Abstract
This study shows that DKK-1, a member of the Dickkopf family and a regulator of the Wnt pathways, represents a novel target of statins which, through the inhibition of HMG-CoA reductase and of non-steroidal isoprenoid intermediates, exert extra-beneficial effect in preventing atherosclerosis beyond their effect on the lipid profile. We found that atorvastatin downregulates DKK-1 protein (−88.3 ± 4.1%) and mRNA expression (−90 ± 4.2%) through the inhibition of Cdc42, Rho and Rac geranylgeranylated proteins. Further, a combined approach based on the integration of label-free quantitative mass spectrometry based-proteomics and gene silencing allowed us to demonstrate that DKK-1 itself mediates, at least in part, statin effects on human endothelial cells. Indeed, DKK-1 is responsible for the regulation of the 21% of the statin-modulated proteins, which include, among others, clusterin/apoJ, plasminogen activator inhibitor type 1 (PAI-1), myristoylated alanine-rich C-kinase substrate (MARCKS), and pentraxin 3 (PTX3). The Gene Ontology enrichment annotation revealed that DKK-1 is also a potential mediator of the extracellular matrix organization, platelet activation and response to wounding processes induced by statin. Finally, we found that plasma level of DKK-1 from cholesterol-fed rabbits treated with atorvastatin (2.5 mg/kg/day for 8 weeks) was lower (−42 ± 23%) than that of control animals. Thus, DKK-1 is not only a target of statin but it directly regulates the expression of molecules involved in a plethora of biological functions, thus expanding its role, which has been so far restricted mainly to cancer.
Introduction
DKK-1, a member of the Dickkopf family, was originally identified in Xenopus as an inhibitor of β-catenin-dependent Wnt signalling and an inducer of head formation during embryogenesis, a phenotype that coined the Dickkopf (German for ‘big head, stubborn’) nomenclature1. Its human homologue was also characterised as a potent Wnt inhibitor2. Thereafter, multiple studies demonstrated that DKK-1 impeded β-catenin-dependent Wnt signalling by binding to the LRP6 co-receptor with high affinity and blocking signalling3,4. More recently, DKK-1 has also been proposed to activate β-catenin-independent Wnt pathways through a mechanism that is not well known but is probably indirect and involves DKK-1 shifting the Wnt signaling balance from the β-catenin-dependent pathway to β-catenin-independent pathways5. Therefore, the effect of DKK-1 on cellular function is rather complex and presumably involves modulation of both canonical and non-canonical Wnt pathways5. Based on the ability of DKK-1 to inhibit β-catenin-dependent Wnt signaling, a pathway that is frequently dysregulated in cancer cells, DKK-1 has been widely investigated in oncology and is now considered an attractive therapeutic target for anti-cancer therapy5. However, the importance of DKK-1 and the other members of the DKK family in the pathophysiology of the arterial wall is beginning to be understood.
Ueland et al.6 reported increased levels of DKK-1 in experimental and clinical atherosclerosis, both systemically and within the atherosclerotic lesion, with particularly high levels in advanced and unstable disease. They showed that platelets and endothelial cells (ECs) are important cellular sources of DKK-1, and provided evidence suggesting that platelet- and endothelial-derived DKK-1 contributes to the inflammatory cross-talk between these cells by inhibiting the canonical β-catenin-dependent Wnt pathway and enhancing NF-kB activation6. In line with these findings, plasma DKK-1 levels were found to be significantly higher in patients with type 2 diabetes mellitus (T2DM) compared to healthy subjects, in association with increased levels of endothelial dysfunction and platelet activation markers. Moreover, DKK-1 was found to be downregulated by ameliorating glycemic control and/or by inhibiting platelet function by low-dose aspirin treatment, which support its involvement in the inflammatory interaction between platelets and ECs in the setting of T2DM7. In addition, elevated concentrations of DKK-1 were observed in patients with atherosclerotic disorders8–10 and in subjects with symptomatic aortic stenosis11,12. Moreover, analyses from the prospective population-based Bruneck Study displayed a significant positive association between the plasma level of DKK-1 and the common carotid artery intima-media thickness (IMT), a surrogate marker of early atherosclerosis13.
Data from animal atherosclerosis studies and in vitro investigations support the observations in humans, suggesting that DKK-1 may be an attractive target for cardiovascular therapy. Di et al. reported that silencing of DKK-1 in ApoE−/− mice attenuated atherogenesis14,15, while overexpression of DKK-1 by means of lentivirus transfection promoted lesion formation and vulnerability and increased EC apoptosis15. In addition, they showed that DKK-1 expression in human umbilical vein endothelial cells (HUVECs) is upregulated by two important proatherogenic factors, perturbation of laminar shear stress14 and exposure to oxidized low-density lipoprotein15, and demonstrated that silencing of DKK-1 prevented the detrimental effects of these factors on endothelial cell function. Cheng et al.16 reported that transduction of cultured aortic bovine ECs with vectors expressing DKK-1 inhibits the expression of EC differentiation markers and promotes endothelial-mesenchymal transition (EndMT), a process of cellular trans-differentiation that has emerged as an important driver of atherosclerosis progression and vascular calcification17,18. In addition, it has been demonstrated that platelet-derived DKK-1 has a unique proinflammatory role since it potentiates neutrophilic infiltration during the innate immune response19 and promotes pathological type-2 cell-mediated inflammation20. Overall, these findings suggest that antagonizing DKK-1 pathway could be a promising strategy in atheroprotection.
We and others have previously shown that inhibition of the mevalonate pathway by statin treatment decreases the expression of DKK-1 in cultured cells, specifically in the immortalised endothelial hybrid cell line EA.hy. 926 and in cancer cells21–23. Herein, we provide evidence that DKK-1 is indeed a target of statin in human primary vascular cells and, by means of a quantitative label-free proteomic approach, we showed that it could mediate several of the pleiotropic effects exerted by this multifaceted class of drugs24.
