Abstract
The major purpose of this article is to evaluate oligochitosan coated cerium oxide nanoparticles (OCCNPs) alginate laden injectable hydrogels and their potential treatment for age-related macular degeneration (AMD). The water soluble OCCNPs were loaded within injectable hydrogels as antioxidative agents. The release of OCCNPs from hydrogel, radical scavenging properties, and biocompatibility were evaluated and calculated in vitro. The effects of OCCNP laden hydrogel downregulating expression of angiogenic proteins and pro-inflammatory cytokines were quantified in human retinal pigment epithlium-19 (ARPE-19) and umbilical endothelium cell lines. The hydrogels behaved with moderate swelling and controllable degradation. The laden OCCNPs were released in a controlled manner in vitro during two months of testing. The OCCNP loaded hydrogels exhibited robust antioxidative properties in oxygen radical absorbance capacity tests and reduced apoptosis in H2O2-induced ARPE-19 cells. Furthermore, OCCNP loaded injectable hydrogels are biocompatible and suppressed the LPS-induced inflammation response in ARPE-19 cells, and inhibited expression of vascular endothelium growth factor in human ARPE-19 and umbilical endothelium cell lines. The alginate-gelatin injectable hydrogel loaded OCCNPs are biocompatible and have high potential in protecting cells from apoptosis, angiogenesis, and production of pro-inflammatory cytokines in AMD cellular models.
Keywords: alginate injectable hydrogel, cerium oxide nanoparticle, AMD, cellular models
1. Introduction
Age-related macular degeneration is a leading cause of blindness in the western world, with a prevalence in people over 55 years of age, and the total number of patients is estimated to be 196 million by the year 20201. AMD may be divided into several stages or types. Over 90% of AMD patients remain in the early or intermediate stage (known as dry AMD) which is caused by accumulation of drusen and degeneration of retinal pigment epithelium (RPE) cells, eventually leading to moderate or progressive vision impairment. Less than 10% of patients have acute neovascularization in choroidal (wet) AMD which may result in legal blindness1,2,3,4. The detailed pathophysiology of AMD remains elusive. However, there is growing evidence that both forms (dry and wet) are correlated with the elevated production of reactive oxygen species (ROS) caused by the chronic inflammation, innate immune dysregulation, and aging that release several active mediators, including inflammatory cytokines, chemotactic molecules, and free radicals2,4,5. These factors may significantly raise the oxidative stress level in RPE cells and lead to inadequate removal of ROS as a result of lower antioxidant gene expression that has built up in the lipid waste between RPE layer and Bruch’s membrane. Subsequently, accumulation of abnormal extracellular material external to the RPE is associated with the development of choroidal neovascularization, which almost always damages the photoreceptor cells6–10.
Currently, there is no effective therapy available for dry AMD. There are no FDA-approved drugs for the dry form of AMD. The Age-Related Eye Disease Study (AREDS) was a major clinical trial sponsored by the NEI to investigate the effects of administration of various nutritional supplementation (vitamin C, vitamin E, beta-carotene, zinc and copper)11. Newer formulations (AREDS2) replace beta-carotene with lutein/zeaxanthin in the original AREDS formula to slow the progression to advanced AMD12,13. Although these supplements showed a ~25% beneficial effect in reducing the risk of progression towards advanced dry AMD, the extremely short half-life, weak activity and high dose of the daily amounts correlated with cancer risk have shown mixed results. Recently, the United States Food and Drug Administration (FDA) has approved several anti-vascular endothelium growth factor (VEGF) drugs (e.g., Lucentis, Eylea, Macugen and Avastin)5,8,9. However, VEGF is a signal protein which supports the growth of new blood vessels in a normal human body, and chronic inhibition of VEGF may cause unwanted adverse effects on the retina. Due to the complex and multi-factorial nature of the disease (oxidative stress, inflammation, lipid and carbohydrate metabolism, and genetic risk), rather than a single, direct cause and effect, a single target may not be able to address the issues, but the problems could potentially be resolved if a therapeutic agent had multi-functionalities (e. g. anti-oxidative damage, anti-inflammation, and anti-angiogenesis)2,5,11,13.
As such, the anti-oxidative strategy has been widely investigated as one promising means of treatment and/or prevention of both dry and wet AMD2,4. However, current dietary antioxidant supplements may not effectively postpone or slow the development of dry AMD. Thus, sustained antioxidants were developed to prevent ROS induced damage11. Nanoceria exists in either a +3 (Ce3+, reduced) or +4 (Ce4+, oxidized) oxidative state. This ability for auto-regeneration (to shift oxidation between the two states) is the most important property in terms of application in therapeutics and diagnostics14,15. Nanoceria has been proven to scavenge a variety of super/peroxide and oxygen radicals in metabolism and remained active for an extended period in vivo16,17. Nanoceria may effectively reduce the ROS in a mouse model by upregulating neuro-protective gene expression and reducing the apoptosis of photoreceptors18. Studies have shown that nanoceria was preferentially retained in the retina with the absence of toxicity in a rat model19. There are also studies using nanoceria for anti-inflammatory purposes by reducing free radicals and downregulating correspondence genes, thus finally eradicating the formation of drusen20.
Although nanoceria has been shown to keep catalytic activities, it may be hindered by low aqueous solubility and poorly controlled release. In addition, the outer environment where nanoceria is applied may significantly change the antioxidant properties (acidic environment, etc.)20,21. Recently, we have developed a water-soluble nanoceria22. To guarantee desirable therapeutic effects, a stable local environment with sustained release is preferred. Thus, injectable hydrogels were considered for this application23–25. Injectable hydrogels are in situ formation of crosslinked hydrophilic networks that provide a stable chemical environment with hydrophilicity, and hydrogels may be utilized as delivery vehicles for drugs, cells, genes, and nanoparticles26,27. The sustained release allows continuous delivery and homogeneous distribution of therapeutic payload, thus enhancing the treatment effects. There are a couple of studies on injectable hydrogels for treatment of AMD, using hydrogels to extend the lifespan of anti-VEGF drugs in intraocular delivery and improve the viability of RPE or photoreceptor cells for regeneration28,29, but, to the best of our knowledge, the combination of antioxidant agents and injectable hydrogels as the carrier for AMD have never been studies together before.
