Abstract
PURPOSE
The purpose of this study was to examine the incremental effect of performance coaching, delivered as part of a multicomponent intervention (Ottawa Model for Smoking Cessation [OMSC]), in increasing rates of tobacco-dependence treatment by primary care clinicians.
METHODS
In a cluster-randomized controlled trial, 15 primary care practices were randomly assigned to 1 of the following active-treatment conditions: OMSC or OMSC plus performance coaching (OMSC+). All practices received support to implement the OMSC. In addition, clinicians in the OMSC+ group participated in a 1.5-hour skills-based coaching session and received an individualized performance report. All clinicians and a cross-sectional sample of their patients were surveyed before and 4 months after introduction of the interventions. The primary outcome measure was rates of tobacco-dependence treatment strategy (Ask, Advise, Assist, Arrange) delivery. Secondary outcomes were patient quit attempts and smoking abstinence measured at 6 months’ follow-up.
RESULTS
Primary care clinicians (166) and patients (1,990) were enrolled in the trial. Clinicians in the OMSC+ group had statistically greater rates of delivery for Ask (adjusted odds ratio [AOR] = 1.69; 95% CI, 1.05-2.72), Assist (AOR = 1.64; 95% CI, 1.08-2.49), and Arrange (AOR = 2.01; 95% CI, 1.22-3.31). Sensitivity analysis found that the rate of delivery for Advise was greater only among those clinicians who attended the coaching session (AOR = 1.65; 95% CI, 1.10-2.49; P = .02). No differences were documented between groups for cessation outcomes.
CONCLUSIONS
Performance coaching significantly increased rates of tobacco-dependence treatment by primary care clinicians when delivered as part of a multicomponent intervention.
Key words: smoking cessation, primary care, audit and feedback, randomized controlled trial, coaching, quality improvement, knowledge translation, Ottawa Model for Smoking Cessation
INTRODUCTION
Smoking cessation is arguably the most powerful preventive intervention available in primary care practice.1-3 The 5 As strategy (Ask, Advise, Assess, Assist, Arrange) is the basis for tobacco-dependence treatment in clinical settings; however, integrating evidence-based tobacco-dependence treatment into clinical practice routines remains a challenge.1-8 The important role of family medicine in addressing tobacco use with patients is well recognized, and multiple international guidelines and reports have identified the need to increase rates of tobacco-dependence treatment in primary care settings.1-9
Strategies, including clinician training, electronic health record (EHR) prompts, and adjunct counseling, have been shown to significantly increase rates of tobacco-dependence treatment in primary care settings.10-14 Meta-analyses show that multicomponent interventions combining several intervention strategies are the reference standard for increasing clinician performance in delivering tobacco-dependence treatment.1 The Ottawa Model for Smoking Cessation (OMSC) is a multicomponent quality improvement intervention for addressing tobacco use with smokers in clinical settings that has been implemented in more than 350 hospitals and primary care practices in Canada (http://www.ottawamodel.ca).15-18 The OMSC supports primary care teams with the introduction of a systematic, team-based approach to addressing tobacco-dependence treatment delivery based on 10 best practices.19 Evaluations of the OMSC in primary care settings have documented a significant increase in clinician delivery of evidence-based tobacco-dependence treatments.14-15 Despite an overall increase in treatment rates, significant variability in rates of tobacco-dependence treatment delivery can exist among individual clinicians exposed to the OMSC—even within the same practice.15-16 This variability suggests that the intervention does not take hold among all clinicians in the same way; clinician-level factors may be responsible for some of the observed variance.20-23
Continuing medical education for tobacco-dependence treatment typically involves a single session using didactic training methods. Tobacco-dependence treatment can be a complex clinical intervention, however, requiring a number of skills; 1-time didactic educational sessions may be inadequate for some clinicians. Active forms of continuing medical education, such as interactive training including audit and feedback, are promising methods for ensuring the implementation of evidence-based guidelines within general practice.24-26 Evidence from the health care quality improvement literature suggests that reinforcement training and peer coaching may improve physicians’ and medical residents’ patterns of practice.27-33
The primary objective of this study was to compare the incremental effectiveness of clinician performance coaching when delivered as part of a multicomponent intervention (OMSC) on rates of tobacco-dependence treatment in family practice, compared with the multicomponent intervention alone. Secondary objectives were assessments of the effect of the intervention on patient quit attempts and smoking abstinence.
