Abstract
Aim:
We aimed to investigate the interaction between genetic risk score of HbA1c and weight changes during and after pregnancy(postpartum weight reduction and gestational weight gain) on long-term glycemic changes in the largest cohort of women with preceding history of gestational diabetes mellitus (GDM).
Methods:
This is a retrospective cohort using the baseline data from the Tianjin Gestational Diabetes Mellitus Prevention Program. A genetic risk score was established by combining 10 HbA1c related single nucleotide polymorphisms (SNPs), which were identified by genome-wide association studies (GWAS). General linear regression models were applied to evaluate the interaction between HbA1c genetic risk score and weight changes during and after pregnancy(postpartum weight reduction and gestational weight gain) on glycemic changes.
Results:
A total of 1156 women with preceding history of GDM were included in this respective cohort study. Statistical differences in pre-pregnancy weight, pre-delivery weight and postpartum weight were evidenced across different groups of postpartum weight reduction. After adjusted for covariates, statistical significance in changes of HbA1c % was only observed in postpartum weight reduction<5 kg/yr group (p= 0.002), and significant interaction between HbA1c genetic risk score and postpartum weight reduction on long-term changes of HbA1c was evidenced (p-interaction=0.01). In the category of postpartum weight reduction≥8kg/y group, women with a lower genetic risk score of HbA1c had a greater decrease of HbA1c.
Conclusions:
A genetic risk score of HbA1c interact with postpartum weight reduction on long-term changes of HbA1c among women with preceding history of GDM.
Keywords: postpartum weight reduction, glycemic changes, genetic risk score, gestational diabetes mellitus
INTRODUCTION
During pregnancy, women will gain about 20 percent or more of body weight 1. Abnormal metabolism of glucose are greatly affected by weight changes during and after pregnancy 2,3. Compelling evidence indicates that low postpartum weight reduction is associated with aberrant glucose metabolism and an increased risk of type 2 diabetes in later life 1,4–7, women with greater postpartum weight retention and preceding history of gestational diabetes mellitus (GDM) are more likely to involve in this metabolic disease8–11.
As a common biomarker, hemoglobin A1c (HbA1c) is regarded as a stable biomarker for hyperglycemia. Genetic susceptibility to higher HbA1c levels had been verified by genome-wide association studies(GWAS) 12,13. However, the interactions between genetic variations of HbA1c and weight changes during and after pregnancy (postpartum weight reduction and gestational weight gain) on long-term changes of glycemic traits remain unclear. We hypothesized that weight changes during and after pregnancy (postpartum weight reduction and gestational weight gain) might modify the genetic variations of HbA1c on glycemic changes.
Taken together, we investigated the interaction between genetic risk score of HbA1c and weight changes during and after pregnancy (postpartum weight reduction and gestational weight gain) on long-term changes of glycemic traits in the largest cohort of women with preceding history of GDM.
2. MATERIALS AND METHODS
2.1. Study samples
Our study is a retrospective cohort using the baseline data from the Tianjin Gestational Diabetes Mellitus Prevention Program14. n accordance with the criteria of World Health Organization) at 26–30 gestational weeks(2005–2009) were recruitedWomen with pregnancy, resided in the 6 central urban districts of Tianjin and with diagnosis of GDM(i to engage in a postpartum survey(2009–2011). Those with chronic diseases, diabetes after delivery, usage of medicine to change the glucose values, pregnant and going to be pregnant in the next two years were excluded. A group of 4644 women with preceding history of GDM (postpartum at 1–5 years) were recruited from the Tianjin Gestational Diabetes Mellitus Prevention Study14–17.
A total of 1263 women with preceding history of GDM finished the questionnaire. Glucose levels, the prevalence of impaired glucose tolerance and diabetes at 26–30 gestational weeks didn’t differ between women who returned the questionnaire and those who did not. The 3-day 24-hour food records were obtained by a dietitian. The final analysis was restricted to a group of 1156 participants with available genotype data. The proposal of this respective cohort study was approved by the Human Subjects Committee of Tianjin Women’s and Children’s Health Center. Informed consent were acquired for all of the women with preceding history of GDM.