Here, we developed an injectable system that utilized natural oligochitosan coated nanoceria (OCCNP) and protein-based hydrogels to perform the studies. The hydrogels are biodegradable, biocompatible, and able to release therapeutic payload in a controllable manner. We chose commonly used retina pigment epithelium 19 (ARPE-19) cells, a human RPE cell line, and the human umbilical vein endothelial cells (HUVEC) as in vitro models of the disease. We demonstrated that the OCCNP laden injectable hydrogels have robust antioxidative and anti-inflammatory properties that may prevent the prevalence and development of early stage AMD. This system may serve as a potential solution for AMD treatment based on the success in clinical trials. In addition, the study would add a new, potentially powerful treatment option for use in treatments for several other diseases, such as neurodegenerative disorders, diabetic retinopathy, and vascular diseases, which are limited by current standard approaches.
2. Methods & Materials
Alginate (sodium salt from brown algae, 4–12 cP, bioreagent), chitosan (50–150 kDa molecular weight, >80% degree of deacetylation, from algae), cerium chloride, calcium chloride, N-hydroxy-succinimide (NHS), Gelatin (type B, from bovine skin), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) were obtained from Sigma-Aldrich. Ammonium hydroxide and hydrogen chloride were obtained from BDH.
2.1 Synthesis of oligochitosan coated nanoceria
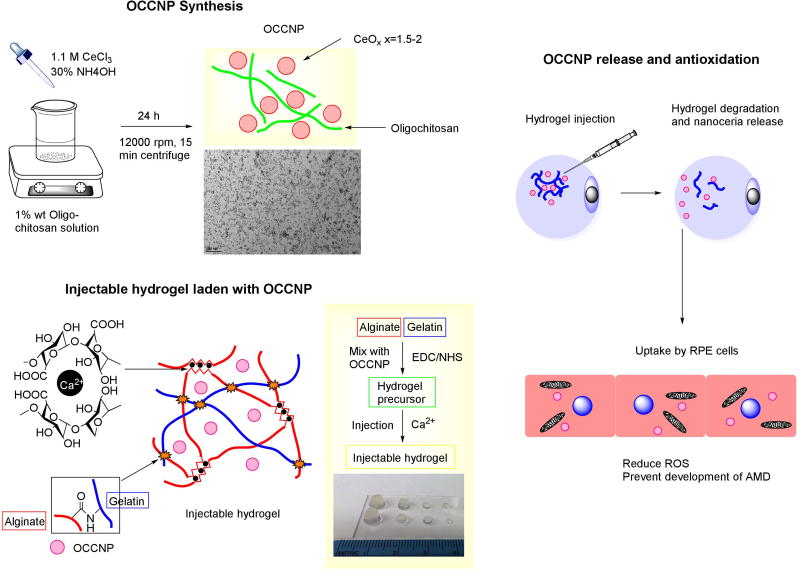
The synthesis of nanoceria was modified from our recent research22. 2 g Chitosan was dissolved in 100 mL 0.5 M HCl Solution at 70°C under constant stirring for 120 min. The reaction was stopped by adding 0.1 M NaOH solution to adjust pH value to 5. The solution was centrifuged at 5,000 rpm for 20 min, and the supernatant was collected and ultrafiltrated (10kDa molecular weight cut-off). 0.2 ml solution of 1.1 M CeCl3 was added to the 5 ml distilled H2O containing 60 mg oligochitosan under constant stirring at room temperature. 0.1 ml 30% w/v ammonium hydroxide was slowly added and then stirred for 24 h at room temperature. The solution was adjusted to pH value 7.4 using acetic acid. Thereafter, the solution was centrifuged at 14,000 rpm for 20 min at room temperature. The precipitate was collected and dispersed in distilled H2O by ultrasonication. The OCCNP solution was ultrafiltrated and weighed, and the OCCNP was stored at ambient temperature.
2.2 Synthesis of alginate-gelatin injectable of hydrogels
The hydrogel synthesis was adopted from our previous research30. 5 mL 2% alginate aqueous solution was mixed with 5 mL 2% gelatin aqueous solution in 20 mL vial. Next, 40 mg EDC and 30 mg NHS were added and reacted at ambient temperature for 24 h. The OCCNP were sonicated and mix with hydrogel precursors at concentrations of 0.1, 0.2, 0.5 or 1 mg/mL in final. The hydrogel precursor was withdrawn through 1 mL medical syringes with 30-gauge needle.100, 25, 10 and 3 µL hydrogel precursor was injected into a mold (1.7 mL centrifuge tube or 0.2 mL PCR tube) and the same volume of 0.05 M CaCl2 solution was injected spontaneously to finish ionic crosslinking. The crosslinking could be done within less than 10 minutes. The graphical illustration is shown in Figure 1. The swelling ratio of hydrogels were determined using following equation:
| (1) |
Where M1 is the mass of hydrogel and M2 is mass of polymer.
2.3 Characterization
The oligo-chitosan coated nanocerias were imaged using transmission electron microscope (TEM) under 80kV. The sample was prepared by dropping 5 µL OCCNP solution on 400 mesh formvar/carbon coated copper grids. The diameter of nanoceria was calculated as the average of 10 randomly measured nanocerias.
Molecular device SpectraMax M5 fluorescence microplate reader was employed to determine the fluorescence intensity at 530 nm by 485 nm excitation for fluorescein detection and absorbance at 570 nm.
The absorption spectra of nanoceria and concentration was determined using a Thermo Scientific Nanodrop 2000c spectrophotometer. The maximum absorption of nanoceria was determined at 295 nm and concentration was determined using standard curve with degraded hydrogel background subtraction (Supporting information S4).
The imaging of cells was conducted using the Carl Zeiss LSM-700 confocal fluorescence microscope and observer D1 fluorescence microscope with Zeiss AxioCam MRC camera. The images were processed by ZEN software.
2.4 Hydrogel degradation and nanoceria release
The hydrogels were formed with 0.2 mL volume with 1 mg/mL OCCNP loaded. The hydrogels were weighted and immersed in 10-fold volume phosphate buffer saline (PBS) solution in 60 mm diameter petri dish and kept at physiological temperature. Hydrogels were weighted and PBS was renewed periodically. The concentration of nanoceria released from hydrogels was measured using method mentioned above, and mass loss of hydrogels was directly weighed in dish.