METHODS
Study Design
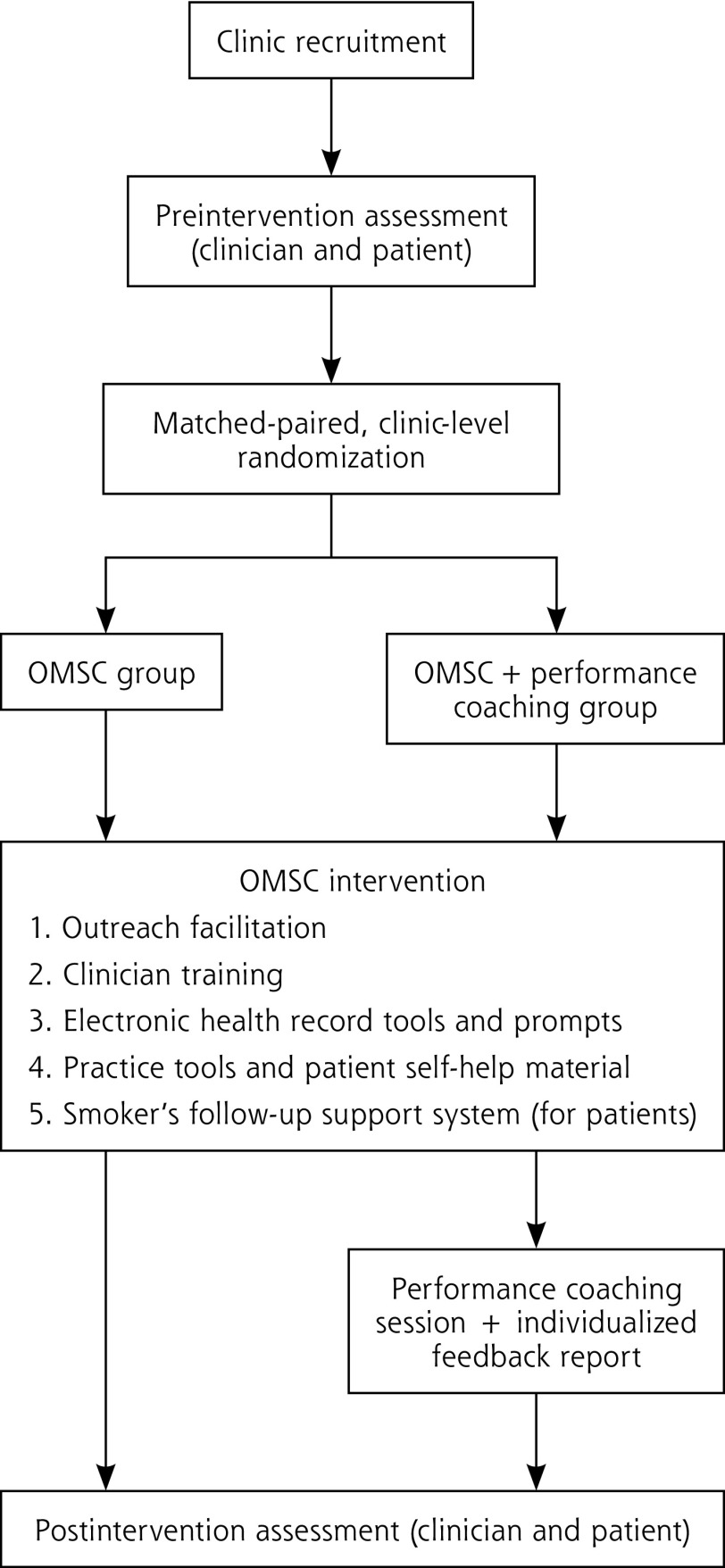
A cluster-randomized controlled trial was undertaken with family health teams (≥5 clinicians) in the province of Ontario, Canada (Figure 1). The complete research protocol for the trial has been published.19 Family medicine practices were matched and randomly assigned to 1 of the following intervention arms: OMSC or OMSC plus clinician performance coaching (OMSC+). From each of the participating practices, a cross-sectional sample of eligible tobacco users was recruited before and after intervention to assess clinicians’ performance in tobacco-dependence treatment delivery (4 As), patient quit attempts, and biochemically verified 7-day point-prevalence abstinence. The trial was approved by the Ottawa Health Science Network Research Ethics Board.
Figure 1.
Randomized controlled trial study design.
OMSC = Ottawa Model for Smoking Cessation.
Randomization and Concealment
The average rate for delivery of the Advise strategy for each practice at baseline was used to match practices before randomization because previous evaluations of the OMSC have shown imbalance in this variable.15,16 Randomization was conducted by the Research Methods Centre (University of Ottawa Heart Institute, Ottawa, Ontario, Canada), which was blind to practice identifiers. Patients and research assistants were blinded to their practice’s group assignment.
Clinical Model
The OMSC is grounded in the latest evidence-based guidelines for tobacco treatment and uses an adaptation of the well-known 5 As model, which includes the following15,16: (1) Asking all patients about their smoking status; (2) delivering personalized Advice to quit smoking to all smokers and offering support with cessation; (3) Assisting patients ready to quit smoking by developing a personalized plan for quitting that involves scheduling a dedicated visit to deliver evidence-based counseling to support cessation, set a quit date, select a quit-smoking pharmacotherapy, and provide self-help material; and (4) Arranging follow-up support, which includes counseling and management of pharmacotherapy to prevent relapse, for 2 to 6 months. Follow-up is typically scheduled monthly after a patient’s quit date. Patients had the option to receive additional telephone-based support between clinic appointments.
Ottawa Model for Smoking Cessation Intervention
The OMSC intervention model supports teams in implementing the 5 As clinical model by using a quality improvement process focused on the introduction of the OMSC 10 Best Practices for delivering tobacco-dependence treatment (Table 1).18 All teams were exposed to this multicomponent intervention that combines several evidence-based strategies including (1) outreach facilitation, (2) clinician training, (3) EHR tools and prompts, (4) practice tools and patient self-help material, and (5) smoker’s follow-up system.5,10,11 Table 2 provides a description of the OMSC intervention components.
Table 1.
OMSC 10 Best Practices
| 1. Clinic task force formed |
| 2. Clinic tobacco-control protocol developed |
| 3. Tobacco use queried and documented for all clinic patients |
| 4. Training in tobacco-dependence treatment completed by clinicians in past year |
| 5. Specific staff identified to provide tobacco-dependence treatment |
| 6. Self-help materials available to patients, family members, and staff |
| 7. EHR or other real-time prompt in place to inform GP/NP of patient smoking status, advice delivery, and quit plan consult forms |
| 8. Process to follow-up tobacco users for at least 2 to 6 months after clinic visit |
| 9. Process to evaluate quality of program implementation in place |
| 10. Process to provide feedback to practices about clinic performance in tobacco-dependence treatment delivery |
EHR = electronic health record; GP = general practitioner; NP = nurse practitioner; OMSC = Ottawa Model for Smoking Cessation.
