Abstract
Background:
Monthly use of the dapivirine vaginal ring has been shown to be safe and effective for HIV-1 prevention in nonpregnant reproductive-aged women. The impact of dapivirine on pregnancy outcomes and infant is not known. We compared pregnancy incidence and outcomes by study arm among HIV-1 uninfected women who became pregnant while participating in MTN-020/ASPIRE.
Methods:
ASPIRE was a randomized, double-blind, placebo controlled phase III safety and effectiveness study of the dapivirine ring for HIV-1 prevention. Sexually active women aged 18–45 from Malawi, South Africa, Uganda, and Zimbabwe were enrolled. Urine pregnancy tests were performed monthly and, if positive, study product was withheld during pregnancy and breastfeeding. Pregnancy-related outcomes included: pregnancy incidence, pregnancy outcomes (live birth, preterm birth, pregnancy loss, congenital anomalies), and infant growth.
Results:
Of 2,629 women enrolled in ASPIRE, 169 became pregnant during follow-up, resulting in 179 incident pregnancies and 181 pregnancy outcomes. No difference in pregnancy incidence by study arm was observed (hazard ratio=0.93; 95% CI 0.68–1.26). The distribution of pregnancy outcomes was similar by study arm, and no difference was noted in the frequency or pattern of congenital anomalies or infant growth parameters by study arm.
Conclusion:
Dapivirine use in the periconception period does not appear to be associated with adverse effects on pregnancy or infant outcomes. Our findings provide support for additional safety studies of the dapivirine ring throughout pregnancy.
Keywords: Dapivirine, pregnancy, HIV prevention, women, Africa
INTRODUCTION
Reproductive-aged women are disproportionately affected by HIV-1, accounting for 56% of adults living with HIV-1 in Sub-Saharan Africa [1]. In addition, pregnancy is a time of heightened HIV-1 risk and women in Sub-Saharan Africa have some of the highest fertility rates in the world [2, 3]. As a result, reproductive-aged women living in this region represent a key target population for HIV-1 prevention interventions. As new biomedical HIV-1 prevention interventions prove efficacious, it is critical to evaluate the safety of these tools in pregnancy, in part so that country regulatory authorities have data to inform conditions of licensure, including for pregnant women.
A vaginal ring containing dapivirine, a novel non-nucleoside reverse transcriptase inhibitor (NNRTI), was well-tolerated and reduced HIV-1 incidence in two phase III randomized trials [4, 5] and represents a promising new HIV-1 prevention technology for reproductive-aged women. Data from animal toxicity studies that evaluated different concentrations of dapivirine vaginal gel, including concentrations substantially higher than the available concentration in the vaginal ring, did not identify any adverse effects on the maternal animals or the developing embryo/fetus [6]. However, the effects of dapivirine exposure in humans during pregnancy are unknown. Despite contraceptive counselling and provision of highly effective contraceptive methods during the conduct of the dapivirine ring trials, pregnancies occurred, resulting in inadvertent exposure to dapivirine during the periconception period. Study product use was stopped once a pregnancy was diagnosed; therefore, investigational product exposure was limited to early pregnancy. The objective of this analysis was to compare pregnancy incidence, pregnancy outcomes, and infant growth among HIV-1 uninfected women randomized to receive the dapivirine ring versus matching placebo in MTN-020/ASPIRE.
METHODS
Study population and procedures
ASPIRE was a phase III, double-blinded, placebo-controlled randomized trial that assessed the safety and effectiveness of the dapivirine vaginal ring for HIV-1 prevention (Clinicaltrials.gov NCT01617096). Detailed study procedures have been described elsewhere [4]. Briefly, 2,629 women from Malawi, South Africa, Uganda, and Zimbabwe were enrolled between 2012 and 2014. Participants were HIV-1 uninfected, between 18 and 45 years of age, not pregnant or breastfeeding, sexually active, and in good health. Use of a highly effective method of contraception at enrollment was a requirement to enroll in the trial. Eligible women were randomly assigned in equal proportions to receive either the dapivirine vaginal ring or placebo ring. Participants provided written informed consent, and applicable local and national ethical and regulatory authorities approved the study protocol.
