Abstract
Cardiac 123I-metaiodobenzylguanidine (MIBG) scintigraphy is a promising biomarker for dementia with Lewy bodies (DLB). However, we experienced a patient with cognitive decline, parkinsonism, and a decreased MIBG uptake who turned out to have HIV dementia. Normal dopamine transporter single-photon emission computed tomography reduced the possibility of comorbid Lewy body pathology causing the patient’s parkinsonism. The decreased MIBG uptake was most likely due to postganglionic sympathetic nerve denervation, which can also be caused by HIV. This case further emphasizes the importance of excluding other causes of autonomic neuropathy, including HIV infection, before interpreting MIBG scans.
Keywords: HIV, HIV dementia, parkinsonism, dementia with Lewy bodies, MIBG scintigraphy
Introduction
Cardiac 123I-metaiodobenzylguanidine (MIBG) scintigraphy is a promising biomarker for dementia with Lewy bodies (DLB). However, we experienced a patient with cognitive decline, parkinsonism, and a decreased cardiac MIBG uptake who turned out to have HIV dementia.
Case Report
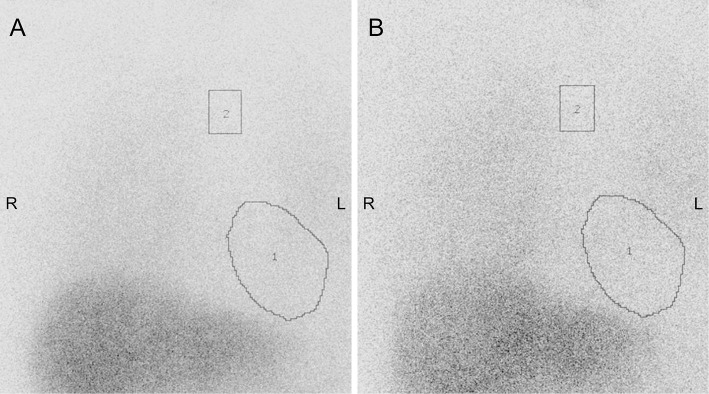
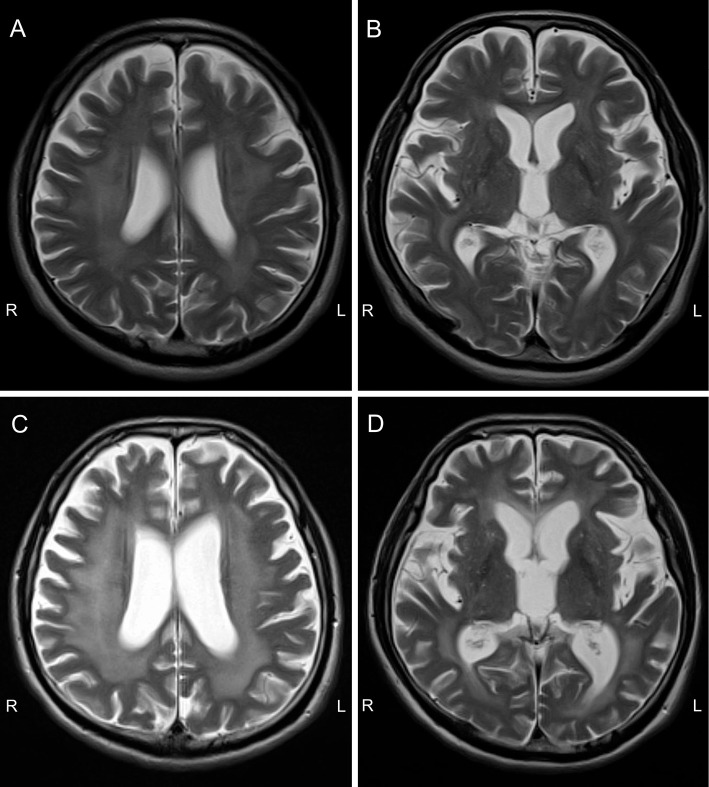
A 58-year-old salesman with a 10-month history of cognitive decline, decreased motivation, bradykinesia, and shuffling gait consulted a neurologist. He made mistakes at work and frequently forgot to take his antihypertensive drugs, but his short-term memory was preserved at the time. His Revised Hasegawa Dementia Scale (HDS-R) score was 27/30. Cardiac 123I-MIBG scintigraphy showed a markedly decreased heart-to-mediastinum ratio (H/M ratio), both in the early and late phases (Fig. 1). The patient had no history of diabetes or cardiac diseases and had been taking no medication other than nifedipine. Brain magnetic resonance imaging (MRI) showed T2 hyper-intense lesions in the white matter, which were considered indicative of leukoaraiosis at that time (Fig. 2A). No obvious abnormalities were noted in either striata (Fig. 2B). DLB was initially suspected based on the cognitive decline, parkinsonism, and decreased cardiac MIBG uptake.
Figure 1.
Cardiac 123I-metaiodobenzylguanidine (MIBG) scintigraphy performed 10 months after the symptom onset. 1) Heart and 2) mediastinum. Early- (A) and late-phase (B) MIBG scans show a decreased heart-to-mediastinum ratio (early phase 1.65; late phase 1.53) (institutional cut-off value, 2.0).
Figure 2.
Brain magnetic resonance imaging (MRI). (A, B) T2-weighted brain MRI performed 10 months after the symptom onset. T2 hyper-intense lesions in the white matter were noted, which were considered indicative of leukoaraiosis at that time. No obvious abnormalities were noted in either striata. (C, D) T2-weighted brain MRI performed 20 months after the symptom onset. The T2 hyper-intense lesions had enlarged with diffuse brain atrophy.