Tianjin gestational diabetes mellitus screening program
The Tianjin Gestational Diabetes Mellitus Prevention Program is ongoing and 2-arm, 4-year randomized controlled trial, which is conducted by Tianjin Women’s and Children’s Health Centre, Tianjin, China. Compared to the usual-care group, six face-to-face sessions with study dietitians in the first year, and two extra sessions and two telephone calls in second year were performed in the intervention group. The detailed information about the study design has been described in elsewhere14. The registered number is NCT01554358 at ClinicalTrials.gov. Among the 1263 women with preceding history of GDM, who finished the questionnaire, a total of 1180 women were randomly assigned (1:1) to receive a 4-year lifestyle intervention or standard care14. Our current analysis is retrospective, the outcomes are changes in glycemic traits from pregnancy to baseline of the intervention trial. Therefore, intervention would not affect our analyses.
Variable definition
All participants completed a baseline self-administered questionnaire. Pre-pregnancy weight, postpartum weight, and weight gain in pregnancy were self-reported. Baseline weight was measured by trained physicians14. Body weight and height were measured using the standardized protocol according to the WHO MONICA project18. Weight was measured to the nearest 0.1 kg and height(without shoes) to the nearest 0.1 cm.
The postpartum weight retention (weight change between pre-pregnancy and 1–5 y postpartum) was classified as gestational weight gain and postpartum weight reduction. The annual post-partum weight reduction was calculated by dividing the difference between postpartum weight and predelivery weight (pre-pregnancy weight plus gestational weight gain) by follow-up years. Gestational weight gain was categorized as inadequate, adequate, and excessive according to 2009 Institute of Medicine guideline19. For the range of pre-pregnancy BMI of <18.5 kg/m2, 18.5–24.9 kg/m2, 25.0–29.9 kg/m2, BMI of ≥30 kg/m2, weight in the range of 12.5 to 18 kg, 11.5 to 16 kg, 7 to 11.5 kg, and 5 to 9 kg were defined as adequate gestational weight gain20. Otherwise under or higher than the cut-off values were taken as inadequate or excessive, separately20. Fasting glucose, 2-hour glucose and HbA1c were measured according to the standard protocols (with analytical machines TBA-120FR and ADAMS A1c HA-8160; Arkray, Japan, respectively). The changes in glycemic traits were computed as the corresponding values at postpartum minus the values at pre-gnancy(GDM diagnosis).
2.2. Calculation of HbA1c genetic risk score
DNA was extracted from the buffy coat fraction of centrifuged blood using a QIAamp Blood Maxi Kit (Qiagen, Chatsworth, CA, USA). SNPs were genotyped by aquantitative real-time TaqMan PCR(Applied Biosystems) with a success rate ≥ 98%. The HbA1c genetic risk score was established by aggregating the HbA1c-increasing alleles according to the HbA1c susceptibility SNPs discovered by GWAS (Table S1) 12,13, and mutual independence were observed for all HbA1c SNPs. Among them, rs1800562 and rs855791 were not in accordance with Hardy-Weinberg equilibrium in our data, thus rs266717 and rs12597579 were used as proxies, respectively(r2=1). GWAS provided the β-coefficients (effect sizes) for the 10 HbA1c susceptibility SNPs 12,13 (Table S1). A genetic risk score was established by summing the 10 effect alleles, weighted by the β-coefficients. We actually transformed the β-coefficients to indicate a higher HbA1c. The HbA1c genetic risk score was divided into tertiles (tertile 1 for the lowest, tertile 2 in the middle, and tertile 3 for the highest).