At enrollment and monthly follow-up visits, standardized face-to-face interviews were conducted to collect data on demographic, clinical, and behavioral characteristics, such as study product use, contraceptive method, reported condom use, and sexual behaviors. Contraceptive methods were provided on-site or could be obtained from local health providers. Women were permitted to change contraceptive method during follow-up. HIV-1 antibody tests were performed monthly and study product was permanently discontinued in the event of HIV-1 seroconversion. Urine β-human chorionic gonadotropin (β-hCG) tests were performed monthly or when clinically indicated. To monitor adherence to study product during the trial, plasma samples were collected quarterly and were tested for dapivirine using a validated liquid chromatography–mass spectrometry assay [4, 7].
All participants were counseled at enrollment regarding that the effect of exposure to the study drugs in pregnancy, including the effect of the study drug on the fetuses of women who use the vaginal ring when pregnant, is uknown. Participants who became pregnant during follow-up were referred to local health services for further care depending on her pregnancy intentions. Study product was withheld for the duration of pregnancy and breastfeeding, and participants whose pregnancies resulted in a live birth were counseled on the benefits of infant breastfeeding in accordance with WHO recommendations and local guidelines [8]. All pregnancies were followed until an outcome could be ascertained. Pregnancy outcomes and infant congenital anomalies identified at the time of delivery were determined by participant report and medical record review, when available, and recorded on case report forms. Product use was resumed after the pregnancy outcome, as evidenced by a negative pregnancy test performed by study staff, and after complete cessation of breastfeeding, as reported by the participant.
Participants who became pregnant in ASPIRE were invited to enroll in MTN-016, a prospective open cohort study designed to assess whether exposure to investigational antiretroviral-based HIV prevention agents impacts pregnancy and infant outcomes during the first year of life [9]. Separate written informed consent was provided for participation in MTN-016. Infants were first assessed within 10 days of birth and followed for one year with follow-up visits at months 1, 6 and 12. Growth was assessed by study staff using serial measurements of length, weight, head/abdominal circumference, and the WHO growth charts at all scheduled study visits [10].
Statistical analysis
Women who reported a bilateral tubal ligation at enrollment, were found to be HIV-1 infected at enrollment, or did not return for follow-up were excluded from the present analysis. In this analysis, pregnancy was defined by a single positive urine pregnancy test. Pregnancy incidence by arm was calculated per 100 person-years of follow-up using Poisson modeling and compared using an Andersen-Gill proportional hazards model with censoring at HIV-1 infection (date of first positive HIV-1 rapid test). Gestational age and estimated date of delivery were assigned using several methods, which may have been used in combination, including date of last menstrual period, ultrasound, or physical examination. The prevalence of each pregnancy outcome and congenital anomalies were summarized by arm using descriptive statistics and compared using Fisher’s exact test. Infant growth parameters were standardized using the WHO Z-score [10]. Two-sample t-tests and linear mixed-effects models with fixed effects for time (age in years, modeled as a flexible restricted cubic spline), and infant gender, were constructed to compare sex-adjusted Z-scores for each growth parameter by study arm of the mother.
A sensitivity analysis was performed categorizing participants in the dapivirine arm based on plasma concentrations of dapivirine in plasma, collected at quarterly visits. Participants with plasma dapivirine levels >95 pg/mL were considered to have had recently used the ring (a level which corresponds to approximately 8 hours of continuous ring use) [4, 7]. Pregnancy and infant outcomes were compared between participants with recent ring use based on the plasma sample at pregnancy detection (or within 6 months prior to pregnancy detection) compared to participants with no recent ring use (≤ 95 pg/mL dapivirine detected in plasma) and participants in the placebo arm using the same methods described above. All analyses were performed using R, version 3.2 (Vienna, Austria).
RESULTS
Population characteristics
Of 2,629 women enrolled in ASPIRE, 78 reported tubal ligation at enrollment, three were determined to be HIV-1 infected at enrollment, and 12 had no follow-up. Therefore, our analysis population included 2,536 women, of whom 1,263 (50%) were assigned to the dapivirine arm and 1,273 (50%) assigned to the placebo arm (Figure 1). There were 179 incident pregnancies among 169 women. Among the 179 pregnancies, 175 pregnancies resulted in a single outcome, 3 pregnancies resulted in two outcomes (twins), and 1 pregnancy had no outcome available, resulting in a total of 181 pregnancy outcomes. Of the 169 women who became pregnant, the median age at enrollment in ASPIRE was 23 years (interquartile range [IQR] 21–27), median number of live births was 2 (IQR 1–2), 65 (38%) were married, and 94 (56%) had secondary education or greater (Table 1).
