Abstract
Objective:
The aim of this work was to investigate determinants of structural myocardial abnormalities in persons living with human immunodeficiency virus (PLWH).
Methods and Results:
We reviewed archived transthoracic echocardiograms (TTEs) performed on PLWH at Duke University Medical Center from 2001 to 2012. The primary outcomes were presence of left ventricular hypertrophy (LVH) or diastolic dysfunction (DD). TTEs for 498 human immunodeficiency virus-infected persons were reviewed (median age 44 years, 38% female, 72% black, 34% with hypertension, 15% with diabetes). Among those with usable images, LVH was detected in 174 of 473 persons (37%) according to LV mass criteria and in 99 of 322 persons (31%) according to American Society of Echocardiography LV mass index criteria. Definite DD was detected in 18 of 224 persons (8%). LVH was more common in PLWH with a CD4 count ≤ 200 cells/mm3 proximal to TTE (adjusted OR 1.68, 95% CI 1.082.62), CD4 nadir ≤ 200 cells/mm3 (adjusted OR 1.63, 95% CI 1.04–2.54) and less common in persons with viral suppression (OR 0.46, 95% CI 0.27–0.80). Lower CD4 nadirs (P = .002) and proximal CD4 counts (P = .002) were also associated with DD.
Conclusions:
Persons with a history of advanced human immunodeficiency virusassociated immune suppression are at higher risk of LVH and DD than infected persons with preserved immune function. (J Cardiac Fail 2018;24:496–503)
Keywords: Diastolic dysfunction, human immunodeficiency virus, left ventricular hypertrophy
Given the remarkable improvement in the life expectancy of persons living with human immunodeficiency virus (PLWH) worldwide, nonacquired immunodeficiency syndrome (AIDS) chronic diseases, such as car-diovascular disease (CVD), have emerged as leading causes of morbidity and mortality in this population.
CVD is now the fourth leading cause of death among PLWH, behind AIDS-related malignancies and opportunistic infections and non-AIDS-related malignancies and liver disease.1 After adjusting for known CVD risk factors, PLWH are at higher risk for major CVD events such as myocardial infarction, stroke, and sudden cardiac death compared with noninfected persons.2,3 Accordingly, efforts to understand determinants of atherosclerotic and nonatherosclerotic CVD in this population have intensified over the past decade.4
Despite our improved understanding of CVD in human immunodeficiency virus (HIV), relatively little is known about the implications of the complex pathobiology of HIV infection on myocardial architecture or the development of myocardial dysfunction in PLWH. In the general population, left ventricular hypertrophy (LVH) is a particularly ominous prognostic sign that may result in heart failure, ventricular arrhythmias, death after myocardial infarction, or decreased left ventricular (LV) ejection fraction (EF).5,6
Diastolic dysfunction, which can go undetected until late in the clinical course, is known to be a leading contributor to clinical heart failure.7 A few cohort studies have characterized echocardiographic abnormalities in asymptomatic PLWH, reporting a high prevalence of diastolic dysfunction (DD) and LVH.8,9 Although these reports have advanced our understanding of myocardial abnormalities in PLWH, most of the studied participants were relatively healthy overall, with demographic characteristics and CVD risk profiles not representative of the general HIV-infected population in the United States, which is increasingly made of non-Hispanic black adults in whom hypertension and LVH are prevalent.5,10,11
In an effort to gain further insight into the determinants of increased risk of CVD among persons living with HIV, particularly in the context of effective antiretroviral therapy (ART), our group reviewed archived transthoracic echocardiograms (TTEs) performed at our medical center in PLWH from 2001 to 2012. The overall objective was to identify HIV-related factors associated with LVH and DD in PLWH, including the impact of HIV immunologic parameters, ART exposure, and hepatitis C coinfection.
Methods
We conducted a retrospective review of all TTEs per-formed in PLWH at Duke University Medical Center from 2001 (the first year that HIV-1 RNA viral quantification was available) to 2012. All participants were selected from a query of the Duke HIV Clinical Database, a clinical record of all HIV-infected patients cared for at Duke since 1988. Results of this query were linked with records from the Duke Echocardiography Lab Database (DELD), a digital archive of all TTEs performed at Duke since 1995 and which has been previously described.12 Clinical data, including sociodemographic characteristics, behavioral risk data, CVD-related diagnoses (hypertension, diabetes, hyperlipidemia), and laboratory data pertinent to CVD risk and HIV infection, were abstracted from medical charts and entered into an electronic database by trained staff. In addition, data on substance abuse, hepatitis C, and antiretroviral exposure (protease inhibitors, abacavir, zidovudine, stavudine, and didanosine) were collected for all study participants. Prevalent heart failure was defined as persons with a billing (International Classification of Disease, Ninth Edition) code of heart failure in the medical record before the date of the echocardiogram analyzed in the study. The study was approved by the Duke University Institutional Review Board, and given the retrospective nature of the study, no informed consents were required.
