Abstract
Objective:
Previous research has shown that a woman’s anxiety about her pregnancy predicts gestational length. Placental corticotrophin-releasing hormone (CRH) is a stress-responsive peptide proposed as a mechanism. We examined placental CRH as a physiological mediator of the association between pregnancy anxiety and gestational length in Latina and non-Latina White women to replicate evidence of associations between pregnancy anxiety, placental CRH and gestational length; test whether placental CRH levels or changes mediate effects of pregnancy anxiety on gestational length; examine ethnic differences in pregnancy anxiety, placental CRH, and gestational length; and explore whether the effects of pregnancy anxiety on gestational length as mediated by placental CRH vary by ethnicity.
Methods:
In a prospective study of 337 pregnant Latina and non-Latina White women, participants completed in-person interviews that included a 10-item measure of pregnancy anxiety and provided blood samples assayed using radioimmunoassay at three timepoints (19, 25, and 31 weeks gestation).
Results:
Pregnancy anxiety at 19 and 31 weeks and levels of placental CRH at 31 weeks predicted gestational length. Tests of indirect effects were consistent with mediation such that both pregnancy anxiety at 19 weeks and increases from 19 to 31 weeks predicted placental CRH at 31 weeks, which in turn predicted gestational length. Tests of moderated mediation by ethnicity showed that the mediated effect of placental CRH at 31 weeks was significant for Latinas only.
Conclusions:
These findings add to growing evidence of the involvement of pregnancy anxiety in the timing of birth and suggest possible ethnic differences.
Keywords: preterm birth, HPA-axis, length of gestation
1. Introduction
Pregnancy anxiety, an emotional state rooted in concerns specific to the current pregnancy, has been implicated in the etiology of preterm birth and shortened length of gestation (Dole, Savitz, Hertz-Picciotto, Siega-Riz, McMahon, & Buekens, 2003; Kramer, et al., 2009; Lobel et al., 2008; Rini, Dunkel Schetter, Wadhwa, & Sandman, 1999; Rini, Dunkel Schetter, Wadhwa, & Sandman, 1999). Pregnancy anxiety refers to worries and fears experienced by pregnant women about their current pregnancy, including concern about the health of the baby, childbirth, health-care experiences, labor and delivery, and the maternal role (Dunkel Schetter, 2010; Guardino & Dunkel Schetter, 2014). Several large prospective studies have shown that this contextually-rooted form of anxiety reliably predicts shorter length of gestation. For example, one prospective study of 2,000 Black and White pregnant women revealed that pregnancy anxiety predicted spontaneous preterm birth (Dole et al., 2003). Another large study assessed many types of acute and chronic stressors in 5,000 pregnant women including relationship strain and job-related stress, and found that only pregnancy anxiety predicted preterm birth in controlled analyses (Kramer et al., 2009).
Although the physiological pathways underlying the associations between pregnancy anxiety and birth outcomes have not been fully determined, the interrelated activities of the hypothalamic-pituitary-adrenal (HPA) axis and especially of placental corticotropin-releasing hormone (CRH) are potential mechanisms explaining this association (Hobel, Dunkel Schetter, Roesch, Castro, &Arora, 1999). In the non-pregnant state, CRH is secreted by the hypothalamus and is not detectable in peripheral blood at concentrations of 10-20 pg/ml (McLean, Bisits, Davies, Woods, Lowry, & Smith, 1995; Smith et al., 2009). During pregnancy, however, the placenta synthesizes and secretes CRH (referred to as placental CRH) into maternal and fetal circulation as early as the seventh week of gestation (McLean et al., 1995). As pregnancy progresses, levels of placental CRH increase exponentially, reaching high levels in maternal and fetal compartments during late pregnancy, and peak concentrations of 1000-10,000 pg/ml at term and in labor (Hillhouse & Grammatopoulos, 2002; Lindsay & Nieman, 2005; McLean et al., 1995). Several studies have shown that circulating CRH acts on CRH receptors in the myometrium to influence contractility (Cong, Zhang, Gao, & Ni, 2009; Jin et al, 2007; & Zhang, et al., 2008; Sandman & Glynn, 2009). Thus, the sharp rise of placental CRH in late pregnancy may initiate a cascade of events ultimately resulting in myometrial activation.
Only one published study has examined whether placental CRH mediates the link between pregnancy anxiety and length of gestation (Mancuso, Dunkel Schetter, Rini, Roesch, & Hobel, 2004). The results of this study showed indirect effects of pregnancy anxiety reported at 28 to 30 weeks on length of gestation via levels of placental CRH measured at 28 to 30 weeks Similar effects were not observed for measures of perceived stress or state anxiety, suggesting that pregnancy anxiety may be a particularly important construct for understanding stress processes underlying preterm birth. These results are consistent with the premise that pregnancy anxiety accelerates placental CRH trajectories during late pregnancy, thereby increasing risk for preterm birth through the triggering of labor and delivery pathways. However, the study did not collect measures of pregnancy anxiety before the time of placental CRH collection, nor did it examine changes in pregnancy anxiety or placental CRH over pregnancy.
Although some of the study samples in prior research on pregnancy anxiety are ethnically diverse, analyses typically adjust for ethnicity rather than examine it as a variable of interest. However, differences in attitudes about child-bearing and motherhood between Latina and nonLatina White women may contribute to differing levels of pregnancy anxiety (Campos, Dunkel Schetter, Walsh, & Shenker, 2007; Engle, Scrimshaw, Zambrana, & Dunkel Schetter, 1990; Fleuriet & Sunil, 2014; Scrimshaw, Zambrana, & Dunkel Schetter, 1997). The authors of one qualitative study on the pregnancy behaviors of Puerto Rican women living in New York and pregnant Mexican immigrant women living in Los Angeles, speculated that Latinas expressed concerns about dying and leaving their babies motherless, and about their infant dying during childbirth (Scrimshaw et al., 1997). However, this study did not investigate these concerns in non-Latina women. In another study, Mexican-immigrant women living in South Texas reported higher levels of pregnancy anxiety compared to Mexican-American women (Fleuriet & Sunil, 2014). The limited available evidence demonstrating that Latinas may experience higher levels of pregnancy anxiety is noteworthy because of the implications for risk of preterm birth1.
However, additional research is needed to better understand how biopsychocultural factors operate in the etiology of preterm birth for the growing subgroup of Latinas in the United States population. In sum, pregnancy anxiety has been shown to predict the length of gestation and has been shown to be higher among Latinas in a few studies. Furthermore, the physiological mechanisms linking pregnancy anxiety and birth outcomes are under active investigation. The present study examined whether pregnancy anxiety differed between Latina and non-Latina White women, tested indirect pathways (mediation) by placental CRH, and considered whether indirect or mediational processes differed between these two ethnic groups.
