Abstract
Dysregulation of the maternal-fetal hypothalamic-pituitary-adrenal axis (HPAA) has been hypothesized to negatively influence various offspring physical and mental health outcomes. Limited data suggest that low maternal socioeconomic status (SES) in pregnancy may disrupt maternal HPAA functioning. Research is needed that examines how maternal SES in childhood may influence maternal HPAA functioning in pregnancy, given evidence that early life adversity can have persistent effects on physiological stress reactivity. In a sample of 343 sociodemographically diverse women, we tested whether indices of life course SES were associated with HPAA functioning across pregnancy reflected in hair cortisol collected within one week after delivery. Mothers were asked whether their parent(s) owned their home across three developmental periods, from birth through adolescence, as an indicator of their childhood SES. Measures of maternal SES in pregnancy included maternal educational attainment, annual household income, and current homeownership. Analyses revealed that indicators of lower maternal SES in childhood and in pregnancy were associated with higher cortisol levels during each trimester. In analyses adjusted for maternal race/ethnicity, pre-pregnancy body mass index, smoking in pregnancy, use of inhaled and topical corticosteroids, and mode of delivery, each indicator of maternal SES in pregnancy fully mediated maternal childhood SES effects on maternal hair cortisol levels in pregnancy. This is the first study to show an association between maternal life course SES and hair cortisol in pregnancy. The results suggest that maternal SES, starting in childhood, may have intergenerational consequences via disruption to the maternal-fetal HPAA in pregnancy. These findings have implications for elucidating mechanisms contributing to health disparities among socioeconomically disadvantaged populations.
Keywords: Hair cortisol, Hypothalamic-pituitary-adrenal (HPA) axis, Socioeconomic status (SES), Childhood, Pregnancy, Maternal
1. Introduction
Dysregulation of the maternal hypothalamic-pituitary-adrenal axis (HPAA) in pregnancy has been hypothesized to impact fetal development negatively, with potentially long-term consequences. Studies have linked prenatal exposure to disrupted cortisol levels in utero to numerous maladaptive outcomes across the lifespan, including poor fetal physical development and child health problems; disrupted HPAA functioning; cortical thinning evident into childhood; poorer cognitive and motor development; elevated negative affectivity and difficult temperament in infancy and toddlerhood; greater emotional and behavior problems in childhood; and increased risk for posttraumatic stress disorder in adulthood (Brand et al., 2006; Davis et al., 2007; Davis et al., 2013; de Weerth et al., 2003; Enlow et al., 2017; Field and Diego, 2008; Zijlmans et al., 2015). Therefore, identifying factors that influence functioning of the maternal HPAA functioning in pregnancy has important public health implications.
Limited research suggests that maternal socioeconomic status (SES) may influence maternal HPAA functioning in pregnancy. A few studies have linked higher cortisol levels with contemporaneous indicators of low SES (education, income) in pregnant women, although these associations do not always hold when models are adjusted for other risk factors (e.g., increased body mass index [BMI], smoking) (Braig et al., 2015; Schreier et al., 2016; Thayer and Kuzawa, 2014; Ursache et al., 2017). In a non- pregnant sample of adults, chronic exposure to low SES from infancy through early adulthood was more consistently predictive of various indicators of disrupted HPAA functioning than SES from any particular developmental period (DeSantis, Kuzawa, et al., 2015). To date, no study has examined whether maternal SES prior to pregnancy influences HPAA functioning during pregnancy, although recent theories of SES effects on health suggest that a life course approach may be appropriate (Yang et al., 2017). A sensitive period model (Yang et al., 2017) suggests that maternal SES in childhood may have lifelong impact on the functioning of the HPAA, including during pregnancy. Data link low SES in childhood with long- term disruption of the functioning of the HPAA as well as other key physiological systems that influence HPAA functioning (e.g., oxidative stress, inflammation) (Bates et al., 2017; Lockwood et al., 2018; Sheridan et al., 2013). Consequently, the effects of low SES in childhood may persist even if SES conditions later improve (Yang et al., 2017). Maternal SES in childhood may also influence maternal HPAA functioning in pregnancy through a pathway model, which posits that childhood SES establishes a trajectory to adulthood SES (Yang et al., 2017). In this model, low SES in childhood increases the likelihood of low SES in adulthood, with low SES in pregnancy influencing HPAA functioning during pregnancy. Thus, in the pathway model, the effects of maternal SES in childhood on maternal HPAA functioning in pregnancy are mediated through maternal SES in pregnancy. Finally, an accumulation of risks model hypothesizes that deleterious effects from maternal low SES in childhood and low SES in pregnancy are compounded such that they have independent, additive effects on HPAA functioning in pregnancy (Yang et al., 2017). Identifying critical windows of effects and the mechanisms by which SES operates to impact offspring health, for example via disrupted maternal-fetal HPAA functioning, will improve our ability to develop interventions to reduce health disparities among socioeconomically disadvantaged populations.
Studies assessing links between maternal HPAA disruption in pregnancy and offspring outcomes have relied largely on salivary or serum measures of cortisol. Such measures represent relatively short- term assessments of HPAA functioning and thus may not provide an accurate depiction of the fetus’s overall cortisol exposure. Fetal cortisol exposure may be better quantified by methods that characterize longer-term HPAA activity during pregnancy (D’Anna-Hernandez et al., 2011; Kalra et al., 2007; Kirschbaum et al., 2009; Russell et al., 2012). Measures of concentration of cortisol in hair are emerging as promising markers of long-term HPAA activity, including in pregnancy (Braig et al., 2015; D’Anna- Hernandez et al., 2011; Kalra et al., 2007; Kirschbaum et al., 2009; Russell et al., 2012; Wosu et al., 2013). Importantly, hair cortisol measures are not affected by factors that influence salivary and serum cortisol protocols (e.g., nonadherence, circadian patterns, invasiveness, stress of sampling) (Braig et al., 2015; Stalder and Kirschbaum, 2012; Wosu et al., 2013). Moreover, a few cross-sectional studies have linked lower SES to greater hair cortisol levels, including among pregnant women (Braig et al., 2015; Gray et al., 2018; Schreier et al., 2016; Ursache et al., 2017).
