Abstract
Objectives:
Assess the validity of Medicare claims for identifying myocardial infarction (MI).
Methods:
We used data from 9,951 Medicare beneficiaries ≥65 years in the REasons for Geographic And Racial Differences in Stroke study. Between 2003-2012, 669 participants had an MI identified and adjudicated through study procedures (i.e., the gold standard), and 552 had an overnight inpatient claim with a code for MI (ICD-9 code 410.x0 or 410.x1) in any discharge diagnosis position.
Results:
Using Medicare claims with a discharge diagnosis code for MI in any position, the positive predictive value (PPV) was 84.3% (95%CI 80.9%−87.3%) and the sensitivity was 49.0% (95%CI 44.9%−53.1%). Sensitivity was lower for men (45.8%) versus women (55.1%), microsize MIs (13.7%) versus other MIs (64.7%), type 2 (30.9%) and 4-5 MIs (11.1%) versus type 1 MIs (76.6%), and MIs occurring in-hospital (28.8%) versus out-of-hospital (66.7%). Using Medicare claims with a code for MI in the primary discharge diagnosis position, the PPV was 89.7% (95%CI 86.3%−92.5%) and sensitivity was 40.1% (95%CI 36.1%−44.2%). The sensitivity of claims with a code for MI in the primary discharge diagnosis position was lower for microsize versus other MIs, type 2 and 4-5 MIs versus type 1 MIs and MIs occurring in-hospital versus out-of-hospital. Hazard ratios for MI associated with participant characteristics were similar using adjudicated MIs identified through study procedures or claims for MI without further adjudication.
Conclusions:
Medicare claims have a high PPV but low sensitivity for identifying MI and can be used to investigate individual-level characteristics associated with MI.
Keywords: myocardial infarction, administrative claims, healthcare, Medicare, methods
INTRODUCTION
Using claims data could be an efficient approach for cardiovascular research.1 However, claims databases are not designed for research and may not identify all cardiovascular events. Also, some events identified as being cardiovascular-related using claims may be due to non-cardiovascular diseases.2,3 These limitations have raised the concern that using claims databases may lead to biased estimates of absolute risk and exposure-outcome associations.4,5
In 2000, highly sensitive biomarkers (i.e., troponin T or I) were recommended for the diagnosis of myocardial infarctions (MI).6,7 However, some laboratories report troponin thresholds to define MI which are incorrectly high.8 Therefore, MIs with small peaks of troponin may be undetected in clinical practice and not be recorded in claims. Also, claims databases may not identify all MI types as defined by the Universal Definition of MI (i.e., MIs secondary to a plaque rupture [type 1], MIs secondary to an ischemic imbalance [type 2], MIs resulting in death without available biomarkers [type 3], and MIs secondary to coronary revascularization [types 4-5]).9 Type 2 MIs occur in the context of other conditions, including heart failure and tachyarrhythmia, which may be the main cause of hospitalization.10 Type 4-5 MIs occur in patients undergoing a coronary revascularization. Therefore, type 2 and 4-5 MIs may be less likely to be identified using claims databases compared with type 1 MIs if only the main cause of hospitalization is recorded.
There are few data available on the validity of using claims for MI identification in the era with highly sensitive biomarkers available. There are also few data on whether estimations of MI risk and exposure-MI associations are similar using primary data collection, claims data, and primary data collection supplemented by claims data. Using claims data may have low sensitivity for MI identification and underestimate MI risk. However, if the positive predictive value (PPV) is high and estimates of exposure-MI associations are similar using claims data and primary data collection, this would support using either approach to study risk factors for MI.
METHODS
The REasons for Geographic And Racial Differences in Stroke (REGARDS) study enrolled a population-based cohort of 30,239 black and white men and women ≥45 years of age from all 48 contiguous US states and the District of Columbia between January 2003 and October 2007.11 REGARDS study participant data were combined with claims from Medicare, a US government health insurance program for adults ≥65 years of age, and younger adults who are disabled or have end-stage renal disease.12 For the current analysis, we excluded 15,278 REGARDS study participants <65 years of age as younger adults with Medicare coverage represent a select sub-population who are disabled or have end-stage renal disease. We further excluded 2,835 participants without data linked to Medicare, 2,082 participants without inpatient fee-for-service coverage and 93 participants without REGARDS study follow-up for MI. Medicare inpatient fee-for-service coverage was defined as having Medicare Part A coverage without Medicare Advantage (a capitated program which does not require submitting claims for reimbursement). After these exclusions were applied, data for 9,951 REGARDS study participants were analyzed (Supplemental Figure 1). The REGARDS study was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided written informed consent, including for the analysis of their Medicare claims.
Baseline assessment
Baseline data collection in the REGARDS study occurred through a telephone interview administered by trained staff and an in-home examination by health professionals.11 Data collected at baseline were used to define participant characteristics, including age, race, sex, geographic region of residence, education, total annual household income, current smoking, diabetes, hypertension, history of coronary heart disease (CHD), statin use, elevated C-reactive protein (CRP), chronic kidney disease (CKD) and cognitive impairment. Definitions for participant characteristics are provided in Supplemental Table 1.
