Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening syndrome that occurs as a complication in many clinical settings. Malignancy-associated HLH develops in patients with hematopoietic neoplasms, particularly in those with lymphoma, and its development in those with myelodysplastic syndrome (MDS) is uncommon. We herein report a case of HLH in a patient with low-risk MDS that was successfully treated with azacitidine. The prevalence of immune abnormalities among MDS patients and the immune effects of azacitidine have recently been elucidated, suggesting that MDS-associated HLH occurs as a result of immune impairment, and azacitidine improves this condition by restoring the immune system.
Keywords: myelodysplastic syndrome, hemophagocytic lymphohistiocytosis, azacitidine
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare and life-threatening syndrome characterized by excessive cytokine release from activated macrophages and T cells and typically presents with a fever, pancytopenia, hepatosplenomegaly, liver dysfunction, and hyperferritinemia (1, 2). Primary HLH is hereditary immune dysregulation with impaired cytotoxic T lymphocyte (CTL) or natural killer (NK) cells (1, 3, 4), and secondary HLH occurs as a complication of many clinical conditions, such as infection, autoimmune disease, immunodeficiency, and malignancy (1-3). Malignancy-associated HLH (M-HLH) develops in patients with hematopoietic neoplasms, particularly in those with lymphoma (2, 5), and its development in those with myelodysplastic syndrome (MDS) is uncommon; therefore, the underlying pathogenesis is poorly understood, and treatment strategies for this condition are not established.
We herein report a case of HLH in a patient with low-risk MDS that was successfully treated with azacitidine.
Case Report
A 68-year-old Japanese man was referred to our department with prolonged pancytopenia for over 2 years. A hematological examination revealed a white blood cell count of 1,100/μL, hemoglobin concentration of 10.5 g/dL, and platelet count of 45,000/μL. Bone marrow aspirate indicated trilineage dysplasia with no excess of blasts (Fig. 1). A chromosomal analysis showed a normal karyotype. Based on these findings, we diagnosed him with MDS with multi-lineage dysplasia according to the International Prognosis Scoring System (IPSS) Int-1, WHO classification-based Prognosis Scoring System Intermediate, and Revised-IPSS Intermediate.
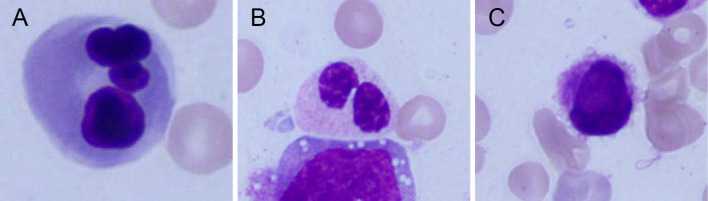
Figure 1.
Bone marrow smear specimen showing the presence of trilineage dysplasia. (A) multinucleated erythroblast, (B) pseudo-Pelger-Huët anomaly in neutrophil, (C) micromegakaryocyte.
We decided to wait and watch because the prognosis scoring systems that indicate a low risk and pancytopenia seem to plateau after two years. One month later, he developed sudden liver injury. A serum chemistry examination demonstrated elevation of hepatic enzyme levels as follows: aspartate aminotransferase (AST) of 217 U/L, alanine aminotransferase (ALT) of 289 U/L, and lactate dehydrogenase (LDH) of 553 U/L. He also had extreme elevation of serum ferritin at 24,316 ng/mL without red blood cell transfusion. In addition, he exhibited mild splenomegaly, slight elevation of soluble interleukin 2 receptor alpha (sIL-2Rα) at 1,025 U/mL, and hypofibrinogenemia at 121 mg/dL, but he was unexpectedly afebrile. These manifestations were indicative of HLH; therefore, we re-examined the bone marrow, and found a small number of hemophagocytic macrophages (Fig. 2). A liver biopsy specimen suggested non-alcoholic steatohepatitis (NASH), but activated macrophages engulfing erythrocytes, a typical finding of HLH in the liver, were not observed, and perivascular lymphoid infiltration was mild. The reactivation of Epstein-Barr virus, cytomegalovirus, and hepatitis B virus was not detected. Of the proposed HLH diagnostic criteria in 2009 (2), our patient exhibited splenomegaly, cytopenia, hepatitis, hemophagocytosis, elevated ferritin, elevated sIL-2Rα, and hypofibrinogenemia. However, it was difficult to judge whether the cytopenia was derived from MDS or HLH. Based on these findings, we suspected HLH, but we were unable to confirm the diagnosis. Furthermore, whether or not MDS was involved in the occurrence of HLH-like manifestations at that time was unclear.