The main aim of the current study was to investigate associations between maternal life course SES and hair cortisol during pregnancy in a large sociodemographically diverse pregnancy cohort. We hypothesized that women who grow up in more socioeconomically disadvantaged circumstances have increased hair cortisol levels in pregnancy compared to women from more advantaged backgrounds. We further hypothesized that lower maternal SES in pregnancy is associated with higher hair cortisol levels. We tested whether maternal SES in pregnancy mediates or has independent effects from associations between maternal SES in childhood and maternal hair cortisol in pregnancy. Finally, we examined whether associations between maternal life course SES and hair cortisol in pregnancy are independent of maternal race/ethnicity, given well-documented links between race and SES as well as evidence for race differences in pregnancy cortisol levels, including in hair (DeSantis, Adam, et al., 2015; Schreier et al., 2016; Schreier et al., 2015). We also considered whether associations between maternal life course SES and hair cortisol in pregnancy are independent of perinatal health, as studies have linked lower SES with perinatal health indicators associated with increased hair cortisol in pregnancy (e.g., increased BMI, smoking) (Braig et al., 2015; Hastert et al., 2016). We hypothesized that maternal SES in childhood has effects independent from maternal SES in pregnancy on hair cortisol in pregnancy, as data support an accumulation of risks model of life course SES effects on health during young to mid-adulthood, i.e., child-bearing years (Yang et al., 2017). We further hypothesized that these effects are not attributable to maternal race or to perinatal health status.
2. Materials and methods
2.1. Participants
Participants were women enrolled in the PRogramming of Intergenerational Stress Mechanisms (PRISM) study, a prospective pregnancy cohort designed to examine the role of maternal and child stress exposures on child development. Between March 2011 and December 2014, pregnant women were recruited from prenatal clinics in urban hospitals and community health centers in the Northeast of the United States. Recruitment sites were chosen given desired heterogeneity in sociodemographic and racial/ethnic characteristics. Eligibility criteria for enrollment in PRISM included: 1) English- or Spanish- speaking; 2) age ≥ 18 years at enrollment; 3) single gestation birth. Exclusion criteria included: 1) maternal endorsement of drinking ≥ 7 alcoholic drinks/week during pregnancy and 2) maternal positive HIV status, as these exposures would influence/confound biomarkers and hypothesized pathways of interest. For the current analyses, additional exclusion criteria included oral steroid use in the past 12 months (n = 8), as usage may influence hair cortisol measures (Braig et al., 2015). Among eligible women, 343 consented to PRISM study participation and provided data relevant for the current analyses. Based on screening data, there were no statistically significant differences in race/ethnicity, education, or income between women who participated and those who declined.
2.2. Measures
2.2.1. Maternal SES in childhood
Because adults are generally able to recall whether their parents owned or rented their homes from an early age when they were growing up, homeownership provides a good retrospective measurement of SES over childhood and adolescence (Cohen et al., 2004). Moreover, previous work indicates that homeownership is associated with greater assets and income and that parental homeownership in childhood is a predictor of health indicators in adulthood, independent of concurrent measures of SES (educational attainment, current homeownership) and parental educational attainment (Cohen et al., 2004). Participants were asked whether their parent(s) owned their home during three developmental periods—early childhood (between birth and 5 years of age), middle childhood (between 6 and 10 years of age), and adolescence (between 11 and 17 years of age)—as an indicator of their family’s SES during their childhood. A composite score was created that scored participants by the number of developmental periods during which their parents owned their home, ranging from 0 to 3. Because relatively small numbers of participants’ families owned their home during one or two developmental periods, these scores were collapsed. The resulting scale scored participants as having no developmental periods, some developmental periods (i.e., one or two), or all developmental periods of family homeownership.
2.2.2. Maternal SES during pregnancy
Three indicators of SES during pregnancy were considered, including maternal educational attainment, annual household income, and current homeownership. Maternal educational attainment was scored as follows: “less than completion of high school,” “completion of high school/GED,” “some college,” “college degree,” or “graduate degree.” Annual household income was scored as follows: less than $10,000, $10,000-$19,999, $20,000-$34,999, $35,000-$49,999, $50,000-$69,999, $70,000-$100,000, or greater than $100,000. Homeownership status was categorized into whether or not the participant owned her home (including having a mortgage).
2.2.3. Hair cortisol in pregnancy
Hair cortisol was assessed from participants’ scalp hair, collected within one week after delivery. A hair strand roughly 3 mm in diameter was cut with scissors as close to the scalp as possible from the posterior vertex, the suggested standard position for hair cortisol collection, given that this region has the most uniform rate of growth, lowest inter-individual variability, and lowest proportion of resting phase in the hair follicle (Stalder and Kirschbaum, 2012). Hair samples were cut into three 3-cm segments, length permitting, with each 3-cm segment corresponding to one trimester based on a hair growth rate of approximately 1 cm/month (Wennig, 2000). As previously described (Braig et al., 2015; Kirschbaum et al., 2009), the 3-cm segment closest to the scalp reflected cortisol levels during the third trimester, and the next two 3-cm segments reflected second and first trimester levels, respectively. Data support the use of cortisol measured from hair collected postpartum as an indicator of maternal HPAA activity during pregnancy (Braig et al., 2015; D’Anna-Hernandez et al., 2011; Kirschbaum et al., 2009).
Hair was stored in manila envelopes at room temperature out of direct sunlight until shipment for analysis. Hair samples were analyzed in the Kirschbaum laboratory at the Technical University of Dresden, Germany. Washing and steroid extraction followed an established protocol (Stalder et al., 2013). Hair was washed in isopropanol, and cortisol was extracted from 7.5 mg of whole nonpulverized hair using methanol in the presence of internal standards. Samples were centrifuged at 15,200 x g, and the supernatant was collected; alcohol was evaporated under a stream of nitrogen and reconstituted with double-distilled water and then injected into a Shimadzu HPLC-tandem mass spectrometry system (Shimadzu, Canby, Oregon) coupled to an AB Sciex API 5000 Turbo-ion-spray triple quadrupole tandem mass spectrometer (AB Sciex, Foster City, CA), with purification by on-line solid-phase extraction (Gao et al., 2013). Lower limits of quantification were 0.1 pg/mg; inter- and intra-assay variabilities were 3.7– 8.8%.
2.2.4. Covariates
Participants reported their race/ethnicity, which was categorized into White, Black, Hispanic, and other. The “other” race/ethnicity group consisted primarily of individuals who self- identified as Asian or multi-racial. Maternal pregnancy health variables that may impact cortisol levels were considered (Braig et al., 2015), including pre-pregnancy BMI, smoking during pregnancy, use of inhaled corticosteroids or topical corticosteroids in the past year, and mode of delivery. BMI was calculated by dividing maternal self-reported pre-pregnancy weight (kg) by height squared (meter). Smoking during pregnancy was categorized as yes/no based on maternal self-report of smoking at baseline and/or in the third trimester. Use of inhaled corticosteroids and topical corticosteroids over the prior year were each categorized as yes/no based on self-report and/or medical records. Mode of delivery was categorized as vaginal or cesarean delivery, based on medical records.
