Abstract
Proteolysis by the ubiquitin-proteasome pathway has pleiotropic effects on both induction and maintenance of long-term synaptic plasticity. In this study, we examined the effect of proteasome inhibition on signaling to the nucleus during late-phase long-term potentiation. When a subthreshold L-LTP induction protocol was used, proteasome inhibition led to a significant increase in phosphorylated CREB (pCREB) in the nucleus. Inhibitors of cAMP-dependent protein kinase/protein kinase A, extracellular signal-regulated kinase and cGMP-dependent protein kinase/protein kinase G all blocked the proteasome-inhibition-mediated increase in nuclear pCREB after subthreshold stimulation. These results lay the groundwork for understanding a novel role for the proteasome in limiting signaling to the nucleus in the absence of adequate synaptic stimulation.
1. Introduction
The ability of the nervous system to change the strength of synapses, or synaptic plasticity, allows it to store information. Short-term synaptic plasticity depends on molecular mechanisms that alter existing proteins [1]. Long-term modification of the synapses requires new gene expression and protein synthesis [2]. Evidence gathered over the last two decades also supports a role for regulated proteolysis by the ubiquitin-proteasome pathway (UPP) in both short-term and long-term synaptic plasticity [3–5]. In this pathway, the proteins to be degraded are marked by enzymatically-mediated covalent attachment of a small protein ubiquitin. To the first ubiquitin a second ubiquitin is attached and thus a polyubiquitin chain forms. The polyubiquitinated protein is then recognized by a multi-subunit proteolytic complex called the proteasome [6]. Monoubiquitination can also serve as a degradation signal especially for short proteins and human proteins with less structural disorder than those that are targeted by polyubiquitination [7–10].
We previously showed that the UPP has differential roles in dendrites and the nucleus using late-phase long-term potentiation (L-LTP) in the murine hippocampus as a model system [11]. We observed that inhibition of the proteasome in dendrites enhances the early, induction phase of L-LTP whereas blockade of proteasome in the nucleus blocks the late, maintenance phase of L-LTP. Through a series of experiments we showed that the enhancement of the induction phase of L-LTP comes about by stabilization of locally translated proteins in dendrites. The inhibition of the late, maintenance phase is caused by inhibition of transcription. Furthermore, our previous data showed that proteasome inhibition stabilizes a CREB repressor called ATF4 (Dong 2008).
There are additional ways in which proteasome inhibition could hinder transcription. One such way would be interference with signaling to the nucleus [12, 13]. Therefore, we investigated this possibility. We found that proteasome inhibition did not block signaling to the nucleus as measured by its effect on phosphorylation of CREB. We then tested whether proteasome inhibition could have the opposite effect and enhance CREB phosphorylation. Indeed, we found that proteasome inhibition significantly increased CREB phosphorylation when we used a subthreshold LTP induction protocol to stimulate the Schaffer collateral pathway in the hippocampus. The increase in CREB phosphorylation was blocked with inhibition of cAMP-dependent protein kinase/protein kinase A (PKA), or extracellular signal-regulated kinase (ERK, also called mitogen-activated protein kinase), or cGMP-dependent protein kinase/protein kinase G (PKG).
2. Materials and Methods
2.1. Animals
Mice (C57/Bl6, male, age 6–12 weeks) were obtained from Charles River (Wilmington, MA) and used for experiments using a protocol approved by the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences. Animals were housed (5 animals maximum per cage) and food and water were available ad libitum. Animal husbandry was done according to the Guide for the Care and Use of Laboratory Animals (8th edition; National Academies Press, Washington, D.C.).
2.2. Electrophysiology
Transverse hippocampus slices (400 μm) were prepared from 6–12-week old mice using a tissue chopper in oxygenated and chilled artificial cerebrospinal fluid (ACSF) containing 125mM NaCl, 3mM KCl, 2.3 mM CaCl2, 1.3 mM MgCl2, 25 mM NaHCO3, 1.25 mM NaH2PO4, and 10 mM glucose, pH 7.4. After recovery for 120 min in ACSF at 32 °C, field excitatory postsynaptic potentials (fEPSP) were recorded in the CA1 region of the hippocampus using a bipolar electrode to stimulate the Schaffer collateral pathway. The stimulation intensity was adjusted to give ~35% of the maximal fEPSP slope and the baseline responses were recorded at this intensity. To examine whether proteasome inhibition affects the phosphorylation of cAMP response element binding protein (CREB), slices were incubated with β-lactone (25 μM) for 30 min. The subthreshold LTP stimulation was given with 2×100 Hz trains spaced 5 min apart. Untreated slices were used as control. Slices were fixed in 4% paraformaldehyde immediately after stimulation and processed for immunohistochemistry and quantification of pCREB by confocal microscopy.