Adapted with permission from Papadakis S, Cole AG, Reid RD, et al. Increasing rates of tobacco treatment delivery in primary care practice: Evaluation of the Ottowa Model for Smoking Cessation. Ann Fam Med. 2016:14(3):235-243.
Table 2.
Summary of OMSC Multicomponent Intervention Components
| Component | Description |
|---|---|
| Outreach facilitation visits | Trained outreach facilitator works with each primary care clinic over a 3-month period to implement the program |
| 7-step facilitation process used to introduce OMSC 10 Best Practices. Facilitators act by supporting clinics as follows: | |
| Review current clinic practices for delivery of evidence-based smoking cessation intervention and complete needs assessment | |
| Provide information and recommendations on integration of evidence-based smoking cessation strategies into clinical practice | |
| Facilitate development of clinic tobacco-dependence treatment protocol for integrating evidence-based smoking cessation strategies into all clinic appointments | |
| Define roles and responsibilities of clinic staff for delivering evidence-based smoking cessation treatments | |
| Support communications and training activities for members of clinic staff | |
| Clinician training | Frontline physicians and nurse practitioners participate in 3-hour training session providing information and skills training for addressing tobacco use with patients in the context of a busy primary care practice setting |
| Key staff responsible for delivering quit plan visits (eg, nurse, nurse practitioner, pharmacist) attend intensive 1-day training session teaching how to conduct quit plan and follow-up visits based on evidence-based practice | |
| Electronic health record tools and real-time prompts | Real time point-of-care reminders (eg, standard smoking-status questions) introduced and embedded in vital-sign screening forms and prompts to document smoking status and deliver brief advice |
| Standardized check-list style smoking cessation consult forms embedded into EHRs to guide tobacco treatment delivery for advice, quit plan, and follow-up visit | |
| Practice tools and patient self-help material | All materials designed to support intervention delivery and reduce amount of face-to-face time required to support tobacco-dependence treatment delivery. Materials include the following: |
| Patient tobacco use survey to document smoking history | |
| Patient self-help quit plan booklet for smokers ready to quit | |
| Patient self-help booklet for smokers not ready to quit | |
| Clinic waiting room posters and materials | |
| Smoker’s follow-up support system | Patients ready to quit referred to smoker’s follow-up system including 5 triage calls or e-mails delivered over a 2-month period (3, 7, 14, 30, 60 days after quit date) by automated program. Patients struggling with quit attempt had additional telephone-based support arranged from trained smoking-cessation counselors, and as required, changes to their quit plan coordinated with primary care clinician |
EHR = electronic health record; OMSC = Ottawa Model for Smoking Cessation.
Adapted with permission from Papadakis S, Cole AG, Reid RD, et al. Increasing rates of tobacco treatment delivery in primary care practice: Evaluation of the Ottowa Model for Smoking Cessation. Ann Fam Med. 2016:14(3):235-243.
Ottawa Model For Smoking Cessation Plus Clinician Performance Coaching
The OMSC+ group received the same multicomponent intervention as the OMSC group. In addition, general practitioners and nurse practitioners received a supplemental 1.5-hour coaching session approximately 4 weeks after the launch of the OMSC at their clinic and received an individualized performance report. The performance coaching intervention was delivered in a group format, at each practice location, by a trained tobacco-dependence treatment specialist using a standardized facilitation guide. Given that clinician self-efficacy (ie, confidence) is associated with rates of tobacco-dependence treatment delivery, this intervention was designed to influence the following 4 factors known to affect self-efficacy: (1) skills training, (2) personal experience, (3) modeling of behaviors, and (4) positive social or environmental supports.34,35 During this session, clinicians identified personal barriers as well as success strategies for tobacco-dependence treatment delivery. The session facilitator introduced 7 techniques that clinicians could use in their practices to address known barriers, with a particular focus on addressing patient resistance, ambivalence, stress, and mental health issues.18 Peer-to-peer exchange and role modeling were used as teaching techniques.34
Data Collection
All participating clinicians and patients provided written informed consent. The characteristics of practices and clinicians were collected at baseline. At each practice, before implementation of the intervention, consecutive patients arriving for appointments were screened for eligibility. Eligible patients were aged ≥18 years, smoked an average of at least 5 cigarettes per day, were scheduled for an annual examination or nonurgent medical appointment with a physician and/or nurse practitioner, and were able to read and understand English or French. Patients were not required to be ready to quit smoking to be eligible to participate. All patient participants completed an exit survey after their clinic visit and were contacted by telephone 6 months (±2 weeks) after their clinic visit to assess cessation outcomes. After practices had implemented the intervention for at least 4 months, postimplementation data were collected from clinicians via a follow-up survey and from a second cross-sectional sample of patients using procedures identical to those used at baseline.