We examined these issues in a sample of pregnant women who identified as Latina or non-Latina White. The first aim was to examine whether pregnancy anxiety, placental CRH, or changes in placental CRH predict the length of gestation. Based on prior research, we hypothesized that higher pregnancy anxiety and higher levels of placental CRH late in pregnancy would predict shorter length of gestation. We further hypothesized that placental CRH in pregnancy (19 and 25 weeks) and rate of change in placental CRH from mid to late pregnancy would predict the length of gestation. Second, we tested for ethnic differences between Latina and non-Latina White women in these variables (pregnancy anxiety, placental CRH at 19, 25, and 31 weeks, and changes in placental CRH from 19 to 31 weeks and 25 to 31 weeks). We expected that Latinas would show higher levels of pregnancy anxiety. No hypotheses were made for ethnic differences in length of gestation or ethnic differences in placental CRH in light of past conflicting evidence (Glynn, Dunkel Schetter, Chicz-DeMet, Hobel, & Sandman, 2007; Ruiz, Fullerton, Brown, & Dudley, 2002; Siler-Khodr et al., 2003). Finally, we examined whether there were indirect pathways between pregnancy anxiety and length of gestation via levels of and changes in placental CRH, and whether ethnicity moderated any indirect effects.
2. Materials and methods
2.1. Protocol
This study utilized data from a prospective longitudinal study on psychosocial and behavioral factors in pregnancy. We recruited participants at two large medical centers, and obtained blood samples and interviewed women at three timepoints in pregnancy separated by six week intervals: T1 (M =19 weeks, SD = .85); T2 (M = 25 weeks, SD = .85); and T3 (M = 31 weeks, SD = .80). Birth outcome data were abstracted from medical charts. Each institution’s Institutional Review Board approved all protocols and procedures.
2.2. Subjects
Eligibility criteria were that a woman had to be 18 years of age or older, English speaking, and carrying a singleton intrauterine pregnancy. Exclusion criteria were no current tobacco, alcohol, or drug use, and no medical conditions involving dysregulated neuroendocrine, cardiovascular, hepatic, or renal functioning. The latter were to collect biomarkers that would be unaffected by these medical conditions.
A total of 63% of the 1,189 women who were screened for the larger study met the eligibility criteria, and 67% of these women consented to participate in the study (N = 498). The primary reasons for declining to participate were having work or school conflict, scheduling difficulties, childcare issues, and lack of interest. For the present study, we consider only the subset of 337 participants who self-identified as Latina (n = 107) or non-Latina White (n = 230).
Among the 107 Latina women, 34 were foreign-born (32%), mainly born in Mexico (n = 23) but also Guatemala (n = 3), El Salvador (n = 3), Cuba (n = 1), the Dominican Republic (n = 1), Panama (n = 1), and other unspecified Latin American countries (n = 2). Foreign-born Latina women had been living in the United States for an average of 18 years (SD = 8.58, Range = 1 to 35).
2.3. Measures
2.3.1. Pregnancy Anxiety
Pregnancy anxiety was measured with the 10-item Pregnancy-Related Anxiety Scale (Rini, Dunkel-Schetter, Wadhaw, & Sandman, 1999). This scale has been used in previous studies of pregnant English and Spanish speaking women (e.g., Rini et al., 1999) with good to excellent reliability and validity in both languages. Participants rated the extent to which they worried or felt concerned about their own health, their baby’s health, labor and delivery, and parenting. Sample items are: “I am concerned or worried about how the baby is growing and developing inside me,” “I am confident of having a normal childbirth,” (reverse scored) “I am worried that the baby might not be normal,” “I am concerned or worried about losing the baby”, and “I am concerned about taking care of a new baby”. Pregnancy anxiety was measured at each timepoint to document changes in levels of pregnancy anxiety throughout pregnancy. Participants responded on a 4-point scale from 1 (not at all or never) to 4 (very much or almost all the time). The Cronbach alpha coefficients were: T1 α = 0.81, T2 α = 0.82, T3 α = 0.84.
2.4. Hormone assays
Levels of placental CRH were measured in samples of maternal blood (25 mL) drawn through antecubital venipuncture (within 20 seconds of venipuncture) and assayed through radioimmunoassay (RIA; Bachem Penisula Laboratories, San Carlos, CA) techniques described in full by Glynn & Sandman (2014). Briefly, withdrawn blood was deposited into siliconized and chilled EDTA (purple top) vacutainers and then centrifuged for 15 minutes at 2000g. The plasma was then decanted into polypropylene tubes containing 500-kallikrein inhibitor units/ml of aprotinin (Sigma Chemical) and stored at −70°C until assayed. CRH concentrations (pg/ml) were determined by radioimmunoassay (RIA; Bachem Penisula Laboratories, San Carlos, CA). CRH was extracted from (1-2mL) plasma samples with three volumes of ice-cold methanol, mixed, allowed to stand (10 minutes, 48°C), and then centrifuged (20 minutes, 1700g, 4°). Pellets were washed with 0.5 mL of methanol, and the combined supernatants were dried in a concentrator (SpeedVac; Savant Instruments, Holbrook, NY). Samples were incubated (100mL/assay tube) for 48 hours with anti-CRH serum (100mL/assay tube) for 48 hours at 4°C followed by a 24hour incubation with iodine-125 -labeled CRH at 4°C. Both labeled and unlabeled CRH were collected by immunoprecipitation, and the aspirated pellets were counted with a gamma counter (Isoflex Gamma Counter; ICN Biomedical, Costa Mesa, CA). Intra-assay and inter-assay coefficients of variance were 5% and 15%, respectively. The placental CRH values were log transformed to reduce the typical skewness of the distribution.
2.5. Birth outcome variables
Birth outcomes and maternal medical risk factors were obtained from medical records abstracted by skilled research staff. Gestation in weeks was estimated during early prenatal visits by the conventional obstetrics method of reported last menstrual period (LMP) and confirmed by pelvic ultrasound.
2.6. Demographic and medical covariates
Variables were selected for inclusion as covariates based on previous studies showing associations with length of gestation (Behrman & Butler, 2007). They were years of completed education, total household income adjusted by household size, age at study entry, parity and medical risk factors. Each was obtained from interviews or medical charts. Twenty-one medical risk conditions were coded based on a woman’s medical history (e.g., diabetes), obgyn history (e.g., previous abortion), and current pregnancy complications (e.g., bacterial vaginosis). A medical risk score was calculated as the total number of conditions present, based on prior research (see Hobel, Youkeles, & Forsythe, 1979; the full list is available from authors).