The stock solutions of pharmacological reagents PKA inhibitor (KT5720), specific PKA inhibitor (PKI), PKG (cGMP-dependent protein kinase) inhibitor KT5823 and MAP kinase inhibitor (U0126) were prepared in dimethylsulfoxide (DMSO) and diluted in ACSF and the final concentration was DMSO was no more than 0.02%. The same concentration of DMSO (without the drugs) was used in controls. The final concentrations of KT5720, U0126 and KT5823 were 1 μM, 20 μM and 2 μM respectively. All these reagents were applied to hippocampal slices before pre-incubation with β-lactone. Thereafter, the subthreshold LTP stimulation was given and slices were fixed for immunohistochemistry.
2.3. Immunohistochemistry (IHC)
IHC was performed as described previously (Dong 2014). Briefly, free-floating 400 μm hippocampal slices were fixed in 4% paraformaldehyde for 1 h and washed six times with PBS at room temperature. A buffer containing 4% normal goat serum (Vector Laboratories, Burlingame, CA), 0.4% Triton-X-100, and 0.05% sodium azide in PBS was used to block hippocampal slices at 4 °C for 6 h. Polyclonal primary antibodies against pCREB (1:1,000, Cell Signaling Technology, Danvers, MA) or CREB (1:1,000, Abcam, Cambridge, MA) in blocking buffer were used to incubate slices at 4 °C overnight. After the overnight incubation, hippocampal slices were washed with 0.2% Triton-X-100 in PBS three times. Secondary Alexa 488- conjugated goat anti-rabbit antibody (1:300, Invitrogen, Grand Island, NY) and To-Pro-3 nuclear stain (1:500, Invitrogen) were then applied at 4 °C for 8 h. Following five washes with 0.2% Triton-X-100 in PBS, slices were mounted with Prolong Gold antifade reagent (Invitrogen) onto glass slides. Carl Zeiss LSM510 laser scanning confocal microscope was used to image the fluorescence and ImageJ (National Institutes of Health, Bethesda, MD) software was used to analyze fluorescence intensity. Hippocampal slices that received subthreshold LTP stimulation and/or chemical treatment were compared to their time-matched controls for quantification of fluorescence. The images were analyzed by an individual who was blind to the experimental conditions.
2.4. Statistical Methods
The sample size was determined by power analysis (power = 0.8) in designing the experiments. The data are represented as mean ± standard error. The sample size (n) in each dataset corresponds to the number of animals (not slices) used to collect the data. We analyzed the data using one-way Analysis of Variance (ANOVA) followed by a post-hoc Tukey test.
3. Results
3.1. Proteasome inhibition increases the amount of phospho-CREB (pCREB) in the CA1 region in response to subthreshold stimulation to induce LTP
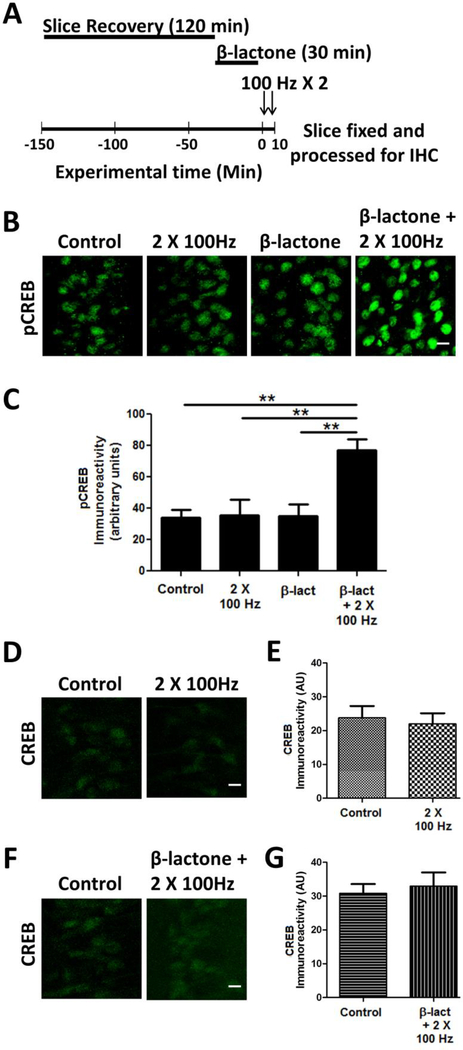
Phosphorylation of CREB on Ser-133 is thought to integrate signaling by multiple kinases to induce gene expression underlying long-term synaptic plasticity [12, 13]. To test the role of proteasome-mediated proteolysis in regulating CREB phosphorylation, we prepared the hippocampal slices and after recovery incubated them in 25 μM clasto lactacystin β-lactone (henceforth β-lactone), an irreversible proteasome inhibitor. We subjected the β-lactone-treated and untreated slices to a subthreshold LTP stimulation protocol (2 × 100 Hz; henceforth referred to as the 2-train protocol). We then examined pCREB levels using immunohistochemistry with an antibody specific for phospho-Ser-133 (Fig. 1A). We found that neither the subthreshold LTP stimulation by itself did cause any significant changes in pCREB immunoreactivity nor did β-lactone alone led to an increase in pCREB immunoreactivity without any stimulation. When the 2-train stimulation and β-lactone were combined, however, we observed a significant increase in pCREB immunoreactivity (Control: 34.2 ± 5.0; 2 × 100 Hz: 35.6 ± 9.7; β-lactone + 2 × 100 Hz: 77.2 ± 6.8; p < 0.01; F(3, 16) = 8.028; n = 5) (Fig. 1 B, C).