Results
Atorvastatin down-regulates DKK-1 biosynthesis in human ECs by inhibiting protein geranylgeranylation
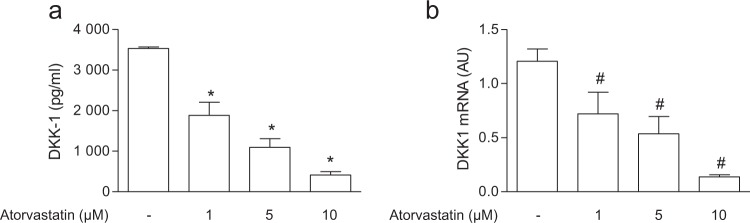
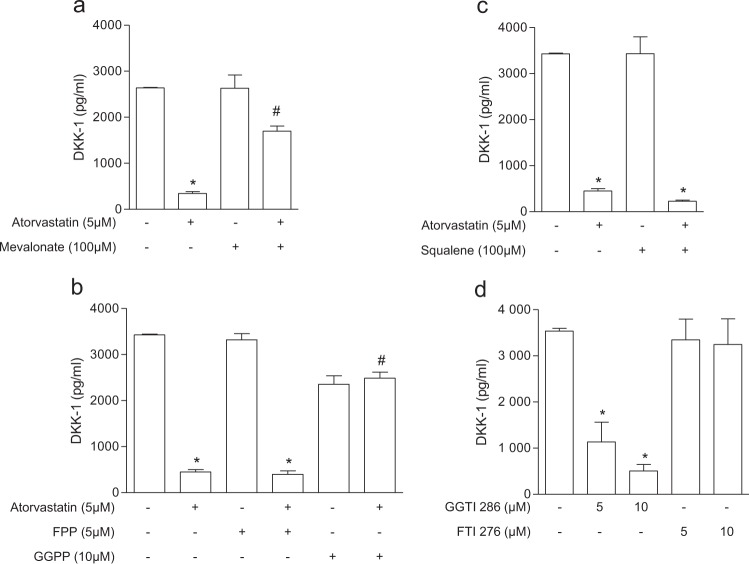
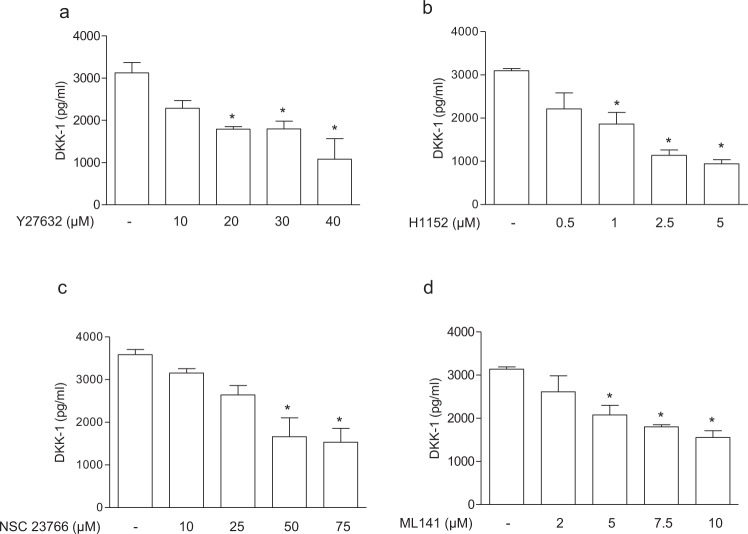
Based on the previous observation that statins decrease DKK-1 in the secretome of the immortalised endothelial hybrid cell line EA.hy. 926, established by fusing a human umbilical vein endothelial cell with a human carcinoma cell line21,25, we addressed the effect of atorvastatin in the primary human endothelial cells HUVECs. We found that atorvastatin reduced DKK-1 biosynthesis in a concentration-dependent manner (Fig. 1). DKK-1 release was reduced also in human aortic smooth muscle cells (AoSMCs) (Supplementary Fig. 1A). To determine whether the effects of atorvastatin on DKK-1 was caused by inhibition of the mevalonate pathway, we evaluated the ability of mevalonate and its isoprenoid derivatives to counteract the inhibitory effect of atorvastatin on DKK-1. Mevalonate, the immediate product of HMG-CoA reductase, and GGPP, the precursor of geranylgeranylated proteins, but not FPP, the precursor of farnesylated proteins, or squalene, the late metabolite of the cholesterol synthesis pathway, prevented the effect of atorvastatin on DKK-1 expression by ECs (Fig. 2), suggesting a crucial role of protein geranylgeranylation on DKK-1 expression. Similarly, fluvastatin decreased DKK-1 in HUVECs by inhibiting geranylgeranylation, which suggests a class effect of statins on DKK-1 regulation (Supplementary Fig. 1B). To further verify this hypothesis, cells were treated with FTI-277 or GGTI-286, selective inhibitors of farnesyltransferase or geranylgeranyltransferase I, respectively. Our results showed that only the treatment with GGTI significantly inhibited DKK-1 (Fig. 2). Inhibition of geranylgeranylation by GGTI was confirmed by assessing ungeranylated RAP1A by immunoblotting (Supplementary Fig. 2). Because the Rho, Rac and cdc42 proteins of the Rho subfamily of GTPases have been involved as mediator of the vascular pleiotropic effects induced by statins26, we investigated the role of the Rho family members on DKK-1 expression by means of selective inhibitors. Treatment of cells with the Rho-associated protein kinase inhibitors Y-27632 and H-1152, the Rac1/2 inhibitor NSC23766, and the Cdc42 inhibitor ML-141, resulted in a significant reduction of DKK-1 release (Fig. 3). Taken together, these results suggest that mevalonate blockade suppresses DKK-1 via inhibition of the Cdc42, Rho and Rac geranylgeranylated proteins.
Figure 1.
Atorvastatin reduces DKK-1 antigen release (a) and mRNA (b) in human endothelial cells. Data are expressed as mean ± SEM of 3 individual experiments performed in duplicate. AU, arbitrary units. *p < 0.001 vs control cells; #p < 0.05 vs control cells.
Figure 2.
DKK-1 downregulation occurs via geranylgeranylation inhibition. DKK-1 antigen was analysed in HUVECs treated with atorvastatin alone and in combination with mevalonate (a), GGPP and FPP (b), or squalene (c), and in HUVECs treated with the prenyltransferases inhibitors, GGTI-286 and FTI-277 (d). Values are the mean ± SEM of 3 individual experiments. *p < 0.01 vs control cells; #p < 0.01 vs statin-treated cells.
Figure 3.
DDK1 release is regulated by geranylgeranylated proteins. DKK-1 antigen was analysed in HUVECs treated with the Rho-associated protein kinase inhibitors, Y-27632 (a) and H-1152 (b), the Rac1/2 inhibitor, NSC23766 (c), and the Cdc42 inhibitor, ML-141 (d). Values are the mean ± SEM of 3 individual experiments. *p < 0.05 vs control cells.
Further studies have demonstrated molecular links between the RhoGTPases and the NFκB pathway27 and a functional crosstalk between NF-kB signaling and Wnt/β-catenin signaling28,29. For example, Swarnkar et al. found that NF-kB activation inhibits osteogenic markers and stimulates the anti-osteogenic factor DKK-130. Therefore, we tested the hypothesis that atorvastatin may affect DKK-1 expression by interfering with NF-kB activity. However, in our experimental conditions, chemical NF-kB inhibitors did not influence DKK-1 (Supplementary Fig. 3).
DKK-1 mediates some statin effects in EC
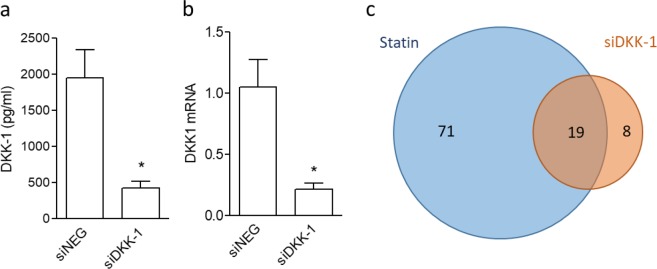
To identify the downstream effects of DKK-1 downregulation mediated by statin we adopted a combined strategy based on proteomics and DKK-1 gene silencing. First, we demonstrated that treatment of HUVECs with siRNA against DKK-1 (siDKK-1) resulted in a 76% reduction of DKK-1 mRNA and antigen secretion in comparison with cells treated with a negative siRNA (siNEG) (Fig. 4a and 4b). In our experimental conditions DKK-1 silencing does not affect cell viability and cell proliferation in HUVECs (Supplementary Fig. 4).
Figure 4.
DKK-1 gene knockdown reduces DKK-1 in HUVECs. HUVECs were transfected with negative siRNA (siNEG) or with DKK-1 siRNA (siDKK-1) and then analysed for DKK-1 antigen release (a) and DKK-1 mRNA levels (b). Values are the mean ± SEM of 3 individual experiments. *p < 0.01 vs. control siRNA-treated cells. (c) Comparison of the effects of DKK-1 silencing and statin treatment on endothelial cell secretome using a Venn diagram generated with the differentially abundant proteins.
Then, we analysed the endothelial secretome derived from DKK1-silenced cells in respect to siNEG-treated cells, and compared the results with those obtained from the analysis of control and statin-treated cells in order to identify unique and common targets. To perform this analysis we employed a label free mass spectrometry based method (LC-MSE), that allows us to simultaneously achieve a qualitative and quantitative analysis of the protein content in the endothelial cells secretome21.
Proteins identified in the secretome in the different experimental conditions are reported in details in Supplementary Table S1 and S2. After treatment with siDKK-1, 6 proteins resulted to be significantly more secreted and 21 less secreted in respect to siNEG-treated cells (Table 1).
Table 1.
