the research team pretested the delivery of surveys and text messages with the assistance of experts in the AIM system (a team of computer scientists and bioinformaticians). This pretesting allowed us to ensure that the surveys and text messages are reachable and readable regardless of the mobile phone or data carrier. We conducted the pretesting initially with our immediate staff and research team. Afterwards, we extended to other staff in one of our departments (Department of Behavioral Science). We conducted an iterative process such that each time an issue was identified by survey testers, it was rectified. Pretesting continued until no issues were reported.
Data collection from the baseline survey ended in July 2017. At the end of each week throughout SMS text message exposure in campaign 1 and campaign 2, participants will complete a manipulation check survey. This weekly manipulation check will ensure that the 8 arms of the study differ with respect to unique features such as perceived emotional level, complexity of the text messages, and framing type. Data collection from the manipulation check survey for the first week of campaign 1 ended in October 2017. Participants will receive a survey regarding their immediate experience with the text messages 7 days PC1 and 7 days PC2. Finally, 2 months PC1 and 2 months PC2, participants will receive a follow-up survey that includes tobacco-related outcome measures.
Survey Measures
All survey measures have been previously tested and validated, with some adaptations (further outlined below). All measures are assessed through Web-based closed surveys. We adhered to the Checklist for Reporting Results of Internet E-Surveys (Multimedia Appendix 3). This checklist will be reported once the study is completed, with the main outcomes of the trial. This paper presents data from the baseline survey and the first weekly manipulation check survey. A detailed description of the main measures and Cronbach alpha values for available data are reported in Multimedia Appendix 4.
Baseline Survey
The baseline survey data for the trial have been collected. With 97 items, baseline information included sociodemographic data such as age, gender, ethnicity, educational attainment, and income [64]. In addition, the baseline survey included questions about factors that may predict perceived risk and tobacco use: mental health status [65], marijuana and alcohol use [64], receptivity to receiving text messages [66], tendency to seek information about tobacco [67], number of friends using tobacco [68], secondhand smoke at home [64], mental health [69], prevention-focus level [70], sensation-seeking level [71], and numeracy ability [72].
Follow-Up Surveys
Follow-up surveys for the trial are ongoing. Weekly manipulation check surveys will assess perceptions of participants about text messages received in the previous week. The perceived message characteristics to be assessed include loss framing [73], message complexity level [74], emotional level of messages [50], credibility [75], message enjoyment [76,77], relevance [78], and message readability.
Surveys completed by participants 7 days after each of the 2 campaigns will assess self-reported attention to the text messages [79], emotional involvement [79], thought provocation [80], motivation to discuss the messages with others [81,82], and recall of actual discussions with others about the messages and tobacco [82].
Two months PC1 and 2 months PC2, we will measure perceived risk of using each tobacco product as the main outcome [83]. As secondary outcomes, we will also measure the status and frequency of tobacco use [84], susceptibility to use tobacco products among nonusers (ie, likelihood to initiate use at some point in the future) [85], perceived addictiveness of products [4], perceived popularity of tobacco use [4], and perceived benefits of tobacco use [86].
Compensation
Participants who complete all survey assessments will be compensated a total of US $135. They received a US $25 gift card for completing the baseline survey and will receive a US $25 gift card for completing each of the surveys administered at 2 months after the campaigns. They will also receive a US $10 gift card for completing each of the surveys administered 7 days after the campaigns and a US $5 gift card for each of the 8 weekly manipulation check surveys throughout the 2 campaigns.
Attrition and Compliance
To the best of our knowledge, no study examining the effects of different communication styles on risk perception among young adults is currently available. On the basis of the results of a study with community college students by Prokhorov and colleagues [61], we expect an acceptable retention rate (beyond 70%) and high compliance (ie, a self-report of paying attention to and reading most or all of the text messages, with a score of 4 or higher out of 5 on message attention).
Sample Size Determination
For sample size determination, we conducted a power calculation using the outcome of change in perceived risk of cigarette smoking from baseline to 2 months after each of the campaigns. The 8 study arms define a 2×2×2 analysis of variance (ANOVA) factorial design. Assuming a balanced design in each of the 8 study arms for the change in perceived risk, with n=70 per arm, we have at least 80% power to detect an effect size of 0.12 in a fixed-effects ANOVA. A total of 560 participants are needed to provide 70 participants per study arm at 2 months PC2, with complete measurements at baseline. We assume 11% attrition between the assessment at 7 days after the program and 2 months after the program and 1.5% attrition between baseline and the assessment at 2 months PC2 (ie, a total of 12.5% attrition). As a result, our retention rate is expected to be 87.5%. This assumes 640 participants randomized to 8 study arms. This sample size was calculated using PASS 2005 (NCSS, LP).
Current Data Analysis
For the currently available baseline data, we used descriptive statistics to summarize sociodemographic characteristics (eg, age, gender, and race), tobacco-related characteristics (tobacco use and number of friends who use tobacco), and primary psychosocial health outcomes (ie, perceived risk of using each tobacco product).
Using the currently available data from the first weekly manipulation check survey, we checked to make sure that the message structures were perceived by participants as intended. Using one-way ANOVA, we examined study arm differences in perceived message loss framing, complexity level, emotional level, credibility, message enjoyment, perceived message relevance, and perceived message readability. STATA version 14 statistical software was used for data analysis.
Planned Data Analysis
Once the trial is complete, we plan to conduct an exploratory analysis to identify which combination of message characteristics (ie, depth, framing, and appeal) most increases perceived risk of using each type of tobacco product. This analysis is exploratory because to date no theoretical framework or empirical evidence has been presented that demonstrates the importance of one message structure over another in the context of tobacco risk communication. We will first conduct, a series of 8 repeated-measures mixed-effects models for each type of tobacco product with the interaction effect (group [one combination vs all other combinations] × time [baseline, 2 months PC1, and 2 months PC2]) to predict perceived risk of using the product. These models will control for past 30-day use of the product at baseline and the crossover group assignment. In addition to the main outcomes analyses, we plan to conduct several moderation analyses, including the examination of different groups such as gender, race, mental health status, and personality types. This analysis will allow us to check if different types of individuals may respond differently to certain structures of text messages. Repeated-measures mixed-effect models with interaction effects will be conducted. For all data analysis, P<.05 is considered statistically significant. We will use STATA version 14 software (StataCorp LLC) for all analyses.
Ethics and Participant Safety
Project Debunk has received full approval from the Research Ethics Board of The University of Texas MD Anderson Cancer Center in Houston, Texas, and it has undergone a local institutional scientific review. To the best of our knowledge, Project Debunk does not pose any significant risks to the physical and psychological safety of participants. Identities of the participants have been coded and only the research team has access to a master list that links names and study codes. This list is kept in a locked file cabinet. Demographic data and assessments of text messages will be stored on secure servers within the institution. Only aggregate data will be reported. We have obtained a Certificate of Confidentiality from the federal government, which will help to protect the privacy of research participants. The certificate protects against the involuntary release of information about participants collected during the course of covered studies.
Results
Status of Results
Data collection is currently underway. Data analysis of change in the main outcomes and writing of the manuscript are expected to be completed in the summer of 2018. We highlight below some of the main baseline findings regarding the study population and measures.
Sociodemographic Characteristics
Table 1 presents sociodemographic characteristics of the respondents. For the entire sample (n=636), the average age was 20.78 years (SD 2.18), about two-thirds (430/636, 67.6%) were male, and most were black or African American (259/636, 40.7%) or white (236/636, 37.1%). With respect to ethnicity, 36.3% (231/636) of participants were Hispanic or Latino. The study arms did not differ in terms of sociodemographic characteristics or mental health status, economic status, planned education level, numeracy ability, prevention-focus level, receptivity to receiving text messages, or sensation-seeking level (Table 1).