2.5 Oxygen radical absorbance capacity (ORAC) assay
The procedure of ORAC was adopted from previously published articles14. 0.15 M 2-2’-azobis(2-amidinopropane)-dihydrochloride (AAPH), and 20 µM (Trolox) was mixed in PBS at pH 7.4 in a 96-well plate in the presence of hydrogels and incubated for 30 min at physiological temperature and 0.2 µM fluorescein sodium salt (FL) was added. The wells were filled up with PBS and fluorescence intensity was measured at 530 nm by 485 nm excitation every minute for 6 h using a microplate reader. The relative fluorescence intensity was measured by the assay. The antioxidant capacity was calculated by measuring the area under curve (AUC) of the time dependent fluorescence intensity from the antioxidant (Trolox and hydrogels) and the blank. The assay contained 120 µL FL, 20 µL Trolox, 60 µL AAPH, and antioxidant (10, 20, or 50 µL Trolox, 20 µL OCCNP solution in 0.1, 0.2, 0.5 or 1 mg/mL or hydrogels in 5, 10, or 20 µL or 10 µL nanoceria laden hydrogels with 0.1, 0.2, 0.5, or 1 mg/mL OCCNP) in various concentration, and the blank samples were at the absence of antioxidant or AAPH. The results were exhibited as the mean of triplicated samples from the following equation:
| (2) |
Where AUC is the area under curve of fluorescence intensity in test and nTrolox is the amount of Trolox.
2.6 Cell culture
All cell lines used in this study were purchased from American Type Culture Collection origin. ARPE-19 cells (10–20 passages) were cultured using Dulbecco's Modified Eagle Medium (DMEM) /F12 (Gibco) media supplied with 1M (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) buffer, 10% fetal bovine serum (FBS), 56 mM final concentration sodium bicarbonate and 1% antibiotic- antimycotic (complete medium)31,32. HUVEC cells were grown in HuMec (Gibco) with supplements and 5% FBS. The incubator was set at 37°C with 5% CO2 supply and medium was renewed every 3 days. The morphology was observed with an inverted phase optical microscope. Monolayers were harvested with 0.05% Trypsin-EDTA. The number of cells were counted using a hemocytometer.
2.7 Nanoparticle uptake, hyperoxia damage, cell viability, anti-angiogenesis, and anti-inflammation test
1 mL 0.5 mg/mL OCCNP was incubated with 5-sulfodicholorphenol (SDP) ester conjugated Alexa Fluor (AF) 488 dye for 8 h in a dark environment. The nanoceria was ultrafiltrated against distilled H2O three times. The dye conjugated nanoceria was added to ARPE-19 cells and cultured on poly-L-lysine coated glass cover for 48 h, then cells were imaged under a confocal fluorescence microscope with 4',6-diamidino-2-phenylindole (DAPI), and AF 555 labeled tubulin antibody.
The harvested ARPE-19 cells were dispensed in serum-free medium, and 2×105 cell/well suspension was seeded on a 6-well plate with 0.1, 0.2, 0.5, or 1 mg/mL OCCNP. Each well was treated with 2 mM H2O2 for hyperoxia damage and cultured for 24 h. The cells were incubated with Live/Dead assay (Life Technologies) in ambient temperature for 10 min. The hyperoxia tests were analyzed using fluorescence imaging and mean of green/red channel histograms were used for live/dead cell comparison. The cell viability assay using MTT assay are in supporting information.
For anti-angiogenesis test, ARPE-19 cells, and HUVEC cells were seeded on a 6-well plate with 1 mg/mL OCCNP, blank hydrogels, and 1 mg/mL OCCNP laden hydrogels. ARPE-19 cells were treated with 0.5 mM H2O2. Cells were cultured for 24 h and harvested for western blotting analysis. For anti-inflammation test, 10µg/mL lipopolysaccharides (LPS) was added to serum-free media and cultured for 24 h before harvested for analysis. The cytokines expression was analyzed using western blotting with F4/80 antibody and cytokines were quantified using real time-polymerase chain reaction (RT-PCR) (Supporting information).
2.8 Statistical method
All experiments were performed in triplicate. All quantitative data were expressed as the mean and standard deviation of triplicates in hydrogel degradation, nanoceria release, ORAC, and live/dead assay. The data were analyzed by one-way and two-way analyses of variance (ANOVA) as appropriate, and a p-value less than 0.05 was considered significant.
3. Result
3.1 Properties of OCCNP and alginate-gelatin injectable hydrogels
The OCCNP was prepared using precipitation of cerium chloride, and oligochitosan served as the matrix to bind with nanoparticles and provide solubility to nanoceria in aqueous phase. The weight ratio of nanoceria in nanocomposite is around 50% by weighing precipitate. The dispersion of OCCNP remained stable in acidic and neutral pH. From TEM images (Figure 1), the nanoceria spheres prepared in oligochitosan solution rendered a 5 nm average dimension for individual nanoparticles, and the chitosan bonded several nanoceria together and formed chitosan-ceria nanocomposites with 135 nm averaged dimension in aqueous phase and dehydrated chitosan coated nanoceria around 100 nm dimension (supporting information S1 and S2). Based on this result, the concentration of 0.5 mg/mL OCCNPs could be estimated to be 0.99 µM based on 7.65 g/cm3 bulk density of ceria14. The results from the particle sizer reflected the overall dimension in the aqueous phase, and oligochitosan rendered stability in ambient temperature for an extended time. The detailed characterization and properties of chitosan coated nanoceria are available in a previous publication22.