Figure 1.
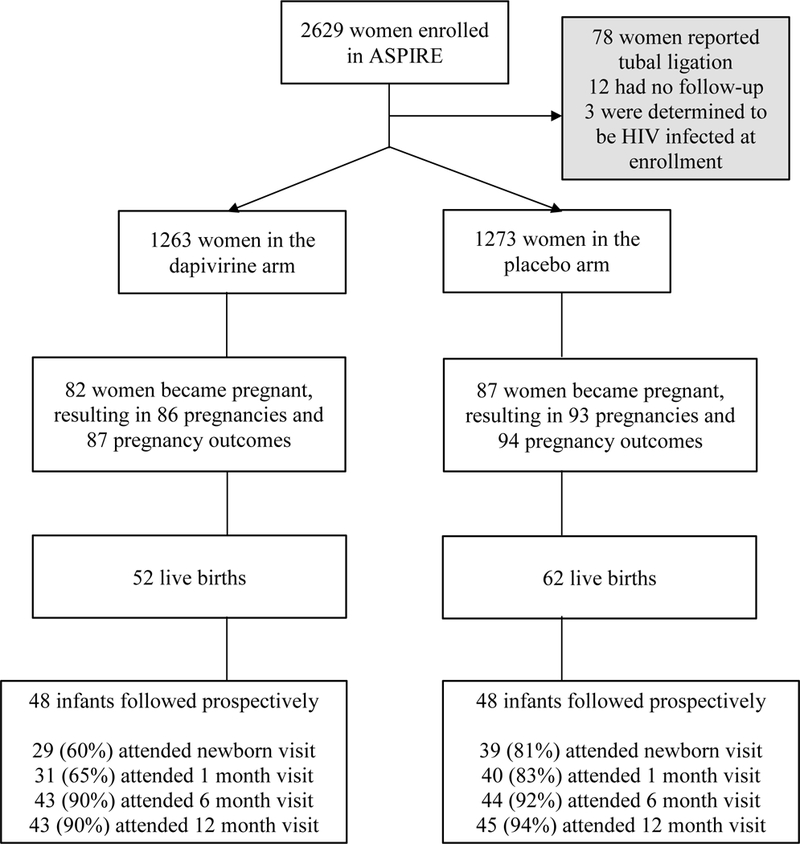
Analysis Population
Table 1.
Baseline characteristics of women who became pregnant in ASPIRE*
| Became pregnant during follow-up | Did not become pregnant during follow-up | |||
|---|---|---|---|---|
| Placebo | Dapivirine | Overall | ||
| n=87 | n=82 | N=169 | N=2,367 | |
| Age | 24 (21, 27) | 23 (20, 27) | 23 (21, 27) | 26 (22, 31) |
| Married | 36 (41%) | 29 (35%) | 65 (38%) | 962 (40%) |
| Secondary education or greater | 50 (57%) | 44 (54%) | 94 (56%) | 1,093 (46%) |
| Number of live births | 2 (1, 2) | 1 (1, 2) | 2 (1, 2) | 2 (1, 3) |
| Country: Malawi | 6 (7%) | 3 (4%) | 9 (5%) | 238 (10%) |
| South Africa | 43 (49%) | 47 (57%) | 90 (53%) | 1,293 (54%) |
| Uganda | 12 (14%) | 13 (16%) | 25 (15%) | 221 (9%) |
| Zimbabwe | 26 (30%) | 19 (23%) | 45 (27%) | 630 (26%) |
| Condom used at last sex act | 56 (64%) | 51 (62%) | 107 (64%) | 1,366 (57%) |
| Any curable STI at enrollment1 | 11 (13%) | 24 (29%) | 35 (21%) | 501 (21%) |
Data presented as N (%) or median (interquartile range)
STI = sexually transmitted infection; this included C. trachomatis, N. gonorroheae, T. vaginalis or syphilis. STIs were treated per local guidelines.