List of differentially abundant proteins in the secretome of HUVECs treated with siDKK-1 identified by LC-MSE.
| Accession | Description | Unique peptides/ Total peptide count | Confidence score | siDKK-1 | Atorvastatin | ||
|---|---|---|---|---|---|---|---|
| fold siDKK-1/siNEG | pvalue | fold atorva/control | pvalue | ||||
| Proteins with an opposite behavior in respect to statin-treated cells | |||||||
| Q9UNN8 | Endothelial protein C receptor | 3/3 | 20,46 | 1,289 | 0,00058 | 0,638 | 6,79E-07 |
| P49327 | Fatty acid synthase | 2/2 | 11,06 | 1,312 | 0,00014 | 0,471 | 2,05E-05 |
| P26022 | Pentraxin-related protein PTX3 | 2/2 | 13,05 | 2,136 | 1,3E-08 | 0,359 | 4,33E-06 |
| P11142 | Heat shock 71 kDa protein | 14/26 | 221,77 | 1,207 | 9,5E-04 | 0,433 | 0,000152 |
| P08238 | Heat shock protein HSP 90-beta | 12/24 | 188,89 | 1,209 | 0,00799 | 0,519 | 9,56E-06 |
| Proteins with the same behavior obtained with statin-treated cells | |||||||
| Q16270 | Insulin-like growth factor-binding protein 7 | 8/8 | 57,56 | 0,796 | 0,00493 | 0,564 | 0,001956 |
| Q8NBS9 | Thioredoxin domain-containing protein 5 | 11/11 | 75,58 | 0,701 | 3,6E-05 | 0,599 | 0,000105 |
| P43121 | Cell surface glycoprotein MUC18 | 2/2 | 11,17 | 0,719 | 0,00024 | 0,585 | 3,33E-05 |
| P30101 | Protein disulfide-isomerase A3 | 18/19 | 140,69 | 0,814 | 0,00306 | 0,674 | 0,000733 |
| P29966 | Myristoylated alanine-rich C-kinase substrate | 2/2 | 12,35 | 0,582 | 0,00302 | 0,437 | 0,060377 |
| P29279 | Connective tissue growth factor | 8/16 | 114,37 | 0,776 | 0,00020 | 0,736 | 0,000162 |
| P21810 | Biglycan | 10/12 | 92,66 | 0,607 | 0,00011 | 0,600 | 1,77E-05 |
| P14625 | Endoplasmin | 10/11 | 67,74 | 0,812 | 0,01037 | 0,621 | 0,007624 |
| P11021 | 78 kDa glucose-regulated protein | 19/22 | 176,57 | 0,743 | 1,6E-06 | 0,637 | 0,00019 |
| P09486 | SPARC | 10/10 | 91,66 | 0,827 | 3,8E-05 | 0,577 | 2,68E-06 |
| P08253 | 72 kDa type IV collagenase MMP2 | 5/5 | 30,86 | 0,572 | 5,3E-06 | 0,799 | 0,000777 |
| P07996 | Thrombospondin-1 | 59/63 | 635,11 | 0,763 | 1,2E-04 | 0,682 | 8,2E-05 |
| P06753 | Tropomyosin alpha-3 chain | 2/8 | 57,15 | 0,810 | 1,1E-05 | 0,575 | 2,84E-05 |
| P05121 | Plasminogen activator inhibitor 1 | 18/18 | 189,33 | 0,403 | 1,4E-06 | 0,816 | 0,00607 |
| P04792 | Heat shock protein beta-1 | 10/10 | 79,48 | 0,829 | 4,2E-03 | 0,644 | 0,000994 |
| P02751 | Fibronectin | 56/56 | 478,89 | 0,820 | 0,00012 | 0,573 | 8,94E-05 |
| P00491 | Purine nucleoside phosphorylase | 2/2 | 11,59 | 0,743 | 0,00020 | 0,571 | 3,18E-06 |
| P13797 | Plastin-3 | 10/10 | 61,67 | 0,822 | 1,2E-05 | 0,456 | 8,36E-05 |
| P10909 | Clusterin | 5/5 | 33,41 | 1,304 | 0,00056 | 1,944 | 6,71E-05 |
| Proteins modulated by siDDK-1 only | |||||||
| P04275 | von Willebrand factor | 4/4 | 22,66 | 0,755 | 0,00028 | 1,001 | 0,960219 |
| P98160 | Basement membrane-specific heparan sulfate proteoglycan core protein | 59/60 | 440,06 | 0,695 | 2,5E-05 | 1,081 | 0,097248 |
| P61769 | Beta-2-microglobulin | 2/2 | 25,87 | 0,704 | 3,5E-03 | 0,997 | 0,984152 |
A comparison of the list of proteins modulated by siDKK-1 with those modulated by atorvastatin, led us to identify 19 proteins with a common behavior (Fig. 4c). In particular, only one protein, namely clusterin, was commonly enhanced by the two treatments, and 18 were reduced by both DKK-1 silencing and atorvastatin treatment. Five proteins were oppositely regulated by statin and DKK-1 silencing (i.e., pentraxin 3, PTX3), as reported in Table 1.
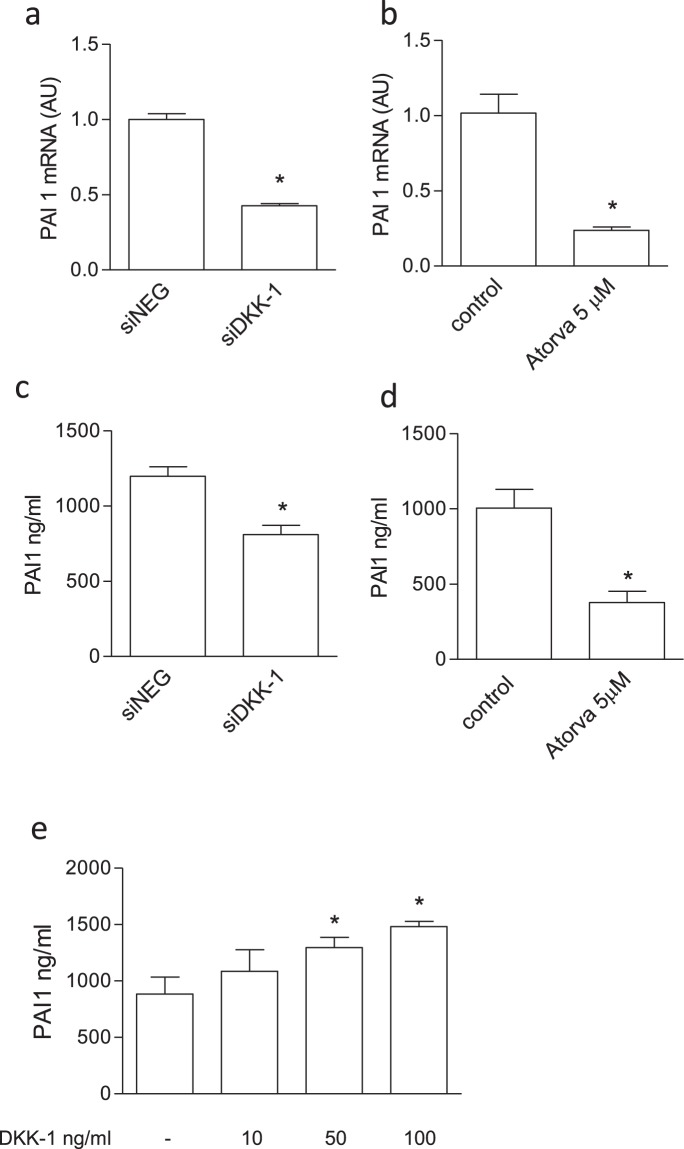
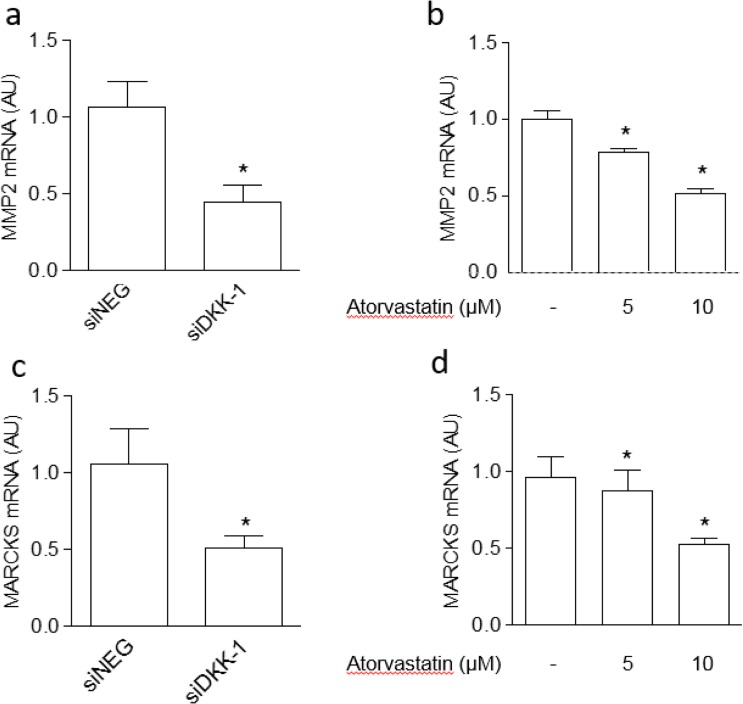
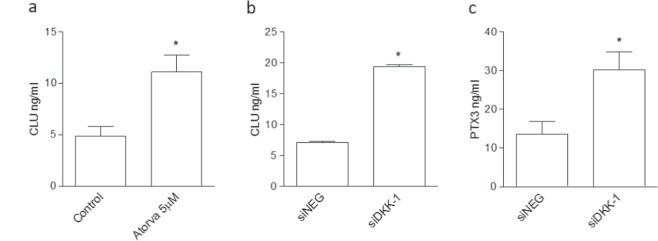