2.3. Statistical analysis
We did a retrospective analysis using the baseline data from Tianjin Gestational Diabetes Mellitus Prevention Program, the final analysis was restricted to a group of 1156 participants with available genotype data. The baseline characteristics across different groups of annual postpartum weight reduction (<5, 5–8, and ≥8 kg/y) and gestational weight gain(inadequate, adequate, excessive) were estimated by chi-square tests and general linear model(GLM), as appropriate. The association of HbA1C genetic risk score and changes in glycemic traits by annual weight reduction groups was tested by GLM. An interaction between the HbA1C genetic risk score and annual postpartum weight reduction on changes in glycemic traits was tested by including an interaction term in the models. The long-term effect of weight changes on the glycemic traits stratified by tertiles of HbA1c genetic risk score were also estimated by GLM. In addition, sensitivity analyses were performed to estimate the effect of follow-up years on the observed association. SAS 9.4 was used to perform all of the analysis with two-sided significance at 0.05 (SAS Institute, Cary, NC).
3. RESULTS
Characteristics across different groups of postpartum weight reduction and gestational weight gain are summarized in Table 1 and Table S2, respectively. Statistical differences in pre-pregnancy weight, pre-delivery weight and postpartum weight were evidenced across different groups of postpartum weight reduction. Others characteristics and HbA1c genetic risk score were not significant across different groups (Table 1).
Table 1.
Characteristics of women with prior gestational diabetes mellitus by annual weight reduction
| Variables | Weight reduction |
||||
|---|---|---|---|---|---|
| <5 kg/year | 5 to 8 kg/year | ≥8 kg/year | P value | ||
| Number | 428 | 396 | 332 | ||
| Age (years) | 32.46 ± 3.45 | 32.79 ± 3.76 | 31.89 ± 3.56 | 0.0548 | |
| Follow-up (years) | 2.71 ± 0.91 | 2.42 ± 0.80 | 1.94 ± 0.78 | 0.1029 | |
| Pre-pregnancy BMI (kg/m2) | 23.27 ± 3.47 | 22.95 ± 2.83 | 22.61 ± 2.96 | 0.0051 | |
| Weight (kg) | |||||
| Pre-pregnancy | 59.74 ± 9.52 | 58.46 ± 8.43 | 58.56 ± 8.00 | 0.0486 | |
| Pre-delivery | 75.94 ± 11.18 | 72.83 ± 9.43 | 78.53 ± 10.39 | 0.0122 | |
| Postpartum | 64.49 ± 10.50 | 64.16 ± 8.39 | 71.14 ± 9.94 | <0.0001 | |
| Fasting glucose (mmol/L) | |||||
| At GDM diagnosis | 5.34 ± 0.81 | 5.23 ± 0.71 | 5.31 ± 0.83 | 0.4442 | |
| Postpartum | 5.13 ± 0.76 | 5.66 ± 0.58 | 5.36 ± 0.95 | 0.4144 | |
| 2-h glucose (mmol/L) | |||||
| At GDM diagnosis | 9.19 ± 1.27 | 8.99 ± 1.16 | 9.06 ± 1.33 | 0.0824 | |
| Postpartum | 6.05 ± 1.03 | 8.30 ± 2.12 | 3.90 ± 1.69 | 0.1982 | |
| HbA1C (%) | |||||
| At GDM diagnosis | 5.84 ± 0.64 | 5.73 ± 0.63 | 5.78 ± 0.59 | 0.1479 | |
| Postpartum | 5.61 ± 0.75 | 5.69 ± 0.67 | 5.69 ± 0.78 | 0.1040 | |
| Family history of diabetes | 140 (32.71%) | 117 (29.54%) | 122 (36.75%) | 0.7282 | |
| Current Smokers | 10 (2.33%) | 0 (0.00%) | 10 (3.01%) | 0.2654 | |
| Alcohol drinker | 99 (23.13%) | 79 (19.95%) | 95 (28.61%) | 0.4069 | |
| Leisure time physical activity | |||||
| 0 min/d | 120 (28.04%) | 320 (80.80%) | 246 (74.10%) | 0.4294 | |
| 1 to 30 min/d | 140 (32.71%) | 68 (17.17%) | 79 (23.79%) | ||
