Abstract
A 65-year-old man was diagnosed with advanced non-small, non-squamous lung cancer. He was treated with chemotherapy containing bevacizumab as well as cisplatin and pemetrexed. After 2 courses of treatment, computed tomography revealed that his abdominal aortic artery was almost occluded by a thrombus; however, he had no ischemic symptoms. Heparin infusion and warfarin reduced the size of the arterial thrombus and the patient was subsequently treated with chemotherapy without bevacizumab. No thrombotic events occurred during the subsequent treatment. We later noticed a small organized abdominal arterial clot and calcification on a computed tomography scan taken before bevacizumab treatment. Atherosclerotic changes should be evaluated before the administration of bevacizumab.
Keywords: non-small, non-squamous lung cancer, bevacizumab, arterial thrombosis
Introduction
Bevacizumab (AvastinⓇ; Genentech, South San Francisco, USA), a humanized mAb that neutralizes vascular endothelial growth factor (VEGF), has been approved for the treatment of many advanced cancers, including non-small cell lung cancer (NSCLC) (1, 2). Combination treatment with bevacizumab and chemotherapy has improved the survival of patients with previously untreated NSCLC (2). It is well-known that an increased incidence of arterial and venous thromboembolism is observed among patients receiving bevacizumab (3). We herein report a case of NSCLC with a massive abdominal aortic thrombus - not angina pectoris or myocardial infarction - that occurred due to the administration of a chemotherapy regimen containing bevacizumab.
Case Report
A 65-year-old man visited the urology department of our hospital to determine the cause an elevated serum prostate specific antigen level. Because left pleural effusion was found on a chest X-ray during a prostate examination (Fig. 1A), the patient was referred to the Department of Pulmonary and Infectious Diseases. The patient was a current smoker with a history of chronic obstructive pulmonary disease (COPD) and atrial fibrillation with medications including aspirin and tiotropium. A physical examination revealed a low body mass index (15.6 kg/m2), no cervical, axillary, or inguinal lymph adenopathy, and decreased left breath sounds on auscultation. No abnormal findings were observed on ear, nose, throat, or abdominal examinations. A peripheral blood cell count revealed the absence of leukocytosis, anemia, and thrombocytosis. The patient's serum carcinoembryonic antigen (CEA) level was elevated to 817 ng/mL (normal range, 0-5 ng/mL).
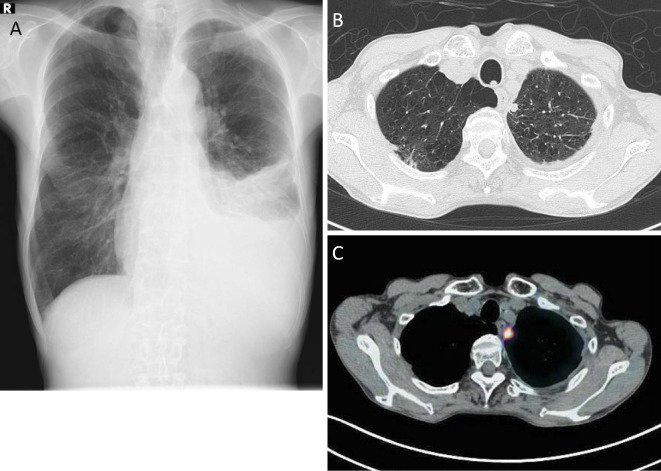
Figure 1.
A: Chest X ray revealed left pleural effusion. B: Chest CT showed emphysematous changes throughout both lung fields, left pleural effusion, and a small nodule of approximately 1 cm in diameter, adjacent to the pleura in the left lung apex. C: A small nodule was the sole location that showed a high uptake on fludeoxyglucose-positron emission tomography CT.