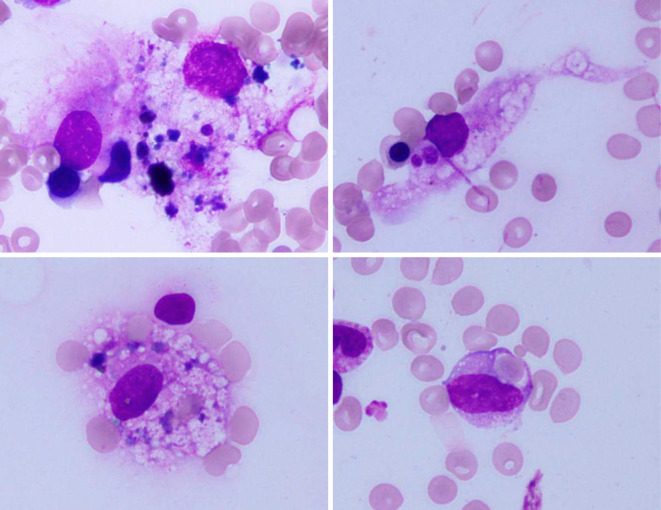
Figure 2.
Bone marrow smear specimen showing hemophagocytic macrophages.
One month after the onset of the HLH-like manifestations, high-dose methylprednisolone (1,000 mg for 3 days) was administered. He showed a slight reduction in serum AST, ALT, and LDH, but his liver dysfunction continued to progress, and the serum ferritin levels increased to 43,639 ng/mL (the clinical course is summarized in Fig. 3). The usefulness of highly elevated ferritin in the diagnosis of HLH has been reported (6). A maximum ferritin level over 10,000 ng/mL was found to have a specificity of 96% for the diagnosis of HLH. It was also shown that the rate of change in ferritin between admission and the maximum level was also higher in patients with HLH. Our patient had a maximum ferritin level far above 10,000 ng/mL and the rate of change in ferritin was 1,136 ng/mL/day. Based on these findings, we established a diagnosis of HLH.
Figure 3.
The clinical course. Sequential changes in the blood cell count (upper line chart), and serum AST, ALT, LDH, and ferritin levels (lower line chart) are shown. Ferritin is presented on a logarithmic scale. The patient received two cycles of HD-mPSL (1,000 mg for 3 days), oral PSL (starting with 60 mg and tapered afterwards), oral CsA (starting with 50 mg and gradually increased up to 150 mg), and 5 cycles of 5-Aza (75 mg per square meter of body-surface area on 7 consecutive days every 4 weeks). Dx: diagnosis, MDS: myelodysplastic syndrome, HLH: hemophagocytic lymphohistiocytosis, HD-mPSL: high-dose methylprednisolone, PSL: prednisolone, CsA: cyclosporine A, 5-Aza: azacitidine, WBC: white blood cell, Hb: hemoglobin, Plt: platelet, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase
Fourteen days after the initial treatment, the administration of cyclosporine A (CsA) was started with upward titration. The serum AST, ALT, LDH, and ferritin levels gradually declined subsequently but did not recede to normal levels. Eight months later, he developed a slight flare-up of HLH. NASH-like findings and a small number of hemophagocytic macrophages were observed in the re-examination of the liver biopsy and bone marrow aspiration, respectively. He received another high dose of methylprednisolone, and the serum ferritin level decreased but only to slightly below 2,000 ng/mL, suggesting difficulty of immunosuppressive treatment. We therefore hypothesized the involvement of MDS in the pathogenesis of HLH.
Decitabine has been reported to be effective against acute myeloid leukemia (AML)-associated HLH (7); we therefore administered azacitidine (75 mg per square meter of body-surface area on 7 consecutive days every 4 weeks) at 1 month after the second corticosteroid treatment. The serum AST, ALT, and LDH levels started to decrease within 10 days, and pancytopenia and hyperferritinemia improved after one cycle of azacitidine. Eventually, four cycles of azacitidine caused the blood cell count, serum AST, ALT, LDH, and ferritin to reach almost normal levels.
Discussion
HLH can accompany diverse malignancies (2, 5). In the present case, a stable patient with low-risk MDS suddenly developed HLH. Repeated treatment with corticosteroids and immunosuppressants was insufficient, whereas the administration of azacitidine resulted in prompt improvement. We may draw two conclusions from the clinical course of this patient.