2.7. Statistical analyses
All analyses statistically controlled for covariates (medical risk factors, level of education completed, adjusted household income, age at study entry, and parity), and gestational week at the time of data collection for the placental CRH and pregnancy anxiety variables. Additionally, multiple imputation (Rubin, 2004; Enders, 2010) was used to account for missing data in the sample to increase power and reduce bias. The primary reason for missing data was a standard procedure involving placental CRH data removed at the time of assay for cases in which the values were greater than 25% deviation from the standard curve. Given the causes of missing data, we assumed that missing data were missing at random (MAR; Little and Rubin, 2002).
One-way repeated measures analyses of covariance (ANCOVA) were conducted to determine if levels of pregnancy anxiety, placental CRH, and change in placental CRH differed between Latina and non-Latina White participants. Next, using eight separate multiple regression analyses, gestational length was regressed on: (a) pregnancy anxiety at each of three times in pregnancy; (b) placental CRH at each of three timepoints; and (c) changes in placental CRH from 19 to 31 weeks gestation and from 25 to 31 weeks gestation of gestational length. All ANCOVAs and regression analyses were performed in R using multiply imputed data2. In these analyses, first, we estimated models with the independent variable (pregnancy anxiety, placental CRH, change in placental CRH), covariates, ethnicity, and the interaction between ethnicity and the corresponding independent variable. If the interaction term was significant, we retained the term in the final models. If the interaction term was not significant, we removed it from the model. For the models without the interaction term, ethnicity was included as a covariate.
Primary Analyses. We then tested the hypothesis that placental CRH, and change in placental CRH, mediate the association between pregnancy anxiety and length of gestation. First, we tested whether ethnicity significantly moderated the hypothesized indirect effects of pregnancy anxiety on the length of gestation via placental CRH, or changes in placental CRH. If significant moderation was found, we then used the moderated mediation model to test the simple mediated effects for Latina and non-Latina White women. Otherwise, we dropped the moderation component from the model and estimated the mediated effect in the full sample with ethnicity included as a covariate. This process was repeated for four separate mediators: placental CRH at 25 weeks, placental CRH at 31 weeks, change in placental CRH from 19 to 31 weeks, and change in placental CRH from 25 to 31 weeks. Additionally, we replicated the mediation analyses presented in Mancuso et al. (2004) using pregnancy anxiety at 31 weeks as the predictor and placental CRH at 31 weeks as the mediator in predicting length of gestation. Each effect was judged to be significant if the corresponding bias-corrected 95% confidence interval did not include zero. The mediation and moderated mediation analyses were conducted in each imputed dataset using the PROCESS macro in SPSS (Preacher and Hayes, 2008) and the bootstrapped estimates and confidence intervals were calculated in R3.
3. Results
3.1. Sample demographics
Table 1 presents the descriptive statistics and tests of ethnic differences for demographic characteristics for the full sample. Women in the present study were on average 30 years old (SD = 5.44) and the majority of the sample was either married to the baby’s father (76%) or cohabitating with him (15%). Compared to the 230 non-Latina White women, the Latina women were significantly younger, had lower adjusted household income and less education, and were more likely to have had a prior birth and to be married or cohabitating with the baby’s father? There were no significant differences between these groups on medical risk.
Table 1.
Descriptive Statistics for key variables in the full sample and by ethnicity.
| Full Sample | Latina | Non-LatinaWhite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 337) | (N = 107) | (N = 230) | |||||||||
| Variable | n | M | SD | n | M | SD | n | M | SD | F orχ2 | p |
| Age at Study Entry | 333 | 30.01 | 5.44 | 105 | 27.80 | 5.68 | 228 | 31.03 | 5.01 | 25.22 | <.001 |
| Adjusted Income | 332 | 25.80 | 16.75 | 105 | 14.69 | 12.26 | 227 | 30.97 | 16.05 | 104.08 | <.001 |
| Medical Risk | 329 | 2.45 | 1.63 | 103 | 2.65 | 1.79 | 226 | 2.36 | 1.54 | 1.97 | .081 |
| Parity (% multiparous) | 337 | 57% | 107 | 39% | 230 | 65% | 19.04 | <.001 | |||
| Education | 337 | 107 | 230 | 67.65 | <.001 | ||||||
| High School or less | 16% | 32% | 9% | ||||||||
| Some college | 37% | 53% | 30% | ||||||||
| Bachelor’s degree | 27% | 11% | 34% | ||||||||
| Graduate degree | 19% | 4% | 27% | ||||||||
| Married or cohabitating (% yes) | 337 | 75% | 107 | 59% | 230 | 83% | 20.73 | <.001 | |||
Note. Adjusted household income was calculated as total annual household income divided by household size, in units of thousands of dollars. Estimates and results for maternal age at study entry, adjusted income, medical risk, and length of gestation are based on multiple
3.2. Ethnic differences in pregnancy anxiety, length of gestation, and placental CRH
Table 2 presents descriptive statistics for pregnancy anxiety, placental CRH (19, 25, and 31 weeks), change in placental CRH (from 19 to 31 weeks and 25 to 31 weeks), and length of gestation, along with correlations among these measures in the full sample, univariate descriptive statistics of these measures by ethnicity, and tests of ethnic differences in these measures. After applying an experiment-wise Bonferroni correction (α = .0063 for eight tests), and after adjustment for covariates, Latina women reported significantly higher pregnancy anxiety at each timepoint in pregnancy compared to non-Latina White women (T1 F(1, 336.6) = 14.47, p < .001, d = 0.66; T2 F(1, 335.2) = 9.43, p = .002, d = 0.61; T3 F(1, 335.9) = 10.60, p = .001, d = 0.62). After adjustment for covariates, there were no ethnic differences in levels of placental CRH, changes in placental CRH, or length of gestation (all F’s < 1.33, p’s > .250). There were also no significant differences between US-born Latinas and foreign-born Latinas in length of gestation, pregnancy anxiety, placental CRH, or change in placental CRH (F < .67, p > .41) after adjustment for covariates.
Table 2.