Chest CT revealed emphysematous changes throughout both lung fields, left pleural effusion, and a small nodule of approximately 1 cm in diameter adjacent to the pleura in the left lung apex (Fig. 1B), which was the sole region that showed a high uptake on fludeoxyglucose-positron emission tomography (FDG-PET)-CT (Fig. 1C). The lactate dehydrogenase and CEA levels of his left pleural fluid were 785 IU/L and 6,660 ng/mL, respectively. A cytological examination of the pleural effusion showed malignant cells, suggesting non-small, non-squamous cell carcinoma; however, a histopathological specimen was not obtained from the nodule in his left lung apex. Upper gastrointestinal endoscopy and colonoscopy demonstrated normal findings. Taken together, we diagnosed the patient with non-small, non-squamous lung cancer cT1aN0M1a, Stage 4 based on the TNM classification (7th edition). No epidermal growth factor mutations were detected in the malignant cells in the pleural effusion. One cycle of cisplatin (80 mg/m2), pemetrexed (500 mg/m2), and bevacizumab (15 mg/kg) was administered as the first-line chemotherapy. After two cycles of the regimen, we evaluated the response on CT and found no change in the size of the left lung nodule or the amount of left pleural effusion. The same CT scan revealed that his abdominal aortic artery was almost occluded by a newly formed thrombus from the level below the renal arteries to the common iliac arteries (Fig. 2A and B). A laboratory analysis revealed thrombocytosis with an elevated D dimer level (1.4 μg/mL). He had had no symptoms of abdominal or limb ischemia (defined as Grade ≥2 according to the Common Terminology Criteria for Adverse Events v4.0). Echocardiography showed a normal left ventricular systolic function without any mural thrombus, which suggested that the aortic thrombosis was solely induced by bevacizumab.
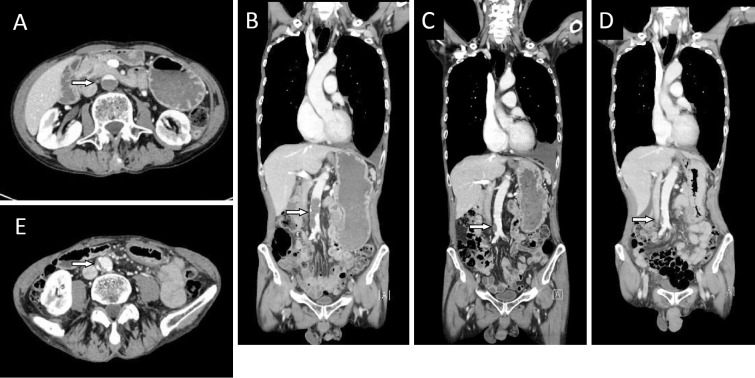
Figure 2.
A: CT (axial view) after 2 courses of bevacizumab-containing chemotherapy showed that the abdominal aortic artery was almost occluded by a newly formed thrombus (arrow) from the level below the renal arteries to the common iliac arteries. B: CT (coronal view) at the same time of 2A demonstrated the newly formed thrombus (arrow). C: After heparin and warfarin therapy, the size of the aortic arterial thrombus (arrow) was reduced. Increased left pleural effusion was found. D: A small mural thrombus with calcification in the abdominal aorta was noted on imaging obtained before the initiation of bevacizumab-containing chemotherapy (arrow). E: CT (axial view) before the initiation of chemotherapy showed a mural thrombus (arrow).
Heparin and warfarin were started immediately after the recognition of the thrombosis, and we confirmed the reduction of the size of the aortic arterial thrombus after the initiation of this treatment (Fig. 2C). We then restarted four courses of chemotherapy with cisplatin and pemetrexed, but without bevacizumab, followed by pemetrexed maintenance therapy. During maintenance therapy, his lung nodule regrew in size and different pleural nodules appeared. However, there was no recurrence of his abdominal aortic thrombus. A retrospective investigation of the imaging findings detected a small mural thrombus and calcification of the abdominal aorta that was present prior to the initiation of the bevacizumab-containing chemotherapy regimen (Fig. 2D and E). He was further treated with nivolumab and warfarin, which maintained a partial response; no recurrence of aortic thrombus was detected.