Figure 1.
β-lactone treatment increases CREB phosphorylation (pCREB).
(A) Schematic outline of the experiment: after the recovery period of 120 min, hippocampal slices were treated with β-lactone for 30 min. The beginning of electrophysiological stimulation is designated as 0 min at which point the slices were subjected to subthreshold LTP induction (2 × 100 Hz). (B) Confocal images of pCREB immunoreactivities in the CA1 region of hippocampal slices without any treatment (control) or after subthreshold LTP induction (2 × 100 Hz) alone, after β-lactone treatment alone, after β-lactone treatment followed by subthreshold LTP induction (β-lactone + 2 × 100 Hz). Scale bars: 20 μm. (D) Quantification of pCREB immunoreactivities shows that β-lactone treatment significantly increases CREB phosphorylation. (D) CREB immunoreactivities in the CA1 region with no treatment (control) or after subthreshold LTP induction (2 × 100 Hz). (F) CREB immunoreactivities in the CA1 region with no treatment (control) or after β-lactone treatment followed by subthreshold LTP induction (β-lactone + 2 × 100 Hz). (E, G) Quantification of CREB immunoreactivities of results shown in panels D & F. **p<0.01 comparison between two groups as indicated by horizontal lines.
To ascertain that the enhanced pCREB immunoreactivity was due to an increase in the amount of phosphorylated CREB rather than an increase in the amount of CREB protein, we carried out immunohistochemistry with anti-CREB antibodies after subjecting the hippocampal slices to the 2-train protocol with or without prior treatment with β-lactone. We did not observe any significant changes in the anti-CREB immunoreactivity (Fig. 1 D, E, F, G) suggesting that there was indeed increase in pCREB amounts.
3.2. Proteasome-inhibition-mediated increase in pCREB depends on PKA
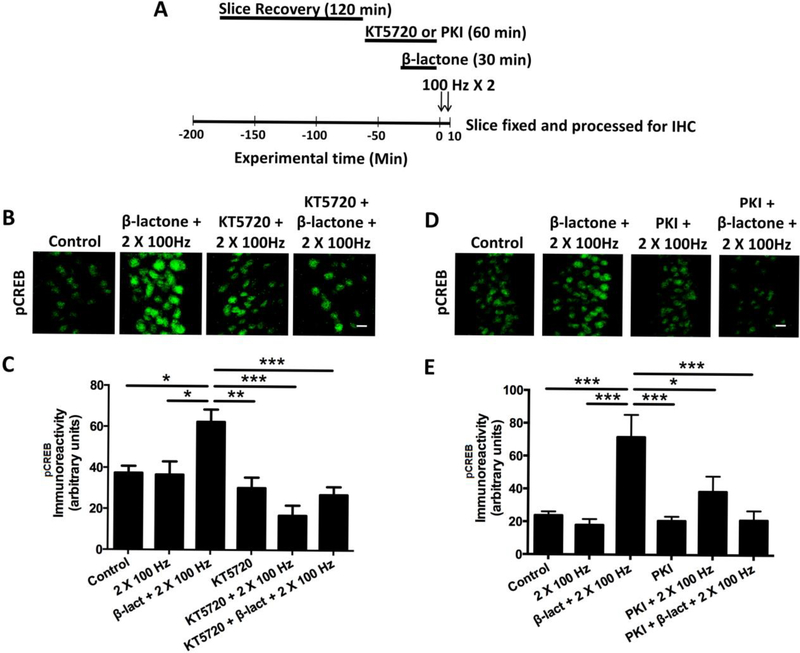
Previous research by many others has shown that PKA is one of main kinases that leads to the phosphorylation of CREB in the nucleus [14]. To test whether PKA plays a role in the enhancement of CREB phosphorylation mediated by proteasome inhibition, we stimulated hippocampal slices with the 2-train LTP protocol after treatment with β-lactone alone or in combination with a PKA inhibitor, KT5720 (1 μM) (Fig. 2 A). As before, β-lactone-treated slices showed a marked increase in CREB phosphorylation whereas in slices that were treated with KT5720 prior to β-lactone exposure, the pCREB immunoreactivity was significantly lower (Fig. 2 B, C) (Control: 37.4 ± 3.4; β-lactone + 2 × 100 Hz: 62.4 ± 6.0; β-lactone + KT5720 + 2 × 100 Hz: 27.0 ± 3.9; p < 0.0001; F(5, 30) = 9.12; n = 6).