Outcome Measures
The primary outcome was rates of clinician tobacco-dependence treatment delivery of 4 of the 5 As (Ask, Advise, Assist, Arrange). We chose not to examine the Assess component in the present study to reduce respondent burden. The study exit survey asked patients whether or not (binary scale: yes, no) at today’s clinic appointment their physician or another member of the team asked them about their smoking status (Ask), advised them to quit smoking (Advise), provided assistance with quitting (Assist), or arranged follow-up support (Arrange). For the Assist strategy, supplemental data were collected regarding the type of assistance (eg, providing self-help materials, setting a quit date, discussing or prescribing smoking-cessation drugs). Patient exit interviews have been used in most previous evaluations of tobacco-dependence treatment interventions in primary care; they have been shown to be more reliable than clinician self-report.36
Patient self-reports of quit attempts lasting 24 hours or more after their index visit, 7-day point-prevalence abstinence (not having smoked even a puff in the past 7 days), and 12-week continuous abstinence (not having smoked even a puff from week 14 up to week 26) were assessed at the 6-month telephone follow-up interview.36 The NicAlert saliva cotinine test was used for biochemical validation (>10 ng/mL) of smoking status.37,38 Participants were mailed the NicAlert test, instruction sheet, and a prepaid package for returning the kit. Patients lost to follow-up were assumed to be active smokers.37
Sample Size and Analysis
Sample-size calculations were adjusted for the cluster-randomized controlled trial design and informed by previous OMSC evaluations.15,16 All calculations were based on a 1-sided test with 80% power, an α level of .05, and intraclass correlation coefficients (ICCs) of 0.05 for 4 As and 0.01 for cessation.39 Sample-size calculations indicated that 10 practices per group with 60 patients per practice were required to detect a minimum difference of 10% between intervention groups for 4 As delivery and 5% for smoking abstinence.
Multilevel models account for the clustered design. A 3-level generalized linear mixed model estimated the effect of the intervention for each outcome measure with the following levels: patients (level 1), clinicians (level 2), and clinics (level 3). Wald tests were used to obtain P values, adjusted odds ratios [AORs], and 95% CIs. Both practice-level ICCs (ie, variation between practices) and clinician-level ICCs (ie, variation between individual clinicians) were calculated. ICC is measured on a scale from 0 to 1, with a value close to 0 indicating that the clusters were similar.38 Data analysis was conducted on an intention-to-treat basis (ie, all clinicians and all patients as allocated to the intervention). Sensitivity analysis examined attendance at the coaching session. All analyses were conducted using SAS 9 (SAS Institute Inc).
RESULTS
Recruitment Flow
Fifteen practices and 166 clinicians were enrolled in the trial. The study was conducted from September 2013 to May 2015. The patient sample comprised 1,123 eligible smokers who participated in the preassessment and a second cross-sectional sample of 867 smokers who participated in the postassessment. Six-month telephone follow-up data were available for ~77% of patients at both the pre- and postassessments, with no between-group differences. The Consolidated Standards of Reporting Trials (CONSORT) study flow diagram is shown in Supplemental Figure 1, http://www.annfammed.org/content/16/6/498/suppl/DC1.
Practice, Clinician, and Patient Characteristics
There were no differences in practice and clinician characteristics between intervention groups (Supplemental Table 1, http://www.annfammed.org/content/16/6/498/suppl/DC1). Patient characteristics are presented in Supplemental Table 2, http://www.annfammed.org/content/16/6/498/suppl/DC1.
Rates of Tobacco-Dependence Treatment Delivery
Both the OMSC and OMSC+ groups documented significant increases in rates of Ask, Advise, and Assist between the pre- and postassessments (Table 3).
Table 3.
Clinician Performance in 4 As Delivery and Patient Outcomes at Postintervention Assessment by Intervention Group
| Parameter | OMSC | OMSC+ | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pre n = 540 |
Post n = 394 |
AOR (95% CI)a |
P Valueb |
Pre n = 583 |
Post n = 473 |
AOR (95% CI)a |
P Valueb |
|
| 4 As delivery | ||||||||
| Ask | 47.3 | 55.9 | 1.45 (1.10-1.92) | .009 | 46.8 | 65.7 | 2.40 (1.83-3.14)c | <.001 |
| Advise | 38.1 | 48.1 | 1.63 (1.20-2.11) | .001 | 42.5 | 53.5 | 1.71 (1.31-2.24) | <.001 |
| Assist | 35.1 | 42.8 | 1.44 (1.09-1.91) | .011 | 33.3 | 53.9 | 2.62 (1.99-3.45) | <.001 |
| Set quit date | 12.4 | 12.1 | 1.03 (0.68-1.55) | .898 | 11.2 | 18.7 | 1.93 (1.33-2.79) | .001 |
| Self-help | 10.0 | 11.1 | 1.19 (0.77-1.84) | .444 | 10.8 | 19.4 | 2.05 (1.42-2.98) | .001 |
| Discuss medications | 25.5 | 26.9 | 1.11 (0.81-1.51) | .517 | 26.1 | 37.1 | 1.87 (1.41-2.50) | <.001 |
| Prescribe medications | 8.7 | 8.8 | 1.11 (0.68-1.80) | .670 | 9.1 | 12.2 | 1.47 (0.96-2.27) | .080 |
| Arrange | 12.2 | 13.4 | 1.10 (0.73-1.66) | .649 | 10.3 | 22.5 | 2.66 (1.84-3.84) | <.001 |
| Patient-level outcomes | ||||||||
| Quit attempts | 29.0 | 30.0 | 1.01 (0.75-1.36)c | .934 | 27.8 | 35.0 | 1.41 (1.07-1.86)c | .015 |
| 7-day point-prevalence abstinence (self-reported) | 4.6 | 9.1 | 2.18 (1.25-3.82)c | .006 | 6.0 | 6.5 | 1.13 (0.65-1.96) | .669 |
| 7-day point-prevalence abstinence (biochemically validated) | 0.0 | 2.8 | … | … | 0.3 | 2.5 | 13.03 (1.65-102.84)c | .015 |
| 6-month continuous abstinence | 4.1 | 6.6 | 1.75 (0.92-3.30) | .086 | 4.0 | 4.8 | 1.30 (0.69-2.45) | .423 |
AOR = adjusted odds ratio; OMSC = Ottawa Model for Smoking Cessation; post = postassessment; pre = preassessment.
