Abstract
Objective
High-deductible health plans (HDHP) have become the predominant commercial health insurance arrangement in the United States. HDHPs require substantial out-of-pocket (OOP) costs for most services but often exempt medications from high cost sharing. We examined effects of HDHPs on OOP costs and utilization of adjuvant hormonal therapy (AHT), which are fundamental care for patients with breast cancer.
Methods
This controlled quasi-experimental study used claims data (2003–2012) from a large national health insurer. We included 986 women with incident early-stage breast cancer, age 25 to 64 years, insured by employers that mandated a transition from low-deductible (<=$500/year) to high deductible (>=$1000/year) coverage, and 3,479 propensity-score matched controls whose employers offered only low-deductible plans. We examined AHT utilization and OOP costs per person-year before and after the HDHP switch.
Results
At baseline, the OOP costs for AHT were $40.41 and $36.55 per person-year among the HDHP and control groups. After the HDHP switch, the OOP costs for AHT were $91.76 and $72.98 per person-year among the HDHP and control groups, respectively. AHT OOP costs increased among HDHP members relative to controls but the change was not significant (relative change: 13.72% [95% CI: −9.25%, 36.70%]). AHT use among HDHP members did not change compared to controls (relative change of 2.73% [95% CI: −14.01%, 19.48%]); the change in AI use was −11.94% (95% CI: −32.76%, 8.88%) and the change in tamoxifen use was 20.65% (95% CI: −8.01%, 49.32%).
Conclusion
We did not detect significant changes in AHT use after the HDHP switch. Findings might be related to modest increases in overall AHT OOP costs, the availability of low-cost generic tamoxifen, and patient awareness that AHT can prolong life and health. Minimizing OOP cost increases for essential medications might represent a feasible approach for maintaining medication adherence among HDHP members with incident breast cancer.
INTRODUCTION
Breast cancer is the most common non-skin malignancy among US women [1]. Guidelines recommend that women with hormone receptor-positive early-stage breast cancer receive adjuvant hormonal therapy (AHT, including tamoxifen and aromatase inhibitors) [2–6]. Taken as oral medications daily for 5–10 years, AHT decrease the risk of breast cancer recurrence and mortality [7].
High-deductible health plans (HDHP) are now the predominant commercial health insurance product in the US.[8] HDHPs require potential annual out-of-pocket spending of approximately $1000-$6000 per person for most non-preventive care including specialist visits. In 2017, 51% of covered workers had deductibles of $1000 or more and 23% had deductibles of $2000 or more [8]. However, the majority of HDHPs have medication cost sharing arrangements (typically, a tiered copayment structure) that are similar to more generous traditional health insurance plans.
Recent research has demonstrated that women in HDHPs experience delays in breast cancer diagnosis and initial treatment [9]. However, little is known about longer-term impacts, especially use of AHT and treatment continuity. Studies suggest that higher patient out-of-pocket (OOP) costs, lower income, as well as Black and Hispanic race/ethnicity are associated with lower rates of appropriate systemic adjuvant therapy [10–12]. Previous research has demonstrated that breast cancer patients with over $30 prescription copayments were 2.1 times more likely to be non-adherent to aromatase inhibitors than patients with less than $10 copayments [6]. It is unclear whether women with early-stage breast cancer facing a general increase in OOP medical costs will reduce the utilization of these necessary medications, given that medication cost sharing structures might be largely unchanged from before to after HDHP enrollment. The purpose of this study was therefore to examine the impact of modern HDHPs on OOP costs for and use of AHT in patients with early-stage breast cancer.