Identification of myocardial infarctions
In the REGARDS study, participants or their proxies are contacted biannually to identify suspected CHD events and deaths.13 Medical records are retrieved for suspected CHD-related hospitalizations. When deaths are identified, interviews with proxies are conducted, and records for hospitalizations in the last year of life, death certificates, and autopsy reports are retrieved. During adjudication, two clinicians independently classified each event as not being an MI, or being a possible, probable or definite MI, following published guidelines.7,9,14,15 Only definite or probable events were considered MIs in the REGARDS study. Definite or probable MIs with a peak troponin level <0.5 μg/L were classified as “microsize” MIs.8 Definite or probable MIs were subsequently classified into types following the Universal Definition and on whether they occurred in-hospital versus out-of-hospital.9
We identified overnight inpatient claims with a discharge diagnosis code for MI (i.e., an International Classification of Diseases, ninth revision [ICD-9] diagnosis code of 410.x0 or 410.x1) in any discharge diagnosis position. We further identified the subgroup of overnight inpatient claims with a discharge diagnosis code for MI in the primary position as this represents the main reason for hospitalization.16 Medicare inpatient claims with a discharge diagnosis code for MI in any position were adjudicated as described above for events identified through REGARDS study procedures. For the current study, definite or probable MIs identified through REGARDS study procedures or Medicare claims, and Medicare claims with a discharge diagnosis code for MI in any position were available through December 31, 2012.
Statistical analysis
We calculated characteristics of participants included in the analysis. The validity of Medicare claims for MI identification was assessed using sensitivity and PPV, following the Centers for Disease Control and Prevention guidelines for evaluating surveillance systems.17 Sensitivity was calculated as the proportion of definite or probable MIs identified and adjudicated through REGARDS study procedures which had a claim for MI in Medicare. The calculation of sensitivity was restricted to participants who had Medicare inpatient fee-for-service coverage on the date of the definite or probable MI adjudicated in the REGARDS study. For participants having multiple definite or probable MI during follow-up, only the first event was used to calculate sensitivity. PPV was calculated as the proportion of Medicare claims for MI which were classified as a definite or probable MI through REGARDS study procedures. The calculation of PPV was restricted to Medicare claims whose adjudication was completed. For participants having multiple Medicare claims for MI, only the first claim was used to calculate PPV. Sensitivity and PPV were calculated for the full population, and in subgroups defined by age, sex, race, region of residence, calendar year of the event, education, income, cognitive impairment and history of CHD. Sensitivity was also calculated for microsize MIs versus other MIs, type of MIs, and MIs occurring in-hospital versus out-of-hospital. PPV was not calculated by MI characteristics because there are no specific ICD-9 diagnosis codes for microsize MI or type of MI. We compared sensitivity and PPV across subgroups using χ2 tests or Fisher’s exact tests, as appropriate. In secondary analyses, sensitivity was calculated after excluding MIs in the last year of life, including adjudicated MIs identified through REGARDS study procedures or through Medicare claims, including recurrent MIs from participants with multiple events during follow-up, and including MIs from participants without Medicare inpatient fee-for-service coverage on the date of their MI (Supplemental methods). In secondary analyses, PPV was calculated after excluding Medicare claims in the last year of life, and including all MI claims for participants with multiple events. To determine whether MIs identified and adjudicated through REGARDS study procedures were not billed to Medicare, we calculated the proportion of events included in the main analysis of sensitivity which did not have an inpatient claim for any reason in Medicare. Calculations were repeated in subgroups defined by age, sex, race, region of residence, calendar year of the event, education, income, cognitive impairment and history of CHD.
We calculated the cumulative incidence by the Kaplan-Meier method and rates of MI using five definitions:
REGARDS: First definite or probable MI identified and adjudicated through REGARDS study procedures. Participants not having this event were censored on their death date, first day with unknown vital status in REGARDS (i.e., the day of the last study contact with the participant), or December 31, 2012, whichever occurred first.
REGARDS-Medicareany: First event identified through REGARDS study procedures or through Medicare claims with a discharge diagnosis code for MI in any position which was subsequently classified as a definite or probable MI in REGARDS. Participants not having this event were censored on their death date, first day with unknown vital status in REGARDS and without Medicare inpatient fee-for-service coverage, or December 31, 2012, whichever occurred first.
REGARDS-Medicareprimary: First event identified through REGARDS study procedures or through Medicare claims with a discharge diagnosis code for MI in the primary position which was subsequently classified as a definite or probable MI in REGARDS. Participants not having this event were censored on their death date, first day with unknown vital status in REGARDS and without Medicare inpatient fee-for-service coverage, or December 31, 2012, whichever occurred first.
Medicareany: First Medicare inpatient claim with a discharge diagnosis code for MI in any position, regardless of adjudication status. Participants not having this event were censored on their death date, loss of Medicare inpatient fee-for-service coverage, or December 31, 2012, whichever occurred first.
Medicareprimary: First Medicare inpatient claim with a discharge diagnosis code for MI in the primary position, regardless of adjudication status. Participants not having this event were censored on their death date, loss of Medicare inpatient fee-for-service coverage, or December 31, 2012, whichever occurred first.