Controlling for clinic-level variance between clusters, patient sex, patient education, and self-reported anxiety or depression; based on inclusion of 15 clinics unless otherwise indicated.
P value based on Wald statistic.
The estimated G matrix for clinic-level variance was not a definite positive, so clinic-level variance was not included in this model.
Clinicians in the OMSC+ group had statistically greater performance in the rates of Ask (AOR = 1.69; 95% CI, 1.05-2.72), Assist (AOR = 1.64; 95% CI, 1.08-2.49), and Arrange (AOR = 2.01; 95% CI, 1.22-3.31) compared with the OMSC group (Table 4). No significant difference was observed in the rates of Advise in the intention-to-treat analysis.
Table 4.
Clinician Performance in Tobacco-Dependence Treatment Delivery at Postassessment by Intervention Group
| Parameter | OMSC (n = 394) |
OMSC+ (n = 473) |
% Delta | AOR (95% CI)a |
P Value | ICC Clinician | ICC Clinic |
|---|---|---|---|---|---|---|---|
| 4 As delivery | |||||||
| Ask | 55.9 | 65.7 | 9.8 | 1.69 (1.05-2.72) | .03 | 0.126b | 0.025 |
| Advise | 48.1 | 53.5 | 5.4 | 1.42 (0.82-2.46) | .22 | 0.129 | 0.045 |
| Assist | 42.8 | 53.9 | 11.1 | 1.64 (1.08-2.49) | .02 | 0.089c | 0.027 |
| Set quit date | 18.9 | 25.7 | 6.8 | 1.70 (1.09-2.65) | .02 | 0.037 | 0.035 |
| Self-help | 11.1 | 19.4 | 8.3 | 2.01 (1.15-3.52) | .02 | 0.072 | 0.043 |
| Discuss medications | 26.9 | 37.1 | 10.2 | 1.75 (1.15-2.65) | .01 | 0.069 | 0.026 |
| Prescribe medications | 8.8 | 12.2 | 3.4 | 1.44 (0.85-2.42) | .18 | 0.002 | 0.026 |
| Arrange | 24.7 | 35.6 | 10.9 | 2.01 (1.22-3.31) | .01 | 0.036 | 0.050 |
| Patient-level outcome | |||||||
| Quit attemptsd | 30.0 | 35.0 | 5.0 | 1.36 (1.00-1.84) | .05 | … | … |
| 7-day point prevalence abstinence (self-reported)d | 9.1 | 6.5 | −2.6 | 0.73 (0.43-1.26) | .26 | … | … |
| 7-day point prevalence abstinence (biochemically validated)d | 2.8 | 2.5 | −0.3 | 1.05 (0.42-2.64) | .92 | … | … |
| Continuous abstinence | 6.6 | 4.8 | −2.2 | 0.82 (0.40-1.67) | .58 | 0.038 | 0.030 |
AOR = adjusted odds ratio; ICC = intraclass correlation coefficient; OMSC = Ottawa Model for Smoking Cessation.
Controlling for clinic-level variance between clusters, patient sex, patient education, and self-reported anxiety or depression; based on inclusion of 15 clinics unless otherwise indicated.
P < .01.
P = .05.
For ICC Clinician and ICC Clinic, the estimated G matrix was not a definite positive; therefore, we could not calculate clinician or clinic ICC.
A total of 34% of clinicians randomized to the OMSC+ group did not attend the performance coaching session, owing to annual leave, maternity leave, or illness. Sensitivity analysis found that rates of Ask (AOR = 1.51; 95% CI, 1.01-2.26), Advise (AOR = 1.65; 95% CI, 1.10-2.49), and Assist (AOR = 1.50; 95% CI, 1.03-2.19) were significantly greater among clinicians who attended the coaching session compared with clinicians who were not exposed to performance coaching (Supplemental Table 3, http://www.annfammed.org/content/16/6/498/suppl/DC1).
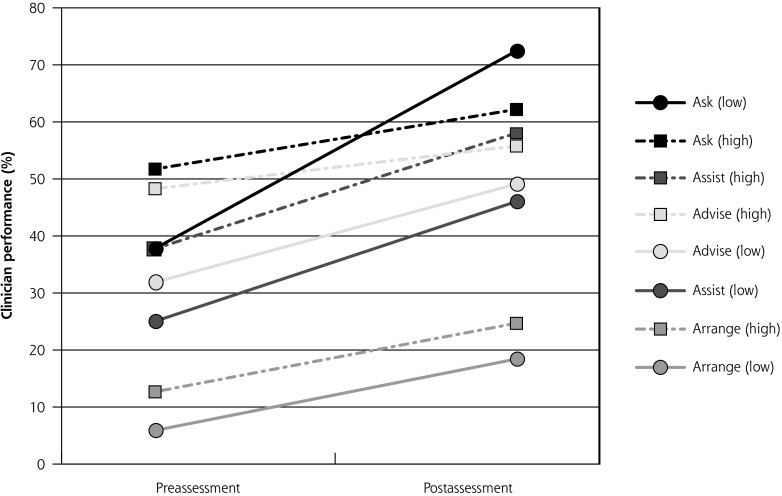
Clinicians who documented lower performance at baseline (<40%) had greater overall increases in rates of Ask and Advise relative to higher-performing clinicians (Figure 2). Changes in rates of Assist and Arrange were found to be similar between low- and high-performing clinicians. Clinicians were more likely to Ask, Advise, and Assist if the patient was seen in the clinic for a first visit or annual examination compared with other types of appointments (Supplemental Table 4, http://www.annfammed.org/content/16/6/498/suppl/DC1). Clinicians’ beliefs about the importance of cessation were associated with rates of Ask (AOR = 4.36; 95% CI, 2.05-9.29; P < .001). Patient readiness to quit and smoking-related illness were also associated with 4 As delivery (Supplemental Table 4).