Figure 2.
Pretreatment with PKA inhibitors, KT5720 and PKI, blocks β-lactone-mediated enhancement in amounts of pCREB.
(A) Schematic outline of the experiment: after the recovery period of 120 min, hippocampal slices were treated with a PKA inhibitor (KT5720 or PKI) for 60 min. For the last 30 min of the PKA inhibitor treatment, β-lactone was added to the incubation solution. After chemical treatment, slices were transferred to the recording chamber and the slices were subjected to the 2 × 100 Hz protocol. Slices were then fixed and processed for immunohistochemistry. (B, D) Confocal images of pCREB immunoreactivities in the CA1 region of hippocampal slices without any treatment (control) or after β-lactone treatment followed by subthreshold LTP induction (β-lactone + 2 × 100 Hz), after PKA inhibitor treatment followed by subthreshold LTP induction (KT5720 or PKI + 2 × 100 Hz), or after PKA inhibitor and β-lactone pretreatment followed by subthreshold LTP induction (KT570 or PKI + β-lactone + 2 × 100 Hz). Scale bars: 20 μm. (C, E) Quantification of pCREB immunoreactivities shows that PKA inhibitor pretreatment blocks the β-lactone-mediated enhancement of CREB phosphorylation after subthreshold LTP. *p<0.05, **p<0.01, ***p<0.001 comparison between two groups as indicated by horizontal lines.
Next, we tested the effect of Myristoylated PKI (200 nM), which is a membrane-permeant peptide that specially inhibits PKA [15]. We found that treatment with PKI before β-lactone treatment greatly reduced pCREB immunoreactivity compared to treatment with β-lactone alone (Fig. 2 D, E) (Control: 23.8 ± 2.3; β-lactone + 2 × 100 Hz: 71.7 ± 11.5; β-lactone + PKI + 2 × 100 Hz: 38.5 ± 8.0; p < 0.0001; F(5, 42) = 9.79; n = 6).
3.3. MAP kinase and PKG play a role in proteasome-inhibition-mediated increase in pCREB
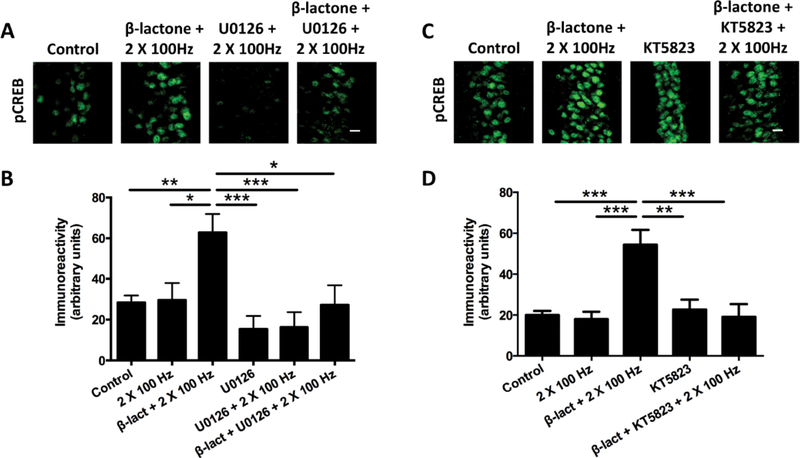
Signaling from synapses to the nucleus that increases phospho-CREB amounts during development of L-LTP does not depend just on PKA. Rather, previous research suggests that there is cross-talk between kinases. MAP kinase (ERK) is one of the main kinases implicated in signal transduction to the nucleus in hippocampal neurons [16]. To test the role for MAP kinase, we carried out the subthreshold L-LTP protocol after pre-treatment of the hippocampal slices with β-lactone or with the MAP kinase inhibitor U0126 (20 μM) followed by application of β-lactone. Enhancement in pCREB immunoreactivity was much lower with ‘U0126 + β-lactone’ compared to treatment with β-lactone alone (Fig 3 A, B) (Control: 28.3 ± 3.4; β-lactone + 2 × 100 Hz: 62.8 ± 9.1; U0126 + β-lactone + 2 × 100 Hz: 23.6 ± 9.6; p < 0.001; F(5, 39) = 5.49; n = 6).