Rate ratios (RR) and 95% confidence intervals (CI) comparing MI rates using the five definitions listed above were calculated using bootstrapping with 1,000 random samples with replacement and the bias-corrected percentile method.18 We used Cox regression models to calculate multivariable-adjusted hazard ratios (HR) for MI using each of the five definitions listed above associated with age, sex, race, region of residence, education, income, smoking, diabetes, hypertension, history of CHD, statin use, elevated CRP, CKD and cognitive impairment, overall and among participants with and without a history of CHD. The statistical significance of differences in HRs for MI definitions was calculated using bootstrapping with 1,000 random samples with replacement and the bias-corrected percentile method.18 Chained equations were used to obtain 50 multiple imputed datasets in Stata 13 (Stata Corp, College Station, TX) to retain participants with missing data (shown in Supplemental Table 2) in the regression models.19,20 All analyses were conducted using a two-sided level of significance <0.05.
RESULTS
The mean age of participants was 72.6 years, 50.0% were male and 32.8% were black (Table 1). Overall, 669 participants had a definite or probable MI identified and adjudicated through REGARDS study procedures, including 596 who had Medicare inpatient fee-for-service coverage on the date of their MI and were included in the calculation of sensitivity. The sensitivity of Medicare claims with a discharge diagnosis code for MI in any and the primary position was 49.0% (95% CI 44.9%−53.1%) and 40.1% (95% CI 36.1%−44.2%), respectively (Table 2). Sensitivity was not statistically significantly different across levels of age, region of residence, calendar year, education, income and cognitive impairment. Sensitivity was lower for men compared with women, participants with versus without a history of CHD, microsize MIs versus MIs with higher peak troponin, type 2 and 4-5 MIs versus type 1 MIs, and MIs occurring in-hospital versus out-of-hospital. Sensitivity was lower for blacks versus whites when using claims with a discharge diagnosis code for MI in the primary position, but not when using claims with a discharge diagnosis code for MI in any position. Compared with the primary analysis, sensitivity was higher when excluding MIs in the last year of life, and including MIs identified through Medicare claims (Supplemental Figure 2). Sensitivity was lower when including recurrent definite or probable MIs from participants with multiple events, and including definite or probable MIs occurring when participants did not have Medicare inpatient fee-for-service coverage.
Table 1.
Characteristics of Study Participants Included in the Current Analysis.
| All participants | |
|---|---|
| Characteristics | (n=9,951) |
| Age, years, mean (SD) | 72.6 (5.8) |
| Age ≥75 years | 34.5 |
| Men | 50.0 |
| Blacks | 32.8 |
| Geographic region of residence* | |
| Stroke buckle | 22.2 |
| Stroke belt | 35.5 |
| Other contiguous US states | 42.3 |
| Less than high school education | 15.0 |
| Total annual household income <$25,000 | 35.5 |
| Current smoking | 9.7 |
| Diabetes | 23.0 |
| Hypertension | 66.4 |
| History of CHD | 25.2 |
| Statin use | 37.6 |
| Elevated CRP | 38.4 |
| Chronic kidney disease | 30.8 |
| Cognitive impairment | 10.1 |
Abbreviations: CHD, coronary heart disease; CRP, C-reactive protein; SD, standard deviation; US, United States.
Notes: Numbers in the table represent column percentages, unless otherwise indicated.
Stroke buckle includes coastal plains of North Carolina, South Carolina and Georgia. Stroke belt includes the remaining parts of North Carolina, South Carolina and Georgia, and Tennessee, Mississippi, Alabama, Louisiana and Arkansas. Other contiguous US states includes the remaining 40 contiguous US states and the District of Columbia
Table 2.
Sensitivity of Medicare Inpatient Claims to Identify Myocardial Infarctions Identified and Adjudicated Through REasons for Geographic And Racial Differences in Stroke Study Procedures.
| Medicare claims with a discharge diagnosis code for MI | |||||||
|---|---|---|---|---|---|---|---|
| Any position | Primary position | ||||||
| Characteristics | Total MIs* | n | Sensitivity (95% CI) | p-value† | n | Sensitivity (95% CI) | p-value† |