2.3. Procedure
Participant sociodemographics were assessed shortly following recruitment (M = 22.7 weeks gestation, SD = 8.9 weeks gestation) via in-person interviews. Within one week after delivery, staff collected maternal hair samples. Study procedures were approved by the relevant institutions’ human studies ethics committees (Brigham and Women’s Hospital/Partners HealthCare, Mount Sinai School of Medicine). Participants provided written informed consent in their preferred language.
2.4. Data Analytic Plan
Hair cortisol values were examined for outliers, with values > 3 standard deviations (SD) above the mean removed from analyses; the remaining values were log-transformed to reduce skewness, as recommended (Braig et al., 2015). Descriptive statistics were calculated to describe the sample. Associations between measures of maternal SES in childhood and during pregnancy were first examined. Analyses then examined associations between the maternal childhood and pregnancy SES variables and maternal hair cortisol during each trimester. Associations between the covariates and hair cortisol were then tested. For all analyses, Pearson’s correlation coefficients were calculated when both variables were continuous and normally distributed; Spearman’s correlation coefficients when both variables were continuous and at least one was non-normally distributed and/or ordinal; point biserial correlations when one variable was dichotomous and the other was continuous; phi correlation coefficients when both variables were dichotomous; Kruskal-Wallis H tests when one variable was categorical and the other continuous; and chi-square (χ2) tests when both variables were categorical. These analyses were conducted using SPSS v23.
2.4.1. SES life course effects models
To test whether any effects of maternal SES in childhood on maternal hair cortisol in pregnancy were independent of or mediated by maternal SES in pregnancy, a series of mediational models were estimated (Muthen, 2017). The effects of maternal SES in childhood on maternal hair cortisol were investigated with or without their indirect effects through maternal educational attainment, annual household income, and homeownership during pregnancy in separate models. Because indirect effects often are non-normally distributed, confidence intervals were estimated using the non-symmetric bootstrap distribution (Efron, 1979). Two sets of models were run. In the first set, analyses were unadjusted; in the second set, analyses controlled for covariates, including maternal race/ethnicity, pre-pregnancy BMI, smoking during pregnancy, use of inhaled corticosteroids, use of topical corticosteroids, and mode of delivery. Covariates were modeled as exerting direct effects on maternal hair cortisol and indirect effects through maternal SES in pregnancy (Muthen, 2017). Missing data were treated as missing in a pairwise manner. Amount of missing data ranged from 0.6% to 2.0% for all but the annual household income variable, which was missing at 7.6%. These analyses were conducted using Mplus v8.
These statistical models allowed for testing of the three theoretical life course models: sensitive period, pathway, and accumulation of risk. Results would support a sensitive period model if only maternal SES in childhood and not maternal SES in pregnancy predicts hair cortisol in pregnancy. Results would support a pathway model if (a) the associations among maternal SES in childhood, maternal SES in pregnancy, and hair cortisol in pregnancy are significant in bivariate analyses, (b) in the model including both maternal SES in childhood and maternal SES pregnancy, the effect of maternal SES in childhood on hair cortisol in pregnancy becomes non-significant whereas the effect of maternal SES in pregnancy on hair cortisol in pregnancy remains significant; and (c) indirect effects are significant. Results would support an accumulation of risk model if both maternal SES in childhood and maternal SES in pregnancy each contribute significantly to predicting hair cortisol in pregnancy when indicators of both SES in childhood and SES in pregnancy are included in the model together.
3. Results
3.1. Descriptive Data
As shown in Table 1, the sample demonstrated variability across sociodemographic characteristics assessed, including maternal race/ethnicity, educational attainment, household income, and marital status. The infants were primarily of normal birthweight (M = 3312 grams, SD = 521 grams; 93% born greater than 2500 grams) and born full-term (M = 39.0 weeks, SD = 1.7 weeks; 90% born 37 weeks or later). Due to varied hair length, valid cortisol data (i.e., ≤ 3 SD from mean) were available for 182 women during the first trimester, 281 during the second trimester, and 3361 during the third trimester. Table 2 presents the correlation coefficients among the predictor variables and covariates.
Table 1.
Sample characteristics (N = 343)
| na | % | M | SD | |
|---|---|---|---|---|
| Maternal age (years) | 30.21 | 5.83 | ||
| Maternal race/ethnicity | ||||
| White | 106 | 31 | ||
| Black | 93 | 27 | ||
| Hispanic | 124 | 36 | ||
| Otherb | 20 | 6 | ||
| Maternal relationship status | ||||
| Married | 179 | 52 | ||
| Living together | 88 | 26 | ||
| Otherc | 74 | 22 | ||
| Maternal educational attainment | ||||
| <High school completion | 79 | 23 | ||
| Graduated high school/GED | 42 | 12 | ||
| Some college | 78 | 23 | ||
| College degree | 65 | 19 | ||
| Graduate degree | 76 | 22 | ||
| Annual household income | ||||
| < $10,000 | 34 | 10 | ||
| $10,000 - $19,999 | 61 | 18 | ||
| $20,000 - $34,999 | 49 | 14 | ||
| $35,000 - $49,999 | 35 | 10 | ||
| $50,000 - $69,999 | 20 | 6 | ||
| $70,000 - $100,000 | 32 | 9 | ||
| $100,000+ | 86 | 25 | ||
| Current homeownership (yes) | 116 | 34 | ||
| Parental homeownership in childhoodd | ||||
| 0 developmental periods | 103 | 30 | ||
| 1–2 developmental periods | 61 | 18 | ||
| 3 developmental periods | 179 | 52 | ||
| Maternal hair cortisol, log-transformed, 1st trimestera | 0.72 | 0.80 | ||
| Maternal hair cortisol, log-transformed, 2nd trimestera | 0.74 | 0.74 | ||
| Maternal hair cortisol, log-transformed, 3rd trimestera | 0.92 | 0.75 | ||
| Maternal pre-pregnancy body mass index (BMI), kg/m2 | 26.14 | 6.02 | ||
| Maternal cigarette smoking during pregnancy (yes) | 60 | 18 | ||
| Maternal use of inhaled corticosteroids | 25 | 7 | ||
| Maternal use of topical corticosteroids | 19 | 6 | ||
| Mode of delivery (cesarean section) | 92 | 27 |
Data were missing for 0% to 8% of participants across study variables, with the exception of hair cortisol data. Hair cortisol data were available for n = 182 in 1st trimester, n = 281 in 2nd trimester, and n = 336 in 3rd trimester.
The majority categorized as “other” race/ethnicity self-identified as Asian or multiracial.