Echocardiographic Measurements
For all study participants, only the first echocardiogram on record after the diagnosis of HIV infection was included in the analysis. All echocardiograms were reviewed independently by an experienced reader (F.A.) blinded to the clinical data. We reviewed only echocardiograms performed after the diagnosis of HIV. The LV end-systolic and end-diastolic volumes were determined by means of the biplane method from apical 2- and 4-chamber views. EF was calculated as the difference between the end-diastolic volume and end-systolic volume divided by the end-diastolic volume. The LV mass was determined by aligned linear measurements of LV cavity dimension and wall thickness and calculated with the use of a standard validated formula.13 The LV mass values were normalized to body surface area for patients with height and weight data available to obtain the LV mass index (LVMI). In addition, patterns in LV mass increase were categorized (concentric remodeling, concentric hypertrophy, or eccentric hypertrophy) with the use of criteria previously described in joint American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging guidelines.13
Outcome Variables and Covariates of Interest
The outcome variables of interest were LVH and DD. For patients for whom anthropomorphic data were available, LVH was defined as LVMI>115 g/m2 for men and>95 g/m2 for women, per ASE criteria.13 When height and weight data were absent, LVH was defined as LV mass>227 g in men and>171 g in women. DD was graded according to 2016 ASE guidelines, using the following criteria: average E/e′ ratio>14, septalé velocity<7 cm/s or lateral velocity<10 cm/s, tricuspid regurgitation velocity>2.8 m/s, and LA volume index>34 mL/m2.14 Patients with 3 or more criteria positive were diagnosed with DD. Patients who had 2 of 4 diagnostic criteria or 1 positive criteria and missing data for 1 or more of the remaining criteria were classified as “indeterminate” for DD. Patients who were not classified in any of the aforementioned groups were deemed to not have DD. Owing to small sample size, DD was modeled as a binary variable (positive/indeterminate or negative). The exposure covariates of interest were proximal CD4 count (most recent CD4 count preceding echocardiogram)<200 cells/mm,3 CD4 nadir<200 cells/mm3 preceding echocardiogram, viral suppression defined as all viral loads in the 6 months before echocardiogram<400 copies/mL, hepatitis C coinfection (positive hepatitis C antibody and documented detectable hepatitis C virus [HCV] viral load before TTE) and any exposure to abacavir, didanosine, stavudine, zidovudine, or protease inhibitors.
Statistical Analysis
Univariable analysis for the association of relevant exposure variables with LVH and DD was done with the use of simple logistic regression. Individual multivariable logistic regression models were fitted separately for 3 HIV immune parameters (proximal CD4 count, nadir CD4 count, and viral suppression), adjusting for basic demographics (age, sex, race, and years since HIV diagnosis), traditional risk factors (hyperlipidemia, diabetes, hypertension, and obesity), substance abuse (cocaine and tobacco use), ART exposure, and hepatitis C. Results are reported as odds ratios (ORs) and 95% confidence intervals (CIs). A significance level of 5% was used throughout the analysis. All statistical analyses were performed with the use of SAS statistical software, version 9.4 (Cary, North Carolina).
Results
A query of the Duke HIV Clinical Database yielded 5215 unique persons with HIV cared for at Duke through December 31, 2012. After matching records with DELD, there were 766 unique PLWH who had a TTE during this time period. Of these, 498 persons had a TTE performed after the diagnosis of HIV infection, making up the final study analysis cohort. The characteristics of the study cohort were as follows: median age at time of TTE 44 years, median age at HIV diagnosis 35 years, 62% male, 72% black/African American. The median time between HIV diagnosis and first TTE was 7.3 years (interquartile range [IQR] 2.7–12.1). A previous diagnosis of hypertension was found in 34% of participants, 17% had hyperlipidemia, and 18% had a previous diagnosis of diabetes. Fifty-seven percent of the study participants were either current or former cigarette users, and 32% had a documented history of cocaine use. Fifteen percent of the study participants had a diagnosis of heart failure at the time of the analysis echocardiogram (Table 1). The median CD4 count just before the TTE was 244 cells/mm3 (IQR 68–487) and the median CD4 nadir among participants was 84 cells/mm3 (IQR 18–247). The mean time between CD4 nadir and baseline echo was 144 days. The median time between last viral load and baseline TTE was 275 days. Viral load data was available for 361 of the 498 study participants. Of these, 44% had a suppressed viral load at the time of the TTE (Table 1).
Table 1.