Pregnancy incidence and outcomes
Overall, we observed 179 pregnancies during 4,334 person-years of follow-up (incidence=4.1 per 100 person-years; 95% confidence interval [CI] 3.5, 4.9). There were 86 pregnancies during 2,162 person years of follow-up in the dapivirine arm (incidence=4.0 per 100 person-years; 95% CI 3.1, 5.1) and 93 pregnancies during 2,172 person-years of follow-up in the placebo arm (incidence = 4.3 per 100 person-years; 95% CI 3.4–5.5), with no difference in pregnancy incidence by study arm (Table 2). Median gestational age at pregnancy detection was 5.4 weeks (interquartile range [IQR] 4.3, 6.8), which also did not differ by arm (dapivirine arm = 5.4 weeks; placebo arm = 5.6 weeks). The proportion of pregnancies by country generally mirrored the proportion of participants enrolled in ASPIRE from the participating countries, with 53% of pregnancies occurring among participants from South Africa, 27% from Zimbabwe, 15% from Uganda, and 5% from Malawi.
Table 2.
Pregnancy incidence and outcomes by study arm
| Placebo | Dapivirine | Overall | |||||
|---|---|---|---|---|---|---|---|
| Pregnancy incidence | |||||||
| Number of pregnancies | 93 | 86 | 179 | ||||
| Pregnancy incidence (95% CI)* | 4.3 | (3.4–5.5) | 4.0 | (3.1–5.1) | 4.1 | (3.5–4.9) | |
| Pregnancy outcomes | N=94** | N=87 | N=181** | ||||
| Full term birth | 53 | (56%) | 52 | (60%) | 105 | (58%) | |
| Preterm birth | 9 | (10%) | 0 | (0%) | 9 | (5%) | |
| Stillbirth/intrauterine fetal demise | 2 | (2%) | 2 | (2%) | 4 | (2%) | |
| Spontaneous abortion | 21 | (22%) | 18 | (21%) | 39 | (22%) | |
| Therapeutic/elective abortion | 8 | (9%) | 14 | (16%) | 22 | (12%) | |
| Ectopic pregnancy | 1 | (1%) | 1 | (1%) | 2 | (1%) | |
| Congenital anomalies | N=59 | N=48 | N=107 | ||||
| Any congenital anomaly | 4 | (7%) | 4 | (8%) | 8 | (7%) | |
Per 100 person-years
175 pregnancies resulted in a single outcome, 3 pregnancies resulted in two outcomes (twins), and 1 pregnancy had no outcome available, resulting in a total of 181 pregnancy outcomes.
The distribution of pregnancy outcomes by arm is presented in Table 2. Of 181 pregnancy outcomes, there were 105 (58%) full term live births, nine (5%) preterm births, 39 (22%) spontaneous abortions, and 22 (12%) elective/therapeutic abortions. There were four stillbirth/intrauterine fetal deaths, two in each arm (2%). The distribution of pregnancy outcomes did not differ by study arm. Of 114 deliveries resulting in a live birth, the majority of deliveries (93%) occurred at a hospital or clinic, with spontaneous vaginal delivery being the most common method of delivery (77%). Caesarean birth occurred in 24 (21%) of deliveries and did not differ by study arm. In sensitivity analyses, no differences were noted by dapivirine drug detection in plasma around the time that pregnancy was detected (Supplemental Table 1).
Infant outcomes and development
Data on congenital anomalies identified around the time of delivery were available for 107 of 114 live births. The frequency and type of reported anomalies by maternal study arm are presented in Table 2. The overall prevalence of anomalies (all structural) was 7% and did not differ by study arm (P > 0.99, Table 3). Ninety-six infants were enrolled into MTN-016 and underwent regular assessments of growth through the first year of life. Overall infant retention was high, with 92% completing the 12-month follow-up visit (Figure 1). Summaries of Z-scores at each study visit and by study arm are presented in Table 4 and Figure 2. Across all visits, we observed no differences in infant weight (mean difference −0.04; 95% CI −0.36, 0.28), length (mean difference −0.20; 95% CI −0.63, 0.28), or head circumference (mean difference 0.15; 95% CI −0.27, 0.57) by study arm, indicating no reductions in infant growth among women with inadvertent exposure to dapivirine in early pregnancy compared to women in the placebo arm. Similar to the pregnancy outcomes analysis, no differences were noted in infant outcomes by dapivirine drug detection in plasma around the time that pregnancy was detected (Supplemental Table 1).
Table 3.