Some proteomics results were validated at mRNA level, by means of qRT-PCR, or at protein level, by means of specific ELISAs. Validation analysis confirmed, for example, that plasminogen activator inhibitor type 1 (PAI-1) was inhibited by both treatments, and it was increased after treatment with recombinant DKK-1 (Fig. 5). Further, both MMP-2 and MARCKS were modulated in the same manner by DKK-1 silencing and atorvastatin (Fig. 6). In addition, we found that clusterin release was increased by both statin treatment and DKK-1 silencing, while PTX3 secretion was enhanced by DKK-1 silencing (Fig. 7) and reduced by atorvastatin, as previously reported31.
Figure 5.
Validation of proteomics results regarding PAI-1 on endothelial cells treated with atorvastatin (Atorva) or by DKK-1 silencing as described in the Material and methods section (n = 3). Effect on PAI-1 mRNA levels exerted by siDKK-1 (a) or atorvastatin (b). PAI-1 was evaluated at antigen levels by ELISA in cells treated with siDKK-1 (c), atorvastatin (d) or recombinant DKK-1 (e). The data are expressed as ng/ml of PAI-1. Values are the mean ± SEM of 3 individual experiments.*p < 0.05 vs siNEG or control cells.
Figure 6.
Validation of proteomics results regarding MMP-2 and MARCKS on endothelial cells treated with atorvastatin or by DKK-1 silencing. Effect on MMP-2 and MARCKS mRNA levels exerted by siDKK-1 (a,c) or atorvastatin (b,d). Values are the mean ± SEM of 3 individual experiments.*p < 0.05 vs siNEG or control cells.
Figure 7.
Validation of proteomics results regarding clusterin (CLU) and PTX3 on endothelial cells treated with atorvastatin or by DKK-1 silencing. (a) Effect of treatment with atorvastatin (a) and siDKK-1 on CLU antigen release. (c) Effect on PTX3 antigen exerted by siDKK-1. Values are the mean ± SEM of 3 individual experiments. *p < 0.05 vs siNEG-treated cells.
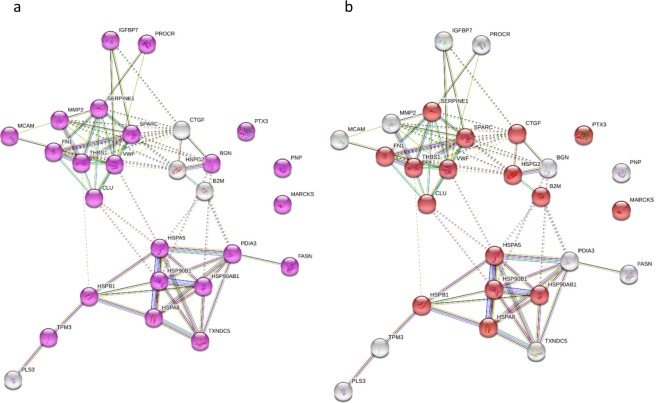
Proteins modulated by siDKK-1 were analysed with STRING for evaluation of protein-protein interactions and gene ontology analysis in order to characterise their localization and function. As shown in Fig. 8, modulated proteins are mainly localised in the extracellular region (Fig. 8a, Supplementary Table S3), and the most over-represented term in the molecular function category is protein binding (Fig. 8b, Supplementary Table S3). Regarding the biological process category, the analysis of the most over-represented terms revealed that 10 proteins are involved in extracellular matrix organization, 11 in response to wounding and 8 in platelet activation (Fig. 9, Supplementary Table S3). Similar results were obtained performing the Gene Ontology analysis using a background containing only secreted proteins (Supplementary Table S4).
Figure 8.
Gene ontology analysis of secreted proteins modulated by DKK-1 silencing in the cellular component and molecular function categories visualised with STRING. (a) Within the cellular component category the GO term related to Extracellular region resulted overrepresented (purple circles). (b) In the molecular function category the GO term protein binding was associated with the highest number of proteins (red circles). Detailed results are reported in Supplementary Table S3.
Figure 9.
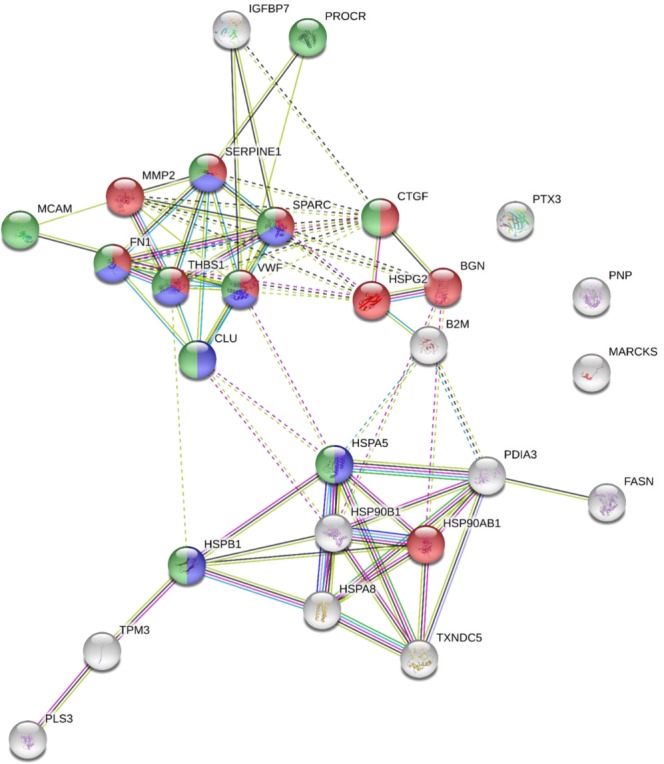
Biological processes of secreted proteins modulated by DKK-1 silencing visualised with STRING. Considering the biological processes category the enriched terms were response to wounding (green circles), extracellular matrix organization (red circles) and platelet activation (blue circles). Detailed results are reported in Supplementary Table S3.