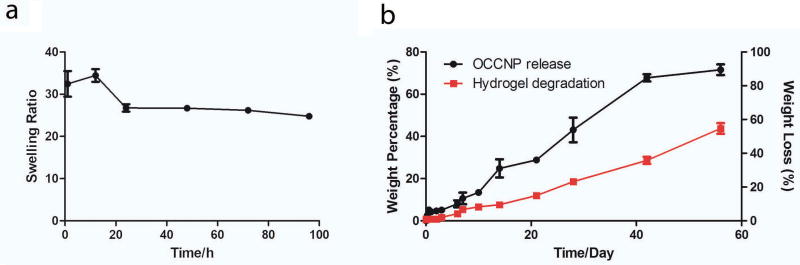
The hydrogels were designed to form in an easy manner with presence and absence of OCCNP using chemical crosslinking of carboxyl acid groups and amino groups and alginate ionic crosslinking (Figure 1). Thus, the hydrogels precursor was synthesized using biocompatible EDC/NHS crosslinking, and injection was completed using precursor and calcium ions. The water content of hydrogel at equilibrium exceeded 95% after 4 days swelling test (Figure 2a), and water content increased with nanoceria content (supporting information S3). The transparent hydrogels could easily form in different volumes (100 µL, 25 µl, 10 µl, and 3 µL) and shapes which may lead to potential for ophthalmic applications in multiple species and use for other tissues with minimal modification (Figure 1). The detailed characterization and properties of standalone alginate-gelatin injectable hydrogels are available in a previous publication30.
The hydrogels were degraded in a controlled manner, with data shown in Figure 2b. The degradation was conducted over a 2-month period, and the profile briefly came in a linear manner (R2=0.961). The hydrogels lost around 50% weight in the degradation test at physiological temperature and pH. The release of nanoceria briefly led to the function of hydrogels degradation in a linear form (R2=0.975), which reached over 70% during a 2-month period. The release profile was close to a zero order release model during the test, and the release of nanoceria was correlated with the degradation of hydrogels in vitro (Pearson’s r=0.9929), indicating that the OCCNPs were mainly released with hydrogels degradation from the release profile.
3.2 Synergistic anti-oxidization of OCCNP laden injectable hydrogels
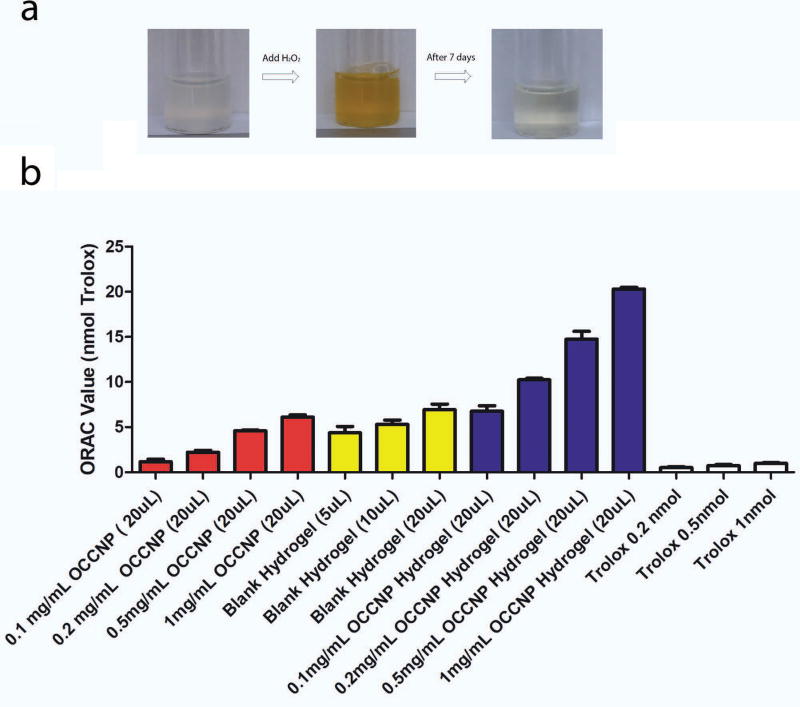
To assess self-regenerative antioxidant properties of OCCNP, we performed a reduction test. H2O2 was dropped into OCCNP solution and the light yellow-colorless was changed to deep yellow, and the solution recovered yellowish-colorless after 7 days as the result of Ce3+ (Figure 3a). The mechanism of reaction was that Ce3+ and Ce4+ were donor-receptor of electrons and the vacancy and defects in ceria crystal lattice surface captured oxygen radicals19. This auto-regenerative property of nanoceria makes it a potential antioxidant and therapeutically useful for oxidative stress-related disorders, such as AMD.
ORAC is a widely used assay that measures antioxidant capacities in biological samples in vitro14. In ORAC test, OCCNP solution achieved strong antioxidant properties. The amount of OCCNP was at the same level of 6-hydroxy-2,5,7,8- tetramethylchromane-2-carboxylic acid (Trolox) (Equation 2). The increase of antioxidant capacity was briefly the function of OCCNP concentration, and 20 µL 1 mg/mL OCCNP equaled 7.1 nmol Trolox, which is estimated to be 355 µL 20 µM Trolox solution. The combination of alginate-gelatin injectable hydrogels and low molecular weight alginate also exhibited the antioxidant properties33,34. The increase of hydrogel volume also improved the radical scavenge capacity, and 20 µL hydrogels equaled 7 nmol Trolox solution. When combining OCCNP and injectable hydrogels, a more powerful antioxidant was created (Figure 3b). The radical scavenge capacity of ceria laden hydrogels was essentially the sum of capacity OCCNP and blank hydrogels, and 20 µL 1 mg/mL OCCNP laden hydrogels equaled over 20 nmol Trolox solution. Therefore, the OCCNP laden hydrogels behaved with robust radical scavenging properties and may provide a long-term antioxidant solution for free radical associated oxidative stress in retinal diseases.