| ≥30 min/d | 168 (39.25%) | 8 (1.49%) | 7 (12.11%) | ||
| Sitting time (h/d) | 3.19 ± 2.15 | 3.51 ± 2.26 | 3.19 ± 1.80 | 0.7509 | |
| Total energy intake (kal/d) | 1679.27 ± 435.85 | 1713.65 ± 438.90 | 1638.34 ± 445.58 | 0.2889 | |
| HbA1C GRS | 10.96 ± 2.07 | 11.11 ± 1.85 | 11.07 ± 1.97 | 0.3602 | |
The distribution of the HbA1c genetic risk score is illustrated in Figure S1. There were no statistical differences for the HbA1c genetic risk score with reference levels of glycemic traits, even adjusted for covariates. The association between HbA1c genetic risk score with changes in glycemic traits stratified by annual postpartum weight reduction and gestational weight gain are summarized in Table 2 and Table S3, respectively. After adjusted for covariates, statistical significance in changes of HbA1c was only observed in postpartum weight reduction<5 kg/yr group (p= 0.002) (Table 2). Significant interactions were evidenced for the association between HbA1c genetic risk score and postpartum weight reduction on changes of HbA1c (p-interaction=0.01). In the category of postpartum weight reduction≥8 kg/y, women with a lower genetic predisposition to high HbA1c might be more susceptible to the improvement of HbA1c (P=0.036). Along with the accumulation of HbA1c genetic risk score, elevated HbA1c was evidenced in the largest postpartum weight loss group (Figure 1C). In the group of postpartum weight reduction<5 kg/yr, HbA1c levels were decreased across tertiles of HbA1c genetic risk score, whereas, HbA1c levels were increased in the group of postpartum weight reduction≥8 kg/y (Figure 1C). We also estimated the tertiles of HbA1c genetic risk score divided by weight reduction (kg/yr) on changes of HbA1c (Table S5). In the category of postpartum weight reduction ≥8kg/y, women with a lower genetic predisposition to high HbA1c showed more[Double check]improvement of HbA1c (p=0.036) (Table S5).We didn’t observe any significant interactions on variations of fasting glucose and 2-hour glucose, as well as in different groups of gestational weight gain (Table S2).
Table 2.
Association between HbA1C genetic risk score and changes in glycemic traits by annual weight reduction
| Changes in glycemic traits | <5 kg/year | 5 to 8 kg/year | ≥8 kg/year |
P for interaction |
||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | |||
| Fasting glucose, mmol/L | ||||||||
| Age-adjusted | 0.163 (0.372) | 0.661 | −0.707 (0.778) | 0.365 | −0.242 (0.316) | 0.445 | 0.371 | |
| Multivariable-adjusted† | 0.182 (0.371) | 0.624 | −0.508 (0.298) | 0.091 | −0.219 (0.320) | 0.493 | 0.428 | |
| 2-h glucose, mmol/L | ||||||||
| Age-adjusted | 0.274 (0.824) | 0.739 | 0.039 (0.093) | 0.966 | 0.094 (0.073) | 0.899 | 0.712 | |
| Multivariable-adjusted† | 0.192 (0.795) | 0.809 | −0.122 (0.089) | 0.174 | −0.364 (0.720) | 0.613 | 0.965 | |
| HbA1C, % | ||||||||
| Age-adjusted | −0.933 (0.322) | 0.003 | −0.226 (0.329) | 0.493 | −0.141 (0.103) | 0.171 | 0.025 | |
| Multivariable-adjusted† | −0.972 (0.323) | 0.002 | −0.125 (0.363) | 0.729 | −0.176 (0.105) | 0.095 | 0.019 | |
Covariates included glycemic traits at GDM diagnosis, age, duration of follow-up, BMI at pre-pregnancy, kal/d for energy intake, time for sitting, smoking, drinking, physical activity and family history of diabetes.