METHODS
Study Population
We drew our study population from commercially insured members in the Optum database (Eden Prairie, MN) enrolled between 1/2003–12/2012. Data comprised all medical and pharmacy claims from members of a large national health plan. We included subjects in the study based on their employers’ health insurance offerings. All members of our sample had continuous pharmacy coverage. As in our previous research [13], we defined employers with low- and high-deductible coverage as those offering exclusively plans with annual deductibles of $0-$500 and $1000 or more, respectively. Both plan types generally cover medications and preventive care (e.g., annual preventive primary care visits) at relatively low out-of-pocket cost. In contrast, HDHP members on average must pay substantially higher OOP costs than traditional plan members for services such as diagnostic testing, procedures, specialist visits, and acute care. Of note, however, approximately 17% of employers offer HDHPs linked to Health Savings Accounts and require that most medications be subject to the deductible [8]. We used a benefits variable available for most smaller employers (approximately ≤100 employees) to estimate employer annual deductibles [9]. For larger employers, we imputed deductible levels using OOP costs among employees who utilized health services, an algorithm that had 96% sensitivity and 87% specificity among employers with at least 100 members. We restricted our analyses to employers with ≥10 enrollees to reduce employer selection based on enrollee health status. We used a full replacement design to minimize self-selection bias; i.e., the study population comprised individuals whose employers offered one year of low-deductible benefits followed by a mandated HDHP switch (HDHP group) or a mandated continuation in low-deductible plans (control group). We defined the beginning of the month of the low-to-high deductible transition as the HDHP-switch month (index date). For HDHP employers, the 12 months before the HDHP switch was the baseline period and the follow-up period was up to a year after the HDHP switch. For control employers, we defined the index date as the beginning of the month when the employer could change health insurance benefits (“anniversary date”), with 12-month baseline and follow-up periods defined in relation to this index date.
Among the HDHP and control employers, we implemented an established claims-based algorithm to identify women with incident early-stage breast cancer; detailed methods are available in the literature [14]. In brief, we defined women as having newly diagnosed early-stage breast cancer if they had a primary diagnosis of breast cancer (malignant or in situ breast cancer [ICD-9-CM diagnosis codes 174.x or 233.x], and had a mastectomy or a lumpectomy/partial mastectomy followed by at least one claim for radiotherapy (see Appendix for codes). The date of the first qualifying procedure was the disease index (incidence) date and we excluded women with breast cancer diagnosis more than 6 months prior to the disease index date, consistent with the established algorithm. We required women to have no use of AHT prior to the disease index date.
We identified rolling cohorts of women with incident early-stage breast cancer in the HDHP and control groups as our populations for analysis. Women entered the denominator in the month that their disease index date occurred if this was in any month of the baseline or follow-up period (a total of 24 months). We required these women to have at least 1 month of enrollment before and 6 months of enrollment after their disease index date. Individuals contributed to study time starting from their disease index date to the end of their enrollment or to the end of the follow-up period, whichever came first. Included study time could therefore range from one month to as long as 24 months per patient. We identified a pre-match sample of 1,012 HDHP members aged 25–64 with newly diagnosed early-stage breast cancer and 10,692 potential controls in low-deductible plans.
Matching Strategy and Covariates
To further minimize potential selection effects, we used a 1:4 propensity score match using member-level and employer-level variables for the propensity to switch to HDHPs. Individuals from HDHP and control groups were categorized by AHT use any time during the study (AI users, tamoxifen users and no use of AHT; individuals who received both AI and tamoxifen were categorized as AI users) and matched within these three strata. Baseline employer-level propensity score predictors included: employer size, mean outpatient copay, percent with mean outpatient copay greater than $15, and an indicator of cost sharing level (ratio of total OOP costs to total standardized care costs greater than 0.2). The member-level variables used in the caliper propensity score match to predict the likelihood of switching to an HDHP included: baseline age, US region of residence, income and education levels, race/ethnicity, HDHP switch month, disease index month, and the following three variables we calculated using the month before the disease index date: monthly mean OOP costs, monthly total standardized costs, and count of distinct drug categories as a proxy for comorbidities.[15] We included a final matched population of 986 women insured by employers that mandated a transition from low-deductible coverage (<=$500/year) to HDHPs (>=$1000/year) – the HDHP group – and 3,479 contemporaneous patients whose employers offered only low-deductible coverage for the baseline and follow-up periods – the control group.
To derive proxy demographic measures, the data vendor linked members’ most recent residential street addresses to their 2000 US Census block group [16]. Census-based measures of socioeconomic status have been validated [17,18] and used in multiple studies to examine the impact of policy changes on disadvantaged populations [19–21]. We classified members as from predominantly white, black, or Hispanic neighborhoods if they lived in a census block group (geocoding) with at least 75% of members of the respective race/ethnicity. We then applied a superseding ethnicity assignment if members had an Asian or Hispanic surname [22], and classified remaining members as from mixed race/ethnicity neighborhoods. This validated approach of combining surname analysis and census data has positive and negative predictive values of approximately 80% and 90%, respectively [23]. Using 2000 US Census block group data and validated methods [17,18], we defined four validated neighborhood income and education levels. Neighborhoods with below-high-school education levels were categorized into 4 education-level strata: <15% and 15%−24.9% defining high-education and 25%−39.9% and >=40% defining low-education. Neighborhoods with below-poverty levels were categorized into 4 income-level strata: <5% and 5%−9.9% defining high-income and 10%−19.9% and >=20% defining low-income. Employer size was defined as 0–99, 100–999, and 1000+ enrollees, and US regions were West, Midwest, South, and Northeast.