Other included never married (n = 37), divorced (n = 4), separated (n = 5), and other relationship status (n = 28).
Developmental periods assessed included early childhood (birth to 5 years old), middle childhood (6 to 10 years old), and adolescence (11 to 17 years old).
Table 2.
Correlation coefficients among predictor and covariate variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Maternal SES, childhood | -- | ||||||||
| 2. Maternal educational attainment, pregnancy | .26*** | -- | |||||||
| 3. Annual household income, pregnancy | .34*** | .74*** | -- | ||||||
| 4. Maternal homeownership, pregnancy | .32*** | .55*** | .65*** | -- | |||||
| 5. Maternal race/ethnicitya | −.36*** | −.60*** | −.67*** | −.45*** | -- | ||||
| 6. Maternal body mass index (BMI), kg/m2 | −.11* | −.26*** | −.27*** | −.29*** | .23*** | -- | |||
| 7. Maternal smoking in pregnancy | .01 | .07 | .09 | −.02 | −.16** | −.04 | -- | ||
| 8. Maternal inhaled corticosteroid use | −.07 | .04 | .03 | .02 | −.01 | −.05 | .05 | -- | |
| 9. Maternal topical corticosteroid use | −.02 | .09 | .09 | .07 | −.03 | .04 | .06 | .13* | -- |
| 10. Mode of deliveryb | −.04 | .07 | .03 | .03 | −.01 | .09 | .03 | −.04 | .11* |
Note. Pearson’s correlation coefficients were calculated when both variables were continuous and normally distributed; Spearman’s correlation coefficients when both variables were continuous and at least one was non-normally distributed and/or ordinal; point biserial correlations when one variable was dichotomous and the other was continuous; and phi correlation coefficients when both variables were dichotomous.
Maternal race dichotomized as 0 = White, 1 = racial/ethnic minority (Black, Hispanic, other).
Mode of delivery dichotomized as 0 = vaginal, 1 = cesarean delivery.
p < 0.05.
p < 0.01.
p < 0.001.
3.2. Maternal SES in Childhood and in Pregnancy
Maternal SES in childhood was associated with all indicators of maternal SES in pregnancy. Number of developmental periods of parental homeownership in childhood was positively correlated with maternal education, rs = .26, p < 0.001, and with household income during pregnancy, rs = .34, p < 0.001. Among mothers whose parents owned their home during all childhood developmental periods, 48% currently owned their home, compared to 30% among those whose parents owned their home during 1–2 periods and 13% among those whose parents did not own their home during any period, χ2(2, N = 341) = 35.14, p < 0.001.
3.3. Maternal SES in Childhood and Hair Cortisol in Pregnancy
The number of developmental periods of maternal parental homeownership was negatively associated with hair cortisol during each trimester of pregnancy: first trimester rs = −.19, p = 0.010; second trimester rs = −.22, p < 0.001; third trimester rs = −.21, p < 0.001. Further analysis indicated that the mean hair cortisol scores for each trimester increased linearly by number of developmental periods of maternal parental homeownership. Additionally, the magnitude of the association of each distinct developmental period (i.e., 0–5 years, 6–10 years, 11–17 years) with hair cortisol levels were not significantly different, suggesting that equally weighting homeownership during each of the developmental periods in the maternal SES in childhood score was appropriate. Together, these data support the validity of the method utilized for scoring maternal SES in childhood.
3.4. Maternal SES in Pregnancy and Hair Cortisol in Pregnancy
Each indicator of maternal SES in pregnancy was associated with hair cortisol levels across pregnancy. Maternal educational attainment was negatively associated with hair cortisol during each trimester: first trimester rs = −.21, p = 0.005; second trimester rs = −.21, p < 0.001; third trimester; rs = - .26, p < 0.001. Household income in pregnancy was also negatively associated with hair cortisol during each trimester: first trimester rs = −.34, p < 0.001; second trimester rs = −.30, p < 0.001; third trimester rs = −.34, p < 0.001. Lower levels of hair cortisol during all three trimesters were associated with owning versus not owning a home during pregnancy: first trimester M = 0.54, SD = 0.75 vs. M = 0.83, SD = 0.80, r = −.18, p = 0.016; second trimester M = 0.56, SD = 0.69 vs. M = 0.85, SD = 0.74; r = −.19, p = 0.001; and third trimester M = 0.66, SD = 0.66 vs. M = 1.05, SD = 0.76, r = −.24, p < 0.001.
3.5. Covariates and Hair Cortisol in Pregnancy
Because hair cortisol levels across trimesters were highly correlated (first and second trimester rs = 0.96; second and third trimester rs = 0.94; first and third trimester rs = 0.89) and the greatest number of samples were available for the third trimester, for the sake of parsimony, mediational models were only run on models considering third trimester hair cortisol levels as the outcome. Thus, associations between covariates and hair cortisol levels were tested only for third trimester hair cortisol.
Third trimester hair cortisol levels varied by maternal race/ethnicity in a Kruskal-Wallis H test, χ2(3) = 52.13, p < 0.001, with the lowest levels among White mothers (M = 0.53, SD = 0.64), then mothers self-identified as other race/ethnicity (M = 0.89 SD = 0.41), then Hispanic mothers (M = 1.03, SD = 0.69), and then Black mothers (M = 1.22, SD = 0.82). Third trimester hair cortisol was positively associated with maternal pre-pregnancy BMI, rs = .17, p = 0.002. Third trimester hair cortisol levels were lower among women who smoked during pregnancy (M = 0.74, SD = 0.67) than among non-smokers (M = 0.96, SD = 0.76), r = −.11, p = 0.047. The association between third trimester hair cortisol and mode of delivery approached significance, r = .11, p = 0.052, with higher cortisol levels among mothers who delivered via cesarean delivery. Third trimester hair cortisol was not associated with use of inhaled corticosteroids, r = −.07, p = 0.221, or topical corticosteroids, r = −.02, p = 0.729. Additionally, third trimester hair cortisol was not associated with infant birthweight, r = .01, p = 0.856, or gestational age, r = .005, p = 0.934.
