Abstract
The laminin family of glycoproteins are major constituents of the basal lamina of blood vessels, and play a fundamental role in promoting endothelial differentiation and blood-brain barrier (BBB) stability. Chronic mild hypoxia (CMH), in which mice are exposed to 8% O2 for two weeks, induces a strong vascular remodeling response in the central nervous system (CNS) that includes endothelial proliferation, angiogenesis, arteriogenesis as well as increased expression of tight junction proteins, suggestive of enhanced vascular integrity. As previous studies highlight an important role for laminin in promoting vascular differentiation and BBB stability, the goal of this study was to determine if CMH influences the expression of the laminins and their cell surface receptors in cerebral blood vessels. Our studies revealed that over a 14 day period of CMH, blood vessels in the brain showed strong upregulation of the specific laminin subunits αl and α4, corresponding to increased expression of laminins 111 and 411 respectively, with no discernible changes in the expression levels of the α2 or α5 laminin subunits. This was accompanied by marked endothelial upregulation of the laminin receptor α6β1 integrin but no alterations in the other laminin receptors α1β1 integrin or dystroglycan. In light of the instructive role for laminins in promoting vascular differentiation and stability, these data suggest that upregulation of the laminin-α6β1 integrin axis is part of the molecular response triggered by mild hypoxia that leads to enhanced BBB stability.
Keywords: Hypoxia, central nervous system, vascular remodeling, blood-brain barrier (BBB), laminin, integrin
1. INTRODUCTION
Blood vessels in the brain are unique in forming the blood-brain barrier (BBB), which confers high electrical resistance and low permeability properties, thus protecting neural cells from potentially harmful blood components (Ballabh et al., 2004; Huber et al., 2001; Pardridge, 2003; Wolburg and Lippoldt, 2002). Extensive evidence suggests that the molecular basis of the BBB depends on three main components: (i) inter-endothelial tight junction proteins, (ii) the influence of astrocyte end-feet and pericytes and (iii) endothelial adhesion to the extracellular matrix (ECM) proteins of the underlying vascular basal lamina (Daneman et al., 2010; del Zoppo and Milner, 2006; Huber et al., 2001; Osada et al., 2011; Pardridge, 2003; Wolburg and Lippoldt, 2002). Laminins are a major component of the basal lamina and have been shown to influence many aspects of cell behavior, including cell migration, proliferation, and differentiation in many different cell types (Grant et al., 1989; Grant and Kleinman, 1997; Timpl, 1989; Timpl and Brown, 1994). Laminins are a family of closely related heterotrimers composed of α, β and γ chains, of which there are 5 α, 3 β and 3 γ chains currently reported, that can combine to form up to 12 different isoforms of laminin (Engvall, 1993; Engvall and Wewer, 1996; Sixt et al., 2001). Several lines of evidence suggest that laminin plays an important instructive role in promoting vascular differentiation and stabilization. First, during blood vessel formation in the developing CNS, there is a gradual switch in the expression of specific ECM proteins, from high-fibronectin and fibronectin receptors during angiogenic modeling to high-laminin and laminin receptors at later times (Milner and Campbell, 2002). Second, in vitro studies have shown that compared to fibronectin, laminin is poor at promoting endothelial proliferation (Wang and Milner, 2006), but potent at inducing endothelial terminal differentiation and the establishment of vascular tubes (Davis and Camarillo, 1995; Grant et al., 1989; Grant and Kleinman, 1997). Third and more recently, a number of studies have shown that transgenic mice deficient in astrocyte or pericyte laminin show defective BBB integrity (Chen et al., 2013; Gautam et al., 2016; Menezes et al., 2014).
During the last decade, a number of studies have demonstrated that hypoxic pre-conditioning (a period of training at a sub-clinical hypoxic level, e.g. 8% O2), protects against pathogenesis in a number of animal models of human neurological disease, including ischemic stroke and multiple sclerosis (MS) (Dore-Duffy et al., 2011; Esen et al., 2016; Miller et al., 2001; Stowe et al., 2011). In trying to understand how this protection is mediated, a number of groups have focused on the influence of chronic mild hypoxia (CMH) on vascular structure and function (LaManna et al., 1992; LaManna et al., 2004; Milner et al., 2008). Remarkably, exposure of mice or rats to chronic mild hypoxia (8% O2) promotes a marked angiogenic response in the CNS, resulting in greater than 50% increased vessel density over a 2 week period. Recent studies have revealed that this hypoxic-induced vascular remodeling is not limited to capillaries, but also includes significant arteriogenic remodeling, demonstrating that CMH promotes vascular remodeling at all stages of the vascular tree (Boroujerdi et al., 2012). Furthermore, CMH also promotes strong upregulation of the endothelial tight junction proteins claudin-5 and ZO-1 at the BBB, implying that CMH also triggers beneficial changes in the vascular integrity of the BBB (Boroujerdi and Milner, 2015; Li et al., 2010a) and this is supported by the finding that CMH strongly attenuates leukocyte infiltration across the BBB in models of ischemic stroke and MS (Dore-Duffy et al., 2011; Stowe et al., 2011). More recently, we described similar hypoxic induction of vascular remodeling in blood vessels of the spinal cord (Halder et al., 2018). In light of the important role of laminin in promoting vascular differentiation and stability in CNS blood vessels, the goal of this study was to determine if CMH influences the expression of laminins and their cell surface receptors in cerebral blood vessels.