Table 1.
Baseline sociodemographic characteristics for the total sample and by treatment arm.
| Characteristics | Statisticsa,b,c | |||||||||
|
|
Total | CGEd | CGRe | CLEf | CLRg | SGEh | SGRi | SLEj | SLRk | |
| Gender at birth (men), n (%) | 430 (67.6) | 53 (65.4) | 52 (67.5) | 54 (74.0) | 60 (74.1) | 56 (65.1) | 56 (70.9) | 44 (57.1) | 55 (67.1) | |
| Race, n (%) |
|
|
|
|
|
|
|
|
|
|
|
|
Hispanic or Latino ethnicity | 231 (36.3) | 31 (38.3) | 29 (37.7) | 24 (32.9) | 28 (34.6) | 30 (34.9) | 34 (43.0) | 22 (28.6) | 33 (40.2) |
|
|
Asian | 99 (15.6) | 12 (14.8) | 15 (19.5) | 13 (17.8) | 9 (11.1) | 14 (16.3) | 7 (8.9) | 16 (20.8) | 13 (15.9) |
|
|
Black or African American | 259 (40.7) | 34 (42.0) | 28 (36.4) | 33 (45.2) | 39 (48.1) | 33 (38.4) | 30 (38.0) | 31 (40.3) | 31 (37.8) |
|
|
White | 236 (37.1) | 31 (38.3) | 29 (37.7) | 21 (28.8) | 29 (35.8) | 36 (41.9) | 31 (39.2) | 24 (31.2) | 35 (42.7) |
|
|
Other | 42 (6.6) | 4 (4.9) | 5 (6.5) | 6 (8.2) | 4 (4.9) | 3 (3.5) | 11 (13.9) | 6 (7.8) | 3 (3.7) |
| Have children, n (%) | 58 (9.1) | 7 (8.6) | 8 (10.4) | 8 (11.0) | 5 (6.2) | 5 (5.8) | 9 (11.4) | 9 (11.7) | 7 (8.5) | |
| Age (years), mean (SD) | 20.92 (2.52) | 20.53 (2.21) | 21.03 (2.10) | 20.75 (2.10) | 20.89 (2.30) | 20.55 (2.06) | 20.97 (2.25) | 20.86 (2.22) | 20.66 (2.22) | |
| Mental health status, mean (SD) | 67.33 (19.18) | 67.80 (18.01) | 64.83 (18.80) | 68.11 (19.16) | 69.43 (19.32) | 67.53 (18.74) | 68.30 (19.02) | 67.17 (20.42) | 65.46 (20.33) | |
| Economic status, mean (SD) | 2.77 (0.92) | 2.67 (0.96) | 2.79 (0.96) | 2.74 (0.96) | 2.68 (0.93) | 2.84 (0.89) | 2.96 (0.81) | 2.77 (0.97) | 2.71 (0.90) | |
| Planned education level, mean (SD) | 3.67 (1.15) | 3.77 (1.10) | 3.75 (1.05) | 3.53 (1.28) | 3.81 (1.16) | 3.66 (1.06) | 3.72 (1.09) | 3.52 (1.25) | 3.61 (1.24) | |
| Numeracy ability, mean (SD) | 5.35 (1.81) | 5.41 (1.61) | 5.51 (1.82) | 5.29 (2.00) | 5.15 (2.04) | 5.56 (1.69) | 5.20 (1.86) | 5.39 (1.73) | 5.27 (1.74) | |
| Prevention-focus level, mean (SD) | 2.81 (0.69) | 2.73 (0.71) | 2.90 (0.73) | 2.86 (0.67) | 2.77 (0.72) | 2.80 (0.70) | 2.80 (0.64) | 2.79 (0.72) | 2.82 (0.68) | |
| Receptivity to receiving text messages, mean (SD) | 0.92 (0.15) | 0.94 (0.12) | 0.90 (0.22) | 0.90 (0.15) | 0.89 (0.18) | 0.92 (0.13) | 0.95 (0.11) | 0.94 (0.10) | 0.93 (0.14) | |
| Sensation-seeking level, mean (SD) | 3.50 (0.83) | 3.50 (0.73) | 3.47 (0.85) | 3.34 (0.91) | 3.45 (0.86) | 3.56 (0.78) | 3.60 (0.84) | 3.51 (0.85) | 3.55 (0.81) | |
aMissing values are not presented in this table.
bParticipants were randomized to one of the 8 treatment arms, describing the type of messages.
cProportions in subsample and percentage are presented for categorical variables, and the mean with SD are presented for continuous variables.
dCGE: complex, gain-framed, emotional.
eCGR: complex, gain-framed, rational.
fCLE: complex, loss-framed, emotional.
gCLR: complex, loss-framed, rational.
hSGE: simple, gain-framed, emotional.
iSGR: simple, gain-framed, rational.
jSLE: simple, loss-framed, emotional.
kSLR: simple, loss-framed, rational.
Tobacco-Related Characteristics
Tobacco-related characteristics of the respondents are presented in Table 2. Of the entire sample, at least once in their lifetime, 45.1% (287/636) have ever used cigarettes, 32.4% (206/636) have ever used cigars, 55.7% (354/636) have ever used hookah, and 26.9% (171/636) have ever used e-cigarettes. In addition, 25.3% (161/636) have ever used marijuana, 47.2% (300/636) have ever used more than one tobacco product, and 43.2% (275/636) have ever used both marijuana and tobacco products.
Table 2.
Tobacco-related characteristics for the total sample and by the group at baseline.