3.6. Life Course Effects of Maternal SES on Hair Cortisol in Pregnancy
The first set of models tested whether indicators of maternal SES in pregnancy (maternal educational attainment, household income, current homeownership) mediated the effects of maternal SES in childhood on hair cortisol in the third trimester, unadjusted for covariates. In the model including maternal educational attainment, partial mediation was evident, with childhood SES exerting both direct effects (b = −0.132, p = 0.011) and indirect effects through maternal educational attainment (sum of indirect effects b = −0.057, p = 0.001) on maternal hair cortisol. In the model including homeownership in pregnancy, partial mediation was also evident, with childhood SES exerting both direct effects (b = - 0.123, p = 0.022) and indirect effects through homeownership in pregnancy (sum of indirect effects b = - 0.064, p < 0.001) on maternal hair cortisol. In the model including household income in pregnancy, full mediation was evident, as the direct effects of maternal SES in childhood on maternal hair cortisol was no longer significant (b = −0.080, p = 0.139) when household income in pregnancy was included in the model, whereas the sum of indirect effects was significant (b = −0.109, p < 0.001). The respective bootstrap distributions for each of these models did not contain zero in the 95% confidence intervals; thus, these findings are likely robust and reflective of true population effects.
The second set of models adjusted for the covariates maternal race/ethnicity, pre-pregnancy BMI, smoking during pregnancy, use of inhaled corticosteroids, use of topical corticosteroids, and mode of delivery. In all three models, full mediation was evident, as the direct effects of maternal SES in childhood on hair cortisol were no longer significant when maternal educational attainment (direct effect of maternal SES in childhood on hair cortisol b = −0.106, p = 0.053; Figure 1a) or homeownership in pregnancy (direct effects of maternal SES in childhood on hair cortisol b = −0.090, p = 0.113; Figure 1b) were included, and the direct effect remained non-significant when household income (direct effects of maternal SES in childhood on hair cortisol b = −0.064, p =.255; Figure 1c) was included. For each of these models, the sum of indirect effects was significant (for the models including maternal educational attainment, homeownership in pregnancy, and household income, respectively, sum of indirect effects: b = −0.022, p = 0.041; b = −0.036, p = 0.009; b = −0.064, p = 0.001). In addition, mode of delivery exerted independent direct effects on maternal hair cortisol in all three of the models (b = 0.114, p = 0.043 in the model with maternal educational attainment, Figure 1a; b = 0.113, p = 0.046 in the model with homeownership during pregnancy, Figure 1b; b = 0.114, p = 0.040 in the model with household income during pregnancy, Figure 1c). Maternal race/ethnicity exerted independent direct effects on maternal hair cortisol in the model including homeownership in pregnancy (b = 0.146, p = 0.002; Figure 1b), but not in the models including maternal educational attainment (b = 0.099, p = 0.053) or household income (b = 0.075, p = 0.133) in pregnancy. Pre-pregnancy BMI, maternal smoking in pregnancy, inhaled corticosteroid use, and topical corticosteroid use did not demonstrate independent direct effects on maternal hair cortisol in any of the models. The respective bootstrap distributions for each of these models did not contain zero in the 95% confidence intervals; thus, these findings are likely robust and reflective of true population effects.
Figure 1.
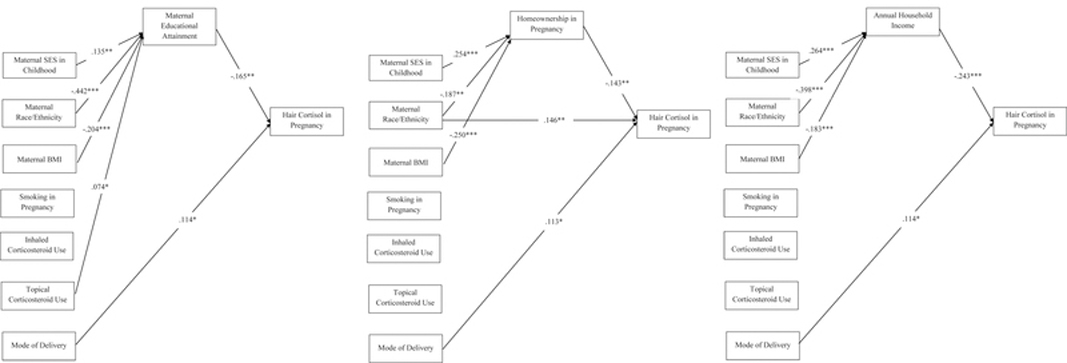
Models testing contributions of maternal socioeconomic status (SES) in childhood and in pregnancy on maternal hair cortisol levels during pregnancy. Maternal SES in childhood was operationalized as the number of developmental periods (early childhood, middle childhood, and adolescence) during which the mother’s parents owned their home. Maternal hair cortisol in pregnancy was operationalized as hair cortisol level during the third trimester, log-transformed to reduce skewness. Independent models tested three indicators of maternal SES in pregnancy: maternal educational attainment (Figure 1a), current homeownership (Figure 1b), and annual household income (Figure 1c). For all models, covariates (maternal race/ethnicity, pre-pregnancy BMI, smoking during pregnancy, use of inhaled corticosteroids, use of topical corticosteroids, mode of delivery) were modeled as exerting direct effects on maternal hair cortisol in pregnancy and indirect effects through maternal SES indicators in pregnancy. For all models, only significant paths are displayed (*p < 0.05, **p < 0.01, ***p < 0.001). The presence of a significant path from maternal SES in pregnancy to maternal hair cortisol in pregnancy in conjunction with the lack of a significant path from maternal SES in childhood to maternal hair cortisol in pregnancy indicates full mediation by the maternal SES indicator in pregnancy.
4. Discussion
The overall goal of this study was to examine whether maternal life course SES predicts maternal HPAA functioning during pregnancy, assessed via hair cortisol, in a sociodemographically diverse pregnancy cohort. There were three main findings: (1) Lower maternal SES in childhood predicted higher levels of hair cortisol across pregnancy trimesters. (2) Each tested indicator of maternal SES in pregnancy (educational attainment, household income, current homeownership) was associated with hair cortisol in every trimester, with lower SES consistently associated with higher cortisol levels. (3) In models adjusted for maternal race/ethnicity, pre-pregnancy BMI, smoking during pregnancy, use of inhaled or topical corticosteroids, and mode of delivery, maternal SES indicators in pregnancy fully mediated the effects of maternal SES in childhood on maternal hair cortisol levels. Additionally, mode of delivery exerted independent direct effects on maternal hair cortisol in all models, and maternal race/ethnicity exerted independent direct effects on maternal hair cortisol in the model including homeownership in pregnancy but not in the models including maternal educational attainment or household income in pregnancy.