Of note, the proteins modulated by DKK-1 silencing are potentially responsible for much of the effects of statin on extracellular matrix organization (14 proteins, p = 1.19E-06 among statin-modulated proteins), on platelet activation response (29 proteins, p = 1.54E-13 among statin-modulated proteins), and on response to wounding (19 proteins, p = 1.54E-13 among statin-modulated proteins).
Effect of atorvastatin on DKK-1 levels in plasma of cholesterol-fed rabbits
Since DKK-1 is a secreted protein whose levels are increased in experimental and clinical atherosclerosis6, to address whether statin-mediated DKK-1 regulation also occurs in vivo, we evaluated DKK-1 levels in plasma of cholesterol-fed rabbits treated with or without atorvastatin 1 mg/kg per day for 6 days. The semi-quantitative evaluation of DKK-1 performed by immunoblotting analysis on these samples showed a trend towards reduced levels of DKK1 in animals treated with atorvastatin compared to controls, although the difference did not reach statistical significance (Supplementary Fig. 5).
Discussion
This study shows that DKK-1 represents a novel target of statins in primary human vascular cells (endothelial cells and aortic smooth muscle cells), and that it potentially mediates several of the pleiotropic effects exerted by this drug. Indeed, we found that atorvastatin downregulates DKK-1 protein and mRNA expression through the inhibition of Cdc42, Rho and Rac geranylgeranylated proteins, and that DKK-1 itself mediates, at least in part, statin effects on ECs. It directly regulates the expression of molecules involved in a plethora of biological functions, thus expanding its role, which has been so far restricted mainly to cancer5,32. Indeed, in two previous papers, Rachner et al.23 and Göbel et al.22 circumstantiated the effects of statins in combination with amino-bisphosphonates on DKK-1 expression in breast cancer cell lines. The rationale for these studies derives from the potential role of DKK-1 as promoter of osteolytic bone lesions through the inhibition of osteoblast functions and, therefore, as a potential adverse marker in multiple cancers. The authors showed that the suppression of DKK-1 by statin and amino-bisphosphonates inhibited the ability of breast cancer cells to block WNT3A-induced production of alkaline phosphates and bone-protective osteoprotegerin in preosteoblastic C2C12 cells.
In this study we took advantage of a proteomics-based approach to compare statin and DKK-1 effects in order to identify unique and common targets. Indeed, rapid recent progress in proteomics instrumentation and software have led to a marked decrease in the duration of a typical proteomics experiment, enabling analysis of thousands of proteins, and thus opening an unexplored opportunity to apply proteomics to many cellular proteomes for cross-comparison33. As described in our recent review, proteomics is also useful to understand the effects of a drug on its protein targets and shed light on the cellular mechanisms resulting in the observed phenotype24. Herein, proteomics allowed us to identify proteins uniquely or commonly regulated by statin and DKK-1, the latter representing the 21% of statin regulated proteins. Further, a functional interpretation of the identified proteins obtained by searching the Gene Ontology (GO) annotations for over-represented terms in the “biological process” category, revealed that DKK-1, besides being a downstream target of statin, is a potential mediator of the extracellular matrix organization, platelet activation and response to wounding processes induced by statin.
The effects exerted by DKK-1 on the endothelial cell proteome are not attributable to any perturbations of the cell viability or proliferation. Among the endothelial proteins commonly regulated by DKK-1 and atorvastatin we found clusterin, also known as apolipoprotein J (apoJ), which is considered one of the most important extracellular chaperones ever found34. It is involved in a broad range of physiological and pathophysiological functions, where it exerts mainly a cytoprotective role34, conferring, for example, resistance to endothelial cells against TNF-α-induced apoptosis35. Further, a potential anti-atherogenic role of exogenously administered clusterin/apoJ, partially attributable to an increased cholesterol efflux from foam cells, was demonstrated in apolipoprotein E-deficient mice and monkeys36,37. The only study available until now in endothelial cells, precisely in primary porcine brain capillary endothelial cells, suggests an importance of clusterin/apoJ in the production of amyloid precursor protein and Amyloid-β peptides transport in the brain38. Thus, our findings deserve further investigations in order to understand the role of clusterin/apoJ in human endothelial cells.
In the attempt to address the potential implications of the inhibitory effect observed in vitro, we performed a semi-quantitative evaluation of DKK-1 by immunoblotting analysis of plasma samples from rabbits treated with atorvastatin 1 mg/kg per day for 6 days, which were available from a previous study39. This dose of atorvastatin may be considered clinically relevant, since the administration of a dose 5 times greater, under comparable experimental conditions, resulted in atorvastatin-equivalent concentrations (mean total plasma concentrations of atorvastatin plus active metabolites) comparable to the concentrations detected in plasma of individuals treated with daily doses of 5–20 mg atorvastatin39,40. Herein, we found that the administration of atorvastatin was associated with a trend towards reduced levels of DKK1 in animals treated with atorvastatin compared to controls. Based on these observations, we believe that statin-mediated DKK-1 regulation can also occurs in vivo. This observation, however, needs further investigation and should be interpreted with caution because of its preliminary nature.
Interestingly, once released, a substantial part of circulating clusterin/apoJ associates to lipoproteins, mainly to HDL, where it suggested to play a relevant role in maintaining their qualitative properties41. Indeed, Riwanto et al. demonstrated that reduced HDL-associated clusterin in patients with coronary artery diseases (CAD) contributes to impair the anti-apoptotic capacity of HDL itself42. Conversely, the combined statin/niacin therapy increases levels of clusterin/apoJ in the HDL3 subpopulation of CAD patients, reverting the lipoprotein profile to the one more closely resembling that of HDL3 in healthy control subjects43.
Regarding the discovery of clusterin/apoJ regulation by DKK-1, it is worth highlighting that, if on the one hand it is well known that this protein is regulated by a wide variety of stimuli44, on the other hand, the signaling molecules responsible for the complex tissue-specific control of the clusterin/apoJ gene have been only partially identified and limited to the NF-κB or Src-Mek-Erk pathways44,45. Therefore, our results add a novel regulator to the list of signaling pathways which regulate clusterin/apoJ.
We also provide the evidence that DKK-1 is a mediator of the negative regulation exerted by atorvastatin on PAI-1. This secretory protein regulates, through potent inhibition of serine proteases tissue-type plasminogen activator (t-PA) and urokinase plasminogen activator, not only the fibrinolytic system, but also functions unrelated to this system, such as cellular processes, including cell adhesion, migration, and proliferation. It is well known that statin treatment of vascular cells results in decreased PAI-1 activity and synthesis, and increased tPA synthesis, expression, activity, and release46 (summarised in47). However, in the complex interplay of signaling pathways responsible for the effect of statin on PAI-1, which includes reduced expression of ROCK (Rho associated protein kinase) activity, a decrease of PTEN (phosphatase and tensin homolog), upregulation of Akt48, and lastly NF-κB, a role for DKK-1 has never been identified49.
In line with the evidence that statins inhibit the secretion of a broad spectrum of metalloproteases, in vascular cells and in foamy macrophages26,50, we confirm that MMP-2 was reduced in the secretome of endothelial cells, and we found that DKK-1 silencing mimicked the statin effect. Although the direct regulation of MMP-2 by DKK-1 in endothelial cells is of novelty, an effect of DKK-1 in the cell matrix remodeling has been observed in epithelial ovarian cancer cells cultured on three-dimensional collagen I gels, likely due to a transcriptional up-regulation of the membrane-tethered collagenase membrane type 1 matrix metalloproteinase51.