| Substance usea | Nb (%) | |||||||||
|
|
Total | CGEc | CGRd | CLEe | CLRf | SGEg | SGRh | SLEi | SLRj | |
| Cigarettes |
|
|
|
|
|
|
|
|
|
|
|
|
Ever | 287 (45.1) | 37 (45.7) | 34 (44.2) | 28 (38.4) | 35 (43.2) | 43 (50.0) | 41 (51.9) | 35 (45.5) | 34 (41.5) |
|
|
p30k | 87 (13.7) | 12 (14.8) | 7 (9.1) | 9 (12.3) | 14 (17.3) | 11 (12.8) | 11 (13.9) | 12 (15.6) | 11 (13.4) |
| Cigars |
|
|
|
|
|
|
|
|
|
|
|
|
Ever | 206 (32.4) | 27 (33.3) | 29 (37.7) | 29 (39.7) | 22 (27.2) | 20 (23.3) | 28 (35.4) | 25 (32.5) | 26 (31.7) |
|
|
p30 | 61 (9.6) | 2 (7.4) | 11 (14.3) | 4 (5.5) | 9 (11.1) | 8 (9.3) | 10 (12.7) | 4 (5.2) | 9 (11.0) |
| Smokeless |
|
|
|
|
|
|
|
|
|
|
|
|
Ever | 33 (5.2) | 2 (2.5) | 1 (1.3) | 6 (8.2) | 3 (3.7) | 2 (2.3) | 9 (11.4) | 6 (7.8) | 4 (4.9) |
|
|
p30 | 5 (0.8) | 1 (1.2) | 1 (1.3) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 2 (2.5) | 0 (0.0) | 0 (0.0) |
| Hookah |
|
|
|
|
|
|
|
|
|
|
|
|
Ever | 354 (55.7) | 42 (51.9) | 43 (55.8) | 34 (46.6) | 46 (56.8) | 52 (60.5) | 46 (58.2) | 44 (57.1) | 47 (57.3) |
|
|
p30 | 116 (18.2) | 16 (19.8) | 15 (19.5) | 8 (11.0) | 15 (18.5) | 18 (20.9) | 15 (19.0) | 15 (19.5) | 14 (17.1) |
| e-Cigarettes |
|
|
|
|
|
|
|
|
|
|
|
|
Ever | 171 (26.9) | 22 (27.2) | 19 (24.7) | 19 (26.0) | 21 (25.9) | 26 (30.2) | 22 (27.9) | 20 (26.0) | 22 (26.8) |
|
|
p30 | 50 (7.9) | 5 (6.2) | 5 (6.5) | 7 (9.6) | 7 (8.6) | 13 (15.1) | 5 (6.3) | 6 (7.8) | 2 (2.4) |
| Marijuana | 161 (25.3) | 21 (25.9) | 24 (31.2) | 15 (20.6) | 21 (25.9) | 22 (25.6) | 24 (30.4) | 17 (22.1) | 17 (20.7) | |
| Poly-tobacco usek | 300 (47.2) | 43 (53.1) | 34 (44.2) | 35 (48.0) | 39 (48.2) | 40 (46.5) | 42 (53.2) | 34 (44.2) | 33 (40.2) | |
| Susceptibility to usel |
|
|
|
|
|
|
|
|
|
|
|
|
Cigarettes | 46 (13.2) | 7 (15.9) | 2 (4.7) | 2 (4.4) | 9 (19.6) | 7 (18.3) | 6 (15.8) | 4 (9.5) | 9 (18.8) |
|
|
Cigars | 96 (22.7) | 14 (24.1) | 4 (7.8) | 7 (14.9) | 19 (29.2) | 18 (26.1) | 10 (16.7) | 10 (18.2) | 14 (23.7) |
|
|
Smokeless | 80 (13.3) | 14 (17.7) | 5 (6.6) | 7 (10.5) | 13 (16.7) | 10 (11.9) | 8 (11.4) | 10 (14.1) | 13 (16.7) |
|
|
Hookah | 85 (30.1) | 19 (48.7) | 6 (17.7) | 12 (30.8) | 10 (28.6) | 11 (32.4) | 8 (24.2) | 6 (18.2) | 13 (37.1) |
|
|
e-Cigarettes | 125 (26.9) | 21 (35.59) | 13 (22.4) | 11 (20.4) | 16 (26.7) | 13 (21.7) | 18 (31.6) | 14 (24.6) | 19 (31.7) |
| Use marijuana and tobacco | 275 (43.2) | 40 (49.4) | 39 (50.6) | 26 (35.6) | 34 (42) | 38 (43.7) | 32 (40.5) | 31 (40.3) | 35 (42.7) | |
| Secondhand smoke in house | 68 (10.7) | 10 (12.3) | 9 (11.7) | 10 (13.7) | 7 (8.6) | 6 (6.9) | 4 (5.1) | 12 (15.6) | 10 (12.2) | |
| Have friends who use tobacco | 566 (89.0) | 76 (93.8) | 67 (87.0) | 60 (82.2) | 68 (84.0) | 80 (92.0) | 69 (87.3) | 72 (93.5) | 74 (90.2) | |
aResults that include Ever product use followed by Past 30 days use (p30).
bRandomization of participants to 8 groups of short message service text messages.
cCGE: complex, gain-framed, emotional.
dCGR: complex, gain-framed, rational.
eCLE: complex, loss-framed, emotional.
fCLR: complex, loss-framed, rational.
gSGE: simple, gain-framed, emotional.
hSGR: simple, gain-framed, rational.
iSLE: simple, loss-framed, emotional.
jSLR: simple, loss-framed, rational.
kRefers to the concurrent use of multiple tobacco products among participants at any time.
lSusceptibility to use is measured with nonusers only.
Among nonusers, 13.4% (47/351) were found to be susceptible to smoking cigarettes, 24.3% (109/449) were susceptible to smoking cigars, 30.4% (86/283) were susceptible to using hookah, and 24.1% (106/440) were susceptible to using e-cigarettes. At baseline, no significant differences between the 8 groups were found with respect to all such tobacco-related characteristics (Table 2).
Manipulation Checks
We first checked to ensure that the messages were perceived by participants as intended after the first week of message exposure (Table 3). Out of 636 participants, 530 (530/636, 83.3%) completed the manipulation check survey. Compared with gain-framed messages, loss-framed messages were significantly more likely to be perceived as presenting a loss, F7,522=13.13, P<.001. Groups receiving CLE, CLR, SLE, and SLR text messages scored higher on perceived message framing as loss than that of groups receiving CGE, CGR, SGE, or SGR text messages. Complex messages were perceived to be significantly more complex than that of simple messages, F7,520=2.04, P=.05. Groups receiving CLE, CLR, CGE, and CGR messages scored higher on perceived message complexity than that of groups receiving SLE, SLR, SGE, or SGR messages. Emotional messages were perceived to be significantly more emotionally involving than rational messages, F7,520=6.46, P<.001. The groups receiving CLE, SLE, CGE, and SGE messages scored higher on the perceived emotional level of their messages than did the groups receiving CLR, CGR, SLR, or SGR messages (Table 3).
Table 3.
Week 1 manipulation check outcomes for the total sample and by treatment arm.
| Outcomesa,b | Mean (SD) | P valuek | ||||||||
|
|
Total | CGEc | CGRd | CLEe | CLRf | SGEg | SGRh | SLEi | SLRj |
|
| Perceived message-framing as a loss (8-point scale) | 5.20 (3.22) | 3.74 (2.23) | 4.39 (3.00) | 5.74 (3.25) | 7.58 (2.86) | 4.07 (2.66) | 4.17 (3.04) | 6.06 (3.33) | 6.00 (3.41) | <.001 |
| Perceived complexity level (8-point scale) | 2.98 (2.02) | 3.07 (2.12) | 2.81 (2.26) | 3.34 (2.33) | 3.60 (2.14) | 2.45 (1.54) | 2.86 (1.95) | 3.05 (1.96) | 2.78 (1.81) | .05 |
| Perceived emotional level (8-point scale) | 3.35 (2.23) | 3.72 (2.13) | 2.24 (1.52) | 4.39 (2.72) | 3.39 (2.33) | 3.62 (2.22) | 2.80 (1.90) | 3.82 (2.28) | 2.86 (1.97) | <.001 |
| Perceived credibility (8-point scale) | 7.57 (2.01) | 7.43 (1.85) | 8.01 (1.78) | 7.60 (1.90) | 7.87 (1.80) | 6.98 (2.45) | 7.70 (1.96) | 7.36 (2.01) | 7.64 (2.09) | .10 |
| Enjoyment of messages (8-point scale) | 5.90 (1.49) | 5.87 (1.46) | 5.78 (1.37) | 6.13 (1.57) | 5.93 (1.46) | 5.93 (1.54) | 5.78 (1.47) | 5.96 (1.56) | 5.80 (1.53) | .90 |
| Perceived relevance (5-point scale) | 2.37 (0.85) | 2.37 (0.87) | 2.40 (0.91) | 2.38 (0.81) | 2.57 (0.79) | 2.22 (0.81) | 2.41 (0.83) | 2.26 (0.94) | 2.40 (0.81) | .40 |
| Perceived readability (5-point scale) | 3.46 (0.78) | 3.41 (0.81) | 3.44 (0.84) | 3.51 (0.75) | 3.45 (0.85) | 3.53 (0.70) | 3.50 (0.75) | 3.36 (0.84) | 3.49 (0.71) | .93 |
aMissing values are not presented in this table. Out of 636 participants, 530 (530/636, 83.3%) completed the manipulation check survey.
bParticipants were randomized to one of the 8 treatment arms, describing the type of messages.
cCGE: complex, gain-framed, emotional.
dCGR: complex, gain-framed, rational.
eCLE: complex, loss-framed, emotional.
fCLR: complex, loss-framed, rational.
gSGE: simple, gain-framed, emotional.
hSGR: simple, gain-framed, rational.
iSLE: simple, loss-framed, emotional.
jSLR: simple, loss-framed, rational.
kSignificance testing with analysis of variance.