2. RESULTS
2.1. Chronic mild hypoxia upregulates laminin expression in the basal lamina of cerebral blood vessels
To examine if chronic mild hypoxia (CMH) influences laminin expression in cerebral blood vessels, 10 week old mice were exposed to normoxia (control) or hypoxia (8% O2) for 2, 4, 7 or 14 days. Frozen brain sections were then analyzed by CD31/laminin dual-immunofluorescence (dual-IF) for the endothelial cell marker CD31 and a polyclonal antibody that recognizes all isoforms of laminin. This revealed that after 4 days CMH, vascular expression levels of total laminin were strongly upregulated on all blood vessels in the brain (Figure 1), with the increase in expression obvious as early as day 4 CMH and maintained at all time-points examined thereafter. Quantification of expression levels by fluorescent densitometry using NIH Image J software showed that following 4 days CMH, laminin expression levels on cerebral blood vessels were significantly increased from 3.87 ± 0.36 fluorescent units under normoxic conditions to 7.08 ± 0.33 fluorescent units (p < 0.01) (Figure 1B) and this expression was maintained high at the later time-points of 7 days (7.71 ± 0.51, p < 0.01) and 14 days (6.66 ± 0.13, p < 0.01). Our studies on brain issue were focused in the medulla oblongata region of the brainstem, but similar changes in laminin expression were observed in all brain regions examined.
Figure 1.
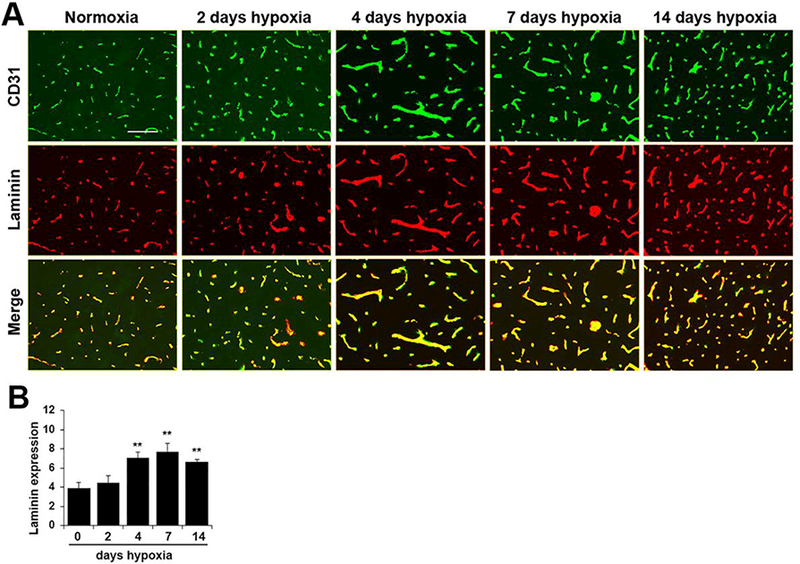
Chronic mild hypoxia (CMH) upregulates laminin expression in cerebral blood vessels. A. Dual-IF was performed on frozen brain sections taken from mice exposed to normoxia or 2, 4, 7, or 14 days CMH (8% O2) using antibodies specific for CD31 (AlexaFluor-488) and total laminin (Cy-3). Scale bar = 100 μm. B. Quantification of total laminin fluorescent signal at different time-points of CMH in the brain. Results are expressed as the mean ± SEM (n = 4 mice/group). Note that after 4 days CMH, total vascular laminin expression was strongly increased, and this enhanced expression was maintained at the later time-points of 7 and 14 days. ** p < 0.01 vs. normoxia (0 days hypoxia) and vs. 2 days hypoxia.
2.2. Chronic mild hypoxia specifically upregulates expression of laminins 111 and 411 in the basal lamina of cerebral blood vessels
As laminins are a large family of more than 12 different members (Engvall, 1993; Engvall and Wewer, 1996), it is important to determine which of these specific isoforms are being upregulated in response to CMH. The vascular basal lamina of CNS blood vessels is a bi-layer structure that during development, is generated both by endothelial cells (luminal side) and astrocytes and leptomeningeal cells (parenchymal side). Previous work from the Sorokin laboratory has shown that endothelial cells secrete and organize the laminin α subunits α4 and laminin β5 (which trimerize with the β1 and γ1 subunits to form laminins 411 and 511 respectively) in the luminal (innermost) layer of the vascular basal lamina, while astrocytes and leptomeningeal cells contribute laminin α1 and α2 (to form the laminins 111 and 211 respectively) to make the parenchymal (outer) layer of the vascular basal lamina (Sixt et al., 2001).
IF studies using a panel of antibodies specific for the α1, α2, α4 and α5 laminin subunits revealed that all isoforms are expressed within the basal lamina of cerebral blood vessels (Figure 2). The α2, α4 and α5 laminin subunits are expressed by all sizes of cerebral blood vessels, including arterioles, capillaries and venules, while in contrast, and confirming previous work from the Sorokin lab (Sixt et al., 2001), expression of the laminin α1 subunit appears to be restricted to the larger diameter arterial and venous vessels and is not expressed by the smaller diameter capillaries.
