Abstract
We herein report a rare case of methotrexate (MTX)-associated intravascular large B-cell lymphoma (IVLBCL) in a man with rheumatoid arthritis. Two episodes of a fever of unknown origin accompanied by elevated levels of serum lactate dehydrogenase and the soluble interleukin-2 receptor occurred within a year, so the patient was suspected of having an MTX-associated lymphoproliferative disorder. His clinical symptoms resolved after the cessation of MTX. However, after treatment with iguratimod, another disease-modified anti-rheumatic drug, markedly similar symptoms recurred, and random skin biopsies resulted in a diagnosis of IVLBCL. The patient received a rituximab-containing chemotherapy and achieved complete remission.
Keywords: methotrexate, intravascular large B-cell lymphoma, rheumatoid arthritis
Introduction
Methotrexate-associated lymphoproliferative disorders (MTX-LPD) are iatrogenic conditions categorized as “other iatrogenic immunodeficiency-associated LPD” (OIIA-LPD) in the revised 4th edition of the World Health Organization’s (WHO) classification. Low-dose MTX is widely known to be capable of causing LPD in patients with rheumatoid arthritis (RA), which is described as MTX-LPD, a major type of OIIA-LPD. Diffuse large B-cell lymphoma (DLBCL) is the most common type of MTX-LPD (35-60%), followed by classical Hodgkin’s lymphoma (12-25%) (1-4).
Intravascular large B-cell lymphoma (IVLBCL), which was first reported by Pfleger et al. (5) in 1959, exhibits the unique pathological characteristic of restricted growth in the blood vessel lumina. This rare type of DLBCL is often difficult to diagnose and displays aggressive behavior (6, 7).
Kikuchi et al. reported the first case of MTX-associated IVLBCL in 2015, in which remission was maintained for two years after chemotherapy (8). Kida et al. described a similar IVLBCL case that might have been caused by MTX (9).
We herein report the third case in which an RA patient developed MTX-associated IVLBCL. Complete remission (CR) was achieved by treating the patient with standard chemotherapy including rituximab.
Case Report
A 76-year-old man with RA, who had been treated with MTX for more than 10 years, was admitted to our hospital complaining of dyspnea and pyrexia. Although IVLBCL was suspected based on his elevated levels of serum lactate dehydrogenase (LDH; 470 U/L) and the soluble interleukin-2 receptor (sIL-2R; 4,220 U/mL) and a decreased platelet count (3.6×104/mm3), skin and bone marrow biopsies did not result in a diagnosis of malignant lymphoma (ML). His symptoms were rapidly relieved, and his laboratory findings were normalized by the discontinuation of MTX and temporary administration of oral prednisolone. Five months later, markedly similar symptoms appeared again just after the resumption of MTX therapy. While bone marrow sampling did not reveal any noteworthy pathological findings, the patient soon recovered after the MTX treatment was stopped.
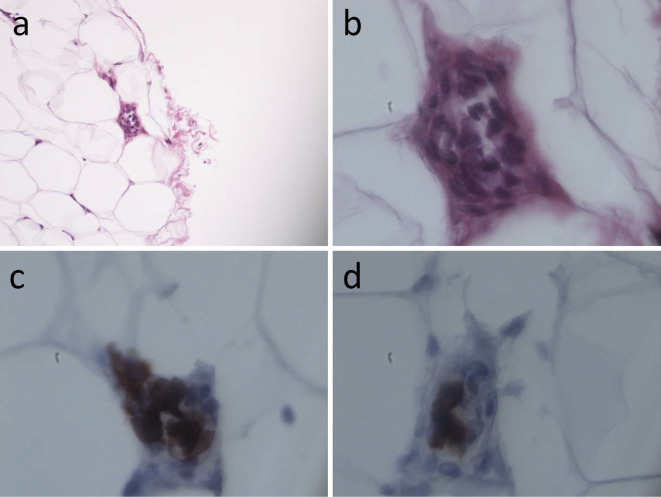
Eight months later, at three months after the start of treatment with the disease-modified anti-rheumatic drug (DMARDS) iguratimod, the patient was readmitted to our hospital due to the recurrence of similar symptoms and abnormal laboratory findings (Table 1). Throughout these periods, neither lymphadenopathy nor hepatosplenomegaly was detected by whole-body computer tomography (CT) scan. Therefore, skin biopsy examinations of the normal-appearing truncal and femoral regions were performed, which detected atypical lymphoid cell proliferation within the small blood vessels in the subcutaneous fat in 1 out of 10 resected specimens (Fig. 1a and b). These cells were positive for CD20 and CD79α (Fig. 1c and d) and negative for CD3 (data not shown). Epstein-Barr virus (EBV) was not detected by in situ hybridization (data not shown). Finally, a diagnosis of IVBCL was made.