We also checked to make sure that the health messages were consistently perceived as credible (Table 3). As expected, there was no significant difference among the treatment arms with regard to the perceived credibility of message content (F7,520=1.70, P=.10). The total mean score on SMS text message credibility was 7.57 (SD 2.01) on an 8-point scale. This confirms that all text message interventions were perceived to be credible sources of information related to tobacco. Similarly, as shown in Table 3, the treatment arms did not differ with regard to the enjoyment of the messages (F7,520=0.41, P=.90), perceived message relevance (F7,517=1.04, P=.40), or perceived message readability (F7,517=0.34, P=.94).
Baseline Treatment Arm Differences in Outcome Measures
At baseline, there were no significant differences among the treatment arms with respect to our risk communication variables: perceived risk of using each tobacco product, perceived personal and general benefits of e-cigarettes, perceived addictiveness of products, or perceived popularity of tobacco use (Table 4).
Table 4.
Baseline risk communication outcomes for the entire sample and by treatment arm.
| Outcomea | Mean (SD) | ||||||||
|
|
Total | CGEb | CGRc | CLEd | CLRe | SGEf | SGRg | SLEh | SLRi |
| Perceived risk of using cigarettes | 3.69 (0.55) | 3.76 (0.49) | 3.68 (0.52) | 3.78 (0.35) | 3.68 (0.6) | 3.57 (0.65) | 3.70 (0.56) | 3.75 (0.47) | 3.60 (0.63) |
| Perceived risk of using cigars | 3.61 (0.56) | 3.67 (0.49) | 3.64 (0.5) | 3.63 (0.52) | 3.62 (0.56) | 3.51 (0.62) | 3.65 (0.56) | 3.62 (0.54) | 3.52 (0.67) |
| Perceived risk of using smokeless tobacco | 3.48 (0.60) | 3.49 (0.57) | 3.44 (0.60) | 3.59 (0.49) | 3.47 (0.68) | 3.45 (0.61) | 3.48 (0.60) | 3.56 (0.53) | 3.38 (0.68) |
| Perceived risk of using hookah | 3.09 (0.8) | 3.00 (0.87) | 3.15 (0.71) | 3.37 (0.72) | 3.09 (0.79) | 2.99 (0.84) | 3.13 (0.79) | 3.11 (0.77) | 3.01 (0.85) |
| Perceived risk of using e-cigarettes | 3.06 (0.83) | 2.98 (0.82) | 3.17 (0.65) | 3.31 (0.77) | 3.08 (0.8) | 2.98 (0.9) | 3.11 (0.84) | 3.04 (0.84) | 2.96 (0.94) |
| Perceived personal benefits of e-cigarettes | 0.88 (0.76) | 0.87 (0.77) | 0.88 (0.73) | 1.02 (0.87) | 0.76 (0.65) | 0.91 (0.8) | 0.85 (0.77) | 0.89 (0.72) | 0.85 (0.74) |
| Perceived general benefits of e-cigarettes | 1.41 (0.69) | 1.47 (0.51) | 1.53 (0.68) | 1.42 (0.69) | 1.39 (0.72) | 1.45 (0.78) | 1.31 (0.77) | 1.37 (0.73) | 1.38 (0.64) |
| Perceived addictiveness of products | 1.23 (0.59) | 1.23 (0.53) | 1.26 (0.56) | 1.33 (0.59) | 1.21 (0.64) | 1.24 (0.55) | 1.18 (0.62) | 1.23 (0.61) | 1.18 (0.61) |
| Perceived popularity of tobacco use | 2.44 (1.13) | 2.57 (1.11) | 2.5 (1.05) | 2.33 (1.21) | 2.36 (1.15) | 2.47 (1.09) | 2.49 (1.06) | 2.28 (1.19) | 2.48 (1.2) |
aParticipants were randomized to one of the 8 treatment arms, describing the type of messages.
bCGE: complex, gain-framed, emotional.
cCGR: complex, gain-framed, rational.
dCLE: complex, loss-framed, emotional.
eCLR: complex, loss-framed, rational.
fSGE: simple, gain-framed, emotional.
gSGR: simple, gain-framed, rational.
hSLE: simple, loss-framed, emotional.
iSLR: simple, loss-framed, rational.
Discussion
Overview
The Project Debunk trial will evaluate a comprehensive campaign delivered by mobile phone for increasing tobacco risk perception among a large sample of young adults in community college, including both tobacco users and nonusers. In particular, the trial will identify which structures of SMS text messages, if any, have the strongest effect on increasing the perceived risk of using conventional tobacco products and NETP. The results of this study will form the basis of an evidence-based resource that future researchers and practitioners could modify for use among their populations of interest.
To the best of our knowledge, this is the first published mHealth protocol for a trial that assesses the effect of a comprehensive and evidence-based mobile phone text messaging campaign for tobacco risk communication. This protocol summarizes the design and describes the planned evaluation of Project Debunk. Going beyond a simple presentation of our future study procedures, the protocol also presents the results from our baseline data. In particular, baseline information confirms that a substantial proportion of young adults at community colleges continue to smoke cigarettes, in addition to using NETP such as e-cigarettes and hookah. There were no differences among the treatment arms with respect to sociodemographic or tobacco-related characteristics. In addition, the treatment arms did not differ at the baseline with respect to the perceived risk of using any tobacco product. Preliminary results also show that we have successfully manipulated the 8-message structure combinations with our study sample. This is evident from treatment arm differences with respect to perceived message loss framing, emotional level, and complexity. All 8-message structure combinations were found to be enjoyable, easy to read, and credible.
Anticipated Results
On the basis of previous pilot data collected by our team [62], we anticipate adequate feasibility and satisfaction among participants. In a previous study that we conducted with young adult college students [61], the recruitment rate was high (80.1%) and participants reported positive changes in their perceived risk of tobacco use. We anticipate similar results in Project Debunk for all groups. We project that all message structure combinations will result in an increase in perceived risk of using tobacco products. As suggested by recent reports [4,87], we expect higher levels of perceived risk of using combustible cigarettes compared with NETP such as e-cigarettes and hookah. In addition, change over time in perceived risk is expected to be lower for combustible cigarettes, compared with e-cigarettes and hookah. We cannot predict or anticipate specific results with respect to which message structure is most effective in improving tobacco risk perception. This study will be the first to provide empirical evidence that highlights the importance of one message structure over another in the context of tobacco risk communication. Once the successful types of text messages have been identified, our future plan is to introduce the messages in the context of an advanced digital intervention that can effectively communicate tobacco risk.
Strengths and Limitations
We will address the anticipated difficulties described in previous studies of mobile phone text messaging in young-adult populations [88-90], such as participant retention, in several ways: regular communication with participants and continuous reminders via phone, and compensation (gift cards) at project completion. This study has a convenience sample. Nevertheless, our sample is representative of the community college population in age, gender, and ethnicity. It also involves a heterogeneous ethnic distribution, with a proportion of tobacco users and demographics that are similar to that of young adults in the state of Texas [91].