3.3 OCCNP laden hydrogels maintain cellular viability and prevent hydrogen peroxide-induced apoptotic cell death in ARPE-19 cells
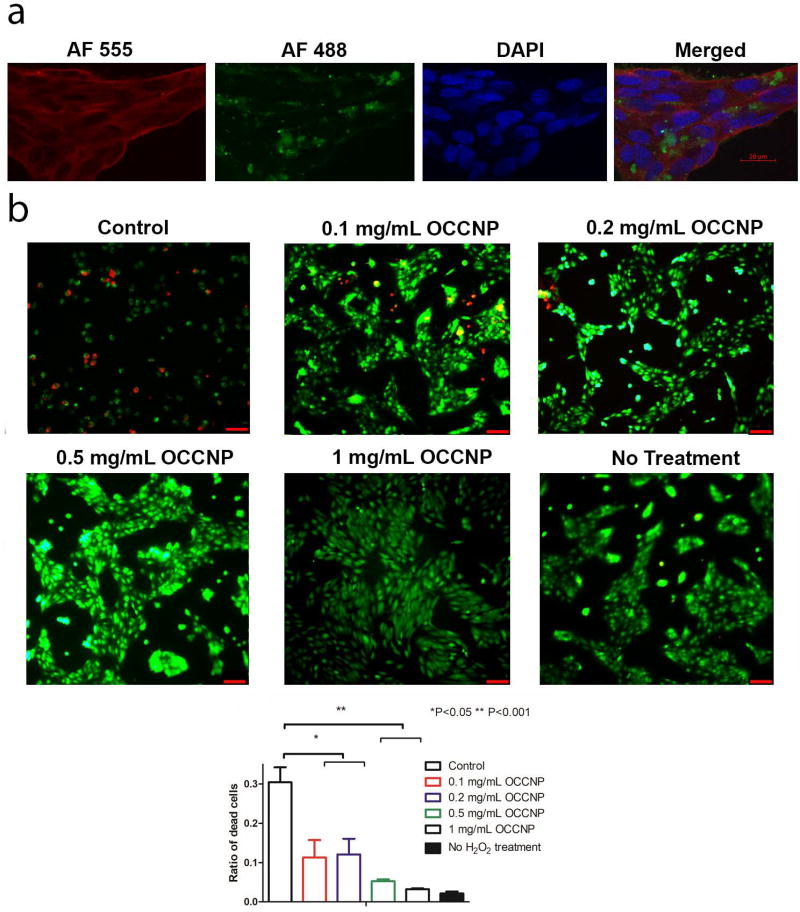
To evaluate the uptake and distribution of the nanoceria, AF488 conjugated OCCNPs were added to ARPE-19 cells. As shown in Figure 4a, the cells took up a large proportion of dye conjugated OCCNP in cytoplasm within 48 h. The blank area in green channel indicated OCCNPs did not enter the nucleus of cells. Water-soluble OCCNPs allowed for easy penetration of plasma membrane according to previous studies14. Chitosan matrix can be degraded by lysosome, and individual nanoceria functions as the mediator of ROS in cells35.
The H2O2 hyperoxia treatment simulated the oxidative stress damage in the in vitro culture of ARPE-19 cells. From the result in Figure 4b, we found severe cell damage and death (over 30%) in the H2O2 groups with the absence of nanoceria. The presence of nanoceria effectively reduced the ratio of dead or damaged cells (P=0.0357, one-way ANOVA), and 0.1 and 0.2 mg/mL OCCNP reduced the number and fraction of dead cells. The evidence of cell death or damage was hard to observe by using 0.5 and 1 mg/mL nanoceria. Moreover, there was no significant morphology change in cells found in hyperoxia treatment using nanoceria.
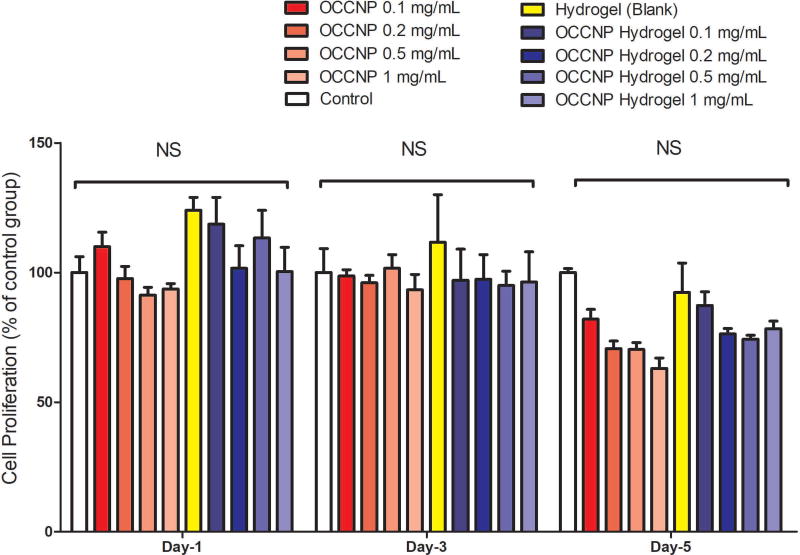
Cell viability of ARPE-19 cells cultured with OCCNP and hydrogels was also characterized (Figure 5). The blank hydrogels and OCCNP laden hydrogels facilitated cell proliferation on the first day, and the number of live cells also kept the similar tendency on day 3. Decreased cell viability was found with higher concentration of OCCNP on 5-day culture, but this effect is not statistically significant (P=0.6227). A possible explanation is that pH changes in a culture environment switch OCCNP to oxidative levels15,20,21. Studies have shown that the antioxidant properties of nanoceria are optimal at physiological pH, but it may behave as oxidative at low pH level. After 5-day culture with standalone OCCNP, the lowered pH rendered higher oxidative level and inhibited the proliferation of ARPE-19 cells. Nonetheless, this phenomenon can be diluted by combining OCCNP with hydrogels (Figure 5). From this aspect, the OCCNP laden hydrogels can be considered biocompatible and non-toxic, which can serve as a desirable sustained delivery vehicle of OCCNP.