Outcome Measures
AHT included in the study were tamoxifen and the three aromatase inhibitors (AIs): exemestane, letrozole, and anastrozole. First, we calculated pharmacy OOP costs defined as the sum of deductibles and copayments per prescription. We estimated monthly and yearly OOP costs per person for AHT as a group and for AIs and tamoxifen separately during the baseline and follow-up periods, adjusting yearly estimates for the length of enrollment. We used winsorizing to reduce the impact of extreme outliers on effect estimates. We capped any member’s AHT OOP cost per fill to the 99% value of OOP costs established from all AHT fills. To estimate AHT utilization, we first created a person-level daily indicator of medication availability for each AHT starting from the disease index date by spreading the days supplied for each dispensing over days that the patient was enrolled in the health plan; generically equivalent products were treated as the same medication. Our AHT utilization measure was numbers of days covered by AHT per person per year for any AHT as well as separately for AIs and tamoxifen during the baseline and follow-up periods, adjusting for the length of enrollment.
Design and Analysis
We first used unadjusted, aggregate monthly time series to display the population-level data. Next, we used a pre-post with control group design with a patient-level difference-in-differences analytic approach to calculate effect estimates between the study groups from before to after employer-mandated transitions to HDHPs and to test the significance of the annualized differences for all outcome measures.
We compared baseline characteristics of our study groups using a standardized differences approach [24]. Continuous measures are reported as mean (SD) and categorical variables are reported as count (percentage). We removed from statistical models the index month and the prior month to reduce bias due to anticipatory changes in utilization before the HDHP switch (and consequent reductions in the month after the switch). Our difference-in-differences analyses compared changes in outcomes among members before and after the mandated HDHP switch versus controls. The term of interest was the two-way interaction between an indicator of HDHP versus control group and an indicator of before versus after the index date. We used a zero-inflated negative binomial distribution for OOP costs for AHT per person-year and a binomial distribution for analyzing AHT utilization days per person-year to account for members without any utilizations or costs [25,26]. Using terms from the regression model, we then used marginal effect methods to calculate mean adjusted baseline and follow-up OOP costs and AHT utilization as well as absolute and relative changes in outcomes[10]. We controlled for covariates described above. All analyses were carried out using the SAS software, version 9.3 (SAS Institute, Cary, NC) and STATA 14 (StataCorp, College Station, TX).
RESULTS
Compared with the unmatched sample (Table 1), our propensity-score matching approach increased the similarity of the HDHP and control groups. After matching, all standardized differences between HDHP and control group characteristics were well below 0.2, indicating minimal differences [24]. The average age of HDHP and control members was 51 years. Approximately 27–29% lived in low-income neighborhoods, 17–18% lived in low-education neighborhoods, and 6–7% were Hispanic. About half (50.8% of HDHP and 54.2% of control) of members were enrolled through mid-sized employers with 100–999 enrollees.
Table 1.