Conclusions
It is evident that young adult tobacco users and nonusers are interested in mHealth programs that help them learn about tobacco risks [62]. Moreover, as a mass media strategy, mHealth programs offer the potential to greatly increase the reach of young adults. If our results suggest that a specific mobile phone text message structure is most effective for helping young adults accurately perceive tobacco risk, this would provide evidence to include such text messages as part of larger technology-based campaigns such as smartphone apps, entertainment-based campaigns, and social media. These findings would also provide a deeper understanding of the factors that drive change in the perceived risk of using tobacco and improve the design of our text messages. Considering the wide variety of tobacco products studied in the trial, the results will highlight any potential differences between the products. With the use of mHealth text messaging, the results of this study will reveal the best strategies to efficiently and widely communicate risk to young adults and ultimately prevent tobacco use in this age demographic.
Acknowledgments
We are grateful to campus leadership at HCC for supporting our research activities and recognizing its importance for the health and safety of students. We appreciate students who volunteered to participate in this study. We would also like to acknowledge the members of the TX TCORS scientific steering committee for their constructive support and valuable recommendations throughout this study. The committee includes Jerome Williams (Prudential Chair in Business, at the Rutgers Business School); Cornelia (Connie) Pechmann (Professor at the Paul Merage School of Business, University of California, Irvine); John Pierce (Professor at the Cancer Prevention Program, Moores Cancer Center, University of California, San Diego, La Jolla, California and the Department of Family Medicine and Public Health, University of California, San Diego); Lisa Hendrickson (Senior Research Scientist at the Stanford Prevention Research Center); and Lois Biener (Senior Research Fellow at the University of Massachusetts Boston). This study was funded by the Tobacco Center of Regulatory Science on Youth and Young Adults (5P50CA180906-02). This project was also partially supported by The University of Texas MD Anderson Cancer Center’s Support Grant (P30CA016672).
Abbreviations
- AIM
assessment, intervention, and measurement
- ANOVA
analysis of variance
- CGE
complex, gain-framed, emotional
- CGR
complex, gain-framed, rational
- CLE
complex, loss-framed, emotional
- CLR
complex, loss-framed, rational
- e-cigarettes
electronic cigarettes
- ELM
elaboration likelihood model
- HCC
Houston Community College
- mHealth
mobile health
- NETP
new and emerging tobacco products
- PC1
postcampaign 1
- PC2
postcampaign 2
- SGE
simple, gain-framed, emotional
- SGR
simple, gain-framed, rational
- SLE
simple, loss-framed, emotional
- SLR
simple, loss-framed, rational
- SMS
short message service
- TX TCORS
Tobacco Center of Regulatory Science on Youth and Young Adults
Peer-review report from the Food and Drug Administration of the National Institutes of Health.
Examples of text messages.
Checklist for Reporting Results of Internet E-Surveys (CHERRIES).
Description of the main measures.
Footnotes
Authors' Contributions: AVP and KSC conceptualized the initial study with numerous valuable contributions by GEK. TCM, SR, KWC, and GCB managed recruitment and will manage follow-up assessments. GEK wrote the preliminary manuscript. GEK and MC conducted the statistical analysis. AVP, CLP, DJV, KSC, TCM, and AP contributed to drafts of the manuscript. AVP approved the final manuscript. Authors GEK and MC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest: None declared.
References
- 1.Schulenberg JE, Johnston LD, O'Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoringthefuture. 2016. [2018-07-10]. College students and adults ages 19-55 http://monitoringthefuture.org/pubs/monographs/mtf-vol2_2016.pdf .
- 2.Pokhrel P, Lam TH, Pagano I, Kawamoto CT, Herzog TA. Young adult e-cigarette use outcome expectancies: validity of a revised scale and a short scale. Addict Behav. 2018 Mar;78:193–9. doi: 10.1016/j.addbeh.2017.11.019.S0306-4603(17)30428-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hair E, Rath JM, Pitzer L, Emelle B, Ganz O, Halenar MJ, Cantrell J, Vallone D. Trajectories of hookah use: harm perceptions from youth to young adulthood. Am J Health Behav. 2017 May 1;41(3):240–7. doi: 10.5993/AJHB.41.3.3. [DOI] [PubMed] [Google Scholar]
- 4.Berg CJ, Stratton E, Schauer GL, Lewis M, Wang Y, Windle M, Kegler M. Perceived harm, addictiveness, and social acceptability of tobacco products and marijuana among young adults: marijuana, hookah, and electronic cigarettes win. Subst Use Misuse. 2015 Jan;50(1):79–89. doi: 10.3109/10826084.2014.958857. http://europepmc.org/abstract/MED/25268294 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozlowski LT, Sweanor DT. Young or adult users of multiple tobacco/nicotine products urgently need to be informed of meaningful differences in product risks. Addict Behav. 2018 Jan;76:376–81. doi: 10.1016/j.addbeh.2017.01.026. https://linkinghub.elsevier.com/retrieve/pii/S0306-4603(17)30053-9 .S0306-4603(17)30053-9 [DOI] [PubMed] [Google Scholar]
- 6.Wong EC, Haardörfer R, Windle M, Berg CJ. Distinct motives for use among polytobacco versus cigarette only users and among single tobacco product users. Nicotine Tob Res. 2017 Dec 13;20(1):117–23. doi: 10.1093/ntr/ntw284.ntw284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redonnet B, Chollet A, Fombonne E, Bowes L, Melchior M. Tobacco, alcohol, cannabis and other illegal drug use among young adults: the socioeconomic context. Drug Alcohol Depend. 2012 Mar 1;121(3):231–9. doi: 10.1016/j.drugalcdep.2011.09.002.S0376-8716(11)00388-7 [DOI] [PubMed] [Google Scholar]
- 8.Mays D, Arrazola RA, Tworek C, Rolle IV, Neff LJ, Portnoy DB. Openness to using non-cigarette tobacco products among U.S. young adults. Am J Prev Med. 2016 Apr;50(4):528–34. doi: 10.1016/j.amepre.2015.08.015. http://europepmc.org/abstract/MED/26549502 .S0749-3797(15)00489-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasza KA, Ambrose BK, Conway KP, Borek N, Taylor K, Goniewicz ML, Cummings KM, Sharma E, Pearson JL, Green VR, Kaufman AR, Bansal-Travers M, Travers MJ, Kwan J, Tworek C, Cheng Y, Yang L, Pharris-Ciurej N, van Bemmel DM, Backinger CL, Compton WM, Hyland AJ. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017 Dec 26;376(4):342–53. doi: 10.1056/NEJMsa1607538. http://europepmc.org/abstract/MED/28121512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loukas A, Murphy JL, Gottlieb NH. Cigarette smoking and cessation among trade or technical school students in Texas. J Am Coll Health. 2008;56(4):401–7. doi: 10.3200/JACH.56.44.401-408.X1627480W11R2P81 [DOI] [PubMed] [Google Scholar]
- 11.O'Brien F, Simons-Morton B, Chaurasia A, Luk J, Haynie D, Liu D. Post-high school changes in tobacco and cannabis use in the United States. Subst Use Misuse. 2018 Jan 2;53(1):26–35. doi: 10.1080/10826084.2017.1322983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Association of Community Colleges. 2017. Mar, Financially challenged: each year almost half of community college students must reply on outside financial sources https://www.aacc.nche.edu/2017/03/16/datapoints-financially-challenged/
- 13.Carpenter CM, Wayne GF, Pauly JL, Koh HK, Connolly GN. New cigarette brands with flavors that appeal to youth: tobacco marketing strategies. Health Aff (Millwood) 2005;24(6):1601–10. doi: 10.1377/hlthaff.24.6.1601.24/6/1601 [DOI] [PubMed] [Google Scholar]
- 14.Ling PM, Glantz SA. Why and how the tobacco industry sells cigarettes to young adults: evidence from industry documents. Am J Public Health. 2002 Jun;92(6):908–16. doi: 10.2105/ajph.92.6.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahreinifar S, Sheon NM, Ling PM. Is snus the same as dip? Smokers' perceptions of new smokeless tobacco advertising. Tob Control. 2013 Mar;22(2):84–90. doi: 10.1136/tobaccocontrol-2011-050022. http://europepmc.org/abstract/MED/21972063 .tobaccocontrol-2011-050022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter OB, Donovan R, Jalleh G. Using viral e-mails to distribute tobacco control advertisements: an experimental investigation. J Health Commun. 2011 Aug;16(7):698–707. doi: 10.1080/10810730.2011.551998.935258912 [DOI] [PubMed] [Google Scholar]
- 17.Perez DA, Grunseit AC, Rissel C, Kite J, Cotter T, Dunlop S, Bauman A. Tobacco promotion 'below-the-line': exposure among adolescents and young adults in NSW, Australia. BMC Public Health. 2012 Jun 12;12:429. doi: 10.1186/1471-2458-12-429. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-12-429 .1471-2458-12-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jane Lewis M, Bover Manderski MT, Delnevo CD. Tobacco industry direct mail receipt and coupon use among young adult smokers. Prev Med. 2015 Feb;71:37–9. doi: 10.1016/j.ypmed.2014.11.030. http://europepmc.org/abstract/MED/25511177 .S0091-7435(14)00473-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson A, Ganz O, Pearson J, Celcis N, Vallone D, Villanti AC. How the industry is marketing menthol cigarettes: the audience, the message and the medium. Tob Control. 2015 Nov;24(6):594–600. doi: 10.1136/tobaccocontrol-2014-051657.tobaccocontrol-2014-051657 [DOI] [PubMed] [Google Scholar]
- 20.Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015 Jul;24(4):341–7. doi: 10.1136/tobaccocontrol-2013-051246.tobaccocontrol-2013-051246 [DOI] [PubMed] [Google Scholar]
- 21.Rainie L, Perrin A. 10 facts about smartphones as the iPhone turns 10. 2017. Jun 28, http://www.pewresearch.org/fact-tank/2017/06/28/10-facts-about-smartphones/
- 22.Reynolds RJ. Camel Snus: We had a feeling you would be stopping 2012. https://snus.tobaccopleasure.com/modules/
- 23.Phillip Morris USA. 2012. [2018-04-23]. Corporate Responsibility http://www.philipmorrisusa.com/en/cms/search/results.aspx?q=social .