Figure 2.
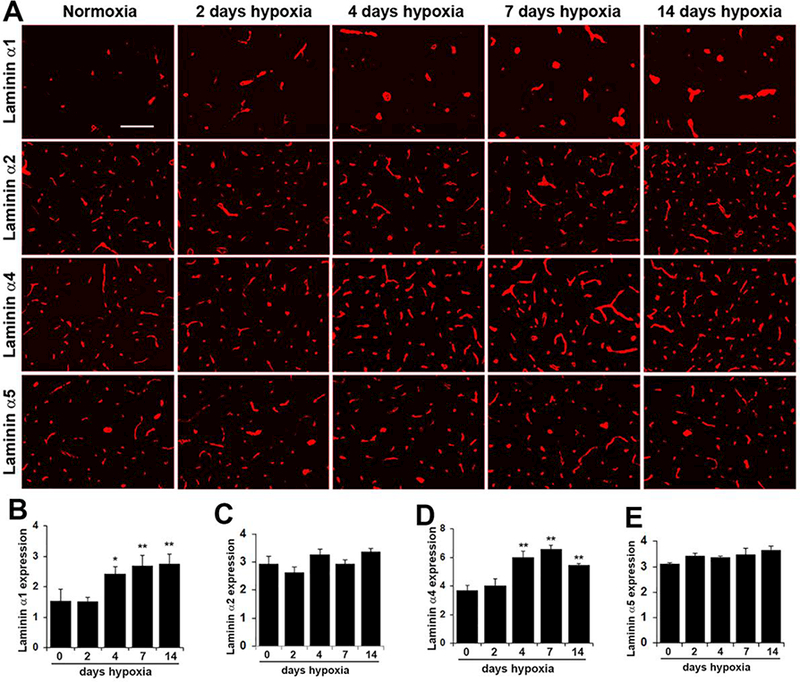
Chronic mild hypoxia (CMH) specifically upregulates the laminin αl and α4 subunits in cerebral blood vessels. A. Frozen brain sections taken from mice exposed to normoxia or 2, 4, 7, or 14 days CMH (8% O2) were stained using antibodies specific for the laminin subunits αl, α2, α4 or α5. Scale bar =100 μm. B-E. Quantification of cerebrovascular expression of the laminin subunits αl (B), α2 (C), α4 (D) and β1 (E) at different time-points of CMH. Results are expressed as the mean ± SEM (n = 4 mice/group). Note that after 4 days CMH, cerebrovascular expression levels of the al and α4 laminin subunits were significantly increased, and this enhanced expression level was maintained at the later time-points of 7 and 14 days. * p < 0.05, ** p < 0.01 vs. normoxia (0 days hypoxia) and vs. 2 days hypoxia.
Interestingly, while vascular expression levels of the α2 and α5 laminin isoforms showed no obvious change after exposure to CMH, expression of the α1 and α4 subunits on cerebral blood vessels showed clear upregulation (Figure 2). Quantification of expression levels by fluorescent densitometry showed that following 4 days CMH, cerebrovascular expression of the laminin α1 subunit was increased from 1.54 ± 0.22 fluorescent units under normoxic conditions to 2.41 ± 0.14 fluorescent units (p < 0.05) (Figure 2B) and this expression was further increased at the later time-points of 7 days (2.68 ± 0.20, p < 0.01) and 14 days (2.76 ± 0.18, p < 0.01). In the same timeframe, 4 days CMH increased cerebrovascular expression of the α4 laminin subunit from 3.68 ± 0.21 fluorescent units under normoxic conditions to 5.99 ± 0.25 fluorescent units (p < 0.01) (Figure 2D) and this expression was further increased after 7 days CMH to 6.59 ± 0.14 (p < 0.01) and maintained high at the 14 day time-point (5.46 ± 0.07, p < 0.01). While our studies in brain issue were focused in the medulla oblongata region of the brainstem, very similar changes in the expression of vascular laminin isoforms was observed in all brain regions examined. Taken together, these results demonstrate that CMH specifically stimulates increased expression of the laminin α1 and a4 subunits within the basal lamina of cerebral blood vessels.