Baseline Characteristics of the High-deductible Health Plan Group and the Control Group, Before and After Propensity Score Match
| Before Propensity Score Match | After Propensity Score Match | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HDHP Group | Control Group | Standardized Difference* | HDHP Group | Control Group | Standardized Difference* | |||||
| Sample Size | (N=1,012) | (N=10,692) | (N=986) | (N=3,479) | ||||||
| Age > 40 on index date, No. (%) | 944 | (93.3) | 9,795 | (91.6) | 0.0632 | 921 | (93.4) | 3,237 | (93.0) | 0.0145 |
| Age on index date, Mean (SD) | 51.6 | (7.7) | 51.0 | (7.9) | 0.0785 | 51.6 | (7.7) | 51.2 | (7.7) | 0.0591 |
| No. (%) living in neighborhoods with below- poverty levels of | ||||||||||
| <5%1 | 461 | (45.6) | 5,405 | (50.6) | 0.1217 | 453 | (45.9) | 1,682 | (48.3) | 0.0494 |
| 5%−9.9%1 | 255 | (25.2) | 2,683 | (25.1) | 249 | (25.3) | 853 | (24.5) | ||
| 10%−19.9%2 | 207 | (20.5) | 1,797 | (16.8) | 200 | (20.3) | 665 | (19.1) | ||
| >=20%2 | 89 | (8.8) | 791 | (7.4) | 84 | (8.5) | 279 | (8.0) | ||
| Missing Poverty | 0 | (0.0) | 16 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| No. (%) living in neighborhoods with below-high-school education levels of | ||||||||||
| <15%3 | 601 | (59.4) | 6,895 | (64.6) | 0.1181 | 589 | (59.7) | 2,128 | (61.2) | 0.0460 |
| 15%−24.9%3 | 224 | (22.1) | 2,201 | (20.6) | 215 | (21.8) | 769 | (22.1) | ||
| 25%−39.9%4 | 150 | (14.8) | 1,239 | (11.6) | 148 | (15.0) | 470 | (13.5) | ||
| >=40%4 | 37 | (3.7) | 341 | (3.2) | 34 | (3.4) | 112 | (3.2) | ||
| Missing Education | 0 | (0.0) | 16 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Race/ethnicity, No. (%)5 | ||||||||||
| Hispanic | 63 | (6.2) | 766 | (7.2) | 0.1182 | 62 | (6.3) | 207 | (5.9) | 0.0165 |
| Asian | 16 | (1.6) | 287 | (2.7) | 16 | (1.6) | 53 | (1.5) | ||
| Black neighborhood | 14 | (1.4) | 259 | (2.4) | 14 | (1.4) | 50 | (1.4) | ||
| Mixed neighborhood | 141 | (13.9) | 1,450 | (13.6) | 136 | (13.8) | 482 | (13.9) | ||
| White neighborhood | 778 | (76.9) | 7,909 | (74.1) | 758 | (76.9) | 2,687 | (77.2) | ||
| Missing Race | 0 | (0.0) | 21 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| United States Region, No. (%) | 0.3265 | 0.0228 | ||||||||
| West | 110 | (10.9) | 1,364 | (12.8) | 109 | (11.1) | 371 | (10.7) | ||
| Midwest | 395 | (39.0) | 3,357 | (31.4) | 384 | (38.9) | 1,355 | (38.9) | ||
| South | 445 | (44.0) | 4,310 | (40.3) | 431 | (43.7) | 1,517 | (43.6) | ||
| Northeast | 62 | (6.1) | 1,656 | (15.5) | 62 | (6.3) | 236 | (6.8) | ||
| Missing Region | 0 | (0.0) | 5 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Outpatient Copayment, Mean $ (SD) | 18 | (7.1) | 16 | (6.7) | 0.3126 | 18 | (7.1) | 18 | (6.3) | 0.0321 |
| Copayment>$15, No. (%) | 650 | (64.2) | 4,826 | (45.1) | 0.3908 | 626 | (63.5) | 2,060 | (59.2) | 0.0879 |
| Employer Size, Mean (SD) | 652 | (1871.2) | 5,567 | (13379.8) | -0.5146 | 663 | (1894.0) | 998 | (4695.1) | -0.0937 |
| Employer Size, No. (%) | ||||||||||
| 0–99 | 392 | (38.7) | 1,522 | (14.2) | 1.1308 | 375 | (38.0) | 1,235 | (35.5) | 0.0676 |
| 100–999 | 510 | (50.4) | 3,086 | (28.9) | 501 | (50.8) | 1,885 | (54.2) | ||
| 1000+ | 110 | (10.9) | 6,084 | (56.9) | 110 | (11.2) | 359 | (10.3) | ||
Abbreviations: HDHP, high-deductible health plan.