- 24.Sears CG, Walker KL, Hart JL, Lee AS, Siu A, Smith C. Clean, cheap, convenient: promotion of electronic cigarettes on YouTube. Tob Prev Cessat. 2017 Apr;3 doi: 10.18332/tpc/69393. http://europepmc.org/abstract/MED/28725876 .10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Kornfield R, Emery SL. 100 million views of electronic cigarette YouTube videos and counting: quantification, content evaluation, and engagement levels of videos. J Med Internet Res. 2016 Mar 18;18(3):e67. doi: 10.2196/jmir.4265. http://www.jmir.org/2016/3/e67/ v18i3e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BinDhim NF, Freeman B, Trevena L. Pro-smoking apps: where, how and who are most at risk. Tob Control. 2015 Mar;24(2):159–61. doi: 10.1136/tobaccocontrol-2013-051189.tobaccocontrol-2013-051189 [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration. 2017. Research Priorities https://www.fda.gov/TobaccoProducts/PublicHealthScienceResearch/Research/ucm311860.htm .
- 28.Ashley DL, Backinger CL, van Bemmel DM, Neveleff DJ. Tobacco regulatory science: research to inform regulatory action at the Food and Drug Administration's Center for Tobacco Products. Nicotine Tob Res. 2014 Aug;16(8):1045–9. doi: 10.1093/ntr/ntu038.ntu038 [DOI] [PubMed] [Google Scholar]
- 29.Muench F, Weiss RA, Kuerbis A, Morgenstern J. Developing a theory driven text messaging intervention for addiction care with user driven content. Psychol Addict Behav. 2013 Mar;27(1):315–21. doi: 10.1037/a0029963. http://europepmc.org/abstract/MED/22963375 .2012-24205-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obermayer JL, Riley WT, Asif O, Jean-Mary J. College smoking-cessation using cell phone text messaging. J Am Coll Health. 2004;53(2):71–8. doi: 10.3200/JACH.53.2.71-78. https://doi-org.ezproxy1.library.usyd.edu.au/10.3200/JACH.53.2.71-78 . [DOI] [PubMed] [Google Scholar]
- 31.Riley W, Obermayer J, Jean-Mary J. Internet and mobile phone text messaging intervention for college smokers. J Am Coll Health. 2008;57(2):245–8. doi: 10.3200/JACH.57.2.245-248.R4883H2681063U21 [DOI] [PubMed] [Google Scholar]
- 32.Sandrick J, Tracy D, Eliasson A, Roth A, Bartel J, Simko M, Bowman T, Harouse-Bell K, Kashani M, Vernalis M. Effect of a counseling session bolstered by text messaging on self-selected health behaviors in college students: a preliminary randomized controlled trial. JMIR Mhealth Uhealth. 2017 May 17;5(5):e67. doi: 10.2196/mhealth.6638. http://mhealth.jmir.org/2017/5/e67/ v5i5e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Head KJ, Noar SM, Iannarino NT, Grant Harrington N. Efficacy of text messaging-based interventions for health promotion: a meta-analysis. Soc Sci Med. 2013 Nov;97:41–8. doi: 10.1016/j.socscimed.2013.08.003.S0277-9536(13)00447-4 [DOI] [PubMed] [Google Scholar]
- 34.Armanasco AA, Miller YD, Fjeldsoe BS, Marshall AL. Preventive health behavior change text message interventions: a meta-analysis. Am J Prev Med. 2017 Mar;52(3):391–402. doi: 10.1016/j.amepre.2016.10.042.S0749-3797(16)30586-4 [DOI] [PubMed] [Google Scholar]
- 35.Latimer AE, Krishnan-Sarin S, Cavallo DA, Duhig A, Salovey P, O'Malley SA. Targeted smoking cessation messages for adolescents. J Adolesc Health. 2012 Jan;50(1):47–53. doi: 10.1016/j.jadohealth.2011.04.013.S1054-139X(11)00146-7 [DOI] [PubMed] [Google Scholar]
- 36.Toll BA, Salovey P, O'Malley SS, Mazure CM, Latimer A, McKee SA. Message framing for smoking cessation: the interaction of risk perceptions and gender. Nicotine Tob Res. 2008 Jan;10(1):195–200. doi: 10.1080/14622200701767803. http://europepmc.org/abstract/MED/18188760 .789473333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biener L, McCallum-Keeler G, Nyman AL. Adults' response to Massachusetts anti-tobacco television advertisements: impact of viewer and advertisement characteristics. Tob Control. 2000 Dec;9(4):401–7. doi: 10.1136/tc.9.4.401. http://tobaccocontrol.bmj.com/cgi/pmidlookup?view=long&pmid=11106710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steward WT, Schneider TR, Pizarro J, Salovey P. Need for cognition moderates responses to framed smoking-cessation messages 1. J Appl Soc Pyschol. 2003 Dec;33(12):2439–64. doi: 10.1111/j.1559-1816.2003.tb02775.x. [DOI] [Google Scholar]
- 39.Wilson DK, Wallston KA, King JE. Effects of contract framing, motivation to quit, and self-efficacy on smoking reduction 1. J Appl Soc Pyschol. 1990 Apr;20(7):531–47. doi: 10.1111/j.1559-1816.1990.tb00426.x. [DOI] [Google Scholar]
- 40.Wilson DK, Purdon SE, Wallston KA. Compliance to health recommendations: a theoretical overview of message framing. Health Educ Res. 1988 Jun 1;3(2):161–71. doi: 10.1093/her/3.2.161. [DOI] [Google Scholar]
- 41.O'Keefe DJ, Jensen JD. The relative persuasiveness of gain-framed and loss-framed messages for encouraging disease prevention behaviors: a meta-analytic review. J Health Commun. 2007;12(7):623–44. doi: 10.1080/10810730701615198.782997291 [DOI] [PubMed] [Google Scholar]
- 42.Burgoon J, Blair JP, Qin T, Nunamaker JF. Detecting deception through linguistic analysis. 2003 Proceedings: Intelligence and Security Informatics Intelligence and Security Informatics: First NSF/NIJ Symposium; Intelligence and Security Informatics Intelligence and Security Informatics: First NSF/NIJ Symposium; June 2-3, 2013; Tuscon, AZ, USA. 2003. Jun, pp. 91–101. [DOI] [Google Scholar]
- 43.Brochet F, Naranjo P, Yu G. Causes and consequences of linguistic complexity in non-US firm conference calls. 2012. Oct, http://nrs.harvard.edu/urn-3:HUL.InstRepos:9709899 .