A novel candidate regulated by both statin and DKK-1 is represented by MARCKS (myristoylated alanine-rich C-kinase substrate), which, as indicated by the name, was originally identified as a major target of protein kinase C. A growing body of work now indicates MARCKS as a downstream effector in various processes including cytoskeletal control, regulation of the cell cycle, chemotaxis and motility, mediation of the inflammatory response, secretion and exocytosis, muscle spreading, neurological function, and development (reviewed in52). To this regard, MARCKS is usually considered a membrane-bound protein, which interacts with the plasma membrane by a combination of independent electrostatic interactions between the phospholipid bilayer and both the N-terminal myristoyl group and its effector domain. Being this interaction reversible, MARCKS can also shuttle back and forth between the membrane and the cytoplasm53. However, MARCKS has also been found in cell secretome of cancer cells54–56, therefore we cannot exclude a biological role for circulating MARCKS56. Recently, it has been indeed demonstrated, in a number of in vivo and in vitro studies, that a synthetic peptide identical to the N-terminus of MARCKS, affects MARCKS function related to vesicular transport, leukocyte degranulation and cell migration57–59. Lastly, an axolotl MARCKS-like protein has been identified as an extracellularly released factor that induces the initial cell cycle response during axolotl appendage regeneration60, raising the possibility that secretion of this protein may be linked to their ability to promote regeneration56.
Interestingly, we found an opposite effect of statin and DKK-1 silencing on PTX3, a major pattern recognition molecule belonging to the superfamily of pentraxins, which plays important roles as modulator of the immune-inflammatory responses61,62. While we confirmed our previous observation on the capacity of statins to inhibit the expression of PTX3 in vascular cells31, we found that DKK-1 silencing behaves in an opposite way upregulating PTX3. This suggests that DKK-1 is indeed a positive regulator of PTX3 and that statin overcomes it resulting in PTX3 inhibition through alternative ways. Of note, we add DKK-1 to the list of the signaling molecules involved in the regulation of PTX3, which actually only includes NF-kB and JNK in alveolar epithelial cells stimulated with TNFα63 and PI3K/Akt in endothelial cells treated with high-density lipoproteins64.
In conclusion, we show here the potential of proteomics to identify drug targets in vitro, a process called drug target deconvolution, that is, the action aimed at identifying the full spectrum of protein targets regulated by a bioactive molecule and the cellular phenotype that it induces. Of relevance, the identification of the proteins targeted by a drug could not only inform in advance about its therapeutic potential, but also predict its possible adverse effects through the identification of potential toxicity targets. Indeed, a large body of postgenomic biological research has progressively revealed that many effective drugs, such as statins, act on multiple rather than single protein targets, potentially having functional roles that go beyond their intended effects26. Finally, proteomics, through the analysis of protein networks, is able to reveal pathways affected by a drug, leading to the identification of molecules which mediate drug action.
Materials and Methods
Materials
The geranylgeranyl transferase I inhibitor GGTI-286, the farnesyl transferase inhibitor FTI-276, the cell permeable IkB kinase inhibitor peptide and its inactive control peptide, the NF-kB inhibitor SN50, the SN50M inactive control peptide, the Rac inhibitor NSC23766, the Rho-associated protein kinase (ROCK) inhibitors, Y- 27362 and H1152, and fluvastatin were purchased from Calbiochem (San Diego, CA, USA). Atorvastatin, mevalonate, squalene, geranylgeranyl pyrophosphate (GGPP), farnesyl pyrophosphate (FPP), Bay 11–7082 were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Cdc42 inhibitor, ML141, was purchased by TOCRIS (Bristol, UK). Human recombinant DKK-1 was from R&D Systems (Minneapolis, MN, USA). Antibody against RAP1A was purchased from Santa Cruz Biotechnology Inc. (Dallas, Texas, USA).
Cell cultures and experimental conditions
Human umbilical vein endothelial cells (HUVECs), human aortic smooth muscle cells (AoSMCs), EGM-2 Medium (Endothelial Cell Growth Medium-2 Bullet Kit) and SmGM-2 Medium (Smooth Muscle Growth Medium-2 Bullet Kit) were purchased from Lonza Walkersville Inc. (Walkersville, MD, USA). Cells were cultured according to Lonza’s Clonetics™ instructions. Experimental procedures have been described in detail in previous investigations31,46. Briefly, confluent cells were incubated for 24 h in complete medium containing atorvastatin or fluvastatin, respectively dissolved in ethanol and dimethyl sulfoxide. Then, the cells were incubated for 16 h in serum-free and phenol-free medium containing statins, with or without mevalonate and isoprenoid intermediates for antigen, mRNA and proteomics analysis. The control cells were treated with the appropriate vehicles. Experiments using chemical inhibitors of the various signaling pathways were performed under the same experimental conditions.
Real-time quantitative RT-PCR
Total cellular RNA was extracted and reverse transcribed (1 µg) as previously described65. Amplification of 18S ribosomal RNA was used to correct for fluctuations in input RNA levels and the efficiency of the reactions. Real-time qRT-PCR was performed in triplicate with 2.5 μL of cDNA incubated in 22.5 μL IQ Supermix containing primers and SYBRGreen fluorescence dye (Bio-Rad Laboratories, Milan, Italy) using the iCycler Optical System (Bio-Rad Laboratories, Milan, Italy). The sequences of primers used as normalizer were: 18S forward: 5′-CGG CTA CCA CAT CCA AGG AA-3′; 18S reverse: 5′-CCT GTA TTG TTA TTT TTC GTC ACT ACC T-3′. The sequences of PTX-3 primers were: forward TGC GAT TCT GTT TTG TGC TC; reverse TGA AGA GCT TGT CCC ATT CC. Other specific primers were purchased from QIAGEN (Hilden, Germany): DKK-1, QT 00009093; PAI-1, QT 00062496; MMP2, QT 00088396; MARCKS, QT 00092694; CLU-1, QT 00054460.
Antigen assays
The concentration of DKK-1, clusterin, PTX3 in the cell supernatant was measured using specific immunoassays from R&D System (Minneapolis, Minnesota, USA). PAI immunoassay was from BIOMEDICA Diagnostics (Stanford, CT, USA).
Cell proliferation and viability assays
Cell Proliferation Kit I (MTT) and Lactate Dehydrogenase (LDH) Activity Assay Kit were from Sigma-Aldrich (St. Louis, MO, USA).
RNA interference and cell transfection
Validated high-performance purity grade small interfering RNAs (siRNA) against human DKK-1 (SI00098546) were synthesised by Qiagen Inc. using the HiPerformance siRNA design algorithm and a proprietary homology analysis tool; siRNA with a non-silencing oligonucleotide sequence that does not recognise any known homology to mammalian genes was also generated as a negative control31.
Secretome analysis
For the analysis of cell secretome by label-free mass spectrometry, the conditioned media were collected from vehicle, statin-treated or silenced cells and processed as described21. Before tryptic digestion, the samples were dissolved in 25 mmol/L NH4HCO3 containing 0.1% w/v RapiGest SF, reduced with 5 mmol/L dithiothreitol, dissolved in 100 mmol/L NH4HCO3, at 60 °C for 15 min, and then carbamidomethylated with 10 mmol/L iodacetamide for 30 min at room temperature. Digestion performed overnight at 37 °C using sequencing grade trypsin (Promega, Milan, Italy) was stopped by the addition of 2% v/v TFA. Quantitative label-free LC-MSE was performed as previously described66,67. The proteins were identified by searching a human species-specific UniProt database (release 2017.1; 20,201 entries), and quantified using Progenesis QIP for proteomics (v3.0, NonLinear dynamics, Newcastle upon Tyne, UK).
Data mining
Data mining was performed the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING 10.5) database, to create network of interactions among differentially abundant proteins68. In order to find out the enriched GO terms in the biological process, molecular function or cellular component categories that can be associated to the proteins in the network, we employed the enrichment widget of STRING, which calculates an enrichment P-value based on the Hypergeometric test and the method of Benjamini and Hochberg for correction of multiple testing (P value cut-off of <0.05).