First, HLH can occur in low-risk MDS patients via a unique mechanism. In general, M-HLH is known to develop in two different clinical situations (5, 8). [1] HLH occurs as a manifestation with disease onset or relapse (malignancy-triggered HLH). For this condition, the involvement of cytokines secreted by malignant cells and viral infections is assumed. [2] HLH occurs during any phase of chemotherapy (HLH during chemotherapy). In this setting, the infection of virus, bacteria, or fungus caused by immunosuppression helps trigger HLH. In our case, the patient had pancytopenia for at least two years before the diagnosis, indicating the presence of MDS for a long time before the occurrence of HLH. In addition, he was completely naïve to chemotherapy. These findings suggest a distinctive pathogenesis of MDS-associated HLH rather than conventional M-HLH. Immune dysregulation has been noted in several cell types derived from MDS patients, including T lymphocytes, NK cells, myeloid-derived suppressor cells, and mesenchymal stromal cells (9-11). For T lymphocytes, a variety of abnormalities have been reported, including numerical anomalies, changes in the repertoire of the T cell receptor, defective cytotoxicity, altered expression levels of Foxp3 or chemokine receptors, and deficiency of human telomerase reverse transcriptase. Furthermore, abnormalities in T cells can occur even if the clinical course of MDS is stable (12). In NK cells from patients with MDS, decreased cytotoxicity, reduced levels of perforin or granzyme B, and defective differentiation have been reported (13-15). To our knowledge, only one report has described MDS-associated HLH, and the patient with MDS-associated HLH had polyclonal expansion of CD8-positive T lymphocytes in the bone marrow (16). In contrast, the proportion of NK cells in our patient increased over time with serial bone marrow aspiration, especially during flare-up, but decreased after azacitidine treatment (Table). These findings indicate the involvement of an NK cell abnormality. A CTL or NK cell abnormality in the bone marrow was likely involved in the pathogenesis of MDS-associated HLH. In primary HLH, the decreased activity of CTL or NK cells constitutes the basis of the pathogenesis (1, 3, 4). These data suggest that MDS-associated HLH occurs via a mechanism similar to that of primary HLH rather than conventional M-HLH.
Table.
The Percentage of CD4-positive T Cells, CD8-positive T Cells, and NK Cells in the Bone Marrow.
| Dx of MDS | Dx of HLH | Flare-up of HLH | Remission of HLH | |
|---|---|---|---|---|
| CD4+T cells | 3.12% | 4.49% | 4.98% | 3.98% |
| CD8+T cells | 3.89% | 5.04% | 5.03% | 5.11% |
| NK cells | 4.42% | 5.81% | 36.7% | 7.04% |
The numerical values were calculated by flow cytometry. The percentage of NK cells was calculated as the difference between the CD2-positive fraction and the CD3-positive fraction. The number of NK cells markedly increased at the time of HLH flare-up and decreased after treatment with azacitidine, whereas the numbers of CD4- and CD8-positive T cells remained almost unchanged.
CD: cluster of differentiation, NK: natural killer, Dx: diagnosis, MDS: myelodysplastic syndrome, HLH: hemophagocytic lymphohistiocytosis
Second, azacitidine is effective against MDS-associated HLH. In general, treatment for primary HLH is performed according to the HLH-2004 protocol (17), and treatment for secondary HLH depends on the underlying conditions. For example, patients with lymphoma-associated HLH receive corticosteroids and CsA along with chemotherapy and often allogeneic hematopoietic stem cell transplantation (3, 5, 8). However, a treatment strategy has not been established for HLH associated with other hematological malignancies, including AML and MDS. AML patients with HLH have a significantly higher mortality rate than those without HLH (18), and nearly all of the case reports describing AML combined with HLH have had poor outcomes (19-21). To our knowledge, this is the first report describing the successful treatment of MDS-associated HLH. In a previous report about an MDS patient with HLH, the administration of corticosteroids and subsequent induction chemotherapy did not lead to improvement (16). The effectiveness of decitabine for HLH associated with AML was reported previously (7). Decitabine is a hypo-methylating agent used for the treatment of high-risk MDS and AML (22). Azacitidine is another hypo-methylating agent used for the treatment of those diseases and is the only hypo-methylating agent available in Japan (22, 23). Azacitidine is known to have multiple effects, including cytotoxicity due to incorporation into DNA, re-activation of silenced tumor suppressor genes via the inhibition of DNA methyltransferase, and effects on the immune system (23). Anti-MDS activity is mainly produced by the first two effects. For MDS-associated HLH, the contribution of the last effect should be considered based on the hypothesis that MDS-associated HLH occurs as a result of immune dysregulation. The immune effects caused by azacitidine include the immune response caused by azacitidine-induced immune genes, the upregulation of endogenous retroviruses in tumor cells, and the inhibition of the nuclear factor kappa B pathway (23). In addition, azacitidine reverses aberrant T cell receptor repertoires observed in AML and MDS patients (24). Azacitidine was also found to reverse the aberrant killer-cell immunoglobulin-like receptor repertoire and restore the impaired cytotoxic activity of NK cells in MDS patients (25). These findings suggest that azacitidine is therapeutically effective for MDS-associated HLH not only due to cytotoxic effects against MDS clones but also due to the immune restoration of CTLs and NK cells.