Additional details on reported congenital anomalies
| Participant | Study arm | Pregnancy outcome | Anomaly |
|---|---|---|---|
| 1 | Dapivirine | Full term live birth | Umbilical hernia, which was reducible |
| 2 | Dapivirine | Full term live birth | Micrognathia and epicanthic folds |
| 3 | Dapivirine | Full term live birth | Inguinal hernia which was repaired |
| 4 | Dapivirine | Full term live birth | Cranio-facial (structural) right frontal skull depression positional plagiocephaly |
| 5 | Placebo | Full term live birth | Reducible umbilical hernia, approximately 4cm in diameter |
| 6 | Placebo | Full term live birth | Umbilical hernia, uncomplicated |
| 7 | Placebo | Full term live birth | Umbilical hernia noted, 1.8 by 2cm, reducible and non-tender |
| 8 | Placebo | Full term live birth | Polydactyly both hands (bilateral) |
Table 4.
Standardized infant z-scores for growth measures by study arm and visit
| Measurement | Visit | Placebo | Dapivirine | |||
|---|---|---|---|---|---|---|
| N | mean (SD) | N | mean (SD) | P | ||
| Head circumference-for-age | Newborn | 39 | 0.29 (1.39) | 29 | 0.83 (1.33) | 0.11 |
| Month 1 | 40 | 0.94 (2.08) | 31 | 0.77 (1.39) | 0.68 | |
| Month 6 | 44 | 0.73 (1.47) | 43 | 0.95 (1.56) | 0.51 | |
| Month 12 | 45 | 0.99 (1.12) | 43 | 1.34 (1.86) | 0.30 | |
| Length-for-age | Newborn | 39 | −0.12 (1.58) | 29 | 0.09 (1.47) | 0.56 |
| Month 1 | 40 | −0.14 (1.38) | 31 | −0.30 (1.45) | 0.64 | |
| Month 6 | 44 | −0.25 (1.68) | 43 | −0.37 (1.74) | 0.95 | |
| Month 12 | 45 | −0.16 (1.64) | 43 | −0.23 (1.43) | 0.83 | |
| Weight-for-age | Newborn | 39 | −0.39 (0.92) | 29 | −0.20 (0.84) | 0.37 |
| Month 1 | 40 | −0.11 (0.85) | 31 | −0.47 (1.15) | 0.16 | |
| Month 6 | 44 | 0.24 (1.52) | 43 | 0.11 (1.01) | 0.66 | |
| Month 12 | 45 | −0.00 (1.28) | 43 | 0.14 (1.03) | 0.51 | |
Figure 2.
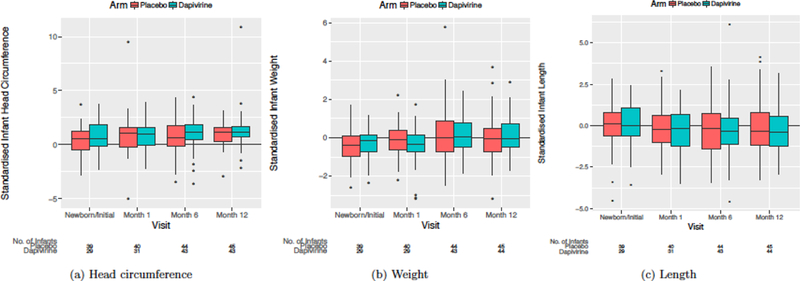
Box plots of standardized infant growth measures by study arm and visit
DISCUSSION
Among women participating in a randomized trial of the dapivirine vaginal ring for HIV-1 prevention, we observed no differences in the incidence of pregnancy between the dapivirine ring and placebo arms, and no adverse effects on pregnancy outcomes, infant congenital anomalies, or infant growth through the first year of life. Although contraception use was a requirement for study eligibility, pregnancies did occur, affording an important opportunity to assess the effect of dapivirine exposure in early pregnancy. To our knowledge, this is the first study to report on pregnancy and infant outcomes among women exposed to the dapivirine vaginal ring during the periconception period. Our findings add to the body of evidence demonstrating the safety of the dapivirine vaginal ring for HIV-1 prevention in reproductive aged women.