3.4 OCCNP laden hydrogels reduce expression of the pro-angiogenic factor VEGF in multiple angiogenesis-related cells
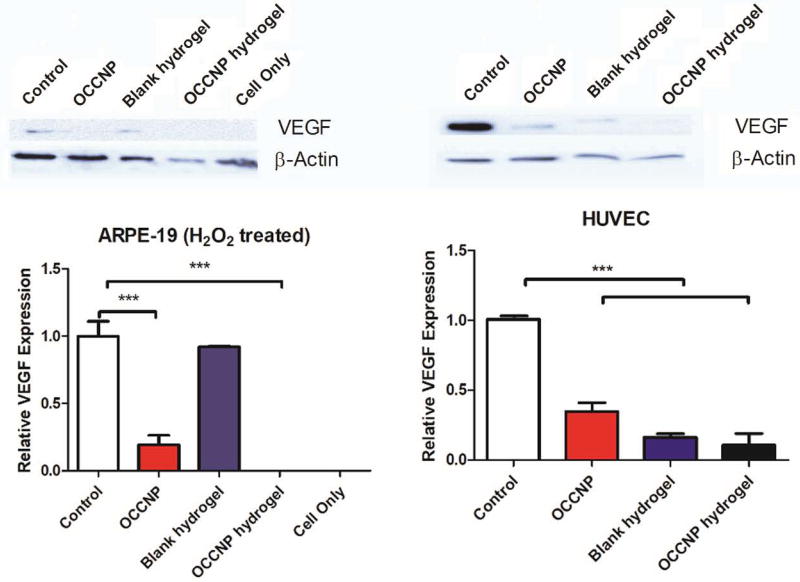
To determine whether OCCNP laden hydrogels have the ability to inhibit angiogenesis, we next investigated whether the treatment affects the stability of VEGF expression in vitro. From western blotting analysis of cells lysate of H2O2 treated ARPE-19 and HUVEC, it was shown that the expression of VEGF was substantially inhibited (P=0.0001 and 0.0003) using nanoceria or the hydrogel with OCCNP (Figure 6), which matches the previous research result that ROS scavenging materials may downregulate the expression of VEGF36. The hydrogel without nanoceria also downregulated VEGF expression in both cell lines. It is likely due to the antioxidative property of the alginate hydrogels, which is linked to their inherent antiangiogenic activity33,36. Consistent with the antioxidative studies, the OCCNP laden hydrogels significantly reduced VEGF expression in H2O2 treated ARPE-19 and untreated HUVEC cell lines.
3.5 Oligochitosan coated nanoceria-laden hydrogels inhibit LPS induced inflammatory response in ARPE-19 cells
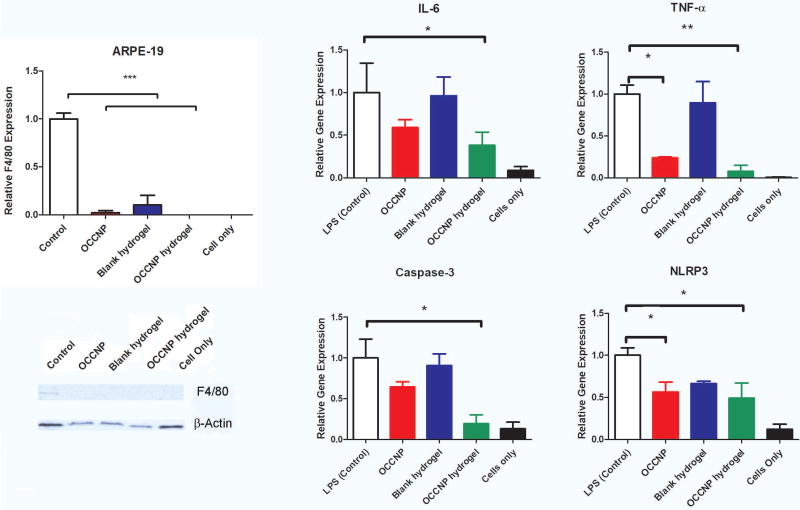
Studies have shown that nanoceria has anti-inflammatory properties37,38. To examine whether OCCNP laden hydrogels have anti-inflammatory effects, we treated ARPE-19 cells with LPS to induce inflammation and found that inflammatory reactions were significantly reduced by the treatment of OCCNP, hydrogels, and/or OCCNP laden hydrogels as revealed by western blot using a macrophage marker, F4/80 (Figure 7, left panel).
The analysis of LPS induced cytokine expressions were also quantified with RT-PCR (Figure 7, right panel). After adding LPS, the amount of IL-6 was reduced ~40% in ARPE-19 cells using OCCNP or injectable hydrogels; the reduction was not statistically significant, but the significance was achieved by OCCNP laden hydrogels (P=0.0420). The downregulation of TNF-α were significant using both OCCNP and OCCNP laden hydrogels. The expression of IL-6 and TNF-α is considered ROS associated, and OCCNP decreased the amount of cytokines produced as a response to oxidative level. The suppression of caspase-3 and NLRP-3 was statistically significant using OCCNP laden hydrogels (P=0.0196 and 0.0179). The OCCNP downregulated the expression of caspase-3 by 36% and NLRP3 over 45%, although the reduction of caspase-3 was not statistically significant. It indicated that the OCCNP and OCCNP laden hydrogels could have good efficacy in reducing apoptosis related protein expression, thus having the potential to protect photoreceptors and RPE cells. The alginate moiety has been reported as an anti-inflammatory agent26. However, the alginate-gelatin hydrogels did not largely decrease the cytokine production induced by LPS. The combination of OCCNP and hydrogels exhibited synergistic antioxidative properties and remarkably downregulated the pro-inflammatory proteins expression.
4. Discussion
The prevention of onset or worsening of AMD requires addressing multiple factors. Nanoceria can assist prevention by controlling ROS, inhibiting VEGF, and mitigating inflammatory reaction19,38,39. Conventionally, the administration of nanoceria via intravitreal injection remains a challenge due to its water-insoluble formulation21,37. The synthesis of nanoceria in the oligo-chitosan solution allows the homogenous dispersion of nanoceria in aqueous phase or hydrogels. The alginate-gelatin hydrogels can be injected and formed in a relatively easy manner, thus providing localized delivery and sustained release of nanoceria, and serving as an antioxidant and free radical scavenger upon injection. The sustained release could render more homogeneous intraocular dispersion of OCCNP and overcome the efficacy issues seen in conventional intravitreal injections.