Figure 1.
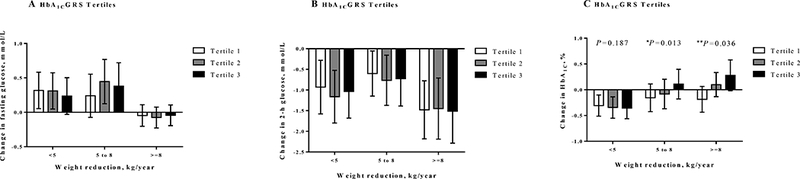
Changes in glycemic traits according to weight reduction and HbA1c genetic risk score.
The HbA1c genetic risk score were divided into tertiles (tertile 1 for the lowest, tertile 2 in the middle, and tertile 3 for the highest). From tertile 1 to tertile 3, the β values for every 1-unit postpartum weight reduction with changes of fasting glucose were 0.182 (P = 0.624), −0.508 (P =0.091), and −0.219 (P = 0.493) mmol/L, respectively (Figure 2A). The β values for every 1-unit postpartum weight reduction with changes of 2-hour glucose were 0.192 (P = 0.809), −0.122 (P =0.174), and −0.364 (P =0.613) mmol/L, respectively (Figure 2B). And the β values for every 1-unit postpartum weight reduction with changes of HbA1c were −0.972% (P = 0.002), −0.124% (P = 0.729) and −0.176% (P = 0.095), respectively (Figure 2C).
Figure 2.
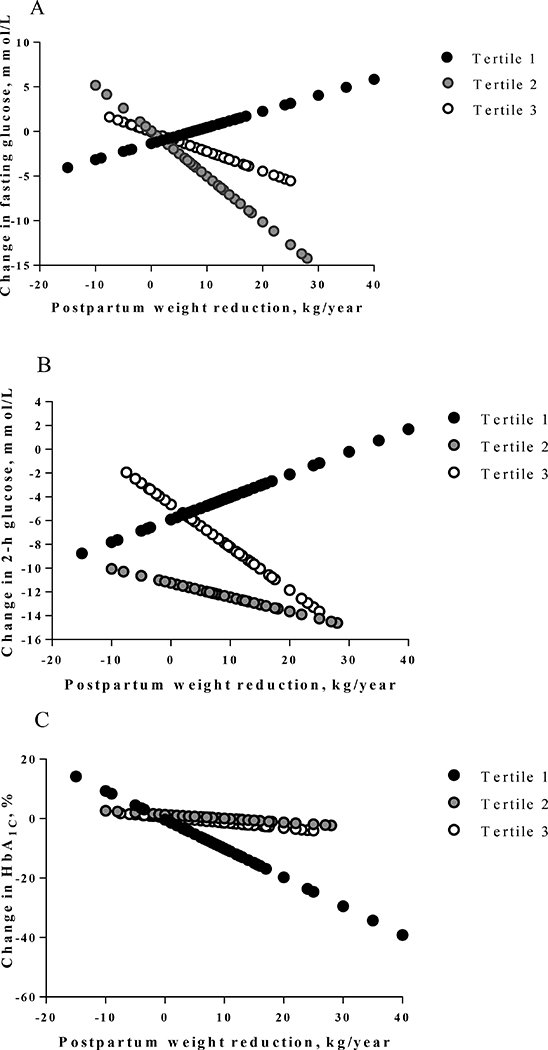
Predicted differences in glycemic traits stratified by HbA1c genetic risk score.
A sensitivity analyses by limiting the study to participants with delivery within the first 1.5 years was also performed(sample size equal 207). No statistical significant interactions between HbA1c genetic risk score and postpartum weight reduction/gestational weight gain on any glycemic changes were observed (Table 3 and Table S4, respectively).