Clinical Characteristics of Patients in Duke HIV Echocardiography Cohort (n = 498)
| Age at first TTE (y) | 44 (37–51) |
|---|---|
| Age at HIV diagnosis (y) | 35 (29–43) |
| Time from HIV diagnosis to first TTE (y) | 7.3 (2.7–12.1) |
| Female | 187 (38) |
| Race/Ethnicity | |
| White non-Hispanic | 108 (22) |
| Black non-Hispanic | 360 (72) |
| Hispanic | 16 (3) |
| Other | 14 (3) |
| Body mass index (kg/m2) | |
| Tobacco use | 26.6 (23.1–31.2) |
| None | 212 (43) |
| Former | 79 (16) |
| Active | 199 (41) |
| Cocaine use | 159 (32) |
| Diabetes | 89 (15) |
| Hyperlipidemia | 84 (17) |
| Hypertension | 169 (34) |
| ACE inhibitor at time of TTE | 101 (20) |
| Beta-blocker at time of TTE | 89 (18) |
| Coronary artery disease | 27 (5) |
| Heart failure | 76 (15) |
| Proximal CD4 count (cells/mm3) | 244 (68–547) |
| Nadir CD4 count (cells/mm3) | 84 (18–247) |
| Viral load 400 copies/mL for 6 months before TTE | 159 (44)[N= 361] |
| Hepatitis C | 91 (18) |
| On antiretroviral therapy | 356 (72) |
| ART exposure by drug/class | |
| Abacavir | 91 (18) |
| Didanosine/stavudine | 131 (26) |
| Zidovudine | 147 (30) |
| Protease Inhibitors | 229 (46) |
Values are presented as median (interquartile range) or n (%). ACE, angiotensin-converting enzyme; ART, antiretroviral therapy; HIV, human immunodeficiency virus; TTE, transthoracic echocardiogram.
For the study cohort, the median LV mass was 182 g (IQR 148–215). The median septal wall thickness was 1.1 cm (IQR 1.0–1.2). The median LVMI for the cohort was 92 g/m2 (IQR 76–113). With the use of LV mass cutoffs alone, 174 of 473 participants (37%) met criteria for LVH. When ASE LVMI criteria were applied, 99 of 322 persons assessed (31%) met echocardiographic criteria for LVH (Table 2). In the unadjusted analysis, hypertension (OR 1.56, 95% CI 1.05–2.31, P = .03) and beta-blocker use (OR 1.76, 95% CI 1.10–2.82; P = .02) were the only risk determinants significantly associated with the diagnosis of LVH. There were, however, trends toward significance for cocaine use (OR 1.45, 95% CI 0.97–2.17; P = .07), proximal CD4 count <200 cells/mm3 (OR 1.38, 95% CI 0.92–2.08; P = .12) and CD4 nadir <200 cells/mm3 (OR 1.46, 95% CI 0.96–2.22; P = .08). Of note, there was a significant negative association between viral load suppression and LVH in the unadjusted analysis (OR 0.56, 95% CI 0.35–0.89; P = .02). In the multivariable analysis, proximal CD4 count <200 cells/mm3 (OR 1.68, 95% CI 1.08–2.62; P = .02), and CD4 nadir <200 cells/ mm3 (OR 1.63, 95% CI 1.04–2.54; P = .03) were independently associated with an increased prevalence of LVH. These associations held when the variables for nadir CD4 count (OR per 50-cell decrease nadir CD4 = 1.09, 95% CI 1.02–1.14; P = .02) and proximal CD4 count (OR per 50-cell decrease proximal CD4= 1.04, 95% CI 1.00–1.09; P=.03) were modeled on continuous scales. Viral load suppression was also found to be independently associated with a decreased prevalence of LVH (OR 0.46, 95% CI 0.27–0.80; P = .01; Tables 3 and 4).
Table 2.
Echocardiographic Parameters and Outcomes of Study Cohort (n = 498)
| LV dimensions and mass | |
|---|---|
| SWT (cm) | 1.1 (1.0–1.2) |
| PWT (cm) | 1.1 (1.0–1.2) |
| LVEDD (cm) | 4.7 (4.3–5.1) |
| LV mass (g) | 182 (148–215) |
| LV mass index (g/m2) | 92 (76–113) [N=322] |
| LV volumes and systolic function | |
| LV end-diastolic volume index (mL/m2) | 112 (99–127) [N=271] |
| LV end-systolic volume index (mL/m2) | 44 (34–60) [N=271] |
| LV ejection fraction 50% | 44 (34–60) [N=271] |
| LV diastolic function | |
| E/A ratio, median (IQR) | 1.35 (1.08–1.67) [N=257] |
| LA volume (mL/m2) | 32 (24–44) [N=247] |
| MV E velocity (m/s) | 85 (77–105) [N=261] |
| MV A velocity (m/s) | 67 (56–78) [N=257] |
| MV deceleration time (m/s) | 190 (160–220) [N=252] |
| E/E′ ratio | 11.6 (8.6–14.7) [N=83] |
| TR velocity (m/s) | 2.0 ± 0.6 [N=244] |
| LVH by LV mass | 174/473 (37) |
| LVH by LV mass index | 99/322 (31) |
| Diastolic dysfunction by ASE criteria | |
| None | 122/224 (54) |
| Indeterminate | 85/224 (38) |
| Definite | 18/224 (8) |
Values are presented as median (IQR), n (%), or mean § standard devia-tion. ASE, American Society of Echocardiography; IQR, interquartile range; LA, left atrial; LV, left ventricular; LVEDD, left ventricular end- diastolic diameter; LVH, left ventricular hypertrophy; MV, mitral valve; PWT, posterior wall thickness; PV, pulmonary valve; SWT, septal wall thickness, TR, tricuspid regurgitation.