Discussion
It is well known that many conditions, including malignancies, hypercoagulative disorders, iatrogenic causes such as aortic catheterization, and infective or genetic disorders of the aortic wall may induce thromboembolic events. Trousseau syndrome is a hypercoagulable state associated with cancer, which induces unexplained thrombotic events that either precede the diagnosis of an occult visceral malignancy or which appear concomitantly with the tumor (4). This patient was not diagnosed with Trousseau syndrome because the thrombus, which almost completely occluded the abdominal aorta, became obvious after the initiation of bevacizumab-containing chemotherapy. Rather, we consider that the patient's atrial fibrillation was partially attributed the formation of the embolism in this case; however, it would have been difficult to predict an embolism based on his low CHADS2 score (0 point), the absence of a thrombus distal to the abdominal aortic thrombus, the absence of a detectable thrombus on echocardiography, and the complete absence of symptoms during the clinical course. A hypercoagulative state with dehydration caused by a loss of appetite during chemotherapy might affect thrombosis formation. The patient was considered to have a high risk of emesis and was treated accordingly; no dehydration or renal dysfunction was observed during the course of treatment. A series of CT scans revealed that the plaque with calcification in the lower abdominal aorta persisted during therapy in the present case. It should be noted that local calcifications might have been - at least in part - the origin of the thrombus formation.
Bevacizumab, a humanized monoclonal antibody that targets VEGF, is approved for the treatment of non-squamous non-small lung cancer (2). It is associated with serious adverse events, including hemorrhage and gastrointestinal perforation, as well as common events, such as hypertension and proteinuria (5). One of the serious complications of bevacizumab is an increased risk of arterial thromboembolic events (3). Študentová et al. demonstrated that anti-VEGF therapy affected the laboratory risk factors for atherosclerosis and resulted in the acceleration of atherosclerosis, in patients with metastatic colorectal carcinoma or metastatic renal cell carcinoma prior to and at 3-month intervals during anti-VEGF treatment (6). In this case, the period of thrombus formation was too short to consider the effects of the acceleration of atherosclerosis by bevacizumab. Meyer et al. reported compelling evidence that complex formation with VEGF and the activation of the platelet FccRIIa receptors directly induced platelet aggregation and granule release in vitro and caused thrombocytopenia and thrombosis in vivo (7). Chemotherapy-induced thrombin generation and endothelial damage might amplify the subclinical changes in the coagulation/fibrinolytic system. Increased thrombin activation and thrombocytosis, which occurred after chemotherapy, could play a synergistic role in triggering thrombotic events (8). To date, the molecular mechanisms involved in bevacizumab-related thromboembolisms remain poorly understood.
Matsumura et al. reported that 6,076 cancer patients had adverse events in association with bevacizumab treatment between January 2004 and January 2015, and that 233 (3.8%) developed arterial thromboembolic events (9). Age ≥70 years and a history of either hypertension or diabetes mellitus were risk factors for arterial thromboembolic events as well as the presence of cancer. The median cumulative times of onset for arterial thromboembolic and venous thromboembolic events were 60 and 80 days, respectively. According to Scappaticci et al., baseline risk factors for the occurrence of an arterial thromboembolic event included exposure to bevacizumab, age ≥65 years, hypertension at baseline, history of an arterial thromboembolic event, history of atherosclerosis and history of myocardial infarction (3). Our patient was 65 years of age, which indicates that had a high risk of developing bevacizumab-induced arterial thromboembolisms. A retrospective analysis of a CT scan of the abdominal aorta before the initiation of bevacizumab-containing chemotherapy showed a small mural thrombus that was probably caused by arteriosclerosis because of his smoking history and the presence of calcification near the thrombus, which was detected by abdominal CT. The clinical course of the present case suggests that bevacizumab accelerate the growth of a pre-existing thrombus to form a massive thrombus. Single nucleotide polymorphisms (SNPs) related to hereditary thrombophilia have been investigated as risk factors for thromboembolism in cancer patients (10). Further studies would be useful for clarifying risk factors for thromboembolism in lung cancer patients for whom chemotherapy with bevacizumab is planned.