These findings provide support for a pathway model of maternal life course SES effects on maternal HPAA functioning during pregnancy (Yang et al., 2017); that is, maternal SES in childhood appeared to establish a trajectory to SES in adulthood such that maternal SES in childhood impacts HPAA functioning during pregnancy through its influence on maternal SES in pregnancy. Support for this conclusion was evident when considering any of the SES indicators in pregnancy as mediators in models adjusted for maternal race/ethnicity and perinatal health covariates. Notably, the unadjusted model considering maternal educational attainment and the unadjusted model considering maternal homeownership in pregnancy as mediators of maternal childhood SES effects on maternal hair cortisol in pregnancy suggested an accumulation risk model of effects. In both models, SES in childhood and in pregnancy exerted direct effects on hair cortisol in pregnancy independent of the mediational effects of childhood SES through maternal SES in pregnancy. However, household income during pregnancy fully mediated the effects of maternal SES in childhood on maternal hair cortisol levels even in the unadjusted model. This pattern of results highlights the importance of the operationalization of SES in research, with different SES variables producing somewhat different findings. The extant literature contains various operationalizations of SES, which likely contributes to inconsistencies in reports of SES effects on health. This study’s findings suggest that measures of SES that are reflective of current financial resources have greatest impact on maternal HPAA functioning in pregnancy. The results also indicate that, once perinatal health variables are considered in conjunction with concurrent SES indicators, the direct effects of maternal SES in childhood on maternal HPAA functioning in pregnancy are no longer apparent.
The mechanisms via which maternal SES influences maternal-fetal HPAA functioning are as yet unknown, and may include increased psychosocial stress, oxidative stress, inflammation, disruptions to autonomic nervous system reactivity, and poorer health/health behaviors (Hatch and Dohrenwend, 2007; Matthews et al., 2010; McEwen, 1998; Seeman et al., 2010). In the current analyses, maternal pregnancy health indicators (pre-pregnancy BMI, smoking during pregnancy, use of corticosteroid medications) did not account for associations between maternal SES and hair cortisol levels, suggesting other mechanisms are responsible. Studies have linked maternal stress during pregnancy to disruptions in HPAA functioning, including a blunted morning response and flatter waking to bedtime rhythm in early to mid- pregnancy and elevated evening cortisol in late pregnancy (Obel et al., 2005; Rothenberger et al., 2011; Suglia et al., 2010). Our group has previously reported associations of both childhood trauma and lifetime stress exposures with maternal hair cortisol during pregnancy in a subsample of the participants included in the current study (Schreier et al., 2016; Schreier et al., 2015). Moreover, higher rates of exposure to stress and trauma have been documented among low-income populations (Myers, 2009). Thus, low SES individuals may experience higher rates of exposure to stressors that disrupt HPAA functioning. Living in poverty/under disadvantaged economic circumstances may represent a stressor in itself that influences HPAA functioning. Notably, a number of studies have documented weak or no associations between maternal stress and cortisol levels during pregnancy (Baibazarova et al., 2013; Davis et al., 2007; DiPietro, 2012; Zijlmans et al., 2015), suggesting that relations among SES, stress, and maternal-fetal HPAA functioning are likely complex.
Our analyses found differences in maternal hair cortisol levels by race/ethnicity. Consistent with prior work (Cohen et al., 2006; Hatch and Dohrenwend, 2007; Roberts et al., 2011; Schreier et al., 2015, 2016), minority women, particularly Black women, demonstrated relatively higher hair cortisol levels. Further, in correlational analyses, White women scored higher than racial/ethnic minority women on all of the measures of SES in childhood and pregnancy. In mediational analyses, maternal race/ethnicity exerted direct effects on maternal hair cortisol levels independent of maternal SES in childhood, maternal homeownership in pregnancy, and perinatal health indicators; however, the direct effects of maternal race/ethnicity on hair cortisol were not significant when maternal educational attainment or household income during pregnancy were considered. Prior studies suggest that racial/ethnic groups may differ in their physiological stress responses, including HPAA reactivity (Brunst et al., 2014; Cohen et al., 2006; Hatch and Dohrenwend, 2007; Roberts et al., 2011; Suglia et al., 2010; Thoits, 2010; Tse et al., 2012). Moreover, SES specifically may have differential effects on cortisol production among different racial/ethnic groups (O’Brien et al., 2013). In addition, epidemiological data indicate that racial/ethnic groups vary in their exposure to risk factors that may disrupt HPAA functioning, including negative life events/trauma, ongoing discrimination stress, and reduced access to buffering resources (Brunst et al., 2014; Cohen et al., 2006; Hatch and Dohrenwend, 2007; Myers, 2009; Roberts et al., 2011). This study’s findings produced mixed results as to whether maternal race/ethnicity exerted effects on HPAA functioning independent of lifetime SES. The extant literature indicates that various indicators of SES may impart different levels of risk/protection among different racial/ethnic groups and thus have different implications for health outcomes (Geronimus et al., 2015). Further exploration of the nature of race/ethnicity effects on maternal HPAA functioning during pregnancy in the context of SES should be pursued in future research, particularly given literature suggesting complex associations among race/ethnicity, SES, and health. Determining how race/ethnicity, in combination with SES, contributes to differences in HPAA functioning in pregnancy may enhance our understanding of sociodemographic health disparities and suggest potential interventions to prevent intergenerational effects.
In all adjusted models, mode of delivery exerted independent direct effects on maternal hair cortisol levels in pregnancy, with cesarean deliveries associated with higher hair cortisol levels than vaginal deliveries. Interestingly, the direction of effects was opposite those found by the one other study examining links between mode of delivery and hair cortisol in pregnancy (Braig et al., 2015). The reasons for associations between mode of delivery and maternal hair cortisol are currently unknown and may include higher levels of obstetric risk and psychosocial stress among mothers requiring a cesarean delivery as well as influences of mode of delivery directly on hair cortisol levels (Braig et al., 2015). Given the discrepancies in findings across the studies, more research is needed to determine how mode of delivery may influence hair cortisol measures and the mechanisms responsible for any effects.
This study offers a number of strengths. It is one of a few studies to explore determinants of maternal hair cortisol in pregnancy and the first to consider maternal life course SES on maternal HPAA functioning during pregnancy. The sample was large and sociodemographically diverse. The study considered a range of maternal SES indictors, critical given inconsistencies in the literature regarding SES effects on health, which may be due in part to different operationalizations/reliance on single indicators of SES across studies (Yang et al., 2017). The importance of exploring maternal SES effects on HPAA functioning in pregnancy is highlighted by the fact that young to middle adulthood (i.e., child-bearing years) is the period when social inequities in health are purported to be largest (Geronimus et al., 2015). Finally, this study responds to recent calls to utilize a life course approach to characterizing SES effects on biophysiological processes underlying health (Yang et al., 2017).