Table 3.
Univariate Odds Ratios (ORs) for Left Ventricular Hypertrophy (LVH) and Diastolic Dysfunction (DD)
| Variable | LVH (n = 448) |
DD (n = 224) |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age, per 10-y increase | 1.10 | 0.92—1.31 | .30 | 1.37 | 1.06—1.76 | .01 |
| Black race | 1.22 | 0.79—1.88 | .38 | 0.76 | 0.43—1.36 | .35 |
| Hypertension | 1.56 | 1.05—2.31 | .03 | 1.21 | 0.71—2.05 | .48 |
| Hyperlipidemia | 1.22 | 0.75—2.00 | .42 | 1.43 | 0.77—2.66 | .25 |
| Diabetes mellitus | 1.33 | 0.83—2.16 | .23 | 1.79 | 0.94—3.44 | .08 |
| Cocaine use | 1.45 | 0.97—2.17 | .07 | 0.88 | 0.50—1.54 | .65 |
| Tobacco use, current | 1.25 | 0.75—2.10 | .37 | 0.49 | 0.29—0.84 | .01 |
| Tobacco use, any | 1.19 | 0.81—1.76 | .39 | 1.40 | 0.66—2.96 | .38 |
| Hepatitis C infection | 0.92 | 0.56—1.52 | .75 | 0.93 | 0.48—1.79 | .82 |
| Time from HIV diagnosis to first TTE, per 5-y increase | 0.99 | 0.85—1.16 | .90 | 1.07 | 0.87—1.31 | .53 |
| ACE inhibitor use | 1.44 | 0.91—2.27 | .12 | 1.30 | 0.72—2.37 | .38 |
| Beta-blocker use | 1.76 | 1.10—2.82 | .02 | 2.01 | 1.09—3.71 | .03 |
| Abacavir exposure | 0.73 | 0.44—1.21 | .22 | 0.86 | 0.46—1.62 | .64 |
| Didanosine/stavudine exposure | 1.05 | 0.68—1.63 | .82 | 1.30 | 0.69—2.44 | .42 |
| Zidovudine exposure | 1.10 | 0.73 — 1.67 | .89 | 0.64 | 0.36—1.15 | .33 |
| PI exposure | 0.87 | 0.59—1.28 | .48 | 0.85 | 0.50—1.44 | .54 |
ACE, angiotensin-converting enzyme inhibitor; CI, confidence interval; PI, protease inhibitor.
Table 4.
Odds Ratios (ORs) for Left Ventricular Hypertrophy (n = 498)
| Variable | Unadjusted |
Adjusted |
|||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | Wald χ (2) | P Value | |
| Proximal CD4 count < 200 cells/mm3 | 1.38 | 0.92–2.08 | .12 | 1.68 | 1.08–2.62 | 5.26 | .02 |
| CD4 nadir < 200 cells/mm3 | 1.46 | 0.96–2.22 | .11 | 1.63 | 1.04–2.54 | 4.60 | .03 |
| VL (most recent) < 400 copies/mL | 0.56 | 0.35–0.89 | .02 | 0.46 | 0.27–0.80 | 7.48 | .006 |
| Abacavir exposure | 0.73 | 0.44–1.21 | .22 | 0.74 | 0.43–1.30 | 1.09 | .30 |
| Didanosine/stavudine exposure | 1.05 | 0.68–1.63 | .82 | 1.17 | 0.72–1.89 | 0.38 | .54 |
| Zidovudine exposure | 1.10 | 0.73–1.67 | .89 | 1.17 | 0.75–1.83 | 0.47 | .79 |
| PI exposure Continuous proximal CD4 count |
0.87 | 0.59–1.28 | .48 | 0.83 | 0.53–1.33 | 0.59 | .44 |
| per 1-cell/mm3 decrease | 1.001 | 1.00–1.001 | .07 | 1.001 | 1.00–1.002 | 4.75 | .03 |
| per 50-cell/mm3 decrease | 1.03 | 1.00–1.06 | 1.04 | 1.00–1.09 | |||
| Continuous nadir CD4 | |||||||
| per 1-cell/mm3 decrease | 1.001 | 1.00–1.002 | .05 | 1.002 | 1.00–1.003 | 5.97 | .02 |
| per 50-cell/mm3 decrease | 1.06 | 1.00–1.12 | 1.09 | 1.02–1.14 | |||
CI, confidence interval; PI, protease inhibitor; VL, viral load.