Table 1.
Laboratory Data at Admission.
| WBC | 5,600 | /uL | TP | 6.6 | g/dL | BNP | 12.4 | pg/mL |
| Neu | 69 | % | Alb | 3.0 | g/dL | Ferittin | 931 | ng/mL |
| Lym | 15 | % | T-Bil | 0.8 | mg/dL | sIL-2R | 1,705 | U/mL |
| Mono | 16 | % | BUN | 40.4 | mg/dL | Anti-CCP antibody | 374 | U/mL |
| Eo | 0 | % | Cr | 1.11 | mg/dL | Rheumatoid factor | 39 | IU/mL |
| Baso | 0 | % | UA | 8.9 | mg/dL | EBV-DNA not detected | ||
| RBC | 407×106 | /uL | AST | 36 | U/L | |||
| Hb | 13.1 | g/dL | ALT | 25 | U/L | IgG | 1,297 | mg/dL |
| Hct | 38.9 | % | LDH | 439 | U/L | IgA | 436 | mg/dL |
| MCV | 95.5 | % | ALP | 318 | U/L | IgM | 86 | mg/dL |
| RET | 0.67 | % | γ-GTP | 15 | U/L | ANA | (-) | |
| Plt | 4.2×104 | /uL | CPK | 16 | U/L | |||
| Na | 137 | mEq/L | ||||||
| PT (INR) | 1.25 | K | 4.5 | mEq/L | ||||
| APTT | 30.5 | sec | Cl | 103 | mEq/L | |||
| Fib | 400 | mg/dL | Ca | 8.4 | mg/dL | |||
| FDP | 2.7 | μg/mL | CRP | 4.29 | mg/dL | |||
| BS | 113 | mg/dL | ||||||
| HbA1c | 5.6 | mg/dL |
BNP: brain natriuretic peptide, CCP: citrullinated protein, EBV: Epstein-Barr virus, ANA: antinuclear antibody
Figure 1.
The pathological findings of the skin biopsy. (a, b) Atypical lymphoid cells had proliferated within the small vessels present in the subcutaneous fat. Immunohistochemical staining showed that these cells were positive for CD20 (c) and CD79α (d). a: ×100, b-d: ×1,000
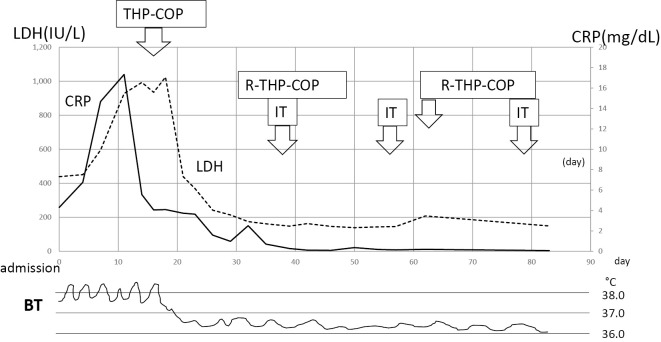
The patient received a total of 6 courses of rituximab, pirarubicin, cyclophosphamide, vincristine, and prednisolone (R-THP-COP) chemotherapy (375 mg/m2 rituximab, 30 mg/body pirarubicin, 500 mg/m2 cyclophosphamide, 1 mg/body vincristine, and 30 mg/body prednisolone for 5 days). He also received 3 rounds of intrathecal MTX (12 mg) plus dexamethasone (4 mg) infusions, followed by 1 course of high-dose MTX (3.5 g/m2) therapy to prevent meningeal or cerebral invasion, as some atypical lymphoid cells were detected in his spinal fluid. He achieved CR after the first course of R-THP-COP treatment, and is still in CR at 2 months after the end of therapy (Fig. 2).