Figure 3.
Pretreatment with inhibitors of ERK (U0126) and PKG (KT5823) blocks β-lactone-mediated pCREB enhancement.
(A) Confocal images of pCREB immunoreactivities in the CA1 region of hippocampal slices without any treatment (control) or after β-lactone treatment followed by subthreshold LTP induction (β-lactone + 2 × 100 Hz), after ERK inhibitor treatment followed by subthreshold LTP induction (U0126 + 2 × 100 Hz), and after ERK inhibitor and β-lactone treatment followed subthreshold LTP induction (β-lactone + U0126 + 2 × 100 Hz). Scale bars: 20 μm. (B) Quantification of pCREB immunoreactivities shows that ERK inhibitor pretreatment blocks the β-lactone-mediated enhancement of CREB phosphorylation after subthreshold LTP. *p<0.05, **p<0.01, ***p<0.001 comparison between two groups as indicated by horizontal lines. (C) Confocal images of pCREB immunoreactivities in the CA1 region of hippocampal slices in without any treatment (control) or after β-lactone treatment followed by subthreshold LTP induction (β-lactone + 2 × 100 Hz), after PKG inhibitor treatment followed by subthreshold LTP induction (KT5823 + 2 × 100 Hz), and after PKG inhibitor and β-lactone treatment followed by subthreshold LTP (β-lactone + KT5823 + 2 × 100 Hz). Scale bars: 20 μm. (D) Quantification of pCREB immunoreactivities shows that PKG inhibitor pretreatment blocks the β-lactone-mediated enhancement of CREB phosphorylation after subthreshold LTP. *p<0.05, **p<0.01, ***p<0.001 comparison between two groups as indicated by horizontal lines.
Another protein kinase that is known to have a role in signaling to the nucleus in hippocampal neurons is PKG [17]. Previous work showed that PKG phosphorylates CREB and increases CREB-mediated transcription in neurons [18]. We tested the possible role of PKG by prior treatment of the slices with a PKG inhibitor, KT5823 (2 μM), before treating with β-lactone. We found that phospho-CREB immunoreactivity was significantly lower when β-lactone-treatment was preceded by application of KT5823 (Fig. 3 C, D) (Control: 19.7 ± 2.0; β-lactone + 2 × 100 Hz: 54.3 ± 7.3; KT5823 + β-lactone + 2 × 100 Hz: 19.1 ± 6.2; p < 0.0001; F(4,37) = 11.29; n = 5).
4. Discussion
As a result of many studies, the role of protein degradation by the UPP in synaptic plasticity is now firmly established [5, 19]. It has also been clear that the role of proteolysis in synaptic plasticity is complex and exact mechanistic functions of the UPP remain to be understood. Our previous work showed differential roles for proteasome-mediated degradation in different parts of the neuron [11]. In dendrites, proteasome limits accumulation of locally translated proteins and therefore proteasome inhibition boosts the early induction part of L-LTP [11, 20]. In contrast, the proteasome facilitates transcription in the nucleus by degrading the negative regulators of transcription such as ATF4 and possibly through modulation of histone modification. Proteasome inhibition leads to blockade of transcription and inhibition of the late, maintenance phase of L-LTP [11].