Variances, Covariances, Correlations, and Comparisons by Ethnicity of Pregnancy Anxiety, pCRH (ln), and ΔpCRH (ln)
| Bivariate Statistics | Pregnancy Anxiety | pCRH (ln) | ΔpCRH (ln) | Gestation Length | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 19 wks | 25 wks | 31 wks | 19 wks | 25 wks | 31 wks | 19-31 wks | 25-31 wks | |||
| Pregnancy Anxiety | 19 wks | 0.26 | .70 | .69 | .05 | .21 | .19 | .14 | .00 | −.02 |
| 25 wks | 0.16 | 0.20 | .76 | .03 | .10 | .08 | .06 | −.01 | .05 | |
| 31 wks | 0.16 | 0.16 | 0.22 | .02 | .08 | .14 | .12 | .06 | −.06 | |
| pCRH (ln) |
19 wks | 0.01 | 0.01 | 0.00 | 0.28 | .35 | .19 | −.48 | −.14 | −.07 |
| 25 wks | 0.07 | 0.03 | 0.03 | 0.13 | 0.47 | .44 | .17 | −.49 | −.07 | |
| 31 wks | 0.07 | 0.03 | 0.05 | 0.07 | 0.22 | 0.53 | .77 | .57 | −.25 | |
| ΔpCRH (ln) | 19-31 wks | 0.06 | 0.02 | 0.04 | −0.21 | 0.09 | 0.46 | 0.67 | .59 | −.18 |
| 25-31 wks | 0.00 | 0.00 | 0.02 | −0.05 | −0.25 | 0.31 | 0.37 | 0.56 | −.17 | |
| Gestation Length | −0.01 | 0.04 | −0.05 | −0.06 | −0.08 | −0.30 | −0.24 | −0.21 | 2.71 | |
| Latina | n | 107 | 95 | 95 | 79 | 41 | 69 | 52 | 33 | 104 |
| M | 2.05 | 1.89 | 1.88 | 2.87 | 3.71 | 5.28 | 2.41 | 1.57 | 38.92 | |
| Min | 1.00 | 1.00 | 1.10 | 2.03 | 2.36 | 3.67 | 0.82 | −0.39 | 32.71 | |
| Max | 3.60 | 3.10 | 3.30 | 4.65 | 5.44 | 7.90 | 4.72 | 3.05 | 42.29 | |
| Non-Latina White | n | 227 | 203 | 204 | 175 | 82 | 128 | 92 | 68 | 217 |
| M | 1.83 | 1.73 | 1.71 | 2.87 | 3.73 | 5.22 | 2.35 | 1.49 | 39.32 | |
| Min | 1.00 | 1.00 | 1.00 | 1.63 | 2.44 | 3.65 | 0.32 | −0.18 | 31.86 | |
| Max | 3.60 | 3.30 | 3.40 | 4.64 | 5.04 | 6.64 | 4.57 | 3.82 | 42.14 | |
| F | 14.47 | 9.43 | 10.60 | 0.78 | 0.60 | 0.33 | 0.06 | 0.06 | 1.33 | |
| p | .000 | .002 | .001 | .377 | .439 | .567 | .811 | .802 | .250 | |
| d | .66 | .61 | .62 | −.20 | −.16 | −.11 | .04 | .04 | −.15 | |
Note. Numbers on the diagonal are variances, those below the diagonal are covariances, and those above the diagonal (italicized) are correlations. Statistical tests of group differences account for maternal age at study entry, parity, medical risk, adjusted income, education, and gestational age(s) at each time point for each dependent variable. Descriptive statistics were not adjusted for covariates. pCRH data were log transformed. Number of non-missing observations (n), minimum (Min) and maximum (Max) are based on the raw data, all other statistics are based on multiple imputation. Effect sizes were estimated using the pooled standard deviation.
3.3. Predictors of length of gestation
Table 3 presents regression coefficients from eight different models predicting gestational length from pregnancy anxiety (one model each for 19 weeks, 25 weeks, and 31 weeks), placental CRH (one each for 19 weeks, 25 weeks, and 31 weeks), and change in placental CRH (one each for 19 to 31 weeks and 25 to 31 weeks). After applying an experiment-wise Bonferroni correction (α = .0063 for eight tests), the interaction of ethnicity with T1 and T3 pregnancy anxiety as predictors of length of gestation was found to be statistically significant. Comparing the simple effects of T1 and T3 pregnancy anxiety for each racial/ethnic group and after applying an experiment-wise Bonferroni correction (α = .0125 for four tests), a significant effect of T3 pregnancy anxiety was identified in Latinas only (F (1, 328.8) = 9.62, p = .002, βx = −0.492). That is, one standard deviation increase in pregnancy anxiety at 31 weeks was associated with 0.492 weeks (3.444 days) shorter gestation in the Latina sample after accounting for covariates. As indicated in Table 3, levels of placental CRH at T1, T2, and T3, and changes in placental CRH from T1 to T3 and from T2 to T3 did not predict the length of gestation after adjusting for covariates. Figure 1 displays placental CRH across three data collection occasions.
Table 3.
Regression coefficients of separate models predicting length of gestation from pregnancy anxiety, pCRH, and ΔpCRH
| Interaction | Full Sample | Latina | Non-Latina White | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | F | p | b | SE | F | p | βx | b | SE | F | p | βx | b | SE | F | p | βx | |
| T1 Pregn ancy Anxiety | −0.99 | 0.35 | 8.01 | .005 | −0.64 | 0.27 | 5.75 | .017 | −.371 | 0.3 5 | 0.23 | 2.28 | .132 | .161 | |||||
| T2 Pregn ancy Anxiety | −0.80 | 0.42 | 3.75 | .054 | 0.03 | 0.21 | 0.0 2 | .883 | .01 4 | ||||||||||
| T3 Pregn ancy Anxiety | −1.14 | 0.39 | 8.35 | .004 | −0.93 | 0.30 | 9.62 | .002 | −.492 | 0.2 0 | 0.25 | 0.64 | .425 | .086 | |||||
| T1 pCRH (ln) | −0.13 | 0.42 | 0.10 | .754 | −0.11 | 0.20 | 0.3 0 | .586 | −.057 | ||||||||||
| T2 pCRH (ln) | −0.10 | 0.40 | 0.06 | .812 | −0.14 | 0.20 | 0.5 2 | .474 | −.096 | ||||||||||
| T3 pCRH (ln) | −0.51 | 0.28 | 3.23 | .074 | −0.58 | 0.16 | 12.54 | .000 | −.420 | ||||||||||
| T1T3 ΔpC RH (ln) | −0.36 | 0.27 | 1.84 | .176 | −0.39 | 0.15 | 6.3 7 | .013 | −.318 | ||||||||||
| T2T3 ΔpC RH (ln) | −0.53 | 0.35 | 2.31 | .130 | −0.37 | 0.19 | 3.8 9 | .050 | −.275 | ||||||||||
Note. βx = partially standardized regression coefficient, standardized using the predictor only. All analyses account for maternal age at study entry, parity, medical risk, adjusted income, education, and gestational age(s) at each time point for the independent variable. pCRH data were log transformed.
Figure 1.