1 Defined as high-income.
2 Defined as low-income.
3 Defined as high-education.
4 Defined as low-education.
5 See manuscript for definition of race/ethnicity categories.
*Lower standardized differences indicate greater similarity
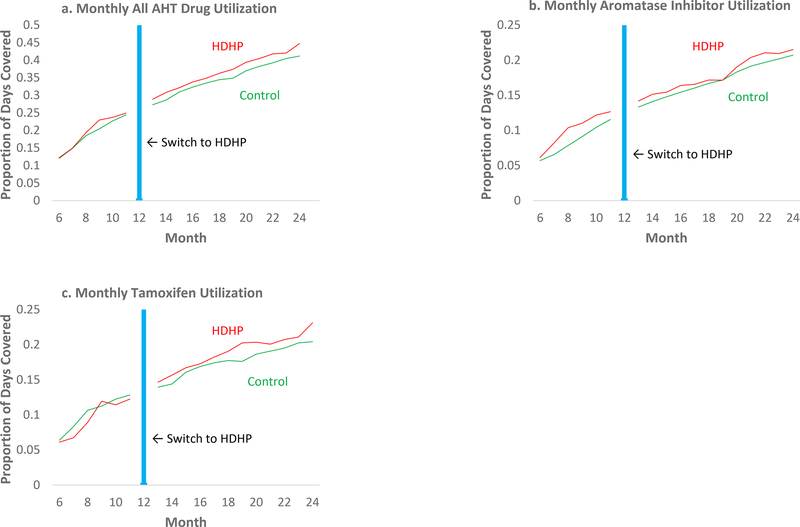
Figure 1A-C presents unadjusted, aggregated time series plots of use of AHT, AIs, and tamoxifen, respectively, by study group from the 6th month of the baseline period (after stable rolling cohort has accumulated) through the 12 months follow-up period after the HDHP switch.
Figure 1a-c.
Monthly proportion of days covered with adjuvant hormonal therapy (all AHT, aromatase inhibitors, and tamoxifen) among women with early breast cancer before and after a mandated switch to HDHPs, compared with contemporaneous propensity score matched members in low-deductible plans.
HDHP members faced an absolute baseline-to-follow-up increase in total OOP costs of approximately $1,205.39 per person-year on average compared to controls during the same periods (p=<0.001). At baseline, the OOP costs for AHT were $40.41 and $36.55 per person-year among the HDHP and control groups, respectively. After the HDHP switch, the OOP costs for AHT were $91.76 and $72.98 per person-year among the HDHP and control groups, respectively. In adjusted difference-in-differences analyses, the absolute change in OOP costs per person-year for AHT among HDHP members compared to controls from baseline to follow-up was not significant ($11.07, 95% CI: −5.61, 27.76; Table 2) and the relative change was also not significant (13.72%, 95% CI: −9.25%, 36.70%). The relative changes in OOP costs per person-year for AIs were 16.32% (95% CI: −14.45% to 47.08%) and for tamoxifen 4.76% (95% CI: −23.42% to 32.95%).
Table 2.
Adjusted difference-in-differences estimates in out-of-pocket costs for AHT per person-year and utilization of AHT (mean days covered per person-year) among HDHP women with early breast cancer and contemporaneous control group members
| HDHP | Control | Absolute Change, HDHP vs. Control (95% CI) | Relative Change, HDHP vs. Control (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |||||
| OOP costs for All Hormonal Therapy | 40.41 | 91.76 | 36.55 | 72.98 | 11.07 | (−5.61, 27.76) | 13.72 | (−9.25, 36.70) |
| ($/person-year) | ||||||||
| Aromatase Inhibitors | 29.71 | 69.23 | 25.84 | 51.77 | 9.71 | (−6.50, 25.93) | 16.32 | (−14.45, 47.08) |
| Tamoxifen | 11.31 | 22.75 | 10.84 | 20.83 | 1.03 | (−4.82, 6.88) | 4.76 | (−23.42, 32.95) |
| Utilization of All Hormonal Therapy | 57.81 | 128.30 | 55.71 | 120.36 | 3.41 | (−16.96, 23.79) | 2.73 | (−14.01, 19.48) |
| (days/person-year) | ||||||||
| Aromatase Inhibitors | 29.81 | 61.43 | 25.55 | 59.80 | -8.33 | (−24.77, 8.11) | -11.94 | (−32.76, 8.88) |
| Tamoxifen | 27.97 | 67.45 | 30.29 | 60.54 | 11.55 | (−1.83, 24.92) | 20.65 | (−8.01, 49.32) |
Abbreviation: HDHP, high-deductible health plan. Utilization of AHT is measured for baseline in average days per person. Units for OOP costs are USD per person time.
In adjusted difference-in-differences analyses, the relative change in mean days covered with AHT per person-year among HDHP members compared to controls from baseline to follow-up was not significant (Table 2; 2.73%, 95% CI:−14.01% to 19.48%); the change in use of AIs was −11.94% (95% CI: −32.76%, 8.88%) and the change in tamoxifen use was 20.65% (95% CI:−8.01% to 49.32%).