Figure 2.
The patient's clinical course during the hospitalization. R-THP-COP: rituximab, pirarubicin, cyclophosphamide, and prednisolone, IT: intrathecal MTX plus dexamethasone infusions
Discussion
The revised 4th edition of the WHO classification proposed the entity of immunodeficiency-associated LPD, along with the following four categories of LPD: LPD with primary immune disorders, LPD associated with human immunodeficiency virus infection, post-transplant LPD, and OIIA-LPDs, which are mainly caused by MTX. It is widely acknowledged that spontaneous regression occurs in up to 59% of cases of MTX-LPD (10) after the withdrawal of MTX, and the relapse of MTX-LPD occurs in around half of cases after LPD regression (3, 10). Based on these clinical features, three distinct patterns of OIIA-LPD have been proposed by Tokuhira et al.: type I, MTX-regressive LPD; type II, MTX-persistent-LPD; and type III, other-mediated LPD (4). The present case exhibited unique behavior and is considered to belong to “type III: other-mediated LPD”, which display clinical manifestations such as a development of fever, infection, or lymphadenopathy after the discontinuation of MTX.
It was stated that LPD can be caused by various immunomodulatory or immunosuppressive drugs, such as azathioprine, bucillamine, salazosulfapyridine, tacrolimus, and tacrolimus+etanercept, all of which were used to control RA activity after the discontinuation of MTX it that study. Furthermore, two out of five cases were diagnosed with DLBCL. Although CR was achieved in three out of five cases, the overall survival of this group was similar to that of MTX-persistent LPD (4). In addition to low-dose MTX, other immunomodulatory agents, such as tumor-necrosis factor, have been reported to be potent inducers of OIIA-LPD (11). Iguratimod, which is a DMARS, suppresses the antibody production by B-lymphocytes, but does not influence the activity of T-lymphocytes (12). Indeed, no cases of iguratimod-induced OIIA-LPD have been reported. Furthermore, Inui et al. found that the use of anti-RA drugs after MTX did not influence the risk of MTX-LPD (13). Therefore, whether or not iguratimod contributed to the clinical course seen in the present case is unclear.
Simon et al. reported that the total pooled standardized incidence ratio of lymphoma in RA was 2.46 (14), and factors such as the RA activity or inflammatory reactions might be related to the development of LPD (15). In contrast, Yoshida et al. showed that MTX-LPD occurred after a relatively short period of RA or MTX treatment (16), as was found by Yamakawa et al. (17) and Tokuhira et al. (3), and mentioned that MTX-LPD occurred independently of inflammation or accumulated drug toxicity. The current case did not involve any significant clinical or laboratory findings that were suggestive of severe RA or inflammation, even after the discontinuation of MTX. In addition to the RA activity and duration of MTX treatment (8 mg/week for 10 years, which was longer than that described in previous reports), aging, which is another risk factor for LPD onset (11), might also play a role in the development of DLBCL after the discontinuation of MTX.
EBV involvement has been detected in around half of the analyzed cases of OIIA-LPD (3, 10, 18), and EBV positivity was found to be predictive of LPD regression (10, 18). As for the histological classification of OIIA-LPD, DLBCL is the most common type (1, 10), and around half of cases of this major subtype are also associated with EBV infection (3). In the present case, EBV was not detected by histological examinations or polymerase chain reaction assays involving whole-blood cells. In the first and second episodes of the patient’s condition, although no definitive diagnosis of LPD or DLBCL was obtained, MTX-regressive LPD was suspected because the patient’s abnormal clinical findings temporarily resolved after the discontinuation of MTX. However, LPD broke out again in the form of DLBCL, possibly due to insufficient immunological surveillance related to the absence of EBV.