Despite treatment with levodopa-carbidopa and donepezil hydrochloride, his cognitive decline and gait disturbance rapidly progressed. He became bedridden within nine months from the first visit and was referred to our hospital. Daily fluctuation in his cognition and visual hallucinations also appeared during the course. He frequently slept in the daytime and woke up at midnight and stated that he had seen numerous insects in his house. He had no history suggesting rapid eye movement sleep behavior disorder. His HDS-R score at admission was 8/30 (orientation to time -4, serial 7 -1, reverse digit span -2, delayed recall -6, object recall -4, word fluency -5). In addition to cognitive decline, bradykinesia, rigidity, and postural instability, a neurological examination revealed moderate muscle weakness, increased deep tendon reflexes in all extremities, and extensor plantar reflexes, which were atypical for DLB. The patient had constipation and urinary dysfunction with residual urine in the absence of prostate hypertrophy.
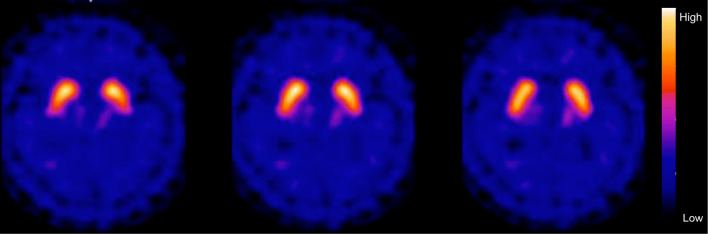
He was seropositive for HIV type 1, with a viral load of 1.1×105 copies/mL, and his CD4 count was 56/μL. The cerebrospinal fluid showed a normal cell count and cytology with an increased IgG index (1.65). Follow-up MRI showed enlargement of the symmetric T2 hyper-intense white matter lesions and diffuse brain atrophy without gadolinium enhancement, which was consistent with HIV dementia (Fig. 2C and D). Dopamine transporter (DAT) SPECT using 123I-ioflupane showed a preserved uptake in both striata (Fig. 3), which was inconsistent with a diagnosis of DLB presenting with parkinsonism. The patient also had massive left pleural effusion that was attributed to malignant lymphoma associated with HIV. Contrast-enhanced computed tomography showed that the lymphoma was restricted to the left pleura, and spinal MRI showed no abnormalities at the spinal cord. The patient received a highly active antiretroviral therapy. Although his cognition and gait partially improved, he remained fully care-dependent and died due to pneumonia one year after the final diagnosis.
Figure 3.
Dopamine transporter (DAT) SPECT using 123I-ioflupan performed 20 months after the symptom onset. DAT SPECT showed a preserved uptake in both striata.
Discussion
The rapid progression of symptoms and white matter lesions revealed by brain MRI suggested that the patient’s symptoms were a manifestation of HIV infection. However, a decreased cardiac MIBG uptake made it difficult to establish an early diagnosis.
HIV is a known cause of dementia and parkinsonism (1) but is also frequently associated with autonomic neuropathy (2). Although the mechanism underlying the autonomic neuropathy in HIV-infected patients is unclear, HIV antigens and inflammatory cells have been detected in the sympathetic ganglia in autopsy studies (3, 4). These reports suggest that HIV can cause sympathetic ganglionitis, which may have contributed to the decreased cardiac MIBG uptake in our patient, although the possibility that coexisting incidental Lewy body disease may have caused the decreased cardiac MIBG uptake cannot be completely ruled out without an autopsy.
Cardiac MIBG scintigraphy reflects postganglionic sympathetic nerve innervation and is useful for differentiating Lewy body diseases from other causes of parkinsonism or dementia (5, 6). It is widely performed in Japan, and since the revised diagnostic criteria for DLB have further emphasized its role (6), the test should be performed in suspected cases of DLB. However, caution should be practiced, as other conditions that cause autonomic neuropathy or cardiac diseases may also be associated with abnormal results (5, 6). Due to the low prevalence of HIV infection in Japan, it has not been widely recognized that patients with HIV may also present with a decreased cardiac MIBG uptake.
HIV infection likely underlies the T2 hyper-intense white matter lesions observed at presentation. These lesions, however, were initially difficult to differentiate from leukoaraiosis. Parkinsonism has been reported in 5-50% of HIV-infected patients and is considered to be associated with atrophy or hypometabolism of the basal ganglia, dopaminergic dysfunction, and subcortical lesions (1). Our patient showed a preserved DAT uptake in both striata, suggesting that the subcortical lesions or post-synaptic dysfunction mainly contributed to the parkinsonism.
In conclusion, although cardiac MIBG scintigraphy is a useful biomarker for DLB, our case emphasizes that other causes of autonomic neuropathy, including HIV infection, which is potentially treatable, should always be taken into account in clinical practice.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Tse W, Cersosimo MG, Gracies JM, Morgello S, Olanow CW, Koller W. Movement disorders and AIDS: a review. Parkinsonism Relat Disord 10: 323-334, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Robinson-Papp J, Sharma SK. Autonomic neuropathy in HIV is unrecognized and associated with medical morbidity. AIDS Patient Care STDS 27: 539-543, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esiri MM, Morris CS, Millard PR. Sensory and sympathetic ganglia in HIV-1 infection: immunocytochemical demonstration of HIV-1 viral antigens, increased MHC class II antigen expression and mild reactive inflammation. J Neurol Sci 114: 178-187, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Chimelli L, Martins AR. Degenerative and inflammatory lesions in sympathetic ganglia: further morphological evidence for an autonomic neuropathy in AIDS. J NeuroAIDS 2: 67-82, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Yoshita M, Taki J, Yokoyama K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology 66: 1850-1854, 2006. [DOI] [PubMed] [Google Scholar]
- 6.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89: 88-100, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]