DISCUSSION
In this rigorous analysis of women with incident early-stage breast cancer who experienced mandated HDHP transitions, we did not detect significant changes in OOP costs for AHT or in AHT use. Other studies have suggested an association between large copayment (>=$90 per 90-day supply) and non-adherence to AIs in both Medicare and commercially insured women [6,27,28]. However, we found no statistically significant changes in AHT use among HDHP members. Findings might be explained by a variety of factors. The average annual increase in OOP costs for these medications – approximately $11.07 per person for HDHP members compared to controls (in the context of an annual increase of $1,205.39 in total OOP costs per person) – was perhaps too small to substantially affect use. In addition, some HDHP women might have substituted low-cost generic tamoxifen for the more expensive AIs, allowing them to preserve AHT use. Patient awareness that AHT can prolong life and health might have made HDHP member reluctant to discontinue these medications. It is also possible that many HDHP members reached their annual deductible amount, after which they would experience low or no cost-sharing and thus less incentive to reduce AHT use. Finally, funds in Health Savings Accounts or Health Reimbursement Arrangements, available to a minority of HDHP enrollees, might play a role in maintaining AHT treatment.
This study has several strengths. We used a large, nationwide sample of women diverse in income, age, and geography. Our study used full replacement employers, strong longitudinal analysis methods, and propensity-score matched controls to reduce bias in estimating HDHP impacts. Prior studies examining the association between copayment amounts and adherence used cross-sectional approaches that do not allow strong causal inference [6]. Our results should generalize well to the rapidly increasing cohort of commercially-insured women with early-stage breast cancer experiencing the major national trend toward HDHP adoption.
There are however several limitations. We were able to determine exact deductible levels for small but not large employers. However, our imputation algorithm for large employers was highly sensitive and specific and our analyses showed that, at the population level, the HDHP group experienced marked relative increases in overall OOP costs. Our study may not be representative of members with very high deductibles or newly insured individuals with no prior insurance experience. We did not have detailed information on tumor stage and we had no information on hormone receptor status that would influence AHT use. We also did not have information on non-cost-related reasons for AHT discontinuation such as toxicity or patient preference. However, these as well as stage and hormone receptor status are unlikely to differ by study group and thus should not influence our findings. Previous research has suggested that women younger than 40 years have higher risk of nonadherence [15] but we did not have sufficient power to conduct this stratified analysis.
Overall, our study adds to the HDHP literature by examining effects of insurance design on the use of AHT, a group of essential and effective medications for patients newly diagnosed with early-stage breast cancer. Future analyses with larger sample sizes should examine HDHP impacts among vulnerable subgroups such as those with low income, racial/ethnic minorities, and individuals covered by HDHPs with Health Savings Accounts that require full drug cost-sharing until deductibles are reached.
Our findings have important implications for employers and policymakers. We studied a natural experiment that approximates “value-based insurance design,” in which OOP costs for a high-value service (AHT in this case) are kept relatively low compared with other services. An increasing body of evidence suggests that when select services have relatively low OOP costs or minimal OOP cost changes (e.g., AHT), use can be preserved despite otherwise large cost sharing increases under HDHPs [13,29–32]. For example, rates of cancer screening [13,30,32,33], chronic disease medications [29,31], and diabetes primary care visits and monitoring [29] are preserved when exempted from high deductibles. Policymakers could use our findings to structure medication cost sharing in a way that continues to preserve guideline-recommended standards of care among patients with life-threatening diseases. Employers offering HDHPs might consider maintaining relatively generous tiered drug copayment plans, purchasing drug riders, or generously funding health savings accounts in order to maintain the health of their employees and families who require life-saving medications.
In conclusion, we did not detect significant changes in AHT use after the HDHP switch. Findings might be related to relatively small increases in AHT OOP costs after the HDHP transition. Minimizing OOP cost increases for essential medications might represent a feasible approach for maintaining medication adherence among HDHP members with incident breast cancer. An increasing number of women at risk for breast cancer will be enrolled in HDHPs over coming years, likely implying that more low-income women will enroll and that many women will enroll in HSA-HDHPs that require high cost sharing. It will therefore be important to continue to monitor AHT use, especially among vulnerable subgroups, as HDHPs and their membership evolve.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the National Cancer Institute and the National Institute of Health Office of the Director under Grant No. R01CA172639 (PI: Wharam). The research protocol was approved by the Harvard Pilgrim Health Care institutional review board. Dr. Zhang and Mr. Xu primarily analyzed the data. We thank Ms. Jamie Wallace, BA for data analysis and coordination, and Ms. Caitlin Lupton, MSc for research assistance and administrative support. The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of the National Cancer Institute and its Board of Governors.