- 44.Barnett T, Hoang H, Furlan A. An analysis of the readability characteristics of oral health information literature available to the public in Tasmania, Australia. BMC Oral Health. 2016 Mar 17;16:35. doi: 10.1186/s12903-016-0196-x. https://bmcoralhealth.biomedcentral.com/articles/10.1186/s12903-016-0196-x .10.1186/s12903-016-0196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S, Lang A. Redefining media content and structure in terms of available resources: toward a dynamic human-centric theory of communication. Commun Res. 2013 May 30;42(5):599–625. doi: 10.1177/0093650213488416. [DOI] [Google Scholar]
- 46.Reilly J, Seibert L. Language and emotion. In: Davidson RJ, Sherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. Oxford: Oxford University Press; 2003. p. 535. [Google Scholar]
- 47.Shimanoff SB. Expressing emotions in words: verbal patterns of interaction. J Commun. 1985 Sep 1;35(3):16–31. doi: 10.1111/j.1460-2466.1985.tb02445.x. [DOI] [Google Scholar]
- 48.Shimanoff SB. Types of emotional disclosures and request compliance between spouses. Commun Monogr. 2009 Jun 2;54(1):85–100. doi: 10.1080/03637758709390217. [DOI] [Google Scholar]
- 49.Carey J. Paralanguage in computer mediated communication. ACL '80 Proceedings of the 18th annual meeting on Association for Computational Linguistics; the 18th annual meeting on Association for Computational Linguistics; June 19 - 22, 1980; Philadelphia. Stroudsburg, PA, USA: Association for Computational Linguistics; 1980. pp. 67–9. https://dl.acm.org/citation.cfm?id=981458 . [DOI] [Google Scholar]
- 50.Vidrine JI, Simmons VN, Brandon TH. Construction of smoking-relevant risk perceptions among college students: The influence of need for cognition and message content. J Appl Soc Pyschol. 2007 Jan 10;37(1):91–114. doi: 10.1111/j.0021-9029.2007.00149.x. [DOI] [Google Scholar]
- 51.Petty RE, Cacioppo JT. Advances in Experimental Social Psychology. New York: Springer; 1986. The elaboration likelihood model of persuasion; pp. 123–205. [Google Scholar]
- 52.O'Keefe DJ. The International Encyclopedia of Communication. New York: John Wiley & Sons; 2008. Mar 28, Elaboration likelihood model; pp. 1–7. [Google Scholar]
- 53.Chen SJ, Lee KP. The role of personality traits and perceived values in persuasion: an elaboration likelihood model perspective on online shopping. Soc Behav Personal. 2008 Nov 1;36(10):1379–1400. doi: 10.2224/sbp.2008.36.10.1379. [DOI] [Google Scholar]
- 54.Lang A, Yegiyan NS. Understanding the interactive effects of emotional appeal and claim strength in health messages. J Broadcast Electron Media. 2008 Aug 8;52(3):432–47. doi: 10.1080/08838150802205629. [DOI] [Google Scholar]
- 55.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. In: MacLean LC, Ziemba WT, editors. Handbook of The Fundamentals of Financial Decision Making: Part I. Singapore: World Scientific Publishing Co Pte Ltd; 2013. pp. 99–127. [Google Scholar]
- 56.Kühberger A. The influence of framing on risky decisions: A meta-analysis. Organ Behav Hum Decis Process. 1998 Jul;75(1):23–55. doi: 10.1006/obhd.1998.2781.S0749597898927819 [DOI] [PubMed] [Google Scholar]
- 57.O'Keefe DJ, Jensen JD. The relative persuasiveness of gain-framed and loss-framed messages for encouraging disease prevention behaviors: a meta-analytic review. J Health Commun. 2007;12(7):623–44. doi: 10.1080/10810730701615198.782997291 [DOI] [PubMed] [Google Scholar]
- 58.Gallagher KM, Updegraff JA. Health message framing effects on attitudes, intentions, and behavior: a meta-analytic review. Ann Behav Med. 2012 Feb;43(1):101–16. doi: 10.1007/s12160-011-9308-7. [DOI] [PubMed] [Google Scholar]
- 59.Committee on Improving the Health, Safety, and Well-Being of Young Adults. Board on Children, Youth, and Families; Institute of Medicine. National Research Council . Young adults in the 21st century. In: Bonnie RJ, Stroud C, Breiner H, editors. Investing in the Health and Well-Being of Young Adults. Washington (DC), USA: National Academies Press (US); 2015. Jan 27, [PubMed] [Google Scholar]
- 60.Houston Community College. [2018-04-24]. Houston Community College Fact Book 2017 https://www.hccs.edu/about-hcc/institutional-research/hcc-fact-book/FactBook2018.pdf .