Limitations may include relying on parental homeownership status as the indicator of maternal SES in childhood. Factors other than SES may contribute to homeownership status (e.g., regional housing availability). Parental homeownership in childhood may be a proxy for other influences on the development of stress physiology systems, including increased access to health care and nutrition, high quality schooling, and community resources, and decreased exposure to chemical toxins, community violence, and other stressors (Cohen, 2004). Lack of parental homeownership may be associated with increased moves and other forms of environmental instability in childhood (Cohen, 2004). Further, homeownership may be an insensitive measure of SES in areas of the country with inflated housing costs such that homeownership does not reliably reflect economic circumstances (Cohen, 2004). Thus, other measures of SES may have stronger associations with HPAA functioning and health outcomes than homeownership in certain geographic regions of the country. Additionally, there is likely wide variability in parental income and education levels represented within homeownership status groups. Thus, our analyses may have underestimated the direct effects of maternal SES in childhood on hair cortisol in pregnancy. The results presented here should be replicated in analyses that consider more comprehensive measures of maternal SES in childhood. Hair growth rates may have varied across participants, influencing the accuracy of the timing of the cortisol assessment. Notably, studies suggest small individual differences in hair growth rates, including between individuals from different racial/ethnic backgrounds (Loussouarn et al., 2005). The mediational analyses focused on third trimester cortisol output to maximize available samples. Cortisol levels were highly correlated across trimesters, suggesting that third trimester levels may represent a reasonable proxy for relative cortisol levels across pregnancy. Additionally, bivariate analyses showed similar associations between childhood and pregnancy SES measures and hair cortisol across all trimesters. These findings suggest that associations between maternal life course SES and HPAA functioning as reflected in hair cortisol are similar across pregnancy. This hypothesis should be tested in future studies to determine whether SES during different periods of life exerts differential effects on HPAA functioning during various stages of pregnancy. This study focused on hair cortisol as an integrated measure of HPAA functioning over long periods (i.e., months). Future work should consider multi-method approaches to determine if other cortisol measures (e.g., diurnal rhythms, stress reactivity and recovery) across the course of pregnancy contribute to our understanding of life course SES effects on maternal-fetal HPAA functioning.
4.1. Conclusions
Our findings suggest that maternal SES in childhood establishes a trajectory to SES in pregnancy that influences maternal-fetal HPAA functioning, reflected in maternal hair cortisol. Prior research indicates that maternal HPAA functioning during pregnancy may influence offspring health and developmental outcomes. The current study suggests that maternal SES history may be mechanistically involved in child health outcomes via maternal HPAA effects on fetal programming and, consequently, contribute to poorer health outcomes among socioeconomically disadvantaged populations. Future research should elucidate the psychosocial and biological factors that mediate links between maternal lifetime SES and maternal-fetal HPAA functioning. Addressing these questions will further our understanding of intergenerational effects of maternal life course SES on offspring physical and mental health, informing the development of interventions to prevent health disparities in vulnerable populations.
Highlights.
First study to link life course SES to maternal hair cortisol in pregnancy.
Lower maternal SES in childhood and pregnancy linked to higher cortisol levels.
Childhood SES effects on pregnancy cortisol were mediated through SES in pregnancy.
Effects were independent of race/ethnicity and pregnancy health measures.
Maternal life course SES may have intergenerational effects via prenatal cortisol.
Acknowledgements
We are grateful for the study families whose generous donation of time made this project possible.
Role of Funding Sources
This work was supported by grants from the National Heart, Lung, & Blood Institute (R01HL095606; R01HL114396), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD082078; R21 HD080359), the National Institute of Environmental Health Sciences (P30ES023515), the Boston Children’s Hospital’s Clinical and Translational Research Executive Committee, and the Program for Behavioral Science in the Department of Psychiatry at Boston Children’s Hospital. None of the funding agencies had any role in the study design, the collection, analysis or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not represent the official views of any granting agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None.
Competing Interests: None.
Seven mothers had hair cortisol data that were measurable and within 3 SD of the mean for the first and/or second trimester but not for the third trimester, producing the final sample size of N = 343 participants with hair cortisol data during any trimester.
References
- Baibazarova E, van de Beek C, Cohen-Kettenis PT, Buitelaar J, Shelton KH, van Goozen SH, 2013. Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology, 38(6), 907–915. 10.1016/j.psyneuen.2012.09.015 [DOI] [PubMed] [Google Scholar]
- Bates R, Salsberry P, Ford J, 2017. Measuring stress in young children using hair cortisol: the state of the science. Biol Res Nurs, 19(5), 499–510. 10.1177/1099800417711583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig S, Grabher F, Ntomchukwu C, Reister F, Stalder T, Kirschbaum C, Genuneit J, Rothenbacher D, 2015. Determinants of maternal hair cortisol concentrations at delivery reflecting the last trimester of pregnancy. Psychoneuroendocrinology, 52, 289–296. 10.1016/j.psyneuen.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Brand SR, Engel SM, Canfield RL, Yehuda R, 2006. The effect of maternal PTSD following in utero trauma exposure on behavior and temperament in the 9-month-old infant. Ann N Y Acad Sci, 1071, 454–458. 10.1196/annals.1364.041 [DOI] [PubMed] [Google Scholar]
- Brunst KJ, Enlow MB, Kannan S, Carroll KN, Coull BA, Wright RJ, 2014. Effects of prenatal social stress and maternal dietary fatty acid ratio on infant temperament: Does race matter? Epidemiology (Sunnyvale), 4(4). 10.4172/2161-1165.1000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP, 2004. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med, 66(4), 553–558. 10.1097/01.psy.0000126200.05189.d3 [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T, 2006. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med, 68(1), 41–50. 10.1097/01.psy.0000195967.51768.ea [DOI] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML, 2011. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav, 104(2), 348–353. 10.1016/j.physbeh.2011.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA, 2007. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry, 46(6), 737–746. 10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, Head K, 2013. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry, 74(9), 647–655. 10.1016/j.biopsych.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar JK, 2003. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev, 74(2), 139–151. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT, 2015. Racial and ethnic differences in diurnal cortisol rhythms: are they consistent over time? Psychosom Med, 77(1), 6– 15. 10.1097/psy.0000000000000131 [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Kuzawa CW, Adam EK, 2015. Developmental origins of flatter cortisol rhythms: socioeconomic status and adult cortisol activity. Am J Hum Biol, 27(4), 458–467. 10.1002/ajhb.22668 [DOI] [PubMed] [Google Scholar]