The high fertility rate in Sub-Saharan Africa contributes to 25% of pregnancies globally [3]. These pregnancies represent a mix of intended and unintended pregnancies. Pregnancy is a particularly vulnerable time for women in many respects, including a time of increased risk for HIV-1 acquisition [2]. Furthermore, women who acquire HIV-1 during pregnancy are more likely to pass the infection on to their infant during pregnancy or breastfeeding [11, 12]. It is imperative that pregnant women have access to safe and effective methods for HIV-1 prevention. Antiretroviral-based strategies represent a promising approach for HIV-1 prevention. For the vast majority of antiretroviral medications, most of which are used solely for HIV-1 treatment, data on teratogenicity related to in utero exposure are available through the Antiretroviral Pregnancy Registry [13]. Data collected to date are generally reassuring, showing no association between antiretroviral medications used for HIV-1 treatment and teratogenicity [14]. However, some evidence suggests that adverse pregnancy outcomes, such as small for gestational age and preterm birth, may be more common among HIV-infected women using certain antiretroviral regimens [15–17]. In addition, recent observational data regarding a potential association between dolutegravir and risk of neural tube defect underscores the need for continued safety assessments of antiretroviral medications used during pregnancy among both HIV-uninfected and HIV-infected women [18].
At present, oral combination emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) is the only antiretroviral medication approved by national regulatory authorities as prophylaxis for the prevention of HIV-1[19, 20]. Prior to being evaluated as an HIV-1 prevention intervention, these medications were widely used in HIV-1 treatment, including among HIV-1 infected pregnant women. As a result, robust data supports the safety of TDF and FTC/TDF use during pregnancy among HIV-1 infected women [21]. In addition, findings from the Partners PrEP trial showed no difference in pregnancy incidence, pregnancy outcomes, congenital anomalies, or infant growth among Kenyan and Ugandan women exposed to TDF or FTC/TDF during the periconception period compared to placebo [22]. These data combined with existing evidence from HIV-1 treatment studies, led the World Health Organization to recommend that oral PrEP should be offered as an additional prevention choice for pregnant women at substantial risk of HIV infection as part of combination prevention approaches [23], including as part of routine antenatal care [24].
In contrast, dapivirine is a novel NNRTI that is not used for treatment and has limited safety data on use in pregnancy. In addition, the vaginal ring is a new method of antiretroviral delivery. Reassuringly, we observed no impact of dapivirine exposure during the periconception period on any pregnancy or infant outcomes. Data on pregnancy and infant outcomes among women at risk for HIV-1 acquisition in resource limited settings are sparse. The overall frequency of pregnancy outcomes and congenital anomalies observed in ASPIRE were similar to those reported in the Partners PrEP trial (ASPIRE preterm births = 5.0% versus Partners PrEP = 5.7%; ASPIRE pregnancy loss= 36.8% versus Partners PrEP = 33.3%; ASPIRE congenital anomalies = 7.4% versus Partners PrEP = 6.7%). Participants in both ASPIRE and Partners PrEP underwent monthly pregnancy testing using highly sensitive urine β-hCG assays in order to detect pregnancies prior to clinical signs or symptoms in order to limit fetal exposure to study product. As a result of such frequent testing, we identified pregnancies that terminated spontaneously prior to clinical recognition (chemical pregnancies), thus increasing the number of pregnancy losses. However, our results are consistent with prior studies that conducted frequent and sensitive pregnancy monitoring, which reported a pregnancy loss rate of 31% [25].
Of note, our pregnancy incidence was substantially lower than what has been reported in other HIV prevention trials with similar contraceptive requirements for enrollment [26]. Efforts to increase uptake of long acting reversible contraceptive methods contributed to the overall lower pregnancy incidence [27], which did not differ by study arm. As new antiretroviral-based HIV-1 prevention interventions are evaluated, such as injectable cabotegravir [28], it is critical that rigorous and expeditious safety assessments be conducted to ensure that novel antiretroviral-based prevention interventions are safe and effective for use during pregnancy.
Our study includes a number of strengths, including leveraging the randomized controlled trial design to assess outcomes by study arm, infant follow-up through 12 months of life, and high retention in the parent study as well as the prospective cohort for mothers and infants. However, several limitations should be considered when interpreting the results. Dapivirine exposure was limited to the periconception period with a short duration of in utero exposure after conception. Future studies should evaluate use of the dapivirine vaginal ring at different points during gestation to evaluate whether ring use is safe throughout pregnancy. In ASPIRE, we enrolled over 2,000 women; however, the number of pregnancies and live births that occurred was relatively small. As a consequence, there were few congenital anomalies; therefore, results should be interpreted with caution given the small numbers and potential for misclassification based on maternal self-report in cases where medical records were not available. Reassuringly, there were no patterns of anomalies that indicated a potential association with dapivirine ring use.