Hydrogels have been studied for delivery of VEGF antibody drugs and delivery of stem cells for retinal regeneration40,41, but it remains a challenging job to completely mitigate worsening vision in AMD patients, especially in early stage AMD. The combination of water soluble long-term antioxidants and controlled delivery vehicles may create a superior approach to treat early/intermediate stage AMD. As AMD is characterized by multifactorial pathological processes, including oxidative stress, inflammation, and angiogenesis which proceed through ROS, the antioxidant therapeutics on multiple pathogenic factors make it a promising drug candidate for the treatment of AMD, and may be superior to anti-VEGF therapeutics which focus on neovascularization only22. The alginate and gelatin contents of hydrogels are biocompatible and biodegradable, and have a large capacity to encapsulate adequate amounts of OCCNP. The self-regenerated nanoceria prevents RPE cells from oxidative damage and accumulation of drusen over a long period, and alginate-based hydrogels may also supply the capacity for oxygen radical scavenging and supplementary anti-inflammatory effects upon injection. Compared to other lipid removal treatment in trials that required repeated administration, the OCCNP injectable hydrogel may be strategically effective over an extended period of time42,
Furthermore, it has been found that the ROS may have direct influence on the secretion of cytokines (e. g. IL-6 and TNF-α) in several types of cell lines31,32,37,38. Inflammation is known to play a clear role in the development of AMD as well. The synergistic anti-oxidative, anti-angiogenesis, and anti-inflammatory properties from both hydrogel and nanoceria can be provided upon injection, and nanoceria may serve as long-term protection of RPE cells. It should be noted that although the use of nanoceria laden hydrogel appears to be an attractive tool for AMD, these tests were displayed in in vitro cell lines (e.g., ARPE-19 and HUVEC). While literatures have shown that ARPE-19 cells express several RPE specific markers (e. g. RPE65 and BEST1)32,43,44, further studies using primary human RPE, stem cell derived RPE, and animal models are anticipated to prove the therapeutic concept that the hydrogel formulations will be useful to treat AMD patient populations. Our goal is to use injectable hydrogel systems that have been developed by the current formulation with modification to serve as an alternative therapy for both dry and wet AMD.
5. Conclusion
This article introduced the combination of OCCNP and alginate-gelatin injectable hydrogels for antioxidative and anti-inflammatory purposes in a potential ophthalmic area. The OCCNP laden hydrogels can be injected, form in an easy manner, and sustain degraded release of the nanoparticles for long term antioxidation. ORAC assay proved that the OCCNP and hydrogels have large capacity to absorb reactive radicals, and nanoceria in 1 mg/mL effectively prevented damage from oxygen free radicals. The OCCNP and hydrogels laden with OCCNP were biocompatible. The OCCNP and hydrogel with OCCNP also inhibited VEGF and inflammatory-related protein expression in AMD associated cell lines. Thus, the OCCNP laden injectable hydrogel has the potential to serve as a treatment option for both dry and wet AMD.
Supplementary Material
Acknowledgments
This work was supported in part by the U.S. National Eye Institute (R21EY024059 & R01EY026564, Z.H.), the Carolina Center of Nanotechnology Excellence (Z.H.), the UNC Junior Faculty Development Award (Z.H.), and the NC TraCS Translational Research Grant (550KR151611, Z.H.). The authors thank Cassandra Janowski Barnhart, M.P.H. (Department of Ophthalmology, University of North Carolina at Chapel Hill) for her critical reading of the manuscript.
Footnotes
Supporting information is available for this publication.
The authors declare no conflicts of interest.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. British Journal of Ophthalmology. 2012;96(5):614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 2.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. The Lancet. 379(9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 3.Congdon N, O'Colmain B, Klaver CCW, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, Hyman L, et al. Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Jager RD, Mieler WF, Miller JW. Age-Related Macular Degeneration. New England Journal of Medicine. 2008;358(24):2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 5.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. New england journal of medicine. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 6.Cano M, Thimmalappula R, Fujihara M, Nagai N, Sporn M, Wang AL, Neufeld AH, Biswal S, Handa JT. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision Research. 2010;50(7):652–664. doi: 10.1016/j.visres.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, Cai J. Age-Related Retinopathy in NRF2-Deficient Mice. PLoS ONE. 2011;6(4):e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ, Grp CR. Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration The CATT Research Group. New England Journal of Medicine. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe GJ. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six–week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Kanagasingam Y, Bhuiyan A, Abràmoff MD, Smith RT, Goldschmidt L, Wong TY. Progress on retinal image analysis for age related macular degeneration. Progress in Retinal and Eye Research. 2014;38:20–42. doi: 10.1016/j.preteyeres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Chew EY, Clemons TE, Agrón E, Sperduto RD, SanGiovanni JP, Kurinij N, Davis MD. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology. 2013;120(8):1604–1611. e4. doi: 10.1016/j.ophtha.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew EY, Clemons TE, SanGiovanni JP, Danis R, Ferris FL, Elman M, Antoszyk A, Ruby A, Orth D, Bressler S. Lutein+ zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA-Journal of the American Medical Association. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 13.Evans JB, Syed BA. New hope for dry AMD? Nat Rev Drug Discov. 2013;12(7):501–502. doi: 10.1038/nrd4038. [DOI] [PubMed] [Google Scholar]
- 14.Lee SS, Song W, Cho M, Puppala HL, Nguyen P, Zhu H, Segatori L, Colvin VL. Antioxidant Properties of Cerium Oxide Nanocrystals as a Function of Nanocrystal Diameter and Surface Coating. ACS Nano. 2013;7(11):9693–9703. doi: 10.1021/nn4026806. [DOI] [PubMed] [Google Scholar]