2.3. Chronic mild hypoxia strongly upregulates endothelial α6β1 integrin in cerebral blood vessels
Having shown that cerebral blood vessels show increased expression of the laminin subunits α1 and α4 with exposure to CMH, next we examined whether CMH affects expression of vascular laminin receptors. First, to define which laminin receptors are expressed by cerebral blood vessels, we performed dual-IF on frozen sections of brain with the endothelial marker CD31 and antibodies against the well-characterized laminin receptors α6β1, α1β1 and dystroglycan. As shown in Figure 3, cerebral blood vessels stained positive for the integrin αl, α6 and β1 subunits as well as β-dystrog1ycan, demonstrating expression of all three laminin receptors. Next, we examined how CMH influences the expression of these receptors. This revealed that CMH increased expression of the integrin α6 and β1 subunits on cerebral blood vessels (Figure 4) but did not appreciably alter expression of the α1 integrin subunit or β-dystroglycan. Quantification by fluorescent densitometry showed that following 4 days CMH, cerebrovascular expression of the α6 integrin subunit was increased from 3.31 ± 0.09 fluorescent units under normoxic conditions to 8.01 ± 0.50 fluorescent units (p < 0.01) (Figure 4C) and this expression was further increased after 7 days CMH to 8.23 ± 0.57 (p < 0.01) and maintained high after 14 days CMH at 7.32 ± 0.26 (p < 0.01). At the same time, 4 days CMH also increased cerebrovascular expression of the β1 integrin subunit from 3.98 ± 0.33 fluorescent units under normoxic conditions to 6.16 ± 0.18 fluorescent units (p < 0.01) and this expression was maintained high at 7 days CMH to 5.86 ± 0.27 (p < 0.01) (Figure 4D). While our studies in brain issue were focused in the medulla oblongata region of the brainstem, very similar changes in the expression of laminin receptors was observed in all brain regions examined. This demonstrates that CMH specifically stimulates increased expression of the α6β1 integrin laminin receptor in cerebral blood vessels but does not affect expression of the alternative laminin receptors, α1β1 integrin or dystroglycan.
Figure 3.
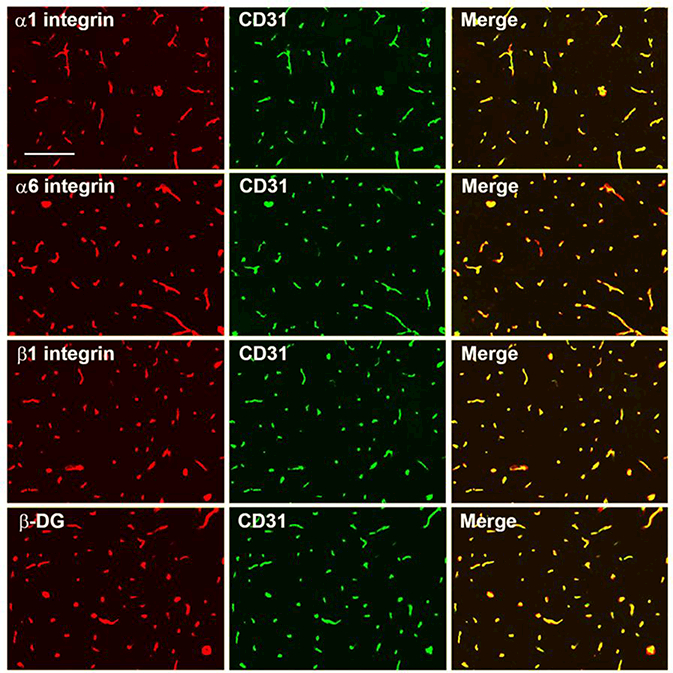
Characterization of laminin receptor expression by cerebral blood vessels. Dual-IF was performed on frozen brain sections using antibodies specific for CD31 (AlexaFluor-488) and antibodies specific for the integrin subunits α1, α6, or β1 or the β-dystroglycan subunit. Scale bar = 100 μm. Note that cerebral blood vessels stain positive for the αl, α6, and β1 integrin subunits as well as for β-dystroglycan.
Figure 4.
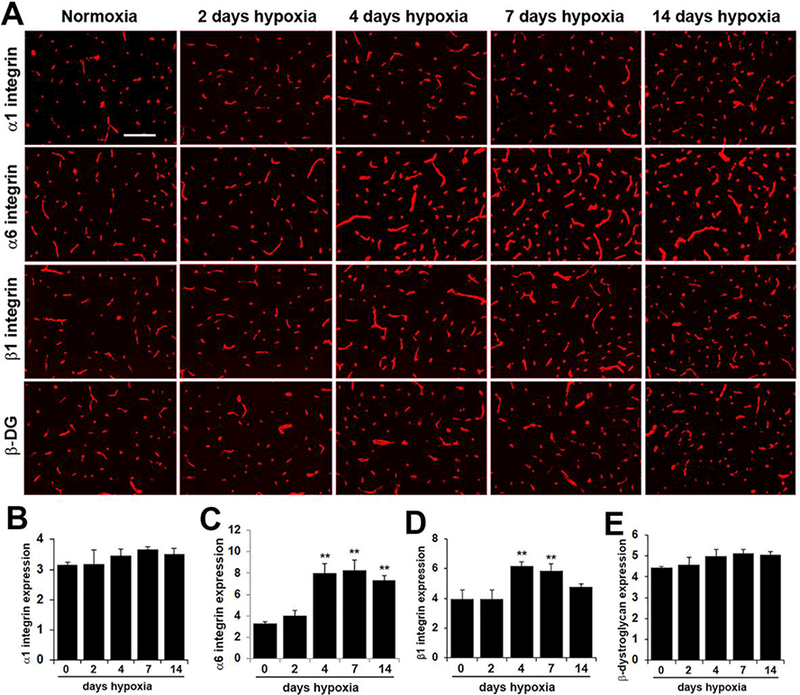
Chronic mild hypoxia (CMH) specifically upregulates α6β1 integrin expression in cerebral blood vessels. A. Frozen sections of brain taken from mice exposed to normoxia or 2, 4, 7, or 14 days CMH (8% O2) were stained using antibodies specific for the integrin subunits α1,α6,or β1 or the β-dystroglycan subunit. Scale bar =100 μm. B -E. Quantification of cerebrovascular expression of the integrin subunits αl (B), α6 (C), β1 (D) or β-dystroglycan (E) at different time-points of CMH. Results are expressed as the mean ± SEM (n = 4 mice/group). Note that cerebrovascular expression levels of the α6 and β1 integrin subunits were significantly increased after 4 and 7 days CMH. ** p < 0.01 vs. normoxia (0 days hypoxia) and vs. 2 days hypoxia. * p < 0.05 vs. 4 days hypoxia.