Figure 2.
Clinician performance in tobacco-dependence treatment delivery at pre- and postassessment in the intervention group according to clinic baseline performance.
Note: Low-performing clinics had a baseline rate of Advise <40.5%; high-performing clinics had a baseline rate of Advise ≥40.5%.
Cessation Outcomes
Multilevel regression analysis showed a borderline effect of intervention group on quit attempts (AOR = 1.36; 95% CI, 1.00-1.84) but no significant effect on patient smoking cessation (Table 4). A small decrease was observed in self-reported 7-day point-prevalence abstinence between the pre- and postassessments in the OMSC+ group. There were no statistically significant differences in rates of smoking abstinence between groups.
Patients were significantly more likely to be abstinent at the follow-up if they were ready to quit smoking in the next 30 days at the index visit, reported high self-efficacy with quitting, did not report anxiety or depression, and had a dedicated smoking cessation visit scheduled at the primary care clinic (Supplemental Table 5, http://www.annfammed.org/content/16/6/498/suppl/DC1).
DISCUSSION
Consistent with earlier evaluations of the OMSC, both of the active-intervention groups increased rates of tobacco-dependence treatment delivery. There was a further incremental increase in the rates of tobacco-dependence treatment delivered by clinicians when the multicomponent intervention was combined with clinician performance coaching. The performance coaching intervention was informed by behavior change theory, quality improvement literature, and previous research. Participants received coaching from both a trained tobacco-dependence treatment specialist and higher-performing peers from their own practice setting. The coaching sessions were well attended, well received, and were easily implemented in the context of general practice. A small number of trials have evaluated the efficacy of reinforcement contact, educational outreach visits, or performance feedback after tobacco-dependence treatment training and found them to be associated with desirable changes in clinician behaviors.40-46 Evidence from the broader primary care literature has documented the value of educational outreach visits, audits, and feedback.26-28 Systematic reviews have found that feedback is most effective when delivered by a respected colleague, presented frequently, and features both specific goals and action plans.26-28 Performance feedback has also been found to be particularly useful in assisting clinicians with low baseline performance.26,27 In the present study, we also observed that clinicians with lower baseline performance with respect to Ask and Advise showed greater overall increases in treatment rates relative to higher-performing colleagues.
The coaching session did not significantly affect 6-month smoking abstinence rates when compared with the OMSC intervention. It is possible that the strength of the intervention program was insufficient in producing clinic-wide patient-level increases in cessation, a finding that has been reported by others.47-49 Smokers enrolled in the present study were heavily nicotine dependent, and it is likely that more-intensive treatment may be needed to support cessation in this patient population. Importantly, and unlike other evaluations in primary care, our study included all smokers and not just those ready to quit smoking. Patients who scheduled for a quit plan visit were significantly more likely to report abstinence from smoking, suggesting that this is an important component of treatment success. Changes in clinician-level behavior observed among practices exposed to the OMSC can be lever aged to facilitate the uptake of evidence-based treatment in future research.
Our findings should be interpreted in light of certain study limitations. First, the study compared 2 active-intervention arms and did not include a control condition. All participating practices were family health teams in the province of Ontario, Canada; the generalizability to other practice models or to other health care systems would require further examination. The assessment was based on patient report of 4 As delivery, which may be subject to reporting bias. Electronic health record data collection for 4 As delivery was not established in clinics at baseline and as such could not be used to examine pre- and post-rates of 4 As delivery. We reported on data for same-day clinic encounters, and as such our findings may not be comparable to studies or reports that use longer time frames (ie, previous 12 months). Our study provides evidence regarding the value of performance coaching when delivered as part of a multicomponent intervention rather than as a stand-alone intervention. We tested a single coaching session, and it is possible that exposure to additional coaching sessions might further increase the likelihood of tobacco-dependence treatment delivery. The optimal amount and frequency of performance coaching is an area for future research.
Identifying and evaluating simple, effective techniques for promoting tobacco-dependence treatment delivery is essential to improving the reach of tobacco-dependence treatment interventions in primary care settings. This study lends support for the integration of performance coaching in the design and delivery of multicomponent interventions to further increase rates of tobacco-dependence treatment delivery, particularly among low-performing clinicians.
Acknowledgments:
The authors thank the staff and clinicians of the primary care clinics involved in this study for their participation. Our thanks to Kelly Cobey and Klea Bertakis for reviewing this manuscript.
Footnotes
Funding support: This study was funded by a grant-in-aid from the Heart and Stroke Foundation of Canada (Grant #NA7193).
Conflicts of interest: R.D.R. has received speaker and consulting fees from Pfizer and Johnson & Johnson; K-A.M. has received speaker fees from Pfizer; A.L.P. has received speaker and consulting fees from Pfizer and Johnson & Johnson. All others report none.
To read or post commentaries in response to this article, see it online at http://www.AnnFamMed.org/content/16/6/498.