Table 3.
Association between HbA1C genetic risk score and changes in glycemic traits by weight reduction within 1.5 years
| Changes in glycemic trait |
<5 kg/year (N=129) | 5 to 8 kg/year (N=44) | ≥8 kg/year (N=34) |
P for interaction |
|||
|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | ||
| Fasting glucose, mmol/L | |||||||
| Age-adjusted | 0.024 (0.489) | 0.960 | −0.249 (0.446) | 0.585 | −0.857 (0.864) | 0.328 | 0.604 |
| Multivariable-adjusted† | 0.130 (0.498) | 0.793 | −0.130 (1.214) | 0.915 | −1.290 (1.046) | 0.090 | 0.723 |
| 2-h glucose, mmol/L | |||||||
| Age-adjusted | 0.137 (1.135) | 0.903 | 1.363 (0.611) | 0.185 | 4.859 (1.714) | 0.700 | 0.088 |
| Multivariable-adjusted† | 0.200 (1.102) | 0.856 | −1.283 (1.549) | 0.454 | −6.149 (1.912) | 0.343 | 0.097 |
| HbA1C, % | |||||||
| Age-adjusted | −0.032 (0.376) | 0.931 | −0.656 (0.572) | 0.273 | −0.879 (0.625) | 0.168 | 0.332 |
| Multivariable-adjusted† | −0.019 (0.379) | 0.958 | −0.607 (1.216) | 0.643 | −1.191 (0.667) | 0.084 | 0.287 |
Covariates included glycemic traits at GDM diagnosis, age, duration of follow-up, BMI at pre-pregnancy, kal/d for energy intake, time for sitting, smoking, drinking, physical activity and family history of diabetes.
4. CONCLUSION
To our knowledge, significant interaction between genetic risk score of HbA1c and postpartum weight reduction on changes of HbA1c was evidenced in currently the largest cohort of women with preceding history of GDM. Our data demonstrated that across different categories of postpartum weight reduction, the genetic risk score of HbA1c differed significantly on changes of HbA1c. Interestingly, In the group of postpartum weight reduction<5 kg/yr, HbA1c levels were decreased across tertiles of HbA1c genetic risk score, whereas HbA1c levels were increased in the group of postpartum weight reduction≥8 kg/y. Our data indicated that postpartum weight reduction might modify the genetic risk score of HbA1c on long-term HbA1c changes, the “vulnerability genes” of HbA1c may act as “plasticity genes”21, with some women more susceptible to postpartum weight reduction, others not. In the category of postpartum weight reduction≥8 kg/y, women with a lower genetic predisposition to high HbA1c might be more susceptible to the improvement of HbA1c (P=0.036).
Women with greater postpartum weight retention are more susceptible to insulin resistance 22. Improvement in glucose metabolism in women with GDM was observed after moderate postpartum weight loss9. Low postpartum weight reduction contributes significantly to the long-term risk of type 2 diabetes in later life, especially in groups of women with preceding history of GDM 1,4–7. Therefore, postpartum weight reduction is a great concern in this population 23,24.
To the best of our knowledge, our findings demonstrated that genetic predisposition to high HbA1c interacted significantly with postpartum weight reduction on postpartum changes of HbA1c. This study brings novel perspectives to understand the role of postpartum body weight management in glycemic improvement under the genetic background. Furthermore, we created the HbA1c genetic risk score using widely accepted methods to sum up the genetic variations weighted by their effect sizes 25,26. Individual genetic variants explain a very small fraction of the overall variation, and a combination of a higher number of included variants with weak to moderate effect sizes into a genetic risk score can explain a larger proportion of heritability 27,28.