3. DISCUSSION
The mouse model of chronic mild hypoxia (CMH), in which mice are exposed to 8% O2 for two weeks, induces a strong vascular remodeling response in the CNS and as such it represents a good model to study the cellular and molecular events underlying this process (LaManna et al., 1992; LaManna et al., 2004; Milner et al., 2008). Over the last few years, we have described marked changes in the expression of several ECM proteins within cerebral blood vessels of mice exposed to CMH, including fibronectin and vitronectin, and highlighted a specific role for the fibronectin-α5β1 integrin axis in driving the CMH-induced angiogenic response (Li et al., 2010b; Li et al., 2012b; Milner et al., 2008). In addition to ECM proteins that drive endothelial proliferation and vascular remodeling, such as fibronectin, other ECM proteins present in the vascular basal lamina act to promote maturation and stabilization of blood vessels. One such class of proteins are the laminins, a major constituent of the vascular basal lamina, and which have been shown to play a fundamental role in promoting endothelial differentiation and blood-brain barrier (BBB) stability (Chen et al., 2013; Gautam et al., 2016; Grant et al., 1989; Grant and Kleinman, 1997; Menezes et al., 2014). In support of this, recent studies have revealed that transgenic mice deficient in astrocyte or pericyte laminins show cerebrovascular defects ranging from BBB leakage to outright haemorrhage (Chen et al., 2013; Gautam et al., 2016; Menezes et al., 2014). Building on these observations, the goal of the current study was to determine if CMH influences the expression of laminins or their cognate cell surface receptors within remodeling cerebral blood vessels, and thus gain some insight as to whether laminin-mediated signaling might be important for the vascular remodeling process. Our studies revealed that over a 14 day period of CMH, cerebral blood vessels showed the following changes: (i) increased expression of laminins within the vascular basal lamina, (ii) more specifically, CMH led to upregulated expression of the laminin α1 and α4 subunits (present in laminins 111 and 411 respectively), with no discernible changes in the expression levels of the α2 or α5 laminin subunits, and (iii) this was accompanied by marked upregulation of the laminin receptor α6β1 integrin but no alteration in the expression level of the other laminin receptors α1β1 integrin or dystroglycan. In light of the instructive role for laminins in promoting vascular differentiation and stability, these data suggest that upregulation of the laminin-α6β1 integrin axis might be part of the molecular response triggered by exposure to mild hypoxia that leads to enhanced vascular stability in the CNS.
A clear outcome from our studies was that exposure to CMH triggered increased expression of the laminin αl and α4 subunits (present in laminins 111 and 411 respectively) within the basal lamina of cerebral blood vessels, with no discernible changes in the expression levels of the α2 or α5 laminin subunits. Previous studies from the Sorokin lab have shown that of the two laminin α subunits expressed by endothelium (α4 and α5), expression of α4 is the most dynamic, having the highest rate of turnover and is most strongly upregulated after activation by pro-inflammatory stimuli such as lipopolysaccharide (LPS) or cytokines such as TNF-α and IL-1 (Sixt et al., 2001). Consistent with this, we found that CMH triggered strong upregulation of the α4 but not the α5 laminin subunit. At the same time, we found that while leptomeningeal expression of the α1 laminin subunit, whose expression is restricted to arterial and venous blood vessels, was increased by CMH, expression of the α2 subunit, which is expressed by astrocytes, showed no discernible change in expression level. Interestingly, while expression of the α6 integrin subunit was maintained high at all time-points examined, expression of the β1 integrin subunit was significantly elevated at days 4 and 7 of CMH, but by day 14 had reduced back towards baseline levels, creating a mismatch between levels of α6 and β1 integrin subunits. One reason that might explain this is that the total pool of all β1 integrin subunit in endothelial cells partners with several different a subunits that include αl, α2 and α5 in addition to α6, and as α5 integrin levels peak after 4 days CMH but then decline quickly back to baseline (Li et al., 2010a; Milner et al., 2008), it seems likely that the apparent fall in total β1 integrin could due to sharp reduction in α5 levels, but that the absolute amount of β1 subunit that partners with α6 subunit remains the same.