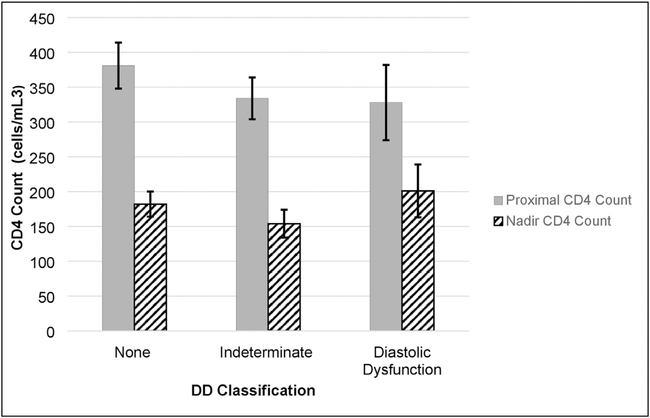
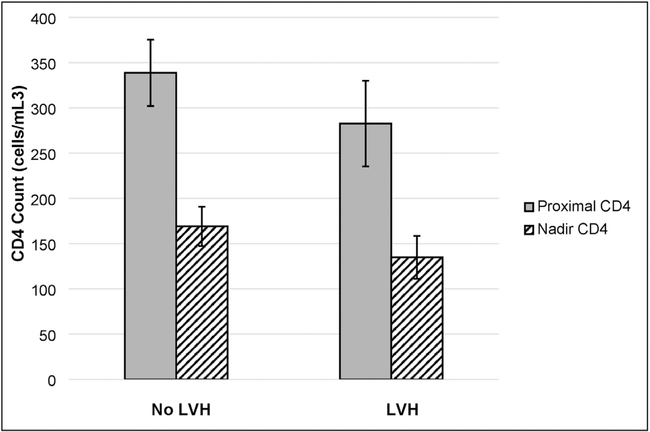
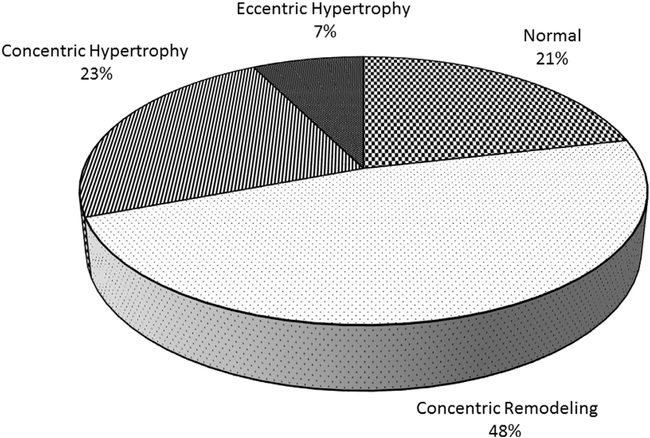
There were 224 patients who had studies of sufficient quality for interpretation for DD according to the 2016 ASE criteria. Among these, 18 (8%) met criteria for DD and an additional 85 (38%) had at least 1 criterion for DD detected on TTE. In the unadjusted analysis, only age (OR 1.37, 95% CI 1.061.76; P = .01), current versus never tobacco use (OR 0.49, 95% CI 0.29–0.84; P = .01), and beta-blocker use (OR 2.01, 95% CI 1.09–3.71; P = .03) had significant associations with the detection of indeterminate or definite DD in our study cohort, although there was a strong trend toward significance for diabetes (OR 1.79, 95% CI 0.94–3.44; P = .08) as well. Results from our multivariable regression analysis did not show an association between proximal CD4 count, CD4 nadir <200 cells, and viral suppression and DD, although there was a trend toward an association between proximal CD4 count and DD (OR 1.69, 95% CI 0.883.26; P = .12; Table 5). How ever, when modeled as a continuous variables, decreases in proximal CD4 count (OR per 50-cell decrease = 1.31 95% CI 1.10–1.56) and decreases in CD4 nadir (OR per decrease = 1.37, 95% CI 1.12–1.67) were both associated with increased prevalence of DD (Table 5) There was no significant difference in CD4 counts by DD class (Fig. 1). Overall, PLWH with LVH did have slightly lower proximal CD4 counts and CD4 nadirs than those without LVH (Fig. 2). Finally, in classifica-tions of LVH by relative wall thickness, only 21% of participants had no evidence of ventricular remodeling. Forty-eight percent had evidence of concentric remodeling, and 30% had some evidence of left ventricular hypertrophy (Fig. 3).