- 61.Prokhorov AV, Yost T, Mullin-Jones M, de Moor C, Ford KH, Marani S, Kilfoy BA, Hein JP, Hudmon KS, Emmons KM. “Look at your health”: outcomes associated with a computer-assisted smoking cessation counseling intervention for community college students. Addict Behav. 2008 Jun;33(6):757–71. doi: 10.1016/j.addbeh.2007.12.005. http://europepmc.org/abstract/MED/18280668 .S0306-4603(07)00352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prokhorov AV, Machado TC, Calabro KS, Vanderwater EA, Vidrine DJ, Pasch KP, Marani SK, Buchberg M, Wagh A, Russell SC, Czerniak KW, Botello GC, Dobbins MH, Khalil GE, Perry CL. Developing mobile phone text messages for tobacco risk communication among college students: a mixed methods study. BMC Public Health. 2017 Dec 31;17(1):137. doi: 10.1186/s12889-017-4027-z. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-017-4027-z .10.1186/s12889-017-4027-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khalil GE, Calabro KS, Crook B, Machado TC, Perry CL, Prokhorov AV. Validation of mobile phone text messages for nicotine and tobacco risk communication among college students: a content analysis. Tob Prev Cessation. 2018 Feb;4 doi: 10.18332/tpc/84866.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE, Miech RA. Monitoring the future national survey results on drug use, 1975-2015: Overview, key findings on adolescent drug use. Bethesda, MD: National Institute on Drug Abuse; 2016. [2018-09-14]. https://deepblue.lib.umich.edu/handle/2027.42/137913 . [Google Scholar]
- 65.Rumpf HJ, Meyer C, Hapke U, John U. Screening for mental health: validity of the MHI-5 using DSM-IV Axis I psychiatric disorders as gold standard. Psychiatry Res. 2001 Dec 31;105(3):243–53. doi: 10.1016/s0165-1781(01)00329-8.S0165-1781(01)00329-8 [DOI] [PubMed] [Google Scholar]
- 66.Kebede M, Zeleke A, Asemahagn M, Fritz F. Willingness to receive text message medication reminders among patients on antiretroviral treatment in North West Ethiopia: a cross-sectional study. BMC Med Inform Decis Mak. 2015 Aug 13;15:65. doi: 10.1186/s12911-015-0193-z. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-015-0193-z .10.1186/s12911-015-0193-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahlor L, Dunwoody S, Griffin RJ, Neuwirth K. Seeking and processing information about impersonal risk. Sci Commun. 2016 Aug 18;28(2):163–94. doi: 10.1177/1075547006293916. [DOI] [Google Scholar]
- 68.Mason M, Cheung I, Walker L. Substance use, social networks, and the geography of urban adolescents. Subst Use Misuse. 2004;39(10-12):1751–77. [PubMed] [Google Scholar]
- 69.Berwick DM, Murphy JM, Goldman PA, Ware JE, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care. 1991 Feb;29(2):169–76. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Fellner B, Holler M, Kirchler E, Schabmann A. Regulatory focus scale (RFS): development of a scale to record dispositional regulatory focus. Swiss J Psychol. 2007 Jun;66(2):109–16. doi: 10.1024/1421-0185.66.2.109. [DOI] [Google Scholar]
- 71.Stephenson MT, Hoyle RH, Palmgreen P, Slater MD. Brief measures of sensation seeking for screening and large-scale surveys. Drug Alcohol Depend. 2003 Dec 11;72(3):279–86. doi: 10.1016/j.drugalcdep.2003.08.003.S0376871603002382 [DOI] [PubMed] [Google Scholar]
- 72.Nelson W, Reyna VF, Fagerlin A, Lipkus I, Peters E. Clinical implications of numeracy: theory and practice. Ann Behav Med. 2008 Jun;35(3):261–74. doi: 10.1007/s12160-008-9037-8. http://europepmc.org/abstract/MED/18677452 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toll BA, O'Malley SS, Katulak NA, Wu R, Dubin JA, Latimer A, Meandzija B, George TP, Jatlow P, Cooney JL, Salovey P. Comparing gain- and loss-framed messages for smoking cessation with sustained-release bupropion: a randomized controlled trial. Psychol Addict Behav. 2007 Dec;21(4):534–44. doi: 10.1037/0893-164X.21.4.534. http://europepmc.org/abstract/MED/18072836 .2007-18113-012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.See YH, Petty RE, Evans LM. The impact of perceived message complexity and need for cognition on information processing and attitudes. J Res Pers. 2009 Oct;43(5):880–9. doi: 10.1016/j.jrp.2009.04.006. [DOI] [Google Scholar]
- 75.Eastin MS. Credibility assessments of online health information: the effects of source expertise and knowledge of content. J Comput-Mediat Comm. 2001;6(4) doi: 10.1111/j.1083-6101.2001.tb00126.x. [DOI] [Google Scholar]
- 76.Cyr D, Head M, Ivanov A. Perceived interactivity leading to e-loyalty: Development of a model for cognitive-affective user responses. Int J Hum Comput Stud. 2009 Oct;67(10):850–69. doi: 10.1016/j.ijhcs.2009.07.004. [DOI] [Google Scholar]
- 77.Nysveen H, Pedersen P, Thorbjørnsen H. Intentions to use mobile services: antecedents and cross-service comparisons. JAMS. 2005 Jun;33(3) doi: 10.1177/0092070305. [DOI] [Google Scholar]
- 78.Coursaris CK, Sung J. Antecedents and consequents of a mobile website's interactivity. New Media Soc. 2012 Apr 26;14(7):1128–46. doi: 10.1177/1461444812439552. [DOI] [Google Scholar]
- 79.Green MC, Brock TC. The role of transportation in the persuasiveness of public narratives. J Pers Soc Psychol. 2000;79(5):701–21. doi: 10.1037/0022-3514.79.5.701. [DOI] [PubMed] [Google Scholar]
- 80.Khalil G. Fear and happiness in Re-Mission: teasing out emotional gaming events responsible for cancer risk perception. In: Schouten B, Fedtke S, Bekker T, Schijven M, Gekker A, editors. Games for Health. The Netherlands: Springer Vieweg, Wiesbaden; 2013. pp. 27–44. [Google Scholar]
- 81.Khalil GE, Rintamaki LS. A televised entertainment-education drama to promote positive discussion about organ donation. Health Educ Res. 2014 Apr;29(2):284–96. doi: 10.1093/her/cyt106. http://europepmc.org/abstract/MED/24399264 .cyt106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelder SH, Prokhorov AV, Murray N, Shegog R, Conroy JL, Agurcia C. ASPIRE, project design of a CD-ROM-based smoking prevention and cessation curriculum for urban youth. Proceedings of the 131st Annual Meeting of the American Public Health Association; the 131st Annual Meeting of the American Public Health Association; 2003; San Francisco, CA. 2003. [Google Scholar]
- 83.Johnston L, O'Malley PM, Bachman J, Schulenberg J. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings. Bethesda, MD: National Institute on Drug Abuse; 2009. [Google Scholar]
- 84.Rath JM, Villanti AC, Abrams DB, Vallone DM. Patterns of tobacco use and dual use in US young adults: the missing link between youth prevention and adult cessation. J Environ Public Health. 2012;2012:679134. doi: 10.1155/2012/679134. doi: 10.1155/2012/679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pierce JP, Choi WS, Gilpin EA, Farkas AJ, Merritt RK. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol. 1996 Sep;15(5):355–61. doi: 10.1037//0278-6133.15.5.355. [DOI] [PubMed] [Google Scholar]
- 86.Velicer WF, DiClemente CC, Prochaska JO, Brandenburg N. Decisional balance measure for assessing and predicting smoking status. J Pers Soc Psychol. 1985 May;48(5):1279–89. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- 87.Pepper JK, Emery SL, Ribisl KM, Rini CM, Brewer NT. How risky is it to use e-cigarettes? Smokers' beliefs about their health risks from using novel and traditional tobacco products. J Behav Med. 2015 Apr;38(2):318–26. doi: 10.1007/s10865-014-9605-2. http://europepmc.org/abstract/MED/25348584 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haug S, Meyer C, Dymalski A, Lippke S, John U. Efficacy of a text messaging (SMS) based smoking cessation intervention for adolescents and young adults: study protocol of a cluster randomised controlled trial. BMC Public Health. 2012;12:51. doi: 10.1186/1471-2458-12-51. http://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-12-51 .1471-2458-12-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bock BC, Barnett NP, Thind H, Rosen R, Walaska K, Traficante R, Foster R, Deutsch C, Fava JL, Scott-Sheldon LA. A text message intervention for alcohol risk reduction among community college students: TMAP. Addict Behav. 2016 Dec;63:107–13. doi: 10.1016/j.addbeh.2016.07.012.S0306-4603(16)30259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scull TM, Kupersmidt JB, Malik CV, Keefe EM. Examining the efficacy of an mHealth media literacy education program for sexual health promotion in older adolescents attending community college. J Am Coll Health. 2018 Apr;66(3):165–77. doi: 10.1080/07448481.2017.1393822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bandiera FC, Loukas A, Li X, Wilkinson AV, Perry CL. Depressive symptoms predict current e-cigarette use among college students in texas. Nicotine Tob Res. 2017 Sep 1;19(9):1102–6. doi: 10.1093/ntr/ntx014.2982066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer-review report from the Food and Drug Administration of the National Institutes of Health.
Examples of text messages.
Checklist for Reporting Results of Internet E-Surveys (CHERRIES).
Description of the main measures.