Having described marked upregulation of the laminin subunits α1 and α4 in the vascular basal lamina of cerebral blood vessels in response to CMH, this raises the question of what advantage does this confer? As laminins play a key role in promoting endothelial differentiation and vascular integrity, particularly within the CNS (Chen et al., 2013; Gautam et al., 2016; Grant et al., 1989; Grant and Kleinman, 1997; Menezes et al., 2014), this would suggest that enhanced expression of these important proteins might act to increase either the structural or functional integrity of cerebral blood vessels. Endothelial cell adhesion to the underlying basal lamina proteins is a key component of the BBB (Osada et al., 2011), implying that tight endothelial adhesion would lead to higher vascular integrity. At the structural level, the increased deposition of laminin isoforms could simply be increasing the thickness of the vascular basal lamina and thereby contribute to an increased barrier. However, as laminins provide instructive cues to direct vascular cell behavior (Chen et al., 2013; Gautam et al., 2016; Grant et al., 1989; Grant and Kleinman, 1997; Menezes et al., 2014), it is more likely that the increased levels of αl and α4 laminin subunits are providing specific signals that promote alterations in endothelial cell function, to enhance endothelial differentiation and promote increased expression of downstream effector proteins that play important roles in conferring enhanced vascular integrity. In this light, it is interesting that CMH also results in marked elevation of endothelial tight junction proteins (Boroujerdi and Milner, 2015; Li et al., 2010a), which are critical determinants of vascular integrity (Huber et al., 2001; Pardridge, 2003; Wolburg and Lippoldt, 2002). The recent finding that ablation of astrocyte laminin leads to reduced BBB integrity and downregulation of tight junction protein expression further supports the idea that laminin plays a critical role in promoting BBB integrity (Yao et al., 2014). In future studies, we aim to further investigate this link by examining CMH-induced vascular remodeling in transgenic mice deficient in the laminin isoforms αl and α4, as well as in mice lacking the laminin receptor α6β1 integrin. While the current analysis was performed in female mice, in light of the described role for estrogens in protecting BBB integrity (Bake and Sohrabji, 2004; Burek et al., 2010; Kang et al., 2006), it will also be interesting to examine if CMH has similar effects on cerebrovascular laminin expression in male mice.
Interestingly, using standard markers such as albumin or fibrinogen leakage, we have never observed any increased permeability of cerebral blood vessels as a result of CMH at the level of 8 or 10% oxygen. We have seen very transient expression of the MECA-32 marker (Milner et al., 2008), which is not expressed on mature brain endothelium but is expressed on immature brain vessel prior to embryonic day 15 in the mouse (Hallman et al., 1995), and also on leaky cerebral vessels following cerebral ischemia (Li et al., 2012a). Based on this finding, we believe it is possible that for a brief period of time, remodeling blood vessels in the brains of mice exposed to CMH could be mildly leaky, but any leakiness is quickly compensated for by other mechanisms. Interestingly, CMH promotes marked upregulation of the tight junction proteins claudin-5 and ZO-1 (Boroujerdi and Milner, 2015; Li et al., 2010a), critical functional molecules in contributing to BBB tightness, suggesting that CMH may conceivably be enhancing barrier integrity by stimulating increased tight junction protein expression. Shedding light on the relationship between laminin expression, tight junction protein expression and BBB integrity, a recent study showed that transgenic mice with deleted laminin expression in astrocytes, show marked reduction in tight junction protein expression, thus establishing a direct link between laminin expression and tight junction expression/BBB integrity (Yao et al., 2014).
In summary, CMH (8% O2 for 2 weeks) promotes a strong vascular remodeling response in the CNS, culminating in greater than 50% increased vessel density after two weeks CMH (LaManna et al., 1992; LaManna et al., 2004; Milner et al., 2008). As laminins play central roles in promoting vascular differentiation and stability in cerebral blood vessels (Chen et al., 2013; Gautam et al., 2016; Grant et al., 1989; Grant and Kleinman, 1997; Menezes et al., 2014), the goal of this study was to determine if blood vessels in the hypoxic brain alter their expression of laminins and their cell surface receptors. Our studies revealed that over a 14 day period of CMH, cerebral blood vessels showed strong upregulation of the specific laminin subunits α1 and α4 subunits, corresponding to increased expression of laminins 111 and 411 respectively. This was accompanied by marked endothelial upregulation of the laminin receptor α6β1 integrin but no alterations in the other laminin receptors α1β1 integrin or dystroglycan. Taken with the instructive role for laminins in promoting vascular differentiation and stability, these data suggest that upregulation of the laminin-α6β1 integrin axis is part of the molecular response triggered by mild hypoxia that leads to enhanced vascular stability in the CNS.
4. EXPERIMENTAL PROCEDURES
4.1. Animals
The studies described have been reviewed and approved by The Scripps Research Institute Institutional Animal Care and Use Committee. Wild-type female SJL/J mice were purchased from JAX labs and maintained under pathogen-free conditions at The Scripps Research Institute (TSRI).
4.2. Chronic Hypoxia Model
Wild-type female SJL/J mice, 10 weeks of age (with 4 mice per cage) were placed into a hypoxic chamber (Biospherix, Redfield, NY) maintained at 8% O2 for periods up to 14 days. Littermate control mice were kept in the same room under similar conditions except that they were kept at ambient sea-level oxygen levels (normoxia, approximately 21% O2 at sea-level) for the duration of the experiment. Every few days, the chamber was briefly opened for cage cleaning and food and water replacement as needed.