Table 5.
Odds Ratios (ORs) for Diastolic Dysfunction (n = 498)
| Variable | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | Wald χ (2) | P value | |
| Proximal CD4 count < 200 cells/mm3 | 1.42 | 0.80–2.52 | .23 | 1.69 | 0.88–3.26 | 2.46 | .12 |
| CD4 nadir < 200 cells/mm3 | 1.33 | 0.77–2.31 | .31 | 1.43 | 0.78–2.63 | 1.31 | .25 |
| VL (most recent) < 400 copies/mL | 0.78 | 0.45–1.37 | .39 | 0.64 | 0.33–1.27 | 1.63 | .20 |
| Abacavir exposure | 0.87 | 0.47–1.64 | .67 | 0.79 | 0.38–1.65 | 0.40 | .52 |
| Didanosine/stavudine exposure | 1.31 | 0.70–2.48 | .40 | 1.45 | 0.69–3.05 | 0.94 | .33 |
| Zidovudine exposure | 0.64 | 0.36–1.15 | .14 | 0.66 | 0.34–1.28 | 1.50 | .22 |
| PI exposure Continuous proximal CD4 count |
0.83 | 0.49–1.41 | .50 | 0.69 | 0.36–1.35 | 1.17 | .28 |
| per 1-cell/mm3 decrease | 1.001 | 1.00–1.001 | .17 | 1.005 | 1.002–1.01 | 9.32 | .002 |
| per 50-cell/mm3 decrease | 1.03 | 0.99–1.08 | 1.31 | 1.10–1.56 | |||
| Continuous nadir CD4 | |||||||
| per 1-cell/mm3 decrease | 1.001 | 1.00–1.001 | .35 | 1.006 | 1.00–1.01 | 9.28 | .002 |
| per 50-cell/mm3 decrease | 1.03 | 0.96–1.11 | 1.37 | 1.12–1.67 | |||
CI, confidence interval; PI, protease inhibitor; VL, viral load.
Fig. 1.
Median proximal and CD4 counts by diastolic dysfunction (DD) classification.
Fig. 2.
Median proximal and CD4 counts by left ventricular hypertrophy (LVH) classification.
Fig. 3.
Left ventricular hypertrophy classification based on relative wall thickness.
Discussion
In this analysis of TTEs conducted at a large academic medical center, we found that low CD4 nadirs and low current CD4 counts are associated with an increased prevalence of LVH and DD as detected by echocardiography. Interestingly, these associations spanned the scale of CD4 counts and, in the case of DD, were not necessarily restricted to the presence of severe immunosuppression. Consistently with previous reports in PLWH, we also found a high prevalence of increased LV mass in our study cohort.8,9 Notably, when current guidelines for the definition of DD were applied, we discovered a much lower prevalence of frank DD than previously reported.
Cohort studies of PLWH have demonstrated that after adjusting for traditional CVD risk factors, PLWH are at higher risk for acute coronary syndrome and sudden cardiac death than uninfected persons.2,3,15 Imaging studies have also shown a higher prevalence of subclinical changes to myocardial architecture in persons living with HIV16. A cardiac magnetic resonance imaging study of 90 asymptom- atic PLWH and 39 uninfected control subjects without - known coronary artery disease showed a significantly higher rate of patchy myocardial fibrosis among the HIV , infected participants than in the control subjects. In the same study, myocardial lipid levels were also 47% higher 50-cell in HIV-infected participants than in uninfected control sub . jects.16 These findings suggest a unique correlation between chronic HIV infection and myocardial fibrosis and steatosis, processes that are known to be associated with increased LV stiffness, and in turn DD.17,18 Silent ischemic events (and associated myocardial remodeling) in the context of unstable coronary plaque also may play an accessory role in the development of LVH in PLWH.19
Hsue et al9 first reported on the impact of HIV infection on LVH and DD. In their study, 50% of patients with asymptomatic HIV had evidence of mild DD on TTE (compared with 29% of control subjects). Those authors also reported an association of CD4 nadir, but not current CD4 count or elevated HIV-1 viral load, with increased LVMI. In a subsequent multicenter study of 656 asymptomatic PLWH, Mondy et al8 reported a DD prevalence of 26%. However, in their analysis the presence of neither DD or LVH on TTE was significantly associated with elevated HIV viremia, CD4 nadir, or CD4 count at the time of the TTE. The observed disparity in the reported prevalence of DD between these 3 studies is not surprising, given the well documented complexity of echocardiographic diagnoses of true DD.20 The prevalence estimates of DD in our cohort were much lower (8%) than in the aforementioned studies. The most likely reason for this discrepancy is our use of the 2016 ASE guidelines for the definition of DD. The 2016 ASE guidelines impose a stricter criteria to be assigned a diagnosis of DD while simultaneously allowing an “indeterminate” classification.14 The previous reports used the 2009 ASE guidelines for the classification of DD, which allowed a significantly less stringent requirement for average e/é ratio (≥8 vs ≥14 in 2016 guidelines), a less stringent requirement for septalé velocity (<8 cm/s vs <7 cm/ s), and no TR velocity requirement.8,9,21,22 Overall, 38% of our study cohort met 50% of criteria for DD and were therefore classified as “indeterminate DD.” To our knowledge, this study is one of the first to report on DD using the more discriminative 2016 ASE criteria, and future reports of DD in this population should be based on that diagnostic standard. Nevertheless, even with these more selective criteria for the diagnosis of DD, previously reported associations of CD4 nadir and current CD4 count with the DD remain true.