The present study began as an attempt to test whether proteasome inhibition blocks signaling to the nucleus and contributes to blockade of transcription, which we had observed earlier [11]. To assess signaling to the nucleus, we measured accumulation of phospho-Ser-133 CREB (pCREB) in the nuclei in the CA1 region of the hippocampus. Previous studies by others have established that the 4-train protocol (4 × 100 Hz, 5 min apart) causes an increase in nuclear pCREB [17]. Our pilot studies also showed an increase in pCREB when the 4-train protocol (4 × 100 Hz, 5 min apart) was used to induce L-LTP, but prior proteasome inhibition with β-lactone revealed no adverse effect on quantities of pCREB (data not shown). Therefore, we concluded that hindrance of signaling to the nucleus is not likely to be the reason for the decrease in transcription that we had previously observed with proteasome inhibition [11]. During the course of investigation, we tested whether proteasome inhibition can enhance pCREB levels. With subthreshold 2-train stimulation protocol (2 × 100 Hz, 5 min apart), we consistently found an increase in nuclear pCREB levels in the presence of a highly specific proteasome inhibitor, β-lactone. Our data also show that under these conditions, the amount to total CREB is not changed. Although CREB is known to be a substrate for the proteasome based on the studies in non-neuronal cells [21, 22], in the induction stage of subthreshold L-LTP perhaps it is not phosphorylated within the DSVDTS motif (which is separate from the sequence within which Ser-133 resides) that targets it to the proteasome. It is likely that initially, when conditions are adequate to induce gene expression through CREB in hippocampal neurons, CREB is phosphorylated on Ser-133, which leads to transcriptional activation. Once transcription is completed, CREB is phosphorylated on a serine residue within the DSVTDS motif and is degraded by the proteasome. The time window for transcription required for L-LTP maintenance is 2 hours [23]. Therefore CREB degradation, if it were to occur, would happen at much later time point than we investigated. In addition, it is possible that CREB degradation does not occur under subthreshold L-LTP conditions. Therefore, it is unlikely that proteasome inhibition is stabilizing CREB leading to an increase in pCREB.
Another possibility for increase in pCREB is that blockade of the proteasome prevents degradation of pCREB. In other words, if basal pCREB were to turn over rapidly, in principle, β-lactone could prevent this from happening and thus cause accumulation of pCREB. This is also highly unlikely because inhibiting protein kinases such as PKA, ERK and PKG prior to proteasome inhibition blocks the increase in pCREB caused by proteasome inhibition. Therefore, it is highly likely that proteasome inhibition is enhancing signaling to the nucleus by PKA, ERK and PKG. Whether these kinases act in series or parallel is not known and will have to await detailed future investigations. It must be noted that we chose not to test another kinase implicated in L-LTP, namely calcium-calmodulin-dependent kinase IV [24] because at present drugs that only inhibit CaMKIV (and not other CaM kinases such as CaMKII) are not available.
How does proteasome inhibition cause an increase in nuclear signaling? The most likely possibility is that proteasome normally limits one or more components of the pathway(s) that links NMDA receptor activation to phosphorylation of CREB. These components could be neurotransmitter receptors themselves or any of the downstream components of the kinase signaling cascade.
Proteasome could in principle limit signaling to the nucleus by limiting accumulation of a critical signaling protein that is locally translated in dendrites. Although this scenario is possible, we think the simplest mechanistic explanation for our results is that proteasome inhibition stabilizes AMPA and NMDA receptors. Stabilizing the AMPA receptor should facilitate opening of NMDA receptor channels and stabilization of the NMDA receptors should further enhance signaling to the nucleus via a cascade initiated by the elevation of Ca2+ entering through the NMDA receptor channels.
Both AMPARs and NMDARs are known to be degraded by the UPP [25, 26]. The number of AMPARs in the plasma membrane is determined by the rate of its turnover. AMPARs are endocytosed in after attachment of ubiquitin. It is thought that linkage to one, two, or three ubiquitin molecules traffics the endocytosed AMPARs to the lysosome whereas attachment of four or more ubiquitin molecules (polyubiquitination) causes AMPARs to be degraded by the proteasome [27]. Polyubiquitination of AMPARs can be reversed by the action of two deubiquitinating enzymes USP8 and USP46 [28, 29]. Removal of polyubiquitin tag caused the AMPARs to be recycled back to the plasma membrane which increases the overall number of the AMPARs in the postsynaptic membrane. We favor the idea that in the presence of a proteasome inhibitor, the 2-train protocol leads to insertion of additional AMPARs in the postsynaptic membrane thus setting the stage for an increase in NMDAR-mediated signaling.
NMDARs are known to be targets of ubiquitin-proteasome-mediated proteolysis. NMDARs are retrotranslocated and degraded by the UPP in an activity-dependent fashion [30]. Ubiquitination of the NR1 subunit of NMDARs by an F-box protein called Fbx2 is critical for this process [30] suggesting that an SCF-type ligase targets the NR1 subunits for ubiquitination. Subsequent studies showed that another NMDAR subunit NR2B is targeted for ubiquitination by an E3 ligase called Mind bomb-2 in a phosphorylation-dependent manner [31].