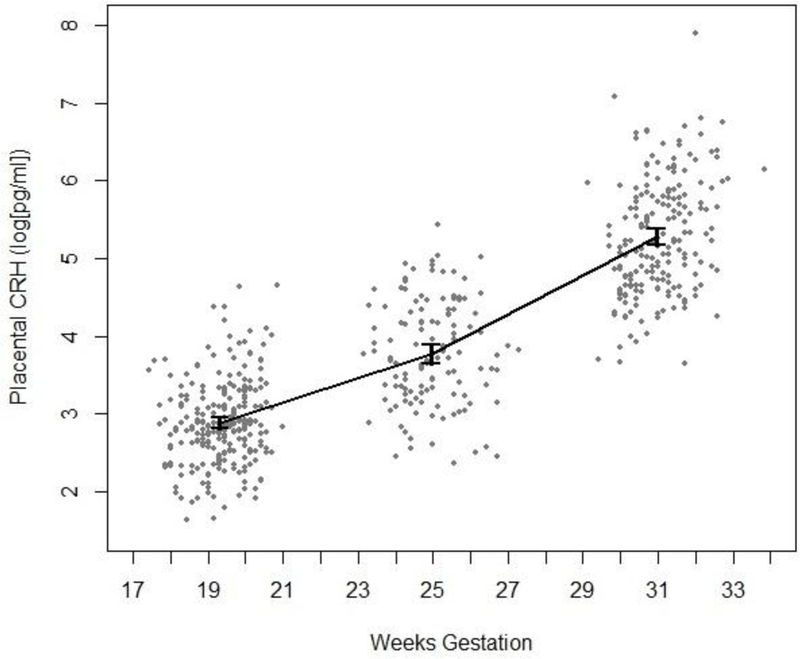
Placental CRH across three data collection occasions: 17-21 weeks gestation, n = 252; 23-29 weeks gestation, n = 123; 29-34 weeks gestation, n = 197. Error bars indicate 95% confidence intervals ( = ±1.96 * SE) for each measurement occasion.
3.4. Mediation and moderated mediation analyses
Table 4 shows the full results of the moderated mediation analyses, with ethnicity moderating the mediated effect of pregnancy anxiety at 19 weeks on length of gestation, mediated by (a) placental CRH at 25 weeks, (b) placental CRH at 31 weeks, (c) change in placental CRH from 19 to 31 weeks, or (d) change in placental CRH from 25 to 31 weeks. When placental CRH at 31 weeks was used as the mediator, the difference in the mediated effects for Latina and non-Latina White women was consistent with moderated mediation, CI = (−0.83, 0.05). However, when placental CRH at 25 weeks, or change in placental CRH from 19 to 31 weeks or from 25 to 31 weeks, was tested as the mediator, the index of moderated mediation was not significant, indicating that there was no difference between the mediated effects for Latina and non-Latina White women for those mediators.
Table 4.
Bootstrapped analyses of mediation and moderated mediation predicting length of gestation from T1 pregnancy anxiety
| Index of Moderated Mediation | Indirect Effect Full Sample | Indirect Effect White | Indirect Effect Latina | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mediat or | Es t | LL CI | UL CI | Es t | LL CI | UL CI | Estx | Es t | LL CI | UL CI | Es tx | Es t | LL CI | UL CI | Es tx |
| T2 pCRH(ln) | 0.03 | −0.28 | 0.35 | −0.05 | −0.15 | 0.04 | −0.02 | ||||||||
| T3 pCRH(ln) | −0.39 | −0.84 | −0.06 | −0.14 | −0.28 | −0.13 | −0.07 | −0.03 | −0.16 | 0.05 | −0.01 | −0.42 | −0.86 | −0.10 | −0.24 |
| ΔpCRH (ln) T1-T3 | −0.18 | −0.52 | 0.04 | −0.07 | −0.18 | −0.06 | −0.03 | ||||||||
| ΔpCRH (ln) T2-T3 | −0.19 | −0.64 | 0.05 | 0.01 | −0.01 | 0.09 | >0.01 | ||||||||
Note. LLCI = lower limit of confidence interval; ULCI = upper limit of confidence interval; Estx = partially standardized indirect effect, standardized using the predictor only. Confidence intervals are bias-corrected and based on 5000 bootstrapped samples for each of 100 imputed datasets. Significant mediating relationships bolded and indicated by 95% confidence intervals that do not include zero. All analyses account for maternal age at study entry, parity, medical risk, adjusted income, education, and gestational age(s) at each time point for pregnancy anxiety and pCRH.
Figure 2 displays the model and path coefficients for moderated association between pregnancy anxiety and length of gestation mediated by placental CRH at 31 weeks. Follow-up analyses using the moderated mediational model indicated that placental CRH at 31 weeks mediated the effect of pregnancy anxiety at 19 weeks on the length of gestation for Latina women CI = (−0.86, −0.09), Estx = −0.24. In this model, the conditional direct effect of pregnancy anxiety at 19 weeks on length of gestation for Latina women, which quantifies the direct effect of pregnancy anxiety at 19 weeks on length of gestation after controlling for placental CRH at 31 weeks and covariates, was not statistically significant (b = 0.49, F(1, 1065.5) = 3.13, p = .077). The calculated effect size indicates that one standard deviation increase in pregnancy anxiety at 19 weeks, as partially mediated by placental CRH at 31 weeks, was associated with 0.24 weeks (1.68 days) shorter gestation in Latina women. No such mediated effect was observed for nonLatina White women, CI = (−0.16, 0.05), Estx =0.01. These results for Latinas remained significant after controlling for place of birth (US-born vs. foreign-born).
Figure 2.
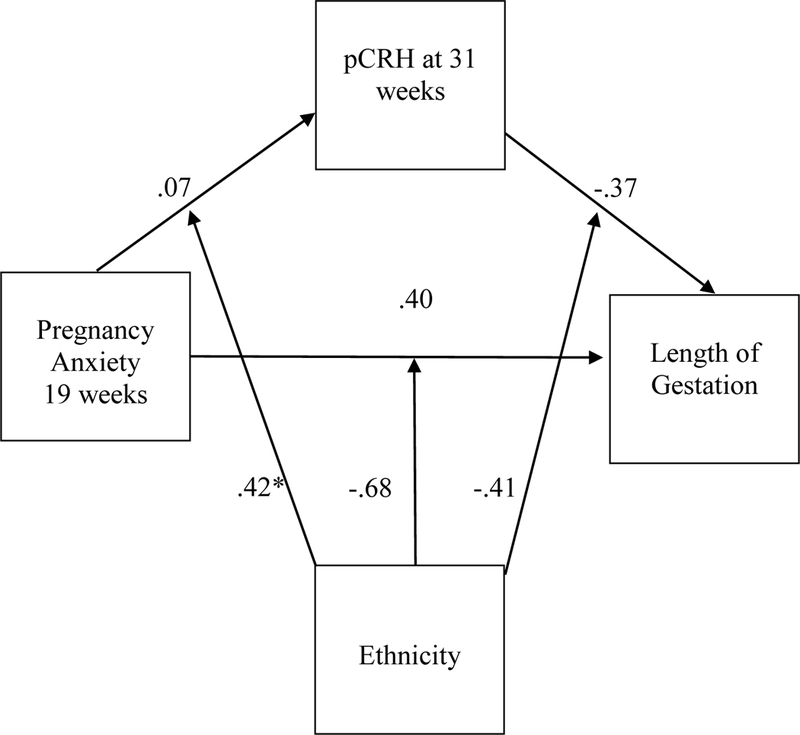
(a) Path model of pregnancy anxiety (19 weeks), pCRH (31 weeks), and length of gestation, moderated by ethnicity (n = 337). * p < .05.