4.3. Immunohistochemistry and antibodies
Immunohistochemistry was performed as described previously (Milner and Campbell, 2002) on 10 μm frozen sections of cold phosphate buffered saline (PBS) perfused brains taken from mice subject to either normoxia (control) or hypoxic conditions. Our studies on brain issue were focused specifically in the medulla oblongata region of the brainstem, though the alterations in the expression of vascular laminins and their cell surface receptors we observed in the medulla were apparent in all brain regions examined. The following antibodies were used in this study: rat monoclonal antibodies reactive for CD31 (clone MEC13.3) and the integrin subunits α6 (clone GoH3) and β1 (clone 9EG7) the hamster monoclonal reactive for the integrin α1 subunit (clone Ha31/8) (all from BD Pharmingen, La Jolla, CA), hamster anti-CD31 (clone 2H8) from Abcam, Cambridge, MA, mouse anti-β-dystroglycan from Novocastra, Newcastle upon-Tyne, England, and rabbit anti-laminin from Sigma, St. Louis, MO. The rat monoclonal antibodies against laminin α1 (clone 200) and α5 (clone 4G6A211) and the rabbit polyclonal specific for the laminin α4 subunit (377b) were kind gifts from Dr. Lydia Sorokin (University of Munster, Germany). The rat monoclonal antibody against laminin α2 (clone 4H8–2) was obtained from Sigma. Secondary antibodies used included goat anti-rabbit Cy3, goat anti-rat Cy3 and goat anti-mouse Cy3 (all from Jackson Immunoresearch, Baltimore, PA) and anti-rat Alexa Fluor 488 and anti-hamster Alexa 488 (both from Invitrogen).
4.4. Image analysis
Images were taken using a 10X or 20X objective on a Zeiss Imager M1.m microscope. All analysis was performed in the medulla oblongata area of the brain. For each antigen, images of three randomly selected areas were taken at 10X or 20X magnification, and three sections per brain analyzed to calculate the mean for each subject. For each antigen in each experiment, exposure time was set to convey the maximum amount of information without saturating the image. Exposure time was maintained constant for each antigen across the time-course of hypoxic exposure. To quantify the expression level of all antigens, NIH Image J software was used to measure the total fluorescent signal per field of view. Each experiment was performed with four different animals per condition, and the results expressed as the mean ± SEM. Statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post-hoc test, in which p < 0.05 was defined as statistically significant.
Chronic mild hypoxia (CMH; 8% 02 for 2 weeks) upregulated laminin expression within the basal lamina of cerebral blood vessels
Specifically, CMH led to enhanced expression of the laminin αl and α4 subunits, with no alteration in the α2 or α5 subunits
This correlated with increased endothelial expression of the laminin receptor α6β1 integrin
Suggests that upregulation of the laminin-α6β1 integrin axis may act to enhance vascular stability in the hypoxic CNS
Acknowledgements
This work was supported by the NIH R56 (NS095753) and R21 (NSO96524) grants (RM). We are very grateful to Dr. Lydia Sorokin of the University of Munster, Germany, for her kind gifts of the antibodies against specific laminin isoforms. This is manuscript number 29588 from The Scripps Research Institute. SH performed the CMH studies and all the histological analysis and quantification, and contributed to drafting the manuscript. RK assisted with the CMH experiments and contributed to drafting the manuscript. RM conceived the study, helped in interpreting the data and drafted the manuscript. All authors read and approved the final manuscript.
ABBREVIATIONS
- BBB
Blood-brain barrier
- CMH
Chronic mild hypoxia
- CNS
Central nervous system
- ECM
Extracellular matrix
- IF
Immunofluorescent
- IL-1
Interleukin 1
- LPS
Lipopolysaccharide
- MS
Multiple sclerosis
- PBS
Phosphate buffered saline
- SEM
Standard error of mean
- TNF
Tumor necrosis factor
- ZO-1
Zona Occludens-1
Footnotes
Declaration of interest
The authors declare that they have no conflict of interest.