Previous presentation: Partial results of this paper were presented at the Society for Research on Nicotine and Tobacco Annual Meeting; March 8-11, 2017; Florence, Italy.
Clinical trial registry: NCT01603524.
Supplementary materials: Available at http://www.AnnFamMed.org/content/16/6/498/suppl/DC1/.
References
- 1.McIvor A, Kayser J, Assaad JM, et al. Best practices for smoking cessation interventions in primary care. Can Respir J. 2009; 16(4): 129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER Package. Geneva, Switzerland: WHO; 2008. http://www.who.int/tobacco/mpower/mpower_report_full_2008.pdf. [Google Scholar]
- 3.Vardavas CI, Symvoulakis EK, Lionis C. Dealing with tobacco use and dependence within primary health care: time for action. Tob Induc Dis. 2013; 11(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Report on the Global Tobacco Epidemic, 2017: Monitoring Tobacco Use and Prevention Policies. Geneva, Switzerland: WHO; 2017. http://apps.who.int/iris/handle/10665/255874. [Google Scholar]
- 5.Van Schayck OCP, Williams S, Barchilon V, et al. Treating tobacco dependence: guidance for primary care on life-saving interventions. Position statement of the IPCRG. NPJ Prim Care Respir Med. 2017; 27(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 7.European Network for Smoking and Tobacco Prevention. European Smoking Cessation Guidelines: The Authoritative Guide to a Comprehensive Understanding of the Implications and Implementation of Treatments and Strategies to Treat Tobacco Dependence. Brussels, Belgium: European Network for Smoking and Tobacco Prevention; 2012. http://ensp.network/wp-content/uploads/2016/12/ENSP-ESCG_FINAL.pdf. [Google Scholar]
- 8.Schauer GL, Wheaton AG, Malarcher AM, Croft JB. Health-care provider screening and advice for smoking cessation among smokers with and without COPD: 2009-2010 National Adult Tobacco Survey. Chest. 2016; 149(3): 676-684. [DOI] [PubMed] [Google Scholar]
- 9.Royal College of Physicians. Hiding in plain sight: treating tobacco dependency in the NHS. London, UK: RCP, 2018. https://www.rcplondon.ac.uk/projects/outputs/hiding-plain-sight-treating-tobacco-dependency-nhs. Published Jun 26, 2018 Accessed Jul 2018. [Google Scholar]
- 10.Anderson P, Jané-Llopis E. How can we increase the involvement of primary health care in the treatment of tobacco dependence? A meta-analysis. Addiction. 2004; 99(3): 299-312. [DOI] [PubMed] [Google Scholar]
- 11.Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A. Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis. Prev Med. 2010; 51(3-4): 199-213. [DOI] [PubMed] [Google Scholar]
- 12.Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database Syst Rev. 2014(12): CD008743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013; 173(6): 458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piper ME, Baker TB, Mermelstein R, et al. Recruiting and engaging smokers in treatment in a primary care setting: developing a chronic care model implemented through a modified electronic health record. Transl Behav Med. 2013; 3(3): 253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadakis S, McDonald PW, Pipe AL, Letherdale ST, Reid RD, Brown KS. Effectiveness of telephone-based follow-up support delivered in combination with a multi-component smoking cessation intervention in family practice: a cluster-randomized trial. Prev Med. 2013; 56(6): 390-397. [DOI] [PubMed] [Google Scholar]
- 16.Papadakis S, Cole AG, Reid RD, et al. Increasing rates of tobacco treatment delivery in primary care practice: evaluation of the Ottawa Model for Smoking Cessation. Ann Fam Med. 2016;14(3):235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid RD, Mullen KA, Slovinec D’Angelo ME, et al. Smoking cessation for hospitalized smokers: an evaluation of the “Ottawa Model”. Nicotine Tob Res. 2010; 12(1): 11-18. [DOI] [PubMed] [Google Scholar]
- 18.Mullen KA, Manuel DG, Hawken SJ, et al. Effectiveness of a hospital-initiated smoking cessation programme: 2-year health and healthcare outcomes. Tob Control. 2017; 26(3): 293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadakis S, Pipe AL, Reid RD, Tulloch H, Mullen KA, Assi R, et al. Effectiveness of performance coaching for enhancing rates of smoking cessation treatment delivery by primary care providers: study protocol for a cluster randomized controlled trial. Contemp Clin Trials 2015; 45(Pt B): 184-190. [DOI] [PubMed] [Google Scholar]
- 20.Vogt F, Hall S, Marteau TM. General practitioners’ and family physicians’ negative beliefs and attitudes towards discussing smoking cessation with patients: a systematic review. Addiction. 2005; 100(10): 1423-1431. [DOI] [PubMed] [Google Scholar]
- 21.Young JM, Ward JE. Implementing guidelines for smoking cessation advice in Australian general practice: opinions, current practices, readiness to change and perceived barriers. Fam Pract. 2001; 18(1): 14-20. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb NH, Guo JL, Blozis SA, Huang PP. Individual and contextual factors related to family practice residents’ assessment and counseling for tobacco cessation. J Am Board Fam Pract. 2001; 14(5): 343-351. [PubMed] [Google Scholar]
- 23.Vaughn TE, Ward MM, Doebbeling BN, Uden-Holman T, Clarke WT, Woolson RF. Organizational and provider characteristics fostering smoking cessation practice guideline adherence: an empirical look. J Ambul Care Manage. 2002; 25(2): 17-31. [DOI] [PubMed] [Google Scholar]