Safe and effective HIV-1 prevention interventions are urgently needed for women during the full course of their reproductive life, including during pregnancy, when HIV incidence is higher than in non-pregnant women of the same age. The dapivirine vaginal ring represents an exciting new HIV-1 prevention technology and assessing its safety for use in pregnancy is of critical importance. Our findings provide important evidence supporting the safety of dapivirine use in early pregnancy and provide support for additional studies of the dapivirine ring at different gestational ages in order to confirm the safety of dapivirine ring use throughout pregnancy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contributions of the women who participated in MTN-
020/ASPIRE and MTN-016. The authors express their sincere appreciation to the study teams for their dedicated work on community engagement and data and sample collection and to the MTN Statistical and Data Management Center for their work on data management. The dapivirine vaginal ring, which was evaluated in the ASPIRE trial, was developed by the International Partnership for Microbicides.
FUNDING
The Microbicide Trials Network (MTN) is funded by National Institutes of Allery and Infectious Disease (NIAID) (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Institute of Mental Health (NIMH), all components of the US National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
ASPIRE Study Team Leadership: Jared Baeten, University of Washington (Protocol Chair); Thesla Palanee-Phillips, Wits Reproductive Health and HIV Institute (Protocol Co-chair); Elizabeth Brown, Fred Hutchinson Cancer Research Center (Protocol Statistician); Lydia Soto-Torres, US National Institute of Allergy and Infectious Diseases (Medical Officer); Katie Schwartz, FHI 360 (Clinical Research Manager)
Study sites and site Investigators of Record:
Malawi, Blantyre site (Johns Hopkins University, Queen Elizabeth Hospital): Bonus Makanani
Malawi, Lilongwe site (University of North Carolina, Chapel Hill): Francis Martinson
South Africa, Cape Town site (University of Cape Town): Linda-Gail Bekker
South Africa, Durban – Botha’s Hill, Chatsworth, Isipingo, Tongaat, Umkomaas, Verulam sites (South African Medical Research Council): Vaneshree Govender, Samantha Siva, Zakir Gaffoor, Logashvari Naidoo, Arendevi Pather, and Nitesha Jeenarain
South Africa, Durban, eThekwini site (Center for the AIDS Programme for Research in South Africa): Gonasagrie Nair
South Africa, Johannesburg site (Wits RHI): Thesla Palanee-Phillips
Uganda, Kampala site (John Hopkins University, Makerere University): Flavia Matovu
Zimbabwe, Chitungwiza, Seke South and Zengeza sites (University of Zimbabwe College of Health Sciences Clinical Trials Unit): Nyaradzo Mgodi
Zimbabwe, Harare, Spilhaus site (University of Zimbabwe College of Health Sciences Clinical Trials Unit):): Felix Mhlanga
Data management was provided by The Statistical Center for HIV/AIDS Research & Prevention (Fred Hutchinson Cancer Research Center, Seattle, WA) and site laboratory oversight was provided by the Microbicide Trials Network Laboratory Center (Pittsburgh, PA).
POTENTIAL CONFLICTS OF INTEREST
All authors declare no commercial or other associations that might pose a conflict of interest relevant to the submitted work.
REFERENCES
- 1.UNAIDS, The Global AIDS Update. http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf; accessed 15 November 2017, 2016.
- 2.Drake AL, et al. , Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med, 2014. 11(2): p. e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sedgh G, Singh S, and Hussain R, Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann, 2014. 45(3): p. 301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, et al. , Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med, 2016. 375(22): p. 2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nel A, et al. , Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. N Engl J Med, 2016. 375(22): p. 2133–2143. [DOI] [PubMed] [Google Scholar]
- 6.Microbicides I.P.f., Investigator’s Brochure: Dapivirine Vaginal Ring (13 July 2011). 2011. [Google Scholar]
- 7.Seserko LA, et al. , The development and validation of an UHPLC-MS/MS method for the rapid quantification of the antiretroviral agent dapivirine in human plasma. Bioanalysis, 2013. 5(22): p. 2771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO, Infant and young child feeding. http://www.who.int/mediacentre/factsheets/fs342/en/; accessed 15 November 2017, 2017.