Two large GWAS analyzed the common genetic determinants of HbA1c levels with larger sample sizes and independent replications, the identified SNPs reached genome-wide significance levels (p<5×10−8), thus providing more reliable estimates12,29. Currently, the construction of GRS use the weighted published effect size from GWAS identified SNPs that achieved genome-wide significance30,31. However, the SNPs in our study didn’t reach the stringent levels, therefore, we used the weighted β-coefficients from the published two large GWAS12,29, and the results were similar when we used the un-weighted GRS scores. Besides, genetic architectures for HbA1c and fasting glucose were partially overlapped, GWAS of fasting glucose suggested that SNPs near three loci (G6PC2, MTNR1B, and GCK) were also associated with HbA1c levels13. Therefore, we not only investigated the interaction between HbA1c genetic risk score and postpartum weight reduction on HbA1c changes, but also on changes of fasting glucose and 2-h glucose.
The results should be mentioned cautiously, our findings may not be generalized to other populations, therefore different studies are required to verify our findings. The sample size of women in the sensitivity analysis was comparatively small, thus the interaction effect was not significant in this sub-group of analysis. The genetic susceptibility of HbA1c SNPs were primarily originated from European ancestry12,29, which may not be representative of the genetic susceptibility of Chinese women with GDM.
Taken together, we found postpartum weight reduction interacted with genetic predisposition to HbA1c on long-term changes of HbA1c. Contradictory to women with a higher genetic risk score, women with a lower genetic risk score demonstrated a more distinct association between postpartum weight reduction and HbA1c improvement. In women genetically predisposed to high HbA1c, postpartum weight management is of great concern.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all participants in the study for their dedication and contribution to the research.
Funding information
The study was supported by grants from the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly Program for Collaborative Research between China and Europe, the Tianjin Public Health Bureau, Natural Science Foundation of Zhejiang Province (LY17H260002), National Natural Science Foundation of Ningbo (2017A610219), Ningbo Scientific Innovation Team for Environmental Hazardous Factor Control and Prevention (2016C51001), and K.C.Wong Magna Fund in Ningbo University. Lu Qi is supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, and HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, and DK078616), the Boston Obesity Nutrition Research Center (DK46200), and the United States-Israel Binational Science Foundation (Grant 2011036). G.H. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100790) and the National Institute of General Medical Sciences (U54GM104940).
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose
Author Contributions
LH, DD, SZ, WL, LW, HL, JL, NL, XS, GH and LQ: designed the research, acquisition of data, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the manuscript. DD: analyzed the data. GH and LQ: involved in the collection and analysis of data and funding of the initial project. GH and LQ: conducted the research, administration, material support, and study supervision. LQ: had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the final manuscript.
References
- 1.Kew S, Ye C, Hanley AJ, et al. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes care. 2014;37: 1998–2006. [DOI] [PubMed] [Google Scholar]
- 2.Saldana TM, Siega-Riz AM, Adair LS,Suchindran C. The relationship between pregnancy weight gain and glucose tolerance status among black and white women in central North Carolina. Am J Obstet Gynecol. 2006;195: 1629–1635. [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM,Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. Bmj. 2017;356: j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser A, Tilling K, Macdonald-Wallis C, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). The American journal of clinical nutrition. 2011;93: 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. Jama. 1995;273: 461–465. [DOI] [PubMed] [Google Scholar]
- 6.Manson JE, Rimm EB, Colditz GA, et al. Parity and incidence of non-insulin-dependent diabetes mellitus. The American journal of medicine. 1992;93: 13–18. [DOI] [PubMed] [Google Scholar]
- 7.Collins VR, Dowse GK,Zimmet PZ. Evidence against association between parity and NIDDM from five population groups. Diabetes care. 1991;14: 975–981. [DOI] [PubMed] [Google Scholar]