Left ventricular hypertrophy was significantly more prevalent in our cohort than DD. Overall, 31% of patients in our cohort had an LVMI that met criteria for LVH. This rates is more consistent with previous reports.8,9 Given the relative young age of the cohort (median age 44 years), our study population may have captured individuals before the development of detectable myocardial relaxation abnormalities. Interestingly, our analysis showed that only 21% of patients in the cohort had no evidence of LV wall thickening. This observation further demonstrates the degree of risk among PLWH who do not meet diagnostic criteria for LVH, suggesting that a majority of patients in our cohort may already be on the clinical continuum of LV wall thickening to LVH to DD. These questions necessitate a longitudinal echocardiographic analysis of PLWH with increased LV mass to improve our understanding of the natural history of DD, and potentially heart failure with preserved EF, in this population. In addition, our analysis supports the hypothesis that immune dysfunction, as measured by CD4 nadir and current CD4 counts, is an independent risk factor for increased LV mass, LVH, and DD, which is consistent with the findings of Hsue et al.23,24 In addition, we found an inverse association between viral suppression and the presence of LVH, an observation that has not been previously reported. Taken together, these data support an association of severe immunosuppression with alterations in myocardial architecture likely to increase the risk of clinical heart failure in PLWH.
Our findings of the association of HIV viremia with LVH support the cardiotoxicity of HIV-1. Although many early reports validated the presence of HIV-induced cardiomyopathy, many of those cases presented as symptomatic dilated cardiomyopathy.25 Autopsy studies of the HIV-infected hearts have shown the presence of HIV-induced myocarditis in as many as one-third of specimens studied, highlighting the cardiotrophic potential of the virus.26 Finally, our cohort, which included 72% non-Hispanic black patients, more closely represents the current HIV epidemic than previous reports did. According to the Centers for Disease Control and Prevention, non-Hispanic black patients represent 41% of PLWH and 44% of new infections in the United States.11
Study Limitations
Several limitations of our study must be acknowledged. Owing to the retrospective study design, our analysis was limited to data available in the clinical archive. It is likely that there was some selection bias because only individuals with clinical indications were referred to the echocardiography laboratory. However, our detected prevalence of LVH is consistent with previous reports in PLWH and higher than previously reported prevalence estimates in the literature.27,28 In addition, because our study was directed toward evaluating the contribution of HIV-associated determinants to DD and LVH, we did not include a contemporaneous group of HIV-noninfected control subjects for comparison. By prospectively enrolling PLWH with and without DD, as well as a non-HIV DD control group, the ongoing Characterizing Heart Function on Antiretroviral Therapy (CHART) study is well positioned to examine associations of LVH, DD, and immune status independently from traditional risk factors. That study aims to establish clinical phenotypes of DD in persons with chronic HIV.29 Finally, the cross-sectional nature of the present study does not address the potential evolution of ventricular dysfunction among PLWH and limits our ability to evaluate the future risk of the evolution of incident clinical heart failure. Although future studies of our cohort will focus on monitoring its members longitudinally to investigate the natural history of ventricular dysfunction in this group, a prospectively identified and followed longitudinal cohort is needed to explore and fully characterize the evolution and determinants of cardiac structural alterations in PLWH. Non-Hispanic black patients are also overrepresented in our cohort compared with the population of HIV-infected persons nationally. Although this may limit the generalizability of our findings presently, non-Hispanic black patients account for 54% of all new infections in the South and 44% nationally, making the study of cardiovascular disease in this segment of the population of added importance.11 To date, this demographic has been underrepresented in previous reports.8,9,22 Finally, our study did not have a contemporaneous group of HIV-noninfected control subjects for comparison
Conclusion
The present review of a large archive of echocardiograms demonstrates an association between low CD4 count and both LVH and DD in a demographically representative sample of PLWH. In addition, with the use of updated 2016 ASE diagnostic guidelines for DD, we found a lower prevalence of DD than in previous reports.14 Our study is the first to characterize the epidemiology of echocardiographic abnormalities in non-Hispanic black patients, a critical HIV risk demographic. Our findings also suggest a strong association of HIV-related immunosuppression with LVH and DD. Whether these myocardial changes are reversible is unclear, but our findings further validate the importance of treating PLWH regardless of CD4 count and as soon after diagnosis as possible in accordance with current Department of Health and Human Services guidelines.30 Findings from our analysis also corroborate the consensus of previous reports that traditional CVD risk factors are strongly associated with the presence of echocardiographic abnormalities in PLWH.8,9 Comprehensive strategies to optimize both traditional and HIV-related risk determinants of heart disease are likely needed to prevent an increase in the burden of heart failure among persons living with HIV.