In the full sample, the mediation results were also consistent with mediation by placental CRH at 31 weeks of the effect of pregnancy anxiety at 19 weeks on the length of gestation, CI = (−0.36, −0.13), Estx = −0.07, and by change in placental CRH from 19 to 31 weeks, CI = (−0.20, 0.06), Estx = −0.03. Our results were also consistent with placental CRH at 31 weeks mediating the effect of pregnancy anxiety at 31 weeks on the length of gestation, CI = (−0.242, −0.076), Estx = −.046, replicating the finding of Mancuso et al. (2004). As with the previous results, the direct effect of pregnancy anxiety on length of gestation, adjusting for placental CRH and covariates, was not significant in either model (F < .79, p > .373). There were no significant mediated effects of placental CRH at 25 weeks or change in placental CRH from 25 to 31 weeks in the full sample, nor was there evidence of moderated mediation for those mediators.
4. Discussion
In this prospective and longitudinal study of pregnant women, we tested a biopsychosocial model with pregnancy anxiety as the psychological predictor, placental CRH (a stress-responsive peptide) as the biological mediator, length of gestation as the outcome, and ethnicity as the moderator of the indirect or mediated effect. We found that pregnancy anxiety at 19 and at 31 weeks and levels of CRH of placental origin at 31 weeks each significantly predicted length of gestation. These results are consistent with prior research and make an important contribution given the specifics of this sample and study design.
The tests of indirect effects of pregnancy anxiety at 19 weeks on gestational length via placental CRH at 31 weeks in the full sample were consistent with partial mediation. These results replicate and extend an earlier finding from the only other study to test these specific processes (Mancuso et al., 2004), which found that anxiety tied to fears and worries surrounding a woman’s current pregnancy predicts the timing of delivery is at least partially explained by altered neuroendocrine function. However, the current study measured pregnancy anxiety and placental CRH at different times in pregnancy than the previous study. Taken together, these two studies on separate cohorts with assays of placental CRH done in separate labs strengthen the evidence that CRH plays a role in stress processes during pregnancy that have been shown to influence timing of delivery. These results also suggest that mid- to late pregnancy may be a sensitive period during which the effects of pregnancy anxiety and placental CRH are particularly salient for effects on gestational length. Further research can further probe the issue of timing.
In addition, this study extends the previous findings by examining whether changes in placental CRH during pregnancy might mediate the relationship between pregnancy anxiety and gestational length. We found evidence consistent with partial mediation in that changes in placental CRH from 19 to 31 weeks partially mediated the association between pregnancy anxiety at 19 weeks and the length of gestation in the full sample. These findings are the first to indicate that levels of pregnancy anxiety at mid-pregnancy are associated with changes in placental CRH from mid to late pregnancy, with implications for the timing of delivery. Further experimental evidence of changes in this neuropeptide and the effects would be valuable.
Placental CRH has been associated with maturation of the human fetus and with postnatal child behavioral and brain development, highlighting the importance of testing this specific biomarker (Class et al., 2009; Howland, Sandman, Glynn, Crippen, & Davis, 2016; Sandman et al., 2018). Although the full physiological pathways explaining the association between pregnancy anxiety and placental CRH have not been fully identified, evidence points to a potential direct or indirect association of maternal stress with activation of the HPA axis during gestation (Kane, Dunkel Schetter, Glynn, Hobel, & Sandman, 2014). It is hypothesized that prenatal maternal stress is related to stress-induced secretion of catecholamines by brain arousal and sympathetic systems, which is thought to lead to maternal cortisol secretion, which in turn stimulates the production of placental CRH (Goldstein, 2003; Valsamakis et al., 2017). Understanding the mechanisms through which maternal stress disrupts the normative physiological processes of pregnancy may have implications for identification of women who might benefit from intervention. For example, assessment of placental CRH levels might be used as an indicator of risk for impending preterm delivery (Hill et al., 2008; Korebrits et al., 1998), which could then be used to guide clinical decision making, ensuring that antenatal glucocorticoids are administered in the optimal time frame (Kamath-Rayne, Rozance, Goldenberg, & Jobe, 2016).
This is also the first study to explore whether ethnicity moderates the indirect pathways from pregnancy anxiety to placental CRH and to gestational length at birth. Tests of moderated mediation by ethnicity indicated significant moderation; that is, pregnancy anxiety at 19 weeks predicted CRH at 31 weeks, which predicted timing of birth for Latina women and not for the non-Latina White women. The indirect effect via changes in placental CRH from 19 to 31 weeks was not significantly moderated by ethnicity. It is important to note that placental CRH level at 31 weeks and change in placental CRH from 19 to 31 weeks were strongly correlated (r = .77) which makes it difficult to compare the two sets of tests and calls for additional research on larger samples. Taken together, these findings contribute to the growing body of research on pregnancy anxiety and its role in the timing of birth and contribute to our understanding of the biological mechanisms involved.
Additionally, we identified significant differences between Latinas and non-Latina Whites in pregnancy anxiety after controlling for medical risk factors, level of education completed, adjusted household income, age at study entry, and parity. Cultural differences may in part explain these group differences. Unique cultural-based sources of anxiety in pregnancy have been identified in a few studies (Campos, Dunkel-Schetter, Walsh, & Schenker, 2007; Engle et al., 1990; Scrimshaw et al., 1997). Furthermore, qualitative research on myths about pregnancy has suggested that Mexican-American women may show heightened concerns about dying and infant death during childbirth (Scrimshaw et al., 1997). In many Latin American cultures, childbearing is a highly-valued aspect of the female role, even to the extent that men and women might believe that a woman cannot be fulfilled until she is a mother (MaldonadoDuran, Munguía-Wellman, Lubin, & Lartigue, 2002). Other research suggests that Latino families in the United States treat pregnancy as a time of privileged social status (Fleuriet, 2009; Fleuriet & Sunil, 2014). Latina women in past research also have reported more positive attitudes toward pregnancy as compared to African-American women (Zambrana, Dunkel Schetter, Collins, & Scrimshaw, 1999). However, studies have not tested whether this privileged status contributes to experiences of pregnancy anxiety in Latina women because pregnancy represents a major responsibility to the family (Fleuriet, 2009). Future research directly measuring cultural variables can help illuminate the contributors of ethnic differences in pregnancy outcomes (Betancourt & López, 1993).