Footnotes
The authors have no conflicts of interest or financial disclosures to report.
Drs. Lu and Zhang had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: All authors
Acquisition of data: Wharam
Analysis and interpretation of the data: All authors
Drafting of the manuscript: Lu, Callahan
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Zhang, Xu
Obtained funding: Wharam, Lu
Administrative, technical, or material support: Wharam, Callahan, Zhang
Study supervision: Wharam, Lu
REFERENCES
- 1.American Cancer Society (2016) Breast Cancer Facts & Figures 2015–2016. American Cancer Society, Inc. 2015, Atlanta, GA [Google Scholar]
- 2.Alexander FE, Anderson TJ, Brown HK, Forrest AP, Hepburn W, Kirkpatrick AE, McDonald C, Muir BB, Prescott RJ, Shepherd SM, et al. (1994) The Edinburgh randomised trial of breast cancer screening: results after 10 years of follow-up. Br J Cancer 70 (3):542-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson I, Aspegren K, Janzon L, Landberg T, Lindholm K, Linell F, Ljungberg O, Ranstam J, Sigfusson B (1988) Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. BMJ 297 (6654):943-948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ, Cancer Intervention Surveillance Modeling Network (CISNET) Collaborators (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353 (17):1784-1792. doi: 10.1056/NEJMoa050518 [DOI] [PubMed] [Google Scholar]
- 5.Cronin KA, Feuer EJ, Clarke LD, Plevritis SK (2006) Impact of adjuvant therapy and mammography on U.S. mortality from 1975 to 2000: comparison of mortality results from the cisnet breast cancer base case analysis. J Natl Cancer Inst Monogr (36):112-121. doi: 10.1093/jncimonographs/lgj015 [DOI] [PubMed] [Google Scholar]
- 6.Sedjo RL, Devine S (2011) Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat 125 (1):191-200. doi: 10.1007/s10549-010-0952-6 [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386 (10001):1341-1352. doi: 10.1016/S0140-6736(15)61074-1 [DOI] [PubMed] [Google Scholar]
- 8.Claxton G, Rae M, Long M, Damico A, Foster G, Whitmore H (2017) The Kaiser Family Foundation and Health Research & Educational Trust Employer Benefits 2017 Annual Survey. The Kaiser Family Foundation, Menlo Park, California [Google Scholar]
- 9.Wharam JF, Zhang F, Lu CY, Wagner AK, Nekhlyudov L, Earle CC, Soumerai SB, Ross-Degnan D (2018) Breast Cancer Diagnosis and Treatment After High-Deductible Insurance Enrollment. J Clin Oncol 36 (11):1121-1127. doi: 10.1200/JCO.2017.75.2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu XC, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J (2012) Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol 30 (2):142-150. doi:JCO.2011.36.8399 [pii] 10.1200/JCO.2011.36.8399 [DOI] [PubMed] [Google Scholar]
- 11.Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI, Neugut AI, Fehrenbacher L, Thompson B, Coronado GD (2012) Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat 131 (2):607-617. doi: 10.1007/s10549-011-1762-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, Keating NL (2011) The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer 117 (1):180-189. doi: 10.1002/cncr.25542 [DOI] [PubMed] [Google Scholar]
- 13.Wharam JF, Zhang F, Landon BE, LeCates R, Soumerai S, Ross-Degnan D (2016) Colorectal Cancer Screening in a Nationwide High-deductible Health Plan Before and After the Affordable Care Act. Med Care 54 (5):466-473. doi: 10.1097/MLR.000000000000052100005650-201605000-00008 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Nattinger AB, Laud PW, Bajorunaite R, Sparapani RA, Freeman JL (2004) An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res 39 (6 Pt 1):1733-1749. doi: 10.1111/j.1475-6773.2004.00315.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu CY, Adams AS, Ross-Degnan D, Zhang F, Zhang Y, Salzman C, Soumerai SB (2011) Association between prior authorization for medications and health service use by Medicaid patients with bipolar disorder. Psychiatr Serv 62 (2):186-193. doi: 10.1176/ps.62.2.pss6202_018662/2/186 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Bureau of the Census (1994) Geographical Areas Reference Manual U.S. Bureau of the Census, Washington, D.C. [Google Scholar]