In conclusion, the present case demonstrated the occurrence of HLH in low-risk MDS and showed that azacitidine is effective against MDS-associated HLH. These findings suggest that HLH results from immune dysregulation and that azacitidine improves the disease by restoring the immune system in MDS patients. More clinical cases are needed to understand the pathogenesis and develop treatment strategies for MDS-associated HLH.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Yusuke Morita and his colleagues in the Department of Hepatology and Pancreatology, Kyushu University Hospital for treating the present patient at the beginning of CsA administration.
References
- 1.Aricò M, Danesino C, Pende D, Moretta L. Pathogenesis of haemophagocytic lymphohistiocytosis. Br J Haematol 114: 761-769, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology Am Soc Hematol Educ Program 127-131, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood 125: 2908-2914, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Cetica V, Pende D, Griffiths GM, Aricò M. Molecular basis of familial hemophagocytic lymphohistiocytosis. Haematologica 95: 538-541, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Xiong L, Tang W, Zhou Y, Li F. A systematic review of malignancy-associated hemophagocytic lymphohistiocytosis that needs more attentions. Oncotarget 8: 59977-59985, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen CE, Yu X, Kozinetz CA, McClain KL. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 50: 1227-1235, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Mulay S, Bauer F, Boruchov A, Bilgrami S. Successful resolution of acute myelogenous leukemia-associated hemophagocytic lymphohistiocytosis with decitabine. Leuk Lymphoma 52: 341-343, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Lehmberg K, Nichols KE, Henter JI, et al. Consensus recommendations for the diagnosis and management of hemophagocytic lymphohistiocytosis associated with malignancies. Haematologica 100: 997-1004, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fozza C, Longinotti M. Are T-cell dysfunctions the other side of the moon in the pathogenesis of myelodysplastic syndromes? Eur J Haematol 88: 380-387, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Gañán-Gómez I, Wei Y, Starczynowski DT, et al. Deregulation of innate immune and inflammatory signaling in myelodysplastic syndromes. Leukemia 29: 1458-1469, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glenthøj A, Ørskov AD, Hansen JW, Hadrup SR, O'Connell C, Grønbæk K. Immune mechanisms in myelodysplastic syndrome. Int J Mol Sci 17: 944, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sand KE, Rye KP, Mannsåker B, Bruserud O, Kittang AO. Expression patterns of chemokine receptors on circulating T cells from myelodysplastic syndrome patients. Oncoimmunology 2: e23138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiladjian JJ, Bourgeois E, Lobe I, et al. Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia 20: 463-470, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Epling-Burnette PK, Bai F, Painter JS, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 109: 4816-4824, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hejazi M, Manser AR, Fröbel J, et al. Impaired cytotoxicity associated with defective natural killer cell differentiation in myelodysplastic syndromes. Haematologica 100: 643-652, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuji T, Yamasaki H, Arima N, Tsuda H. Hemophagocytic lymphohistiocytosis associated with myelodysplastic syndromes. Int J Hematol 92: 547-549, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 48: 124-131, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Delavigne K, Bérard E, Bertoli S, et al. Hemophagocytic syndrome in patients with acute myeloid leukemia undergoing intensive chemotherapy. Haematologica 99: 474-480, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Matsugama M. Refractory hemophagocytic syndrome in a patient with acute myelocytic leukemia. Blood 121: 2820, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Akyay A, Celkan T, Iskender G, Tükün A, Ogan C, Olcay L. Two acute myeloblastic leukemia cases concomitant with hemophagocytic lymphohistiocytosis and review of the literature. Ann Clin Lab Sci 43: 85-90, 2013. [PubMed] [Google Scholar]
- 21.Yamada A, Moritake H, Sawa D, et al. Refractory acute myeloid leukemia developed malignancy-associated hemophagocytic lymphohistiocytosis during treatment of invasive fungal infection. Rinsho Ketsueki (Jpn J Clin Hematol) 54: 383-387, 2013(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 22.Griffiths EA, Gore SD. Epigenetic therapies in MDS and AML. Adv Exp Med Biol 754: 253-283, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diesch J, Zwick A, Garz AK, Palau A, Buschbeck M, Götze KS. A clinical-molecular update on azanucleoside-based therapy for the treatment of hematologic cancers. Clin Epigenetics 8: 71, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fozza C, Corda G, Barraqueddu F, et al. Azacitidine improves the T-cell repertoire in patients with myelodysplastic syndromes and acute myeloid leukemia with multilineage dysplasia. Leuk Res 39: 957-963, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Sohlberg E, Pfefferle A, Andersson S, Baumann BC, Hellström-Lindberg E, Malmberg KJ. Imprint of 5-azacytidine on the natural killer cell repertoire during systemic treatment for high-risk myelodysplastic syndrome. Oncotarget 6: 34178-34190, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]