4.1. Strengths and limitations
The strengths of this research include its longitudinal design, repeated measurements of psychological (e.g., pregnancy anxiety) and biological variables (placental CRH), and inclusion of key sociodemographic factors in the prediction of an objective health outcome of major public health significance. Studies with both psychosocial and biological data collected at multiple times throughout the course of pregnancy on samples of sufficient size to test these questions are rare, in part due to the difficulty of sample recruitment and retention. Such work contributes to understanding how changes in these psychological and biological factors co-occur to influence gestational length at birth.
It is important to note that the association between placental CRH and shortened gestation is based on longitudinal correlational data, and is not conclusive causal evidence of mediation. Future research is needed to test the hypothesized causal pathway between pregnancy anxiety and preterm birth as mediated by placental CRH, ideally with experimental designs. Moreover, these findings require replication in larger samples. Also, this sample of Latina women may not be representative of Latina women in the United States population because participants had to speak sufficient English to be interviewed, which skewed the sample toward more acculturated women and underrepresented foreign-born women. We surmise that the effects of ethnicity would have been stronger if fewer acculturated and more foreign-born women had been included, especially given that pregnancy anxiety has been shown to be higher in less acculturated women (Fleuriet & Sunil, 2014). Furthermore, we did not examine changes in pregnancy anxiety across gestation as predictors of the mediator or outcome, which was beyond the scope of the current report. Future research on trajectories in pregnancy anxiety would be valuable. Finally, we did not examine HPA activity markers such as cortisol or ACTH as mediators, or any other biological markers such as pro-inflammatory cytokines, which are implicated in preterm birth pathways (e.g., Christian, 2012; Coussons-Read et al., 2012; Wadhwa et al., 2001).
4.2. Conclusion
In this study, we found that women who reported higher levels of anxiety regarding their pregnancies at mid and late gestation were at risk for earlier delivery. In addition, Latina women in this sample reported higher pregnancy anxiety during most of pregnancy compared to nonLatina White women, despite being more acculturated on average than a representative sample of Latinas would be. Future research can build on these findings to determine if pregnancy anxiety differs by ethnic group and can incorporate broader conceptualizations of culture. These findings highlight the HPA axis as one potential mechanism explaining the association between pregnancy anxiety and the length of gestation, as well as the role of ethnicity in moderating such effects. Why Latina ethnicity moderated the mediational pathway remains to be determined. Nonetheless, this work represents a relatively rare test of a full biopsychosocial mechanistic model and can inform future research.
Highlights.
Levels of placental CRH at 31 weeks and pregnancy anxiety at 19 and 31 weeks predicted gestational length.
The association between pregnancy anxiety at 19 weeks with gestational length was consistent with partial mediation by placental CRH at 31 weeks, and with mediation by changes in placental CRH from 19 to 31 weeks.
Tests of moderated mediation by ethnicity were consistent with mediation by placental CRH at 31 weeks for Latinas only.
Latinas reported higher pregnancy anxiety at each time point during pregnancy compared to non-Latina White women.
Acknowledgments:
This research was supported by a NIMH pre-doctoral fellowship to the first author (T32MH015750) on Biobehavioral Issues in Mental and Physical Health, and by research grants from NIH (HD 40967) and from NIH (NS 41298)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
In a prior study of a subset of the present cohort examining familism, social support, perceived stress, pregnancy anxiety, and infant birthweight, there was an incidental finding on ethnic differences in pregnancy anxiety, which were not the focus of that study (Campos et al., 2008). The present study uses a larger cohort and different predictors and outcomes. Only the analysis of ethnic differences in pregnancy anxiety is common between them.performed in R using multiply imputed data2
The R package mice (R Core Team, 2016; Buuren & Groothuis-Oudshoorn, 2011) was used to generate 100 imputations using regression imputation for continuous variables and logistic/multinomial imputation for categorical variables. To account for slight differences in gestational week at the time of placental CRH measurement, gestational week at the time of placental CRH measurement was included as an auxiliary variable for imputation (Enders, 2010). Unless otherwise noted, all parameter estimates, standard errors, and significance tests were calculated by pooling the model results across imputations in R using Rubin’s pooling rules (Rubin, 2004), and all F tests used the D1 multiple imputation significance test (Enders, 2010).
Bios-corrected bootstrapped confidence intervals were used to test the mediated effects and index of moderated mediation. We adapted the approach described in Schomaker and Heumann (2016), which calculates confidence intervals based on the bootstrapped estimates from all imputations.
References
- Behrman RE, & Butler AS (Eds.). (2007). Preterm birth: Causes, consequences, and prevention. National Academies Press. [PubMed] [Google Scholar]
- Betancourt H, & López SR (1993). The study of culture, ethnicity, and race in American psychology. American Psychologist, 48(6), 629. [Google Scholar]
- Buuren S, & Groothuis-Oudshoorn K (2011). mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, 45(3). [Google Scholar]
- Campos B, Dunkel Schetter C, Walsh JA, & Schenker M (2007). Sharpening the focus on acculturative change: ARSMA-II, stress, pregnancy anxiety, and infant birthweight in recently immigrated Latinas. Hispanic Journal of Behavioral Sciences, 29(2), 209–224. [Google Scholar]
- Campos B, Dunkel Schetter CD, Abdou CM, Hobel CJ, Glynn LM, & Sandman CA (2008). Familialism, social support, and stress: Positive implications for pregnant Latinas. Cultural Diversity and Ethnic Minority Psychology, 14(2), 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM (2012). Psychoneuroimmunology in pregnancy: immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neuroscience & Biobehavioral Reviews, 36(1), 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Buss C, Davis EP, Gierczak M, Pattillo C, Chicz-DeMet A, & Sandman CA (2009). Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Developmental Neuroscience, 30(6), 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong B, Zhang L, Gao L, & Ni X (2009). Reduced expression of CRH receptor type 1 in upper segment human myometrium during labour. Reproductive Biology and Endocrinology, 7(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, & Cole S (2012). The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain, Behavior, and Immunity, 26(4), 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, & Buekens P(2003). Maternal stress and preterm birth. American Journal of Epidemiology, 157(1), 14–24. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C (2010). Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annual Review of Psychology, 62, 531–558. [DOI] [PubMed] [Google Scholar]