Immunoblotting analysis of DKK-1 in plasma of cholesterol-fed rabbits
The levels of DKK-1 were analysed by immunoblotting in plasma samples from cholesterol-fed rabbits treated with or without atorvastatin (1 mg/kg/day given by oral gavage for 6 days), which were available from a previous in vivo dose-finding experiment with atorvastatin39. Plasma samples were diluted in Laemmli buffer containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Equal volume of plasma (40 µl of diluted plasma 1:100) were separated on 12% SDS-polyacrylamide gels, transferred to nitrocellulose membrane and processed as previously described69. The antibody used was a HRP-conjugated rabbit polyclonal antibody raised against the AA 207–247 portion of human DKK1 (ABIN678167 antibodies-online Inc., Atlanta, USA), which display a 95% homology with its rabbit counterpart, as verified by the alignment of protein sequence from UniProt, accession numbers O94907 and Q6PVU5. Bands were visualized by enhanced chemiluminescence using the ECL kit (Amersham Corp.) and acquired by a densitometer (GS800 Biorad). In each analysis a plasma sample was loaded as control for normalization. Bands detected by ECL were quantitated by densitometry of exposed film using image analysis software QuantityOne (version 4.5.2) from Biorad. Results obtained as ratio of density versus the sample used as normalization control are expressed as arbitrary unit.
Statistical analysis
In in vitro experiments, data were expressed as mean values ± SEM and were statistically compared using Student’s t-test or ANOVA for repeated measures, followed by Tukey’s test (n = the number of individual experiments performed in duplicate), after normality assessment by Kolmogorov– Smirnov and Shapiro–Wilk tests. P values of <0.05 were considered significant. In vivo data were analyzed with Mann-Whitney non parametric test.
Electronic supplementary material
Acknowledgements
This work was supported by the Italian Ministry of Health, Rome, Italy (Ricerca Corrente 2011 BIO 32 ID 2101581). We thank Prof. Alberto Corsini (Dipartimento di Scienze Farmacologiche e Biomolecolari, Università degli Studi di Milano) for suggestions and comments, and for kindly providing rabbit plasma samples.
Author Contributions
M.P. acquired, analysed, interpreted data; M.B. acquired, analysed, interpreted data and wrote the manuscript; R.B. analysed, interpreted data and wrote the manuscript; S.G. acquired, analysed data; C.B. designed study, interpreted data, wrote the manuscript and obtained funding; all authors reviewed the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35119-7.
References
- 1.Glinka A, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 2.Fedi P, et al. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999;274:19465–19472. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
- 3.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 4.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 5.Kagey, M. H. & He, X. Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br J Pharmacol (2017). [DOI] [PMC free article] [PubMed]
- 6.Ueland T, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 7.Lattanzio, S. et al. Circulating dickkopf-1 in diabetes mellitus: association with platelet activation and effects of improved metabolic control and low-dose aspirin. J Am Heart Assoc3 (2014). [DOI] [PMC free article] [PubMed]
- 8.Kim KI, et al. A novel biomarker of coronary atherosclerosis: serum DKK1 concentration correlates with coronary artery calcification and atherosclerotic plaques. J Korean Med Sci. 2011;26:1178–1184. doi: 10.3346/jkms.2011.26.9.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seifert-Held T, et al. Circulating Dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis. 2011;218:233–237. doi: 10.1016/j.atherosclerosis.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Martin A, et al. Relationship of Dickkopf1 (DKK1) with cardiovascular disease and bone metabolism in Caucasian type 2 diabetes mellitus. PLoS One. 2014;9:e111703. doi: 10.1371/journal.pone.0111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Askevold ET, et al. Secreted Wnt modulators in symptomatic aortic stenosis. J Am Heart Assoc. 2012;1:e002261. doi: 10.1161/JAHA.112.002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motovska Z, et al. Serum Dickkopf-1 signaling and calcium deposition in aortic valve are significantly related to the presence of concomitant coronary atherosclerosis in patients with symptomatic calcified aortic stenosis. J Transl Med. 2015;13:63. doi: 10.1186/s12967-015-0423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu B, et al. A Cytokine-Like Protein Dickkopf-Related Protein 3 Is Atheroprotective. Circulation. 2017;136:1022–1036. doi: 10.1161/CIRCULATIONAHA.117.027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, et al. Upregulation of Dickkopf1 by oscillatory shear stress accelerates atherogenesis. J Mol Med (Berl) 2016;94:431–441. doi: 10.1007/s00109-015-1369-9. [DOI] [PubMed] [Google Scholar]
- 15.Di M, et al. Dickkopf1 destabilizes atherosclerotic plaques and promotes plaque formation by inducing apoptosis of endothelial cells through activation of ER stress. Cell Death Dis. 2017;8:e2917. doi: 10.1038/cddis.2017.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng SL, Shao JS, Behrmann A, Krchma K, Towler DA. Dkk1 and MSX2-Wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1679–1689. doi: 10.1161/ATVBAHA.113.300647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen PY, et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125:4514–4528. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109:564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y, et al. Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood. 2015;126:2220–2229. doi: 10.1182/blood-2015-02-622233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chae WJ, et al. The Wnt Antagonist Dickkopf-1 Promotes Pathological Type 2 Cell-Mediated Inflammation. Immunity. 2016;44:246–258. doi: 10.1016/j.immuni.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brioschi M, Lento S, Tremoli E, Banfi C. Proteomic analysis of endothelial cell secretome: a means of studying the pleiotropic effects of Hmg-CoA reductase inhibitors. J Proteomics. 2013;78:346–361. doi: 10.1016/j.jprot.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Gobel A, et al. Potentiated suppression of Dickkopf-1 in breast cancer by combined administration of the mevalonate pathway inhibitors zoledronic acid and statins. Breast Cancer Res Treat. 2015;154:623–631. doi: 10.1007/s10549-015-3624-8. [DOI] [PubMed] [Google Scholar]
- 23.Rachner TD, et al. Dickkopf-1 is regulated by the mevalonate pathway in breast cancer. Breast Cancer Res. 2014;16:R20. doi: 10.1186/bcr3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banfi C, Baetta R, Gianazza E, Tremoli E. Technological advances and proteomic applications in drug discovery and target deconvolution: identification of the pleiotropic effects of statins. Drug Discov Today. 2017;22:848–869. doi: 10.1016/j.drudis.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Emeis JJ, Edgell CJ. Fibrinolytic properties of a human endothelial hybrid cell line (Ea.hy 926) Blood. 1988;71:1669–1675. [PubMed] [Google Scholar]
- 26.Oesterle A, Laufs U, Liao JK. Pleiotropic Effects of Statins on the Cardiovascular System. Circ Res. 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong, L. & Tergaonkar, V. Rho protein GTPases and their interactions with NFkappaB: crossroads of inflammation and matrix biology. Biosci Rep34 (2014). [DOI] [PMC free article] [PubMed]
- 28.Sun J, et al. Crosstalk between NF-kappaB and beta-catenin pathways in bacterial-colonized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G129–137. doi: 10.1152/ajpgi.00515.2004. [DOI] [PubMed] [Google Scholar]
- 29.Du Q, et al. Wnt/beta-catenin signaling regulates cytokine-induced human inducible nitric oxide synthase expression by inhibiting nuclear factor-kappaB activation in cancer cells. Cancer Res. 2009;69:3764–3771. doi: 10.1158/0008-5472.CAN-09-0014. [DOI] [PubMed] [Google Scholar]