| Full population | 596 | 292 | 49.0 (44.9–53.1) | 239 | 40.1 (36.1–44.2) | ||
| Age | |||||||
| <75 years | 350 | 173 | 49.4 (44.1–54.8) | 0.80 | 143 | 40.9 (35.7–46.2) | 0.65 |
| ≥75 years | 246 | 119 | 48.4 (42.0–54.8) | 96 | 39.0 (32.9–45.4) | ||
| Sex | |||||||
| Women | 205 | 113 | 55.1 (48.0–62.1) | 0.03 | 92 | 44.9 (37.9–52.0) | 0.08 |
| Men | 391 | 179 | 45.8 (40.8–50.9) | 147 | 37.6 (32.8–42.6) | ||
| Race | |||||||
| White | 433 | 217 | 50.1 (45.3–54.9) | 0.37 | 184 | 42.5 (37.8–47.3) | 0.05 |
| Black | 163 | 75 | 46.0 (38.2–54.0) | 55 | 33.7 (26.5–41.6) | ||
| Geographic region of residence‡ | |||||||
| Stroke buckle | 142 | 70 | 49.3 (40.8–57.8) | (ref) | 59 | 41.5 (33.3–50.1) | (ref) |
| Stroke belt | 220 | 108 | 49.1 (42.3–55.9) | 0.97 | 89 | 40.5 (33.9–47.3) | 0.84 |
| Other contiguous US states | 234 | 114 | 48.7 (42.2–55.3) | 0.91 | 91 | 38.9 (32.6–45.5) | 0.61 |
| Calendar year | |||||||
| 2003–2006 | 186 | 86 | 46.2 (38.9–53.7) | (ref) | 70 | 37.6 (30.7–45.0) | (ref) |
| 2007–2009 | 238 | 116 | 48.7 (42.2–55.3) | 0.61 | 94 | 39.5 (33.2–46.0) | 0.70 |
| 2010–2012 | 172 | 90 | 52.3 (44.6–60.0) | 0.25 | 75 | 43.6 (36.1–51.4) | 0.25 |
| Education | |||||||
| Less than high school | 102 | 54 | 52.9 (42.8–62.9) | 0.38 | 41 | 40.2 (30.6–50.4) | 0.98 |
| High school or higher | 492 | 237 | 48.2 (43.7–52.7) | 197 | 40.0 (35.7–44.5) | ||
| Total annual household income | |||||||
| <$25,000 | 178 | 95 | 53.4 (45.8–60.9) | 0.08 | 76 | 42.7 (35.3–50.3) | 0.29 |
| ≥$25,000 | 351 | 159 | 45.3 (40.0–50.7) | 133 | 37.9 (32.8–43.2) | ||
| Cognitive impairment | |||||||
| No | 381 | 191 | 50.1 (45.0–55.3) | 0.27 | 160 | 42.0 (37.0–47.1) | 0.55 |
| Yes | 48 | 20 | 41.7 (27.6–56.8) | 18 | 37.5 (24.0–52.6) | ||
| History of CHD | |||||||
| No | 303 | 165 | 54.5 (48.7–60.2) | 0.005 | 139 | 45.9 (40.2–51.7) | 0.002 |
| Yes | 284 | 122 | 43.0 (37.1–48.9) | 95 | 33.5 (28.0–39.3) | ||
| Peak troponin level§ | |||||||
| <0.5 μg/L (microsize MI) | 183 | 25 | 13.7 (9.0–19.5) | <0.001 | 17 | 9.3 (5.5–14.5) | <0.001 |
| ≥0.5 μg/L | 405 | 262 | 64.7 (59.8–69.3) | 218 | 53.8 (48.8–58.8) | ||
| MI type|| | |||||||
| Type 1 MIs | 256 | 196 | 76.6 (70.9–81.6) | (ref) | 183 | 71.5 (65.5–76.9) | (ref) |
| Type 2 MIs | 288 | 89 | 30.9 (25.6–36.6) | <0.001 | 52 | 18.1 (13.8–23.0) | <0.001 |
| Type 3 MIs | 4 | 2 | 50.0 (6.8–93.2) | 0.24** | 1 | 25.0 (0.6–80.6) | 0.08** |
| Type 4–5 MIs | 45 | 5 | 11.1 (3.7–24.1) | <0.001 | 3 | 6.7 (1.4–18.3) | <0.001** |
| Place of occurrence | |||||||
| In-hospital | 278 | 80 | 28.8 (23.5–34.5) | (ref) | 43 | 15.5 (11.4–20.3) | (ref) |
| Out-of-hospital | 318 | 212 | 66.7 (61.2–71.8) | <0.001 | 196 | 61.6 (56.0–67.0) | <0.001 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; MI, myocardial infarction; ref, reference.
Definite or probable myocardial infarctions identified and adjudicated through REasons for Geographic And Racial Differences in Stroke study procedures.
P-values were calculated using a χ2 test, unless otherwise indicated.
Stroke buckle includes coastal plains of North Carolina, South Carolina and Georgia. Stroke belt includes the remaining parts of North Carolina, South Carolina and Georgia, and Tennessee, Mississippi, Alabama, Louisiana and Arkansas. Other contiguous US states includes the remaining 40 contiguous US states and the District of Columbia.
There are 8 MIs with missing peak troponin level.
There are 3 MIs with missing MI type.
Calculated using the Fisher’s exact test
In total, 552 participants had a Medicare claim with a code for MI in any discharge diagnosis position, including 523 (94.7%) for whom the adjudication has been completed. Also, 411 participants had a Medicare claim with a code for MI in the primary discharge diagnosis position, including 398 (96.8%) for whom the adjudication has been completed. The PPV of Medicare claims with a code for MI in any and in the primary discharge diagnosis position was 84.3% (95% CI 80.9%−87.3%) and 89.7% (95% CI 86.3%−92.5%), respectively (Table 3). PPV was not statistically significantly different across levels of age, sex, race, region of residence, calendar year, education, income and cognitive impairment, but was lower among participants with versus without a history of CHD. Compared with the primary analysis, the PPV was higher after excluding Medicare claims in the last year of life (Supplemental Figure 3).
Table 3.