- DiPietro JA (2012). Maternal stress in pregnancy: considerations for fetal development. J Adolesc Health, 51(2 Suppl), S3–8. 10.1016/j.jadohealth.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B (1979). Bootstrap methods: Another look at the jackknife. Annals of Statistics, 7, 1–26. [Google Scholar]
- Enlow MB, Devick KL, Brunst KJ, Lipton LR, Coull BA, Wright RJ, 2017. Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity. Infancy, 22(4), 492–513. 10.1111/infa.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, 2008. Cortisol: the culprit prenatal stress variable. Int J Neurosci, 118(8), 1181 10.1080/00207450701820944 [DOI] [PubMed] [Google Scholar]
- Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C, 2013. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci, 928, 1–8. 10.1016/j.jchromb.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Pearson JA, Linnenbringer E, Schulz AJ, Reyes AG, Epel ES, Lin J, Blackburn EH, 2015. Race-ethnicity, poverty, urban stressors, and telomere length in a Detroit community-based sample. J Health Soc Behav, 56(2), 199–224. 10.1177/0022146515582100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NA, Dhana A, Van Der Vyver L, Van Wyk J, Khumalo NP, Stein DJ, 2018. Determinants of hair cortisol concentration in children: A systematic review. Psychoneuroendocrinology, 87, 204–214. 10.1016/j.psyneuen.2017.10.022 [DOI] [PubMed] [Google Scholar]
- Hastert TA, Ruterbusch JJ, Beresford SA, Sheppard L, White E, 2016. Contribution of health behaviors to the association between area-level socioeconomic status and cancer mortality. Soc Sci Med, 148, 52–58. 10.1016/j.socscimed.2015.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch SL, Dohrenwend BP, 2007. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: a review of the research. Am J Community Psychol, 40(3–4), 313–332. 10.1007/s10464-007-9134-z [DOI] [PubMed] [Google Scholar]
- Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G, 2007. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med, 30(2), E103–107. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L, 2009. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34(1), 32–37. 10.1016/j.psyneuen.2008.08.024 [DOI] [PubMed] [Google Scholar]
- Lockwood KG, John-Henderson NA, Marsland AL, 2018. Early life socioeconomic status associates with interleukin-6 responses to acute laboratory stress in adulthood. Physiol Behav, 188, 212–220. 10.1016/j.physbeh.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loussouarn G, El Rawadi C, Genain G, 2005. Diversity of hair growth profiles. Int J Dermatol, 44 Suppl 1, 6–9. 10.1111/j.1365-4632.2005.02800.x [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC, Taylor SE, 2010. Are psychosocial factors mediators of socioeconomic status and health connections? A progress report and blueprint for the future. Ann N Y Acad Sci, 1186, 146–173. 10.1111/j.1749-6632.2009.05332.x [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1998. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci, 840, 33–44. [DOI] [PubMed] [Google Scholar]
- Muthen B, Muthen L, Asparouhov T, 2017. Regression and mediation analysis using Mplus Los Angeles, CA: Muthen & Muthen. [Google Scholar]
- Myers HF, 2009. Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. J Behav Med, 32(1), 9–19. 10.1007/s10865-008-9181-4 [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Tronick EZ, Moore CL, 2013. Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress Health, 29(4), 337–344. 10.1002/smi.2475 [DOI] [PubMed] [Google Scholar]
- Obel C, Hedegaard M, Henriksen TB, Secher NJ, Olsen J, Levine S, 2005. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology, 30(7), 647–656. 10.1016/j.psyneuen.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC, 2011. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment- seeking for post-traumatic stress disorder in the United States. Psychol Med, 41(1), 71–83. 10.1017/s0033291710000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberger SE, Resch F, Doszpod N, Moehler E, 2011. Prenatal stress and infant affective reactivity at five months of age. Early Hum Dev, 87(2), 129–136. 10.1016/j.earlhumdev.2010.11.014 [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S, 2012. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5), 589–601. 10.1016/j.psyneuen.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Schreier HM, Enlow MB, Ritz T, Coull BA, Gennings C, Wright RO, Wright RJ, 2016. Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi- racial/ethnic sample of pregnant women. Stress, 19(1), 45–52. 10.3109/10253890.2015.1117447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier HM, Enlow MB, Ritz T, Gennings C, Wright RJ, 2015. Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. J Epidemiol Community Health, 69(12), 1169–1174. 10.1136/jech-2015-205541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS, 2010. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci, 1186, 223–239. 10.1111/j.1749-6632.2009.05341.x [DOI] [PubMed] [Google Scholar]
- Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA, 2013. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Dev Sci, 16(5), 665–675. 10.1111/desc.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, 2012. Analysis of cortisol in hair--state of the art and future directions. Brain Behav Immun, 26(7), 1019–1029. 10.1016/j.bbi.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Alexander N, Bornstein SR, Gao W, Miller R, Stark S, Bosch JA, Fischer JE, 2013. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab, 98(6), 2573–2580. 10.1210/jc.2013-1056 [DOI] [PubMed] [Google Scholar]
- Suglia SF, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards JW, Wright RJ, 2010. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychol Trauma, 2(4), 326–334. 10.1037/a0018953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer ZM, Kuzawa CW, 2014. Early origins of health disparities: material deprivation predicts maternal evening cortisol in pregnancy and offspring cortisol reactivity in the first few weeks of life. Am J Hum Biol, 26(6), 723–730. 10.1002/ajhb.22532 [DOI] [PubMed] [Google Scholar]
- Thoits PA, 2010. Stress and health: major findings and policy implications. J Health Soc Behav, 51 Suppl, S41–53. 10.1177/0022146510383499 [DOI] [PubMed] [Google Scholar]
- Tse AC, Rich-Edwards JW, Koenen K, Wright RJ, 2012. Cumulative stress and maternal prenatal corticotropin-releasing hormone in an urban U.S. cohort. Psychoneuroendocrinology, 37(7), 970– 979. 10.1016/j.psyneuen.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A, Merz EC, Melvin S, Meyer J, Noble KG, 2017. Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology, 78, 142–150. 10.1016/j.psyneuen.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennig R, 2000. Potential problems with the interpretation of hair analysis results. Forensic Sci Int, 107(1–3), 5–12. [DOI] [PubMed] [Google Scholar]
- Wosu AC, Valdimarsdottir U, Shields AE, Williams DR, Williams MA, 2013. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Ann Epidemiol, 23(12), 797–811 e792 10.1016/j.annepidem.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Gerken K, Schorpp K, Boen C, Harris KM, 2017. Early-life socioeconomic status and adult physiological functioning: A life course examination of biosocial mechanisms. Biodemography Soc Biol, 63(2), 87–103. 10.1080/19485565.2017.1279536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans MA, Riksen-Walraven JM, de Weerth C, 2015. Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neurosci Biobehav Rev, 53, 1– 24. 10.1016/j.neubiorev.2015.02.015 [DOI] [PubMed] [Google Scholar]