Acknowledgments
Funding: Duke Center for AIDS Research (P30 AI064518) and the Division of Cardiology, Duke University.
Footnotes
Disclosures
The authors have no conflicts of interest to report.
References
- 1.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014;3:241–8. [DOI] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;1:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr 2014;6:160–6. [DOI] [PubMed] [Google Scholar]
- 4.Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol 2013;6:511–23. [DOI] [PubMed] [Google Scholar]
- 5.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005;4:124–9. [DOI] [PubMed] [Google Scholar]
- 6.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998;3:1454–9. [DOI] [PubMed] [Google Scholar]
- 7.Braunwald E Heart failure. JACC Heart Fail 2013;1:1–20. [DOI] [PubMed] [Google Scholar]
- 8.Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, et al. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis 2011;5:378–86. [DOI] [PubMed] [Google Scholar]
- 9.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail 2010;3:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension 19882008. JAMA 2010;3:2043–50. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. HIV surveillancereport, volume 26: Diagnoses of HIV infection in the United States and dependent areas, 2014. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2014-vol-26.pdf.
- 12.Velazquez EJ, Samad Z, Al-Khalidi HR, Sangli C, Grayburn PA, et al. The Mitraclip and survival in patients with mitral regurgitation at high risk for surgery: a propensity-matched comparison. Am Heart J 2015;1:1050–9. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;2:1–39. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;2:277–314. [DOI] [PubMed] [Google Scholar]
- 15.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;9:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 2013;1:814–22. [DOI] [PubMed] [Google Scholar]
- 17.van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic and hypertensive-diabetic heart disease. Circulation 1990;8:848. [DOI] [PubMed] [Google Scholar]
- 18.Jain A, Avendano G, Dharamsey S, Dasmahapatra A, Agarwal R, Reddi A, Regan T. Left ventricular diastolic function in hypertension and role of plasma glucose and insulin. Comparison with diabetic heart. Circulation 1996;9:1396. [DOI] [PubMed] [Google Scholar]
- 19.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, Grinspoon SK. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS 2013;2:1263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossman W Defining diastolic dysfunction. Circulation 2000;1:2020–1. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Appleton CP, Gillbert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;2:107–33. [DOI] [PubMed] [Google Scholar]
- 22.Secemsky EA, Scherzer R, Nitta E, Wu AH, Lange DC, Deeks SG, et al. Novel biomarkers of cardiac stress, cardiovascular dysfunction and outcomes in HIV-infected individuals. JACC Heart Fail 2015;3:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004;1:1603–8. [DOI] [PubMed] [Google Scholar]
- 24.Ho JE, Scherzer R, Hecht FM, Maka K, Selby V, Martin JN, et al. The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. AIDS 2012;2:1115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sliwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J 2012;3:866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson DW, Virmani R, Reilly JM, O’Leary T, Cunnion RE, Robinowitz M, et al. Prevalent myocarditis at necropsy in the acquired immunodeficiency syndrome. J Am Coll Cardiol 1988;1:792–9. [DOI] [PubMed] [Google Scholar]
- 27.Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England screening study: a population based study. Lancet 2001;3:439–44. [DOI] [PubMed] [Google Scholar]
- 28.Stritzke J, Markus MR, Duderstadt S, Lieb W, Luchner A, Doring A, Keil U. et al. ; MONICA/KORA Investigators. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (Monitoring of Trends and Determinations in Cardiovascular Disease/Cooperative Research in the Region of Augsburg) study. J Am Coll Cardiol 2009;5:1982–9. [DOI] [PubMed] [Google Scholar]
- 29.Butler J, Kalogeropoulos AP, Anstrom KJ, Hsue PY, Kim RJ, Scherzer R, et al. Diastolic dysfunction in individuals with human immunodeficiency virus infection: literature review, rationale and design of the Characterizing Heart Function on Anti-Retroviral Therapy (CHART) study. J Card Fail 2018;24:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panel on Antiretroviral Guidelines for Adults and Adolescents, Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.