Positive Predictive Values of Medicare Inpatient Claims for Identifying Myocardial Infarctions Adjudicated Through REasons for Geographic And Racial Differences in Stroke Study Procedures.
| Characteristics | Number of Medicare claims with a discharge diagnosis code for MI in any position | Adjudicated as definite or probable MI in REGARDS | Number of Medicare claims with a discharge diagnosis code for MI in the primary position | Adjudicated as definite or probable MI in REGARDS | ||||
|---|---|---|---|---|---|---|---|---|
| n | PPV (95% CI) | p-value* | n | PPV (95% CI) | p-value* | |||
| Full population | 523 | 441 | 84.3 (80.9–87.3) | 398 | 357 | 89.7 (86.3–92.5) | ||
| Age | ||||||||
| <75 years | 309 | 266 | 86.1 (81.7–89.7) | 0.18 | 236 | 213 | 90.3 (85.7–93.7) | 0.66 |
| ≥75 years | 214 | 175 | 81.8 (75.9–86.7) | 162 | 144 | 88.9 (83.0–93.3) | ||
| Sex | ||||||||
| Women | 215 | 175 | 81.4 (75.5–86.4) | 0.12 | 161 | 139 | 86.3 (80.0–91.2) | 0.07 |
| Men | 308 | 266 | 86.4 (82.0–90.0) | 237 | 218 | 92.0 (87.8–95.1) | ||
| Race | ||||||||
| White | 364 | 313 | 86.0 (82.0–89.4) | 0.11 | 281 | 254 | 90.4 (86.3–93.6) | 0.48 |
| Black | 159 | 128 | 80.5 (73.5–86.4) | 117 | 103 | 88.0 (80.7–93.3) | ||
| Geographic region of residence† | ||||||||
| Stroke buckle | 121 | 104 | 86.0 (78.5–91.6) | (ref) | 97 | 87 | 89.7 (81.9–94.9) | (ref) |
| Stroke belt | 190 | 164 | 86.3 (80.6–90.9) | 0.93 | 142 | 132 | 93.0 (87.4–96.6) | 0.37 |
| Other contiguous US states | 212 | 173 | 81.6 (75.7–86.6) | 0.31 | 159 | 138 | 86.8 (80.5–91.6) | 0.49 |
| Calendar year | ||||||||
| 2003–2006 | 141 | 116 | 82.3 (74.9–88.2) | (ref) | 103 | 90 | 87.4 (79.4–93.1) | (ref) |
| 2007–2009 | 202 | 169 | 83.7 (77.8–88.5) | 0.73 | 160 | 140 | 87.5 (81.4–92.2) | 0.98 |
| 2010–2012 | 180 | 156 | 86.7 (80.8–91.3) | 0.28 | 135 | 127 | 94.1 (88.7–97.4) | 0.07 |
| Education | ||||||||
| Less than high school | 112 | 89 | 79.5 (70.8–86.5) | 0.09 | 78 | 70 | 89.7 (80.8–95.5) | 0.98 |
| High school or higher | 408 | 351 | 86.0 (82.3–89.2) | 319 | 286 | 89.7 (85.8–92.8) | ||
| Total annual household income | ||||||||
| <$25,000 | 192 | 155 | 80.7 (74.4–86.1) | 0.06 | 141 | 123 | 87.2 (80.6–92.3) | 0.16 |
| ≥$25,000 | 267 | 233 | 87.3 (82.7–91.0) | 208 | 191 | 91.8 (87.2–95.2) | ||
| Cognitive impairment | ||||||||
| No | 319 | 274 | 85.9 (81.6–89.5) | 0.12 | 250 | 224 | 89.6 (85.1–93.1) | 0.45 |
| Yes | 43 | 33 | 76.7 (61.4–88.2) | 34 | 29 | 85.3 (68.9–95.0) | ||
| History of CHD | ||||||||
| No | 272 | 240 | 88.2 (83.8–91.8) | 0.008 | 216 | 200 | 92.6 (88.2–95.7) | 0.03 |
| Yes | 246 | 196 | 79.7 (74.1–84.5) | 177 | 152 | 85.9 (79.9–90.6) | ||
Abbreviations: CHD, coronary heart disease; CI, confidence interval; MI, myocardial infarction; PPV, positive predictive value; REGARDS, REasons for Geographic And Racial Differences in Stroke.
P-values were calculated using the χ2 test.
Stroke buckle includes coastal plains of North Carolina, South Carolina and Georgia. Stroke belt includes the remaining parts of North Carolina, South Carolina and Georgia, and Tennessee, Mississippi, Alabama, Louisiana and Arkansas. Other contiguous US states includes the remaining 40 contiguous US states and the District of Columbia.
Overall, 55 (9.2%) of the 596 definite or probable MIs included in the main analysis of sensitivity did not have an inpatient claim in Medicare. The proportion of MIs without an inpatient claim in Medicare was similar across subgroups defined by participant characteristics, except for sex (Supplemental Table 3). The proportion of MIs without a Medicare inpatient claim was higher among men versus women.
Risk for myocardial infarction
The cumulative incidence for MI was higher when definite or probable MIs identified and adjudicated through REGARDS study procedures (REGARDS definition) were supplemented with definite or probable MIs identified through Medicare claims with a discharge diagnosis code for MI in any (REGARDS-Medicareany) and in the primary position (REGARDS-Medicareprimary) (Supplemental Figure 4 and Supplemental Table 4). Compared with using the REGARDS definition, the cumulative incidence for MI was lower when using Medicare claims with a discharge diagnosis code for MI in any (Medicareany) and in the primary position (Medicareprimary), without further adjudication. Compared with the REGARDS definition, Medicareany and Medicareprimary definitions underestimated the rate of MI by 8% (RR 0.92, 95% CI 0.85-0.98) and 32% (RR 0.68, 95% CI 0.62-0.74), respectively (Table 4, top panel). REGARDS-Medicareany and REGARDS-Medicareprimary definitions resulted in a 12% (ratio 1.12, 95% CI 1.08-1.15) and 6% (ratio 1.06, 95% CI 1.04-1.10) higher rates of MI, respectively, compared with the REGARDS definition. RR for MI comparing MI definitions among participants with and without a history of CHD are shown in Table 4, middle and bottom panels, respectively.