Based on our results, we envisage the following scenario. In the absence of adequate stimulation, the turnover of both AMPARs and NMDARs is high and thus the signaling to the nucleus is weak. Therefore, CREB phosphorylation occurs at basal levels. This situation is mimicked by our 2-train protocol. With sufficient stimulation, however, the AMPARs and NMDARs are stabilized and signaling to the nucleus is strengthened and phosphorylation of CREB is increased. Experimentally this situation occurs with the 4 × 100 Hz protocol, which induces L-LTP, which depends on transcription mediated by CREB. With prior proteasome inhibition (as with application of β-lactone in this study) in hippocampal slices AMPARs and NMDARs should be stabilized and even a weak subthreshold (2 × 100 Hz) stimulation should increase CREB phosphorylation which indeed is what we observed. Although we have focused on phosphorylation of CREB in this study, proteasome inhibition may also affect phosphorylation of other transcription factors.
It is possible that proteasome inhibition also boosts signaling to the nucleus by having an effect directly on the kinases. All the three kinases we tested PKA, ERK and PKG have been implicated in L-LTP [32–35]. In addition, cross-talk between PKA and ERK [16] as well as PKG and ERK [17] have been reported. Therefore, at present no simple explanation exists as to how proteasome blockade might enhance kinase function and therefore must await future research.
5. Conclusion
Our investigations suggest a hitherto unknown role for proteasome-mediated proteolysis, namely, regulation of signaling for CREB-phosphorylation in the nucleus. These initial results form the basis for further exploration of proteasome’s role which might include controlling nuclear signaling to modulate other transcription factors.
HIGHLIGHTS.
We studied proteasomal regulation of nuclear signaling in synaptic plasticity.
We used subthreshold long-term potentiation as our experimental model.
We examined phosphorylation of cAMP-responsive element binding protein (CREB).
Inhibition of the proteasome increased phosphorylated CREB (pCREB) in the nucleus.
Blocking plasticity-related protein kinases prevented increase in pCREB.
Acknowledgements
This work was supported by a grants to A.N.H. from National Institute of Neurological Disease and Stroke (NINDS) (NS066583 & NS 098405) and startup funds from Georgia College and State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sossin WS, Defining memories by their distinct molecular traces, Trends Neurosci, 31 (2008) 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Byrne JH, Baxter DA, Buonomano DV, Cleary LJ, Eskin A, Goldsmith JR, McClendon E, Nazif FA, Noel F, Scholz KP, Neural and molecular bases of nonassociative and associative learning in Aplysia, Ann. N. Y. Acad. Sci, 627 (1991) 124–149. [DOI] [PubMed] [Google Scholar]
- [3].Jarome TJ, Helmstetter FJ, Protein degradation and protein synthesis in long-term memory formation, Front Mol Neurosci, 7 (2014) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fioravante D, Byrne JH, Protein degradation and memory formation, Brain Res. Bull, 85 (2011) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hegde AN, Proteolysis, synaptic plasticity and memory, Neurobiol Learn Mem, 138 (2017) 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Glickman MH, Ciechanover A, The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction, Physiol Rev, 82 (2002) 373–428. [DOI] [PubMed] [Google Scholar]
- [7].Shabek N, Herman-Bachinsky Y, Buchsbaum S, Lewinson O, Haj-Yahya M, Hejjaoui M, Lashuel HA, Sommer T, Brik A, Ciechanover A, The size of the proteasomal substrate determines whether its degradation will be mediated by mono- or polyubiquitylation, Mol Cell, 48 (2012) 87–97. [DOI] [PubMed] [Google Scholar]
- [8].Braten O, Livneh I, Ziv T, Admon A, Kehat I, Caspi LH, Gonen H, Bercovich B, Godzik A, Jahandideh S, Jaroszewski L, Sommer T, Kwon YT, Guharoy M, Tompa P, Ciechanover A, Numerous proteins with unique characteristics are degraded by the 26S proteasome following monoubiquitination, Proc Natl Acad Sci U S A, 113 (2016) E4639–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ronai ZA, Monoubiquitination in proteasomal degradation, Proc Natl Acad Sci U S A, 113 (2016) 8894–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Livneh I, Kravtsova-Ivantsiv Y, Braten O, Kwon YT, Ciechanover A, Monoubiquitination joins polyubiquitination as an esteemed proteasomal targeting signal, Bioessays, 39 (2017). [DOI] [PubMed] [Google Scholar]
- [11].Dong C, Upadhya SC, Ding L, Smith TK, Hegde AN, Proteasome inhibition enhances the induction and impairs the maintenance of late-phase long-term potentiation, Learn. Mem, 15 (2008) 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johannessen M, Delghandi MP, Moens U, What turns CREB on?, Cell Signal, 16 (2004) 1211–1227. [DOI] [PubMed] [Google Scholar]