- 8.Huopio H, Hakkarainen H, Paakkonen M, et al. Long-term changes in glucose metabolism after gestational diabetes: a double cohort study. BMC pregnancy and childbirth. 2014;14: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich SF, Hedderson MM, Quesenberry CP Jr., et al. Post-partum weight loss and glucose metabolism in women with gestational diabetes: the DEBI Study. Diabetic medicine : a journal of the British Diabetic Association. 2014;31: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baptiste-Roberts K, Barone BB, Gary TL, et al. Risk factors for type 2 diabetes among women with gestational diabetes: a systematic review. The American journal of medicine. 2009;122: 207–214 e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim C Gestational diabetes mellitus in korean women: similarities and differences from other racial/ethnic groups. Diabetes & metabolism journal. 2014;38: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pare G, Chasman DI, Parker AN, et al. Novel association of HK1 with glycated hemoglobin in a non-diabetic population: a genome-wide evaluation of 14,618 participants in the Women’s Genome Health Study. PLoS genetics. 2008;4: e1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soranzo N, Sanna S, Wheeler E, et al. Common variants at 10 genomic loci influence hemoglobin A(1)(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59: 3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu G, Tian H, Zhang F, et al. Tianjin Gestational Diabetes Mellitus Prevention Program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes research and clinical practice. 2012;98: 508–517. [DOI] [PubMed] [Google Scholar]
- 15.Hill HE, Kornetsky CH, Flanary HG,Wikler A. Studies on anxiety associated with anticipation of pain. I. Effects of morphine. A.M.A. archives of neurology and psychiatry. 1952;67: 612–619. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Liu H, Zhang S, et al. Obesity index and the risk of diabetes among Chinese women with prior gestational diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2014;31: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Dong L, Zhang CP, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabetic medicine : a journal of the British Diabetic Association. 2011;28: 652–657. [DOI] [PubMed] [Google Scholar]
- 18.Pajak A, Kuulasmaa K, Tuomilehto J,E. R. Geographical variation in the major risk factors of coronary heart disease in men and women aged 35–64 years. The WHO MONICA Project. World health statistics quarterly. Rapport trimestriel de statistiques sanitaires mondiales. 1988;41: 115–140. [PubMed] [Google Scholar]
- 19.Medicine I o. (National Academies Press, Washington, DC, 2009). [Google Scholar]
- 20.Institute of M,National Research Council Committee to Reexamine I O M P W G. in Weight Gain During Pregnancy: Reexamining the Guidelines (eds Rasmussen KM,Yaktine AL) (National Academies Press; (US: ) National Academy of Sciences., 2009). [PubMed] [Google Scholar]
- 21.Belsky J,Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135: 885–908. [DOI] [PubMed] [Google Scholar]
- 22.Kahn SE, Hull RL,Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444: 840–846. [DOI] [PubMed] [Google Scholar]
- 23.Cyganek K, Hebda-Szydlo A, Skupien J, et al. Postpregnancy glycemic control and weight changes in type 1 diabetic women. Diabetes care. 2013;36: 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold A E, Reilly C,Walker J D. Transient improvement in glycemic control. The impact of pregnancy in women with IDDM. Diabetes care. 1998;21: 374–378. [DOI] [PubMed] [Google Scholar]
- 25.Qi Q, Chu AY, Kang JH, et al. Sugar-sweetened beverages and genetic risk of obesity. The New England journal of medicine. 2012;367: 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi Q, Chu AY, Kang JH, et al. Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. Bmj. 2014;348: g1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGovern DP, Kugathasan S,Cho JH. Genetics of Inflammatory Bowel Diseases. Gastroenterology. 2015;149: 1163–1176 e1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power Dudbridge F. and predictive accuracy of polygenic risk scores. PLoS genetics. 2013;9: e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soranzo N, Sanna S, Wheeler E, et al. Common variants at 10 genomic loci influence hemoglobin A(1)(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59: 3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith J, Ware E, Middha P, Beacher L,Kardia S. Current Applications of Genetic Risk Scores to Cardiovascular Outcomes and Subclinical Phenotypes. Curr Epidemiol Rep. 2015;2: 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drenos F The use of genetic information in the prediction of Type 2 diabetes. Per Med. 2015;12: 483–496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