IVLBCL is a rare and aggressive type of DLBCL with non-specific clinical features. It presents as a systemic disease involving a fever or malaise and can be life-threatening if the diagnosis is delayed (6). Ferreri et al. reported that 24-40% of IVLBCL patients have skin lesions, such as red nodules and/or plaque formation, on their legs or trunk (7). In the first reported case of MTX-LPD, because spreading painful erythema was seen on the patient’s legs, a pathological diagnosis of IVBCL was speedily made based on a biopsy examination of the rash (8). However, more than half of IVLBCL cases lack any dermatological manifestations, similar to our case. In such cases, a random skin biopsy serves as an important diagnostic tool for obtaining a diagnosis of IVLBCL. Pongpudpunth et al. recommended a 4 mm-deep punch biopsy involving a minimum of three specimens in order to achieve high sensitivity (19). Higashi et al. suggested that obtaining biopsy specimens at three different locations (the upper arm, thigh, and abdomen) is ideal for improving the diagnostic reliability (20). Pathologists should also be aware of the presence of IVLBCL inside the lumina of the capillaries or arterioles present in the subcutaneous fat in the deep dermis. Furthermore, immunohistochemical staining is particularly helpful for detecting atypical B-lymphoid cells. In the present case, lymphoma cell infiltration was only detected in 1 of the 10 random skin biopsy specimens, suggesting that multiple skin biopsies are required to establish a diagnosis if certain clinical findings, such as a fever of unknown origin together with an elevated LDH and/or sIL-2R level, are observed. Besides a pathological diagnosis, an early clinical diagnostic strategy might be another way of making decisions, as time is a limiting factor for most IVLBCL cases (21).
Thus far, only two cases of MTX-associated IVLBCL have been reported, and they are summarized in Table 2. In all three-cases recorded so far (the two previous and the present), the diagnosis was made by a skin biopsy, and combined chemotherapy was successful for achieving CR. EBV infections were not detected in any of the three cases, but central nervous system (CNS) invasion was noted in two cases. The brain is another common site of IVLBCL, and as many as one-third of cases present with neurological symptoms at the diagnosis (7). In addition, CNS involvement is detected on neuroimaging only in around half of examined cases. In our case, no specific neurological symptoms were observed, altough a cytological examination of the patient’s spinal fluid suggested the possibility of meningeal invasion. Shimada et al. reported that skin involvement at the initial diagnosis was a predictive factor of CNS recurrence (22). Therefore, consolidative therapy using high-dose MTX was applied to reduce the risk of recurrence, especially that of CNS lesions.
Table 2.
MTX-induced IVLBCL in the Literature.
| Sex, age | Symptoms | CNS invasion | Diagnosis | EBV infection | Anti-Rheumatoid drugs | Interval from MTX to LPD | Therapy | Response | References |
|---|---|---|---|---|---|---|---|---|---|
| F, 50Y | Fever, eruption | Y | Skin | N | MTX+Etanercept | 3 Y | R-CHOP+IT | CR | 8 |
| F, 75Y | Fever | Y | Skin | N | MTX | 2M | R-HyperCVAD/MA | CR | 9 |
| M,77Y | Fever | N | Skin | N | MTX, Iguratimod | 10Y | R-THP-COP | CR | Present case |
R-CHOP: rituximab, doxorubicin, cyclophosphamide, vincristine, and prednisolone, R-THP-COP: rituximab, pirarubicin, cyclophosphamide, vincristine, and prednisolone, CVAD/MA: cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate and cytarabine, CR: complete remission
Shimada et al. summarized the prognosis of IVLBCL in Japanese patients and demonstrated significantly higher progression-free survival and overall survival in the rituximab-containing chemotherapy group than in the non-rituximab-containing chemotherapy group (23). Nevertheless, the 2-year relapse-free rate of IVLBCL after rituximab-containing chemotherapy is reported to be approximately 40%. As such, further careful observation of our patient is required in the future.