- 30.Swarnkar G, Zhang K, Mbalaviele G, Long F, Abu-Amer Y. Constitutive activation of IKK2/NF-kappaB impairs osteogenesis and skeletal development. PLoS One. 2014;9:e91421. doi: 10.1371/journal.pone.0091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baetta R, et al. Atorvastatin reduces long pentraxin 3 expression in vascular cells by inhibiting protein geranylgeranylation. Vascul Pharmacol. 2015;67-69:38–47. doi: 10.1016/j.vph.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Pirmoradian M, et al. Rapid and deep human proteome analysis by single-dimension shotgun proteomics. Mol Cell Proteomics. 2013;12:3330–3338. doi: 10.1074/mcp.O113.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohne P, Prochnow H, Koch-Brandt C. The CLU-files: disentanglement of a mystery. Biomol Concepts. 2016;7:1–15. doi: 10.1515/bmc-2015-0026. [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, et al. Protective role of clusterin/apolipoprotein J against neointimal hyperplasia via antiproliferative effect on vascular smooth muscle cells and cytoprotective effect on endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:1558–1564. doi: 10.1161/ATVBAHA.109.190058. [DOI] [PubMed] [Google Scholar]
- 36.Navab M, et al. An oral apoJ peptide renders HDL antiinflammatory in mice and monkeys and dramatically reduces atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2005;25:1932–1937. doi: 10.1161/01.ATV.0000174589.70190.e2. [DOI] [PubMed] [Google Scholar]
- 37.Gelissen IC, et al. Apolipoprotein J (clusterin) induces cholesterol export from macrophage-foam cells: a potential anti-atherogenic function? Biochem J. 1998;331(Pt 1):231–237. doi: 10.1042/bj3310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zandl-Lang M, et al. Regulatory effects of simvastatin and apoJ on APP processing and amyloid-beta clearance in blood-brain barrier endothelial cells. Biochim Biophys Acta. 2018;1863:40–60. doi: 10.1016/j.bbalip.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Baetta R, et al. Nitric oxide-donating atorvastatin attenuates neutrophil recruitment during vascular inflammation independent of changes in plasma cholesterol. Cardiovasc Drugs Ther. 2013;27:211–219. doi: 10.1007/s10557-013-6445-1. [DOI] [PubMed] [Google Scholar]
- 40.Stern RH, et al. Pharmacodynamics and pharmacokinetic-pharmacodynamic relationships of atorvastatin, an HMG-CoA reductase inhibitor. J Clin Pharmacol. 2000;40:616–623. doi: 10.1002/j.1552-4604.2000.tb05987.x. [DOI] [PubMed] [Google Scholar]
- 41.Hoofnagle AN, et al. Low clusterin levels in high-density lipoprotein associate with insulin resistance, obesity, and dyslipoproteinemia. Arterioscler Thromb Vasc Biol. 2010;30:2528–2534. doi: 10.1161/ATVBAHA.110.212894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riwanto M, et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 43.Green PS, et al. Combined statin and niacin therapy remodels the high-density lipoprotein proteome. Circulation. 2008;118:1259–1267. doi: 10.1161/CIRCULATIONAHA.108.770669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trougakos IP. The molecular chaperone apolipoprotein J/clusterin as a sensor of oxidative stress: implications in therapeutic approaches - a mini-review. Gerontology. 2013;59:514–523. doi: 10.1159/000351207. [DOI] [PubMed] [Google Scholar]
- 45.Criswell T, et al. Delayed activation of insulin-like growth factor-1 receptor/Src/MAPK/Egr-1 signaling regulates clusterin expression, a pro-survival factor. J Biol Chem. 2005;280:14212–14221. doi: 10.1074/jbc.M412569200. [DOI] [PubMed] [Google Scholar]
- 46.Mussoni L, Banfi C, Sironi L, Arpaia M, Tremoli E. Fluvastatin inhibits basal and stimulated plasminogen activator inhibitor 1, but induces tissue type plasminogen activator in cultured human endothelial cells. Thromb Haemost. 2000;84:59–64. doi: 10.1055/s-0037-1613968. [DOI] [PubMed] [Google Scholar]
- 47.Krysiak R, Okopien B, Herman Z. Effects of HMG-CoA reductase inhibitors on coagulation and fibrinolysis processes. Drugs. 2003;63:1821–1854. doi: 10.2165/00003495-200363170-00005. [DOI] [PubMed] [Google Scholar]
- 48.Kruithof EK. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thromb Haemost. 2008;100:969–975. doi: 10.1160/TH08-04-0269. [DOI] [PubMed] [Google Scholar]
- 49.Iwasaki H, et al. High glucose induces plasminogen activator inhibitor-1 expression through Rho/Rho-kinase-mediated NF-kappaB activation in bovine aortic endothelial cells. Atherosclerosis. 2008;196:22–28. doi: 10.1016/j.atherosclerosis.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 50.Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000;47:648–657. doi: 10.1016/S0008-6363(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 51.Barbolina MV, et al. Matrix rigidity activates Wnt signaling through down-regulation of Dickkopf-1 protein. J Biol Chem. 2013;288:141–151. doi: 10.1074/jbc.M112.431411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fong LWR, Yang DC, Chen CH. Myristoylated alanine-rich C kinase substrate (MARCKS): a multirole signaling protein in cancers. Cancer Metastasis Rev. 2017;36:737–747. doi: 10.1007/s10555-017-9709-6. [DOI] [PubMed] [Google Scholar]
- 53.Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- 54.Barderas R, et al. In-depth characterization of the secretome of colorectal cancer metastatic cells identifies key proteins in cell adhesion, migration, and invasion. Mol Cell Proteomics. 2013;12:1602–1620. doi: 10.1074/mcp.M112.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandi J, et al. Secretome protein signature of human pancreatic cancer stem-like cells. J Proteomics. 2016;136:1–12. doi: 10.1016/j.jprot.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 56.El Amri M, Fitzgerald U, Schlosser G. MARCKS and MARCKS-like proteins in development and regeneration. J Biomed Sci. 2018;25:43. doi: 10.1186/s12929-018-0445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singer M, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 58.Takashi S, et al. A peptide against the N-terminus of myristoylated alanine-rich C kinase substrate inhibits degranulation of human leukocytes in vitro. Am J Respir Cell Mol Biol. 2006;34:647–652. doi: 10.1165/rcmb.2006-0030RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eckert RE, Neuder LE, Park J, Adler KB, Jones SL. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am J Respir Cell Mol Biol. 2010;42:586–594. doi: 10.1165/rcmb.2008-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugiura T, Wang H, Barsacchi R, Simon A, Tanaka EM. MARCKS-like protein is an initiating molecule in axolotl appendage regeneration. Nature. 2016;531:237–240. doi: 10.1038/nature16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 62.Kunes P, Holubcova Z, Kolackova M, Krejsek J. Pentraxin 3(PTX 3): an endogenous modulator of the inflammatory response. Mediators Inflamm. 2012;2012:920517. doi: 10.1155/2012/920517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han B, et al. TNFalpha-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J Immunol. 2005;175:8303–8311. doi: 10.4049/jimmunol.175.12.8303. [DOI] [PubMed] [Google Scholar]
- 64.Norata GD, et al. Long pentraxin 3, a key component of innate immunity, is modulated by high-density lipoproteins in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:925–931. doi: 10.1161/ATVBAHA.107.160606. [DOI] [PubMed] [Google Scholar]
- 65.Banfi C, et al. Tissue factor induction by protease-activated receptor 1 requires intact caveolin-enriched membrane microdomains in human endothelial cells. J Thromb Haemost. 2007;5:2437–2444. doi: 10.1111/j.1538-7836.2007.02759.x. [DOI] [PubMed] [Google Scholar]
- 66.Roverso M, et al. A preliminary study on human placental tissue impaired by gestational diabetes: a comparison of gel-based versus gel-free proteomics approaches. Eur J Mass Spectrom (Chichester) 2016;22:71–82. doi: 10.1255/ejms.1412. [DOI] [PubMed] [Google Scholar]
- 67.Brioschi M, et al. A mass spectrometry-based workflow for the proteomic analysis of in vitro cultured cell subsets isolated by means of laser capture microdissection. Anal Bioanal Chem. 2014;406:2817–2825. doi: 10.1007/s00216-014-7724-9. [DOI] [PubMed] [Google Scholar]
- 68.Franceschini A, et al. STRINGv9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banfi C, et al. Very low density lipoprotein-mediated signal transduction and plasminogen activator inhibitor type 1 in cultured HepG2 cells. Circ Res. 1999;85:208–217. doi: 10.1161/01.RES.85.2.208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.