- Enders CK (2010). Applied missing data analysis. New York, NY: Guilford Press. [Google Scholar]
- Engle PL, Scrimshaw SC, Zambrana RE, & Dunkel-Schetter C (1990). Prenatal and postnatal anxiety in Mexican women giving birth in Los Angeles. Health Psychology, 9(3), 285. [DOI] [PubMed] [Google Scholar]
- Fleuriet KJ (2009). La Tecnología y Las Monjitas. Medical Anthropology Quarterly, 23(3), 212–234. [DOI] [PubMed] [Google Scholar]
- Fleuriet KJ, & Sunil TS (2014). Perceived social stress, pregnancy-related anxiety, depression and subjective social status among pregnant Mexican and Mexican American women in south Texas. Journal of Health Care for the Poor and Underserved, 25(2),546561. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Dunkel Schetter C, Chicz-DeMet A, Hobel CJ, & Sandman CA, (2007). Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides, 28(6), 1155–1161. [DOI] [PubMed] [Google Scholar]
- Glynn LM, & Sandman CA (2014). Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosomatic Medicine, 76(5), 355–362. [DOI] [PubMed] [Google Scholar]
- Goldstein DS (2003). Catecholamines and stress. Endocrine regulations, 37(2), 69–80. [PubMed] [Google Scholar]
- Guardino CM, & Dunkel Schetter C (2014). Understanding pregnancy anxiety: Concepts, correlates, and consequences. Zero to Three, 34(4), 12–21. [Google Scholar]
- Hill JL, Campbell MK, Zou GY, Challis JR, Reid G, Chisaka H, & Bocking AD(2008). Prediction of preterm birth in symptomatic women using decision tree modeling for biomarkers. American Journal of Obstetrics and Gynecology, 198(4), 468.e1–468.e9. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, & Grammatopoulos DK (2002). Role of stress peptides during human pregnancy and labour. Reproduction, 124(3), 323–329. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Youkeles L, & Forsythe A (1979). Prenatal and intrapartum high-risk screening. American Journal of Obstetrics & Gynecology, 135(8), 1051–1056. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, & Arora CP (1999). Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. American Journal of Obstetrics and Gynecology, 180(1), S257–S263. [DOI] [PubMed] [Google Scholar]
- Howland MA, Sandman CA, Glynn LM, Crippen C, & Davis EP (2016). Fetal exposure to placental corticotropin-releasing hormone is associated with child self reported internalizing symptoms. Psychoneuroendocrinology, 67, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, He P, You X, Zhu X, Dai L, He Q, … & Ni X (2007). Expression of corticotropin-releasing hormone receptor type 1 and type 2 in human pregnant myometrium. Reproductive Sciences, 14(6), 568–577. [DOI] [PubMed] [Google Scholar]
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, & Jobe AH (2016). Antenatal corticosteroids beyond 34 weeks gestation: what do we do now?. American Journal of Obstetrics and Gynecology, 215(4), 423–430. [DOI] [PubMed] [Google Scholar]
- Kane HS, Dunkel Schetter C, Glynn LM, Hobel CJ, & Sandman CA (2014).Pregnancy anxiety and prenatal cortisol trajectories. Biological Psychology, 100, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korebrits C, Ramirez MM, Watson L, Brinkman E, Bocking AD, & Challis JRG(1998). Maternal corticotropin-releasing hormone is increased with impending preterm birth. The Journal of Clinical Endocrinology & Metabolism, 83(5), 1585–1591. [DOI] [PubMed] [Google Scholar]
- Kramer M, Lydon J, Séguin L, Goulet L, Kahn S, McNamara H, et al. (2009). Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. American Journal Epidemiology, 169(13), 19–26. [DOI] [PubMed] [Google Scholar]
- Lindsay JR, & Nieman LK (2005). The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocrine Reviews, 26(6), 775–799. [DOI] [PubMed] [Google Scholar]
- Little RJ, & Rubin DB (2014). Statistical analysis with missing data. John Wiley & Sons. [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, & Meyer BA (2008). Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology, 27(5), 604. [DOI] [PubMed] [Google Scholar]
- Maldonado-Duran JM, Munguía-Wellman M, Lubin S, & Lartigue T (2002). Latino families in the perinatal period: Cultural issues in dealing with the health-care system. Great Plains Research, 75–100. [Google Scholar]
- Mancuso RA, Dunkel Schetter C, Rini CM, Roesch SC, & Hobel CJ (2004). Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosomatic Medicine, 66(5), 762–769. [DOI] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, & Smith R (1995). A placental clock controlling the length of human pregnancy. Nature Medicine, 1(5), 460–463. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, & Sandman CA (1999). Psychological adaptation and birth outcomes: The role of personal resources, stress, and sociocultural context in pregnancy. Health Psychology, 18(4), 333. [DOI] [PubMed] [Google Scholar]
- Rubin DB (2004). Multiple imputation for nonresponse in surveys (Vol. 81). John Wiley & Sons. [Google Scholar]
- Ruiz RJ, Fullerton J, Brown CE, & Dudley DJ (2002). Predicting risk of preterm birth: The roles of stress, clinical risk factors, and corticotropin-releasing hormone. Biological Research for Nursing, 4(1), 54–64. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Curran MM, Davis EP, Glynn LM, Head K, & Baram TZ (2018). Cortical Thinning and Neuropsychiatric Outcomes in Children Exposed to Prenatal Adversity: A Role for Placental CRH?. American Journal of Psychiatry, 175(5), 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, & Glynn LM (2009). Corticotropin-releasing hormone programs the fetal and maternal brain. Future Neurology, 4(3), 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomaker M, & Heumann C (2016). Bootstrap Inference when Using Multiple Imputation. arXiv preprint arXiv:1602.07933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimshaw SCM, Zambrana R, & Dunkel Schetter C (1997). Issues in Latino women’s health: Myths and challenges In Ruzek SB, Oleson VL, and Clarke AE (Eds.),Women’s health: Complexities and differences. Ohio University Press. [Google Scholar]
- Siler-Khodr TM, Forthman G, Khodr C, Matyszczyk S, Khodr Z, & Khodr G (2003). Maternal serum corticotropin‐releasing hormone at mid-gestation in Hispanic and White women. Obstetrics & Gynecology, 101(3), 557–564. [DOI] [PubMed] [Google Scholar]
- Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, … & Smith DW (2009). Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. The Journal of Clinical Endocrinology & Metabolism, 94(6), 2066–2074. [DOI] [PubMed] [Google Scholar]
- Valsamakis G, Papatheodorou DC, Chalarakis N, Vrachnis N, Sidiropoulou EJ,Manolikaki M, .& Mastorakos G (2017). In pregnancy increased maternal STAI trait stress score shows decreased insulin sensitivity and increased stress hormones. Psychoneuroendocrinology, 84, 11–16. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, & Glynn L (2001). Stress, infection and preterm birth: A biobehavioural perspective. Paediatric and Perinatal Epidemiology, 15, 17–29. [DOI] [PubMed] [Google Scholar]
- Zambrana RE, Scrimshaw SC, Collins N, & Dunkel-Schetter C (1999). Prenatal health behaviors and psychosocial risk factors in pregnant women of Mexican origin: The role of acculturation. American Journal of Public Health, 87(6), 1022–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LM, Wang YK, Hui N, Sha JY, Chen X, Guan R, & Ni X (2008). Corticotropin-releasing hormone acts on CRH-R1 to inhibit the spontaneous contractility of non-labouring human myometrium at term. Life Sciences, 83(17–18), 620–624. [DOI] [PubMed] [Google Scholar]