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Gion Y, Iwaki N, Takata K, et al. Clinicopathological analysis of methotrexate-associated lymphoproliferative disorders: comparison of diffuse large B-cell lymphoma and classical Hodgkin lymphoma types. Cancer Sci 108: 1271-1280, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maegawa S, Kuroda J, Kobayashi T, et al. Clinical manifestation and prognostic factors of 32 Japanese patients with autoimmune disease-associated diffuse large B-cell lymphoma. Leuk Lymphoma 56: 785-788, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Tokuhira M, Saito S, Okuyama A, et al. Clinicopathologic investigation of methotrexate-induced lymphoproliferative disorders, with a focus on regression. Leuk Lymphoma 59: 1143-1152, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Tokuhira M, Watanabe R, Nemoto T, et al. Clinicopathological analyses in patients with other iatrogenic immunodeficiency-associated lymphoproliferative diseases and rheumatoid arthritis. Leuk Lymphoma 53: 616-623, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Pfleger L, Tappeiner J. [On the recognition of systematized endotheliomatosis of the cutaneous blood vessels (reticuloendotheliosis?)]. Hautarzt 10: 359-363, 1959(in German). [PubMed] [Google Scholar]
- 6.Bogomolski-Yahalom V, Lossos IS, Okun E, Sherman Y, Lossos A, Polliack A. Intravascular lymphomatosis-an indolent or aggressive entity? Leuk Lymphoma 29: 585-593, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant'. Br J Haematol 127: 173-183, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi J, Kaneko Y, Kasahara H, et al. Methotrexate-associated intravascular large B-cell lymphoma in a patient with rheumatoid arthritis. Intern Med 55: 1661-1665, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Kida T, Kohno M, Chinen Y, et al. Intravascular lymphoma in a rheumatoid arthritis patient following short-term methotrexate treatment. Rheumatology (Oxford) 56: 318-320, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa A, Arakawa F, Kiyasu J, et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol 91: 20-28, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto A, Chiba N, Tsuno H, et al. Incidence of malignancy and the risk of lymphoma in Japanese patients with rheumatoid arthritis compared to the general population. J Rheumatol 42: 564-571, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Yamaguchi T, Hara M. Iguratimod for the treatment of rheumatoid arthritis in Japan. Expert Rev Clin Immunol 11: 565-573, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Inui Y, Matsuoka H, Yakushijin K, et al. Methotrexate-associated lymphoproliferative disorders: management by watchful waiting and observation of early lymphocyte recovery after methotrexate withdrawal. Leuk Lymphoma 56: 3045-3051, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther 17: 212, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 54: 692-701, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida Y, Takahashi Y, Yamashita H, Kano T, Kaneko H, Mimori A. Clinical characteristics and incidence of methotrexate-related lymphoproliferative disorders of patients with rheumatoid arthritis. Mod Rheumatol 24: 763-765, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Yamakawa N, Fujimoto M, Kawabata D, et al. A clinical, pathological, and genetic characterization of methotrexate-associated lymphoproliferative disorders. J Rheumatol 41: 293-299, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Niitsu N, Okamoto M, Nakamine H, Hirano M. Clinicopathologic correlations of diffuse large B-cell lymphoma in rheumatoid arthritis patients treated with methotrexate. Cancer Sci 101: 1309-1313, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pongpudpunth M, Rattanakaemakorn P, Fleischer AB Jr. Usefulness of random skin biopsy as a diagnostic tool of intravascular lymphoma presenting with fever of unknown origin. Am J Dermatopathol 37: 686-690, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Higashi Y, Kawai K, Yonekura K, et al. Indication for random skin biopsy for the diagnosis of intravascular large B cell lymphoma. Dermatology 224: 46-50, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Masaki Y, Dong L, Nakajima A, et al. Intravascular large B cell lymphoma: proposed of the strategy for early diagnosis and treatment of patients with rapid deteriorating condition. Int J Hematol 89: 600-610, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Shimada K, Murase T, Matsue K, et al. Central nervous system involvement in intravascular large B-cell lymphoma: a retrospective analysis of 109 patients. Cancer Sci 101: 1480-1486, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol 26: 3189-3195, 2008. [DOI] [PubMed] [Google Scholar]