Ethical standards
All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996, and complied with The Scripps Research Institute Institutional Animal Care and Use Committee. In addition all work described in this article was carried out in accordance with The Code of Ethics described in the EC Directive 86/609/EEC for animal experiments http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm; Uniform Requirements for manuscripts submitted to Biomedical journals http://www.icmje.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bake S, Sohrabji F, 2004. 17beta-estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology. 145, 5471–5475. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M, 2004. The blood-brain barrier: an overview. Structure, regulation and clinical implications. Neurobiology of Disease. 16, 1–13. [DOI] [PubMed] [Google Scholar]
- Boroujerdi A, et al. , 2012. Chronic cerebral hypoxia promotes arteriogenic remodeling events that can be identified by reduced endoglin (CD105) expression and a switch in β1 integrins. J Cereb Blood Flow Metab. 32, 1820–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroujerdi A, Milner R, 2015. Defining the critical hypoxic threshold that promotes vascular remodeling in the brain. Exp. Neurol 263, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burek M, et al. , 2010. Claudin-5 as a novel estrogen target in vascular endothelium. 30 298–304. [DOI] [PubMed] [Google Scholar]
- Chen Z-L, et al. , 2013. Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J. Cell Biol 202, 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, et al. , 2010. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 468, 562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Camarillo CW, 1995. Regulation of endothelial cell morphogenesis by integrins, mechanical forces, and matrix guidance pathways. Exp. Cell Res 216, 113–123. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R, 2006. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler. Thromb. Vasc. Biol 26, 1966–1975. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, et al. , 2011. Chronic mild hypoxia ameliorates chronic inflammatory activity in myelin oligodendrocyte glycoprotein (MOG) peptide induced experimental autoimmune encephalomyelitis (EAE). Adv. Exp. Med. Biol 701, 165–173. [DOI] [PubMed] [Google Scholar]
- Engvall E, 1993. Laminin variants: why, where and when? Kidney Int. 43, 2–6. [DOI] [PubMed] [Google Scholar]
- Engvall E, Wewer UM, 1996. Domains of laminin. J. Cell Biochem 61,493–501. [DOI] [PubMed] [Google Scholar]
- Esen N, et al. , 2016. Endogenous adaptation to low oxygen modulates T-cell regulatory pathways in EAE. J. Neuroinflammation 13, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam J, Zhang X, Yao Y, 2016. The role of pericyte laminin in blood brain barrier integrity maintenance. Sci. Rep 6, 36450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DS, et al. , 1989. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 58, 933–43. [DOI] [PubMed] [Google Scholar]
- Grant DS, Kleinman HK, 1997. Regulation of capillary formation by laminin and other components of the extracellular matrix. EXS. 79, 317–33. [DOI] [PubMed] [Google Scholar]
- Haider SK, Kant R, Milner R, 2018. Chronic mild hypoxia promotes profound vascular remodeling in spinal cord blood vessels, preferentially in white matter, via an a5bl integrin-mediated mechanism. Angiogenesis. 21, 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman R, et al. , 1995. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev. Dyn 202, 325–332. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP,2001. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neourosci. 24, 719–725. [DOI] [PubMed] [Google Scholar]
- Kang HS, et al. , 2006. Effect of estrogen on the expression of occludin in ovariectomized mouse brain. Neurosci Lett. 402, 30–34. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Vendel LM, Farrell RM, 1992. Brain adaptation to chronic hypobaric hypoxia in rats. J. Appl. Physiol 72, 2238–2243. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Chavez JC, Pichiule P, 2004. Structural and functional adaptation to hypoxia in the rat brain. J. Exp. Biol 207, 3163–3169. [DOI] [PubMed] [Google Scholar]
- Li L, et al. , 2010a. In the hypoxic central nervous system, endothelial cell proliferation is followed by astrocyte activation, proliferation, and increased expression of the α6β4 integrin and dystroglycan. Glia. 58, 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Welser JV, Milner R, 2010b. Absence of the ανβ3 integrin dictates the time-course of angiogenesis in the hypoxic central nervous system: accelerated endothelial proliferation correlates with compensatory increases in α5β1 integrin expression. J Cereb Blood Flow Metab. 30, 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. , 2012a. Upregulation of fibronectin and the α5β1 and ανβ3 integrins on blood vessels within the cerebral ischemic penumbra. Exp. Neurol 233, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. , 2012b. An angiogenic role for the α5β1 integrin in promoting endothelial cell proliferation during cerebral hypoxia. Exp Neurol. 237, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes MJ, et al. , 2014. The extracellular matrix protein laminin a2 regulates the maturation and function of the blood-brain barrier. J. Neurosci 12, 15260–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BA, et al. , 2001. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia-reperfusion. Neuroreport. 12, 1663–1669. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL, 2002. Developmental regulation of β1 integrins during angiogenesis in the central nervous system. Mol. Cell. Neurosci 20, 616–626. [DOI] [PubMed] [Google Scholar]
- Milner R, et al. , 2008. Increased expression of fibronectin and the α5β1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol. Cell. Neurosci 38, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, et al. , 2011. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by β1 integrins. J. Cereb. Blood Flow Metab 31, 1972–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, 2003. Blood-brain barrier drug targetting: the future of brain drug development. Mol. Med 3, 90–105. [DOI] [PubMed] [Google Scholar]
- Sixt M, et al. , 2001. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol 153, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe AM, et al. , 2011. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann. Neurol 69, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, 1989. Structure and biological activity of basement membrane proteins. Eur. J. Biochem 180,487–502. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown J, 1994. The laminins. Matrix Biol 14, 275–281. [DOI] [PubMed] [Google Scholar]
- Wang J, Milner R, 2006. Fibronectin promotes brain capillary endothelial cell survival and proliferation through α5β1 and ανβ3 integrins via MAP kinase signaling. J. Neurochem 96, 148–159. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A, 2002. Tight junctions of the blood-brain barrier; development, composition and regulation. Vascular Pharmacology. 38, 323–337. [DOI] [PubMed] [Google Scholar]
- Yao Y, et al. , 2014. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nature Comm. 5, 3413. [DOI] [PMC free article] [PubMed] [Google Scholar]