- 15.Alili L, Sack M, Karakoti AS, Teuber S, Puschmann K, Hirst SM, Reilly CM, Zanger K, Stahl W, Das S. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor–stroma interactions. Biomaterials. 2011;32(11):2918–2929. doi: 10.1016/j.biomaterials.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 16.Pulido-Reyes G, Rodea-Palomares I, Das S, Sakthivel TS, Leganes F, Rosal R, Seal S, Fernández-Piñas F. Untangling the biological effects of cerium oxide nanoparticles: the role of surface valence states. Scientific Reports. 2015;5:15613. doi: 10.1038/srep15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nature nanotechnology. 2006;1(2):142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 18.Kong L, Cai X, Zhou X, Wong LL, Karakoti AS, Seal S, McGinnis JF. Nanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathways. Neurobiology of disease. 2011;42(3):514–523. doi: 10.1016/j.nbd.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong LL, Hirst SM, Pye QN, Reilly CM, Seal S, McGinnis JF. Catalytic Nanoceria Are Preferentially Retained in the Rat Retina and Are Not Cytotoxic after Intravitreal Injection. PLoS ONE. 2013;8(3):e58431. doi: 10.1371/journal.pone.0058431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai X, McGinnis FJ. Nanoceria: a Potential Therapeutic for Dry AMD. In: Bowes Rickman C, LaVail MM, Anderson ER, Grimm C, Hollyfield J, Ash J, editors. Retinal Degenerative Diseases: Mechanisms and Experimental Therapy. Cham: Springer International Publishing; 2016. pp. 111–118. [Google Scholar]
- 21.Kumar A, Das S, Munusamy P, Self W, Baer DR, Sayle DC, Seal S. Behavior of nanoceria in biologically-relevant environments. Environmental Science: Nano. 2014;1(6):516–532. [Google Scholar]
- 22.Mitra RN, Gao R, Zheng M, Wu M-J, Voinov MA, Smirnov AI, Smirnova TI, Wang K, Chavala S, Han Z. Glycol Chitosan Engineered Autoregenerative Antioxidant Significantly Attenuates Pathological Damages in Models of Age-Related Macular Degeneration. ACS Nano. 2017;11(5):4669–4685. doi: 10.1021/acsnano.7b00429. [DOI] [PubMed] [Google Scholar]
- 23.Mawad D, Anne Boughton E, Boughton P, Lauto A. Advances in hydrogels applied to degenerative diseases. Current pharmaceutical design. 2012;18(18):2558–2575. doi: 10.2174/138161212800492895. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Wang R, Zarembinski TI, Doty N, Jiang C, Regatieri C, Zhang X, Young MJ. The application of hyaluronic acid hydrogels to retinal progenitor cell transplantation. Tissue engineering Part A. 2012;19(1–2):135–142. doi: 10.1089/ten.TEA.2012.0209. [DOI] [PubMed] [Google Scholar]
- 25.Ulijn RV, Bibi N, Jayawarna V, Thornton PD, Todd SJ, Mart RJ, Smith AM, Gough JE. Bioresponsive hydrogels. Materials today. 2007;10(4):40–48. [Google Scholar]
- 26.Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Progress in Polymer Science. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overstreet DJ, Dutta D, Stabenfeldt SE, Vernon BL. Injectable hydrogels. Journal of Polymer Science Part B: Polymer Physics. 2012;50(13):881–903. [Google Scholar]
- 28.Yu Y, Lau LCM, Lo AC-y, Chau Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: a 6-month in vivo study. Translational vision science & technology. 2015;4(2):5–5. doi: 10.1167/tvst.4.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballios Brian G, Cooke Michael J, Donaldson L, Coles Brenda LK, Morshead Cindi M, van der Kooy D, Shoichet Molly S. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Reports. 2015;4(6):1031–1045. doi: 10.1016/j.stemcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Nune KC, Misra RDK. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomaterialia. 2016;36:143–151. doi: 10.1016/j.actbio.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Hanus J, Zhang H, Wang Z, Liu Q, Zhou Q, Wang S. Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells. Cell death & disease. 2013;4(12):e965. doi: 10.1038/cddis.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells—Implications for age-related macular degeneration (AMD) Immunology Letters. 2012;147(1–2):29–33. doi: 10.1016/j.imlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Kelishomi ZH, Goliaei B, Mahdavi H, Nikoofar A, Rahimi M, Moosavi-Movahedi AA, Mamashli F, Bigdeli B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food chemistry. 2016;196:897–902. doi: 10.1016/j.foodchem.2015.09.091. [DOI] [PubMed] [Google Scholar]
- 34.Falkeborg M, Cheong L-Z, Gianfico C, Sztukiel KM, Kristensen K, Glasius M, Xu X, Guo Z. Alginate oligosaccharides: enzymatic preparation and antioxidant property evaluation. Food chemistry. 2014;164:185–194. doi: 10.1016/j.foodchem.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 35.Wei L, Cai C, Lin J, Wang L, Zhang X. Degradation controllable biomaterials constructed from lysozyme-loaded Ca-alginate microparticle/chitosan composites. Polymer. 2011;52(22):5139–5148. [Google Scholar]
- 36.Fay J, Varoga D, Wruck CJ, Kurz B, Goldring MB, Pufe T. Reactive oxygen species induce expression of vascular endothelial growth factor in chondrocytes and human articular cartilage explants. Arthritis Research & Therapy. 2006;8(6):R189–R189. doi: 10.1186/ar2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory Properties of Cerium Oxide Nanoparticles. Small. 2009;5(24):2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 38.Karakoti A, Singh S, Dowding JM, Seal S, Self WT. Redox-active radical scavenging nanomaterials. Chemical Society Reviews. 2010;39(11):4422–4432. doi: 10.1039/b919677n. [DOI] [PubMed] [Google Scholar]
- 39.Karakoti AS, Monteiro-Riviere NA, Aggarwal R, Davis JP, Narayan RJ, Self WT, McGinnis J, Seal S. Nanoceria as Antioxidant: Synthesis and Biomedical Applications. JOM (Warrendale, Pa.: 1989) 2008;60(3):33–37. doi: 10.1007/s11837-008-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C-H, Hwang Y-S, Chiang P-R, Shen C-R, Hong W-H, Hsiue G-H. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules. 2011;13(1):40–48. doi: 10.1021/bm2009558. [DOI] [PubMed] [Google Scholar]
- 41.Xu X, Weng Y, Xu L, Chen H. Sustained release of Avastin® from polysaccharides cross-linked hydrogels for ocular drug delivery. International journal of biological macromolecules. 2013;60:272–276. doi: 10.1016/j.ijbiomac.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 42.Cai X, McGinnis JF. Nanoceria: a Potential Therapeutic for Dry AMD. In: Bowes Rickman C, LaVail MM, Anderson RE, Grimm C, Hollyfield J, Ash J, editors. Retinal Degenerative Diseases: Mechanisms and Experimental Therapy. Cham: Springer International Publishing; 2016. pp. 111–118. [DOI] [PubMed] [Google Scholar]
- 43.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light–induced damage to retinal pigmented epithelial cells. Investigative ophthalmology & visual science. 2000;41(7):1981–1989. [PubMed] [Google Scholar]
- 44.Dunn K, Aotaki-Keen A, Putkey F, Hjelmeland L. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Experimental eye research. 1996;62(2):155–170. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.