The efficacy of aspirin in preventing arterial thromboembolism in bevacizumab-containing chemotherapy is controversial. Scappaticci et al. reported that aspirin use was associated with an approximately 1.3-fold increase in the incidence of grade 3 and 4 bleeding events but that the difference did not reach statistical significance (3). Tebutts et al. reported that the rate of arterial thromboembolism was increased in the patients using aspirin. However, the aspirin user group included a higher proportion of patients with cardiac risk factors or a history of arterial thromboembolism (11). The use of low-dose aspirin as prophylaxis against arterial thromboembolic events in high-risk patients is supported by an extensive body of literature (12) and is a recommended standard of care (13). In our patient, aspirin failed to prevent massive aortic thrombosis formation. Low-dose aspirin might not be beneficial as prophylaxis against arterial thromoembolism during bevacizumab containing chemotherapy in high-risk patients.
Robaldo et al. reported a rare case of acute ascending thrombosis of the abdominal and suprarenal aorta who underwent an emergency operation (14). Yoon et al. also reported a case with a large thrombus in the thoracic aorta during the administration of bevacizumab containing chemotherapy (15). In our case, heparin infusion and warfarin prevented the further growth of the thrombus. A partial response was then maintained by treatment with nivolumab, an immune checkpoint inhibitor, without a recurrence of thrombus. Although aortic thrombi may form in various disorders, arterial diseases, including calcification, should be evaluated before the administration of bevacizumab, even when a patient presents no symptoms related to arterial occlusion. If such a condition is observed, the treatment that includes bevacizumab should be carefully considered and should only be administered when the benefit is considered to considerably outweigh the risk of thrombus formation.
In conclusion, bevacizumab can cause unexpected arterial thromboembolic events in high-risk patients. Low-dose aspirin alone might fail to prevent the development of arterial thromboembolisms during treatment with bevacizumab containing chemotherapy. The administration of chemotherapy regimens that include bevacizumab should be carefully administered based on the risks and benefits in high-risk patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335-2342, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542-2550, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 99: 1232-1239, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood 110: 1723-1729, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crino L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol 11: 733-740, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Študentová H, Indráková J, Petrová P, et al. Risk factors of atherosclerosis during systemic therapy targeting vascular endothelial growth factor. Oncol Lett 11: 939-944, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer T, Robles-Carrillo L, Robson T, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost 7: 171-181, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Zecchina G, Ghio P, Bosio S, Cravino M, Camaschella C, Scagliotti GV. Reactive thrombocytosis might contribute to chemotherapy-related thrombophilia in patients with lung cancer. Clin Lung Cancer 8: 264-267, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura C, Chisaki Y, Sakimoto S, Sakae H, Yano Y. Evaluation of thromboembolic events in cancer patients receiving bevacizumab according to the Japanese Adverse Drug Event Report database. J Oncol Pharm Pract 24: 22-27, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Falvella FS, Cremolini C, Miceli R, et al. Variant alleles in factor V, prothrombin, plasminogen activator inhibitor-1, methylenetetrahydrofolate reductase and risk of thromboembolism in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab. Pharmacogenomics J 17: 331-336, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Tebbutt NC, Murphy F, Zannino D, et al. ; Australasian Gastro-Intestinal Trials Group.. Risk of arterial thromboembolic events in patients with advanced colorectal cancer receiving bevacizumab. Ann Oncol 22: 1834-1838, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126: 234S-264S, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Tran H, Anand SS. Oral antiplatelet therapy in cerebrovascular disease, coronary artery disease, and peripheral arterial disease. JAMA 292: 1867-1874, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Robaldo A, Pagliari S, Colotto P. Acute ascending thrombosis of abdominal and suprarenal aorta. Case Rep Surg 2014: 348064, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon S, Schmassmann-Suhijar D, Zuber M, Konietzny P, Schmassmann A. Chemotherapy with bevacizumab, irinotecan, 5-fluorouracil and leucovorin (IFL) associated with a large, embolizing thrombus in the thoracic aorta. Ann Oncol 17: 1851-1852, 2006. [DOI] [PubMed] [Google Scholar]