- 9.Mhlanga Felix G., et al. , Implementation of a prospective pregnancy registry for antiretroviral based HIV prevention trials HIV Clinical Trials, 2017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO, The WHO Child Growth Standards. . http://www.who.int/childgrowth/en/. Accessed 6 July 2017.
- 11.Birkhead GS, et al. , Acquiring human immunodeficiency virus during pregnancy and mother-to-child transmission in New York: 2002–2006. Obstet Gynecol, 2010. 115(6): p. 1247–55. [DOI] [PubMed] [Google Scholar]
- 12.Taha TE, et al. , Association of recent HIV infection and in-utero HIV-1 transmission. AIDS, 2011. 25(11): p. 1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Registry, A.P., The Antiretrovrial Pregnancy Registry. http://www.apregistry.com/HCP.aspx; accessed 15 November 2017.
- 14.Registry, A.P., Antiretroviral Pregnancy Registry International Interim Report. http://www.apregistry.com/forms/exec-summary.pdf; accessed 15 November 2017, 2017.
- 15.Newell ML and Bunders MJ, Safety of antiretroviral drugs in pregnancy and breastfeeding for mother and child. Curr Opin HIV AIDS, 2013. 8(5): p. 504–10. [DOI] [PubMed] [Google Scholar]
- 16.Mesfin YM, Kibret KT, and Taye A, Is protease inhibitors based antiretroviral therapy during pregnancy associated with an increased risk of preterm birth? Systematic review and a meta-analysis. Reprod Health, 2016. 13: p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zash R, et al. , Comparative Safety of Antiretroviral Treatment Regimens in Pregnancy. JAMA Pediatr, 2017. 171(10): p. e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agency, E.M., New study suggests risk of birth defects in babies born to women on HIV medicine dolutegravir. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2018/05/WC500249225.pdf; accessed 13 August 2018, 2018.
- 19.CDC, Pre-exposure prophylaxis for the prevention of HIV infection in the United States 2014; Clinical providers’ supplement. http://www.cdc.gov/hiv/pdf/prepprovidersupplement2014.pdf; accessed 10 July 2015. [Google Scholar]
- 20.WHO, GUIDANCE ON PRE-EXPOSURE ORAL PROPHYLAXIS (PrEP) FOR SERODISCORDANT COUPLES, MEN AND TRANSGENDER WOMEN WHO HAVE SEX WITH MEN AT HIGH RISK OF HIV. http://apps.who.int/iris/bitstream/10665/75188/1/9789241503884_eng.pdf?ua=1; accessed 11 Novemebr 2017, 2012. [PubMed]
- 21.WHO, CONSOLIDATED GUIDELINES ON THE USE OF ANTIRETROVIRAL DRUGS FOR TREATING AND PREVENTING HIV INFECTION. http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1; accessed 12 November 2017, 2016. [PubMed]
- 22.Mugo NR, et al. , Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. JAMA, 2014. 312(4): p. 362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO, PREVENTING HIV DURING PREGNANCY AND BREASTFEEDING IN THE CONTEXT OF PREP. http://apps.who.int/iris/bitstream/10665/255866/1/WHO-HIV-2017.09-eng.pdf?ua=1; accessed 10 November 2017, 2017.
- 24.WHO, WHO recommendations on antenatal care for a positive pregnancy experience. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/anc-positive-pregnancy-experience/en/; accessed 02 April 2018, 2016. [PubMed]
- 25.Wilcox AJ, et al. , Incidence of early loss of pregnancy. N Engl J Med, 1988. 319(4): p. 189–94. [DOI] [PubMed] [Google Scholar]
- 26.Bunge K, et al. , Pregnancy Incidence and Outcomes in Women Receiving Tenofovir-based PrEP in the VOICE Trial International AIDS Society Conference, Vancouver, Canada, 2015. [Google Scholar]
- 27.Bunge K, et al. , Expanding the Mix of Contraceptive Methods in an HIV Prevention Trial . International AIDS Conference, Durban, South Africa, 2016. [Google Scholar]
- 28.Markowitz M, et al. , Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV, 2017. 4(8): p. e331–e340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