Table 4.
Total Number of Myocardial Infarctions, Mean Follow-up and Event Rates Using Five Definitions.
| Myocardial infarctions | Mean follow-up | Events / follow-up | Rate ratio | |
|---|---|---|---|---|
| Definition of myocardial infarction | N | Years | Rate (95% CI)* | Ratio (95% CI) |
| All participants (n=9,951) | ||||
| REGARDS | 669 | 6.30 | 10.7 (9.9-11.5) | 1 (reference) |
| REGARDS-Medicareany | 788 | 6.66 | 11.9 (11.1-12.7) | 1.12 (1.08-1.15) |
| REGARDS-Medicareprimary | 753 | 6.67 | 11.3 (10.5-12.2) | 1.06 (1.04-1.10) |
| Medicareany | 552 | 5.68 | 9.8 (8.9-10.6) | 0.92 (0.85-0.98) |
| Medicareprimary | 411 | 5.71 | 7.2 (6.5-7.9) | 0.68 (0.62-0.74) |
| Without a history of CHD (n=7,311)† | ||||
| REGARDS | 346 | 6.50 | 7.3 (6.5-8.1) | 1 (reference) |
| REGARDS-Medicareany | 407 | 6.88 | 8.1 (7.3-8.9) | 1.11 (1.07-1.17) |
| REGARDS-Medicareprimary | 393 | 6.88 | 7.8 (7.0-8.6) | 1.07 (1.04-1.12) |
| Medicareany | 286 | 5.82 | 6.7 (5.9-7.5) | 0.92 (0.83-1.03) |
| Medicareprimary | 228 | 5.83 | 5.4 (4.7-6.0) | 0.73 (0.66-0.84) |
| With a history of CHD (n=2,458)† | ||||
| REGARDS | 313 | 5.75 | 22.1 (19.7-24.6) | 1 (reference) |
| REGARDS-Medicareany | 371 | 6.04 | 25.0 (22.5-27.5) | 1.13 (1.08-1.20) |
| REGARDS-Medicareprimary | 350 | 6.06 | 23.5 (21.0-26.0) | 1.06 (1.02-1.10) |
| Medicareany | 262 | 5.31 | 20.1 (17.6-22.5) | 0.91 (0.80-1.02) |
| Medicareprimary | 180 | 5.37 | 13.6 (11.6-15.6) | 0.62 (0.54-0.72) |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; REGARDS, REasons for Geographic And Racial Differences in Stroke.
Per 1,000 person-years.
A total of 182 REGARDS study participants had missing data on history of coronary heart disease (see Supplemental Table 2).
The HR for MI among men compared with women was lower using the Medicareany versus the REGARDS definition (Figure 1, left panel and Supplemental Table 5). The HR associated with low income was higher using the Medicareany and Medicareprimary versus the REGARDS definition. Also, the HR associated with elevated CRP was lower using the REGARDS-Medicareprimary versus the REGARDS definition. HRs for MI associated with the remaining participant characteristics were not statistically significantly different when using each MI definition. HRs for MI associated with participant characteristics among participants with and without a history of CHD, separately are shown in Figure 1, middle and right panels and Supplemental Tables 6–7.
Figure 1.
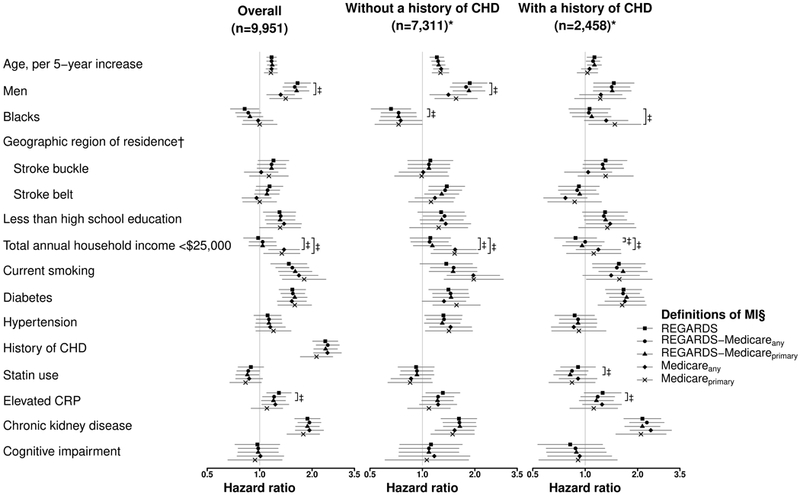
Hazard Ratios for Myocardial Infarction Associated with Participant Characteristics.
Abbreviations: CHD, coronary heart disease; CRP, C-reactive protein; MI, myocardial infarction; REGARDS, REasons for Geographic And Racial Differences in Stroke.
* A total of 182 REGARDS study participants had missing data on history of coronary heart disease (see Supplemental Table 2).