- 24.Gagliardi AR, Alhabib S, the Guidelines International Network Implementation Working Group Trends in guideline implementation: a scoping systematic review. Implement Sci. 2015; 10(1): 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostofian F, Ruban C, Simunovic N, Bhandari M. Changing physician behavior: what works? Am J Manag Care. 2015; 21(1): 75-84. [PubMed] [Google Scholar]
- 26.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012; 6(6): CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau R, Stevenson F, Ong BN, et al. Achieving change in primary care—effectiveness of strategies for improving implementation of complex interventions: systematic review of reviews. BMJ Open. 2015; 5(12): e009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014; 29(11): 1534-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyasere CA, Baggett M, Romano J, Jena A, Mills G, Hunt DP. Beyond continuing medical education: clinical coaching as a tool for ongoing professional development. Acad Med. 2016; 91(12): 1647-1650. [DOI] [PubMed] [Google Scholar]
- 30.Zahid A, Hong J, Young CJ. Coaching experts: applications to surgeons and continuing professional development. Surg Innov. 2018; 25(1): 77-80. [DOI] [PubMed] [Google Scholar]
- 31.Sargeant J, Lockyer JM, Mann K, et al. The R2C2 model in residency education: how does it foster coaching and promote feedback use? Acad Med. 2018; 93(7): 1055-1063. [DOI] [PubMed] [Google Scholar]
- 32.Christopher BA, Grantner M, Coke LA, Wideman M, Kwakwa F. Better care teams: a stepwise skill reinforcement model. J Contin Educ Nurs. 2016; 47(6): 283-288. [DOI] [PubMed] [Google Scholar]
- 33.Larkin A, LaCouture M, Geissel K, et al. Quality improvement in management of acute coronary syndrome: continuing medical education and peer coaching improve antiplatelet medication adherence and reduce hospital readmissions. Crit Pathw Cardiol. 2017; 16(3): 96-101. [DOI] [PubMed] [Google Scholar]
- 34.Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: Freeman; 1997. [Google Scholar]
- 35.O’Loughlin J, Makni H, Tremblay M, et al. Smoking cessation counseling practices of general practitioners in Montreal. Prev Med. 2001; 33(6): 627-638. [DOI] [PubMed] [Google Scholar]
- 36.Pbert L, Adams A, Quirk M, Hebert JR, Ockene JK, Luippold RS. The patient exit interview as an assessment of physician-delivered smoking intervention: a validation study. Health Psychol. 1999; 18(2): 183-188. [DOI] [PubMed] [Google Scholar]
- 37.West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005; 100(3): 299-303. [DOI] [PubMed] [Google Scholar]
- 38.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., III Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003; 5(5): 621-624. [DOI] [PubMed] [Google Scholar]
- 39.Baskerville NB, Hogg W, Lemelin J. The effect of cluster randomization on sample size in prevention research. J Fam Pract. 2001; 50(3): W241-6. [PubMed] [Google Scholar]
- 40.Bentz CJ, Bayley KB, Bonin KE, et al. Provider feedback to improve 5A’s tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine Tob Res. 2007; 9(3): 341-349. [DOI] [PubMed] [Google Scholar]
- 41.Andrews JO, Tingen MS, Waller JL, Harper RJ. Provider feedback improves adherence with AHCPR Smoking Cessation Guideline. Prev Med. 2001; 33(5): 415-421. [DOI] [PubMed] [Google Scholar]
- 42.Leone FT, Evers-Casey S, Graden S, Schnoll R, Mallya G. Academic detailing interventions improve tobacco use treatment among physicians working in underserved communities. Ann Am Thorac Soc. 2015; 12(6): 854-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swartz SH, Cowan TM, DePue J, Goldstein MG. Academic profiling of tobacco-related performance measures in primary care. Nicotine Tob Res. 2002; 4(Suppl 1): S38-S44. [DOI] [PubMed] [Google Scholar]
- 44.Jin M, Gagnon A, Levine M, Thabane L, Rodriguez C, Dolovich L. Patient-specific academic detailing for smoking cessation: feasibility study. Can Fam Physician. 2014; 60(1): e16-e23. [PMC free article] [PubMed] [Google Scholar]
- 45.Goldstein MG, Niaura R, Willey C, et al. An academic detailing intervention to disseminate physician-delivered smoking cessation counseling: smoking cessation outcomes of the Physicians Counseling Smokers Project. Prev Med. 2003; 36(2): 185-196. [DOI] [PubMed] [Google Scholar]
- 46.Richmond R, Mendelsohn C, Kehoe L. Family physicians’ utilization of a brief smoking cessation program following reinforcement contact after training: a randomized trial. Prev Med. 1998; 27(1): 77-83. [DOI] [PubMed] [Google Scholar]
- 47.Joseph AM, Arikian NJ, An LC, Nugent SM, Sloan RJ, Pieper CF. Results of a randomized controlled trial of intervention to implement smoking guidelines in veterans affairs medical centers: Increased use of medications without cessation benefit. Med Care. 2004; 42(11): 1100-1110. [DOI] [PubMed] [Google Scholar]
- 48.Fiore MC, McCarthy DE, Jackson TC, et al. Integrating smoking cessation treatment into primary care: an effectiveness study. Prev Med. 2004; 38(4): 412-420. [DOI] [PubMed] [Google Scholar]
- 49.Thomas D, Abramson MJ, Bonevski B, George J. System change interventions for smoking cessation. Cochrane Database Syst Rev. 2017; 2(2): CD010742. [DOI] [PMC free article] [PubMed] [Google Scholar]