- 17.Krieger N (1992) Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. American Journal of Public Health 82 (5):703-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV (2003) Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures--the public health disparities geocoding project. American Journal of Public Health 93 (10):1655-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ (2006) Relationship between quality of care and racial disparities in Medicare health plans. JAMA 296 (16):1998-2004 [DOI] [PubMed] [Google Scholar]
- 20.Trivedi AN, Rakowski W, Ayanian JZ (2008) Effect of cost sharing on screening mammography in medicare health plans. N Engl J Med 358 (4):375-383. doi: 10.1056/NEJMsa070929 [DOI] [PubMed] [Google Scholar]
- 21.Selby JV, Fireman BH, Swain BE (1996) Effect of a copayment on use of the emergency department in a health maintenance organization. N Engl J Med 334 (10):635-641 [DOI] [PubMed] [Google Scholar]
- 22.Ethnic Technologies (2018) Ethnic Technologies: The Leader in Multicultural Marketing. http://www.ethnictechnologies.com/. Accessed April 27 2018
- 23.Fiscella K, Fremont AM (2006) Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res 41 (4 Pt 1):1482-1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang D, Dalton JE (2012) A unified approach to measuring the effect size between two groups using SAS®. SAS Global Forum 2012—Statistics and Data Analysis, Orlando, FL. [Google Scholar]
- 25.Lambert D (1992) Zero-Inflated Poisson Regression, with an Application to Defects in Manufacturing . Technometrics 34 (1):1-14 [Google Scholar]
- 26.Hall D (2000) Zero-Inflated Poisson and Binomial Regression with Random Effects: A Case Study. Biometrics 56 (4):1030-1039 [DOI] [PubMed] [Google Scholar]
- 27.Hershman DL, Tsui J, Meyer J, Glied S, Hillyer GC, Wright JD, Neugut AI (2014) The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst 106 (11). doi: 10.1093/jnci/dju319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neugut AI, Hillyer GC, Kushi LH, Lamerato L, Leoce N, Nathanson SD, Ambrosone CB, Bovbjerg DH, Mandelblatt JS, Magai C, Tsai WY, Jacobson JS, Hershman DL (2012) Noninitiation of adjuvant chemotherapy in women with localized breast cancer: the breast cancer quality of care study. J Clin Oncol 30 (31):3800-3809. doi: 10.1200/JCO.2012.43.8168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wharam JF, Zhang F, Eggleston EM, Lu CY, Soumerai S, Ross-Degnan D (2017) Diabetes Outpatient Care and Acute Complications Before and After High-Deductible Insurance Enrollment: A Natural Experiment for Translation in Diabetes (NEXT-D) Study . JAMA Intern Med 177 (3):358-368. doi: 10.1001/jamainternmed.2016.8411 2596008 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wharam JF, Graves AJ, Zhang F, Soumerai SB, Ross-Degnan D, Landon BE (2012) Two-year trends in cancer screening among low socioeconomic status women in an HMO-based high-deductible health plan. J Gen Intern Med 27 (9):1112-1119. doi: 10.1007/s11606-012-2057-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiss SK, Ross-Degnan D, Zhang F, Soumerai SB, Zaslavsky AM, Wharam JF (2011) Effect of switching to a high-deductible health plan on use of chronic medications. Health Serv Res 46 (5):1382-1401. doi: 10.1111/j.1475-6773.2011.01252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wharam JF, Graves AJ, Landon BE, Zhang F, Soumerai SB, Ross-Degnan D (2011) Two-year trends in colorectal cancer screening after switch to a high-deductible health plan. Med Care 49 (9):865-871. doi: 10.1097/MLR.0b013e31821b35d8 [DOI] [PubMed] [Google Scholar]
- 33.Wharam JF, Galbraith AA, Kleinman KP, Soumerai SB, Ross-Degnan D, Landon BE (2008) Cancer screening before and after switching to a high-deductible health plan. Ann Intern Med 148 (9):647-655. doi:148/9/647 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.