† Stroke buckle includes coastal plains of North Carolina, South Carolina and Georgia. Stroke belt includes the remaining parts of North Carolina, South Carolina and Georgia, and Tennessee, Mississippi, Alabama, Louisiana and Arkansas. Hazard ratios were calculated using other contiguous US states as the reference. Other contiguous US states includes the District of Columbia.
‡ Indicates that the difference is statistically significant at a two-sided α level <0.05.
§ Markers and error bars indicate hazard ratios and 95% confidence intervals. Analyses were conducted using multiple imputation and including adjustment for all variables in the figure simultaneously.
Hazard ratios for each definition of MI are reported in Supplemental Tables 5–7.
DISCUSSION
In the current analysis, Medicare claims with a discharge diagnosis code for MI had high PPV but low sensitivity for MI identification, using REGARDS study adjudication procedures as the gold standard. Using Medicare claims to supplement MIs identified and adjudicated through REGARDS study procedures detected additional MI cases and resulted in a modestly higher MI rate. In contrast, using Medicare claims with a discharge diagnosis code for MI without further adjudication underestimated the MI rate compared with REGARDS study procedures. Most associations of participant characteristics with MI were similar when events were defined using REGARDS study procedures, REGARDS study procedures supplemented with MIs identified through Medicare claims, and Medicare claims without further adjudication.
In a prior study of Medicare beneficiaries in Pennsylvania in 1999-2000, the PPV of Medicare inpatient claims with an ICD-9 discharge diagnosis code of 410.x1 in the primary position for MI identification was 95.1% (95% CI 94.1%−96.2%).21 In the Cardiovascular Health Study (CHS), which enrolled Medicare beneficiaries, the PPV of hospital records with an ICD-9 discharge diagnosis code of 410.x1 in any and in the primary position from baseline in 1989-1993 through 2012 was 84.7% and 90.6%, respectively.22 Results from the current study are consistent with a high PPV of Medicare claims for MI identification. Taken together, these studies suggest that Medicare claims for MI likely represent an MI that occurred.
In the CHS, sensitivity of hospital records with an ICD-9 discharge diagnosis code of 410.x1 in any and in the primary position was 70.4% and 53.8%, respectively.22 In the current analysis, the sensitivity of Medicare claims with a discharge diagnosis code for MI in any and in the primary position was lower (49.0% and 40.1%, respectively). Results from the current study expand on prior data by showing that the sensitivity of Medicare claims is lower for men versus women, microsize MIs versus other MIs, type 2 and 4-5 MIs versus type 1 MIs, and MIs occurring in-hospital versus out-of-hospital. Several factors may contribute to the lower sensitivity of Medicare claims in the current analysis as compared with hospital records in the CHS. Some hospitalizations among Medicare beneficiaries, including those with Veteran Affairs (VA) benefits, may not be billed to Medicare.23,24 Having VA benefits may also explain the lower sensitivity of Medicare claims for MI among men versus women in the current analysis. Medicare uses a prospective payment system through which reimburses hospitalizations with a fixed amount determined based on the primary reason for hospitalization.25 Therefore, MIs occurring in-hospital, including type 2 and 4-5 MIs, could be less likely to be present in claims versus medical records as they may not change the reimbursement amount. CHS started before (baseline 1989-1993), while the REGARDS study started after (baseline 2003-2007), highly sensitive biomarkers were recommended for the diagnosis of MI in 2000.6 Therefore, the proportion of microsize MIs identified and adjudicated in the REGARDS study may be higher as compared with CHS.
Studies relying on participant or proxy reports to initiate the MI adjudication process may not identify all events and, therefore, may underestimate MI risk. In the Women’s Health Initiative (WHI), which relies on participant or proxy reports for MI identification, supplementing study procedures with Medicare claims with a discharge diagnosis code for MI in any position without adjudication resulted in a 33% increase in the number of participants identified as having an MI.26 In the current analysis, including events identified through Medicare claims with a discharge diagnosis code for MI in any and in the primary position which were subsequently adjudicated as definite or probable MIs increased the MI rate by 12% and 6%, respectively, compared with using the REGARDS study procedures alone. Results from the current study also suggest that Medicare claims may underestimate the risk for MI when used as the sole data source for event identification. Medicare claims with a discharge diagnosis code for MI in any position may be preferred to estimate MI rates as they have higher sensitivity while maintaining high PPV as compared with Medicare claims with a discharge diagnosis code for MI in the primary position.
In the WHI hormone therapy trial, HRs for MI associated with hormone replacement versus placebo were similar using MIs defined through study adjudication procedures and through Medicare claims without adjudication.26 In the CHS, the association of age, sex, race, blood pressure, smoking and diabetes with MI was similar using MIs defined through study adjudication procedures and through hospital records without adjudication.22 Results from the current study expand on these data by showing that HRs for MI associated with many participant characteristics were similar using study procedures, study procedures supplemented with MIs identified through Medicare claims, and Medicare claims without further adjudication.
The current analysis has known and potential limitations. CHD-related hospitalizations were adjudicated using data available in medical records. Therefore, some Medicare claims may have been misclassified as not being a definite or probable MI due to medical records being incomplete, resulting in an underestimation of the PPV. We restricted the analyses to REGARDS study participants ≥65 years of age. Therefore, results may not be generalizable to adults <65 years of age. Having VA benefits or other health insurance programs may contribute to the low sensitivity of Medicare claims for MI identification. However, data from the VA or other health insurance programs were not available in the current analysis. Although the REGARDS study collected MI data from a nationwide, diverse group of hospitals, data on hospital characteristics were not available for the current analysis. Therefore, we were unable to compare the validity of Medicare claims for MI across subgroups defined by hospital characteristics. Finally, many REGARDS study participants were missing data on at least one baseline characteristic, which required the used of multiple imputation to include them in regression models.