- [13].Deisseroth K, Mermelstein PG, Xia H, Tsien RW, Signaling from synapse to nucleus: the logic behind the mechanisms, Curr. Opin. Neurobiol, 13 (2003) 354–365. [DOI] [PubMed] [Google Scholar]
- [14].Gonzalez GA, Montminy MR, Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133, Cell, 59 (1989) 675–680. [DOI] [PubMed] [Google Scholar]
- [15].Walsh DA, Glass DB, Utilization of the inhibitor protein of adenosine cyclic monophosphate-dependent protein kinase, and peptides derived from it, as tools to study adenosine cyclic monophosphate-mediated cellular processes, Methods Enzymol, 201 (1991) 304–316. [DOI] [PubMed] [Google Scholar]
- [16].Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR, Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation, Neuron, 21 (1998) 869–883. [DOI] [PubMed] [Google Scholar]
- [17].Lu YF, Kandel ER, Hawkins RD, Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus, J Neurosci, 19 (1999) 10250–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Paul C, Stratil C, Hofmann F, Kleppisch T, cGMP-dependent protein kinase type I promotes CREB/CRE-mediated gene expression in neurons of the lateral amygdala, Neurosci Lett, 473 (2010) 82–86. [DOI] [PubMed] [Google Scholar]
- [19].Jarome TJ, Helmstetter FJ, The ubiquitin-proteasome system as a critical regulator of synaptic plasticity and long-term memory formation, Neurobiol Learn Mem, 105 (2013) 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dong C, Bach SV, Haynes KA, Hegde AN, Proteasome modulates positive and negative translational regulators in long-term synaptic plasticity, J Neurosci, 34 (2014) 3171–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Taylor CT, Furuta GT, Synnestvedt K, Colgan SP, Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteasome pathway in hypoxia, Proc Natl Acad Sci U S A, 97 (2000) 12091–12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Costes S, Vandewalle B, Tourrel-Cuzin C, Broca C, Linck N, Bertrand G, Kerr-Conte J, Portha B, Pattou F, Bockaert J, Dalle S, Degradation of cAMP-responsive element-binding protein by the ubiquitin-proteasome pathway contributes to glucotoxicity in beta-cells and human pancreatic islets, Diabetes, 58 (2009) 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nguyen PV, Abel T, Kandel ER, Requirement of a critical period of transcription for induction of a late phase of LTP, Science, 265 (1994) 1104–1107. [DOI] [PubMed] [Google Scholar]
- [24].Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S, An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory, Cell, 106 (2001) 771–783. [DOI] [PubMed] [Google Scholar]
- [25].Goo MS, Scudder SL, Patrick GN, Ubiquitin-dependent trafficking and turnover of ionotropic glutamate receptors, Front Mol Neurosci, 8 (2015) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lussier MP, Sanz-Clemente A, Roche KW, Dynamic Regulation of N-Methyl-daspartate (NMDA) and alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications, J Biol Chem, 290 (2015) 28596–28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Clague MJ, Urbe S, Ubiquitin: same molecule, different degradation pathways, Cell, 143 (2010) 682–685. [DOI] [PubMed] [Google Scholar]
- [28].Scudder SL, Goo MS, Cartier AE, Molteni A, Schwarz LA, Wright R, Patrick GN, Synaptic strength is bidirectionally controlled by opposing activity-dependent regulation of Nedd4–1 and USP8, J Neurosci, 34 (2014) 16637–16649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huo Y, Khatri N, Hou Q, Gilbert J, Wang G, Man HY, The deubiquitinating enzyme USP46 regulates AMPA receptor ubiquitination and trafficking, J Neurochem, 134 (2015) 1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kato A, Rouach N, Nicoll RA, Bredt DS, Activity-dependent NMDA receptor degradation mediated by retrotranslocation and ubiquitination, Proceedings of the National Academy of Sciences USA, 102 (2005) 5600–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jurd R, Thornton C, Wang J, Luong K, Phamluong K, Kharazia V, Gibb SL, Ron D, Mind bomb-2 is an E3 ligase that ubiquitinates the N-methyl-D-aspartate receptor NR2B subunit in a phosphorylation-dependent manner, J Biol Chem, 283 (2008) 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Frey U, Huang YY, Kandel ER, Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons, Science, 260 (1993) 1661–1664. [DOI] [PubMed] [Google Scholar]
- [33].Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR, Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus, Neuron, 16 (1996) 973–982. [DOI] [PubMed] [Google Scholar]
- [34].Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R, Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory, Cell, 88 (1997) 615–626. [DOI] [PubMed] [Google Scholar]
- [35].Kelleher RJ III, Govindarajan A, Jung HY, Kang H, Tonegawa S, Translational control by MAPK signaling in long-term synaptic plasticity and memory, Cell, 116 (2004) 467–479. [DOI] [PubMed] [Google Scholar]