In conclusion, Medicare claims have high PPV but low sensitivity for identifying MIs. The sensitivity of Medicare claims for MI identification varied by sex and MI characteristics. Also, using Medicare claims as the only data source underestimates the risk for MI. Despite these limitations, results from the current study supports using primary data collection, claims data, or primary data collection supplemented by claims data to investigate individual-level risk factors for MI.
Supplementary Material
Acknowledgments
Preliminary results from the current study were presented as a scientific poster in the 2017 American Heart Association Epidemiology/Life Style-Epidemiology council meeting in Portland, Oregon. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions and further information about the study can be found at http://www.regardsstudy.org.
Funding
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional support was provided by grants R01 HL080477 and K24 HL111154 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Disclosure of potential conflicts of interest
EBL, HY, MLK, MMS and PM received grant support from Amgen Inc. EBL also served on an advisory board for Amgen Inc. and consulted for Novartis. HY also received grant support from Bristol-Myers Squibb. LDC, JDR and GH have no disclosures.
REFERENCES
- 1.Sorlie PD, Bild DE, Lauer MS. Cardiovascular epidemiology in a changing world--challenges to investigators and the National Heart, Lung, and Blood Institute. Am J Epidemiol 2012;175:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumamaru H, Judd SE, Curtis JR, et al. Validity of claims-based stroke algorithms in contemporary Medicare data: REasons for Geographic And Racial Differences in Stroke (REGARDS) study linked with medicare claims. Circ Cardiovasc Qual Outcomes 2014;7:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick N, Lacaille D, Bhole V, et al. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One 2014;9:e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavrielov-Yusim N, Friger M. Use of administrative medical databases in population-based research. J Epidemiol Community Health 2014;68:283–287 [DOI] [PubMed] [Google Scholar]
- 5.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol 2005;58:323–337 [DOI] [PubMed] [Google Scholar]
- 6.Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959–969 [DOI] [PubMed] [Google Scholar]
- 7.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003;108:2543–2549 [DOI] [PubMed] [Google Scholar]
- 8.Safford MM, Parmar G, Barasch CS, et al. Hospital laboratory reporting may be a barrier to detection of ‘microsize’ myocardial infarction in the US: an observational study. BMC Health Serv Res 2013;13:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–1598 [DOI] [PubMed] [Google Scholar]
- 10.Saaby L, Poulsen TS, Hosbond S, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med 2013;126:789–797 [DOI] [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, et al. The REasons for Geographic And Racial Differences in Stroke Study: objectives and design. Neuroepidemiology 2005;25:135–143 [DOI] [PubMed] [Google Scholar]
- 12.Xie F, Colantonio LD, Curtis JR, et al. Linkage of a Population-Based Cohort With Primary Data Collection to Medicare Claims: The Reasons for Geographic and Racial Differences in Stroke Study. Am J Epidemiol 2016;184:532–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA 2012;308:1768–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prineas RJ, Crow RS, Blackburn H. The Minnesota code manual of electrocardiographic findings: Standards and procedures for measurement and classification Boston, MA: Wright-OSG; 1982 [Google Scholar]
- 15.Prineas RJ, Crow RS, Zhang ZM. Minnesota Code Manual of Electrocardiographic Findings London, England: Springer-Verlag; 2010 [Google Scholar]
- 16.Centers for Medicare & Medicaid Services. Medicare Claims Processing Manual: Chapter 23–Fee Schedule Administration and Coding Requirements. Baltimore, MD: Centers for Medicare & Medicaid Services; 2017 [Google Scholar]
- 17.German RR, Lee LM, Horan JM, et al. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep 2001;50:1–35 [PubMed] [Google Scholar]
- 18.DiCiccio TJ, Efron B. Bootstrap confidence intervals. Statist Sci 1996;11:189–228 [Google Scholar]
- 19.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377–399 [DOI] [PubMed] [Google Scholar]
- 20.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004;148:99–104 [DOI] [PubMed] [Google Scholar]
- 22.Psaty BM, Delaney JA, Arnold AM, et al. Study of Cardiovascular Health Outcomes in the Era of Claims Data: The Cardiovascular Health Study. Circulation 2016;133:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humensky J, Carretta H, de Groot K, et al. Service utilization of veterans dually eligible for VA and Medicare fee-for-service: 1999–2004. Medicare Medicaid Res Rev 2012;2:E1–E21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hynes DM, Koelling K, Stroupe K, et al. Veterans’ access to and use of Medicare and Veterans Affairs health care. Med Care 2007;45:214–223 [DOI] [PubMed] [Google Scholar]
- 25.Mues KE, Liede A, Liu J, et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol 2017;9:267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hlatky MA, Ray RM, Burwen DR, et al. Use of Medicare data to identify coronary heart disease outcomes in the Women’s Health Initiative. Circ Cardiovasc Qual Outcomes 2014;7:157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


