Abstract
The purpose of this meta-analysis was to compare the ability of the qSOFA in predicting short- (≤30 days or in-hospital mortality) and long-term (>30 days) mortality among patients outside the intensive care unit setting. Studies reporting on the qSOFA and mortality were searched using MEDLINE and SCOPUS. Studies were included if they involved patients presenting to the ED with suspected infection and usage of qSOFA score for mortality prognostication. Data on qSOFA scores and mortality rates were extracted from 36 studies. The overall pooled sensitivity and specificity for the qSOFA were 48% and 86% for short-term mortality and 32% and 92% for long-term mortality, respectively. Studies reporting on short-term mortality were heterogeneous (Odd ratio, OR = 5.6; 95% CI = 4.6–6.8; Higgins’s I2 = 94%), while long-term mortality studies were homogenous (OR = 4.7; 95% CI = 3.5–6.1; Higgins’s I2 = 0%). There was no publication bias for short-term mortality analysis. The qSOFA score showed poor sensitivity but moderate specificity for both short and long-term mortality, with similar performance in predicting both short- and long- term mortality. Geographical region was shown to have nominal significant (p = 0.05) influence on qSOFA short-term mortality prediction.
Introduction
Sepsis is a syndrome characterized by a group of clinical signs and symptoms in patients with suspected infection1. It is a significant cause of mortality worldwide; in the last decade, an estimated 31.5 million sepsis patients have been treated globally per year, including 5.3 million deaths due to sepsis2. The diagnosis of sepsis is challenging, as a reliable test for its early confirmation is not available. Given the morbidity and mortality of sepsis, the ability to perform risk stratification in the early phase of patients’ illness is crucial to help physicians manage and improve their outcome.
The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) defined sepsis as “life-threatening organ dysfunction caused by a dysregulated host response to infection”1, previously known as “severe sepsis”3. The Systemic Inflammatory Response Syndrome (SIRS) criteria which was used formerly for early identification of sepsis was considered impractical and inefficient4. As Sepsis-3 definition includes organ dysfunction, the Sequential Organ Failure Assessment (SOFA) has been used to identify life-threatening organ failure, where an acute increase of a score of 2 in SOFA score reflects approximately 10% increase of risk in sepsis mortality in the general population1. The SOFA scoring is sophisticated and time consuming, therefore, Sepsis-3 proposed the parsimonious quick Sepsis-Related Organ Failure Assessment (qSOFA) which depends only on clinical signs to distinguish patients having organ failure in sepsis1. It identifies sepsis patients via presence of two out of the three clinical signs of tachypnoea, altered mental status, and hypotension; in which altered mental status among the three components is emphasized as it reduces the measurement burden with its prediction validity1. Nevertheless, several studies have suggested that qSOFA lacks accuracy for predicting mortality in patients both outside and inside the intensive care unit compared to SOFA, Logistic Organ Dysfunction System (LODS), and other early scoring systems5–7. Ongoing efforts have been directed toward examining the ability of qSOFA to predict poor outcomes in patients with infection7.
The presence of organ failure in sepsis increases the risk of mortality with an average of 28%8. Nevertheless, current therapies for sepsis are aimed to prevent mortality mostly at the acute phase; survival of patients after hospital discharge were rarely followed-up. Only very few studies have investigated long-term mortality of sepsis; and these studies postulated very high mortality rates one-year post sepsis9. A study from Lemay et al. showed that long-term mortality rate for sepsis with organ failure was 30.6% for one year post sepsis and 43% for two years post sepsis, respectively10. Other studies showed similar findings of 51.4%11 for one- and 44.9%12 for two- year mortality, respectively. As qSOFA is a relatively new scoring system, the clinical practicality of this scoring system for predicting short- and long-term sepsis mortality has not been fully evaluated.
The intention of this systematic review and meta-analysis was to evaluate qSOFA as a short- and long-term sepsis mortality predictor in patients presenting outside of the intensive care unit (ICU). We hypothesized that qSOFA can predict short- and long-term mortality in sepsis patients. The prognostic accuracy of qSOFA score for both short- (≤30 days and in-hospital mortality) and long-term (>30 days) mortality was analysed.
Methods
Study Eligibility and Search Strategy
A systematic review and meta-analysis of the literature was conducted to identify relevant studies regarding the role of the qSOFA in mortality prognostication, among patients with suspected infection who presented outside of the ICU after obtaining consent from UKM Research Ethical Committee (UKM PPI/111/8/JEP-2017-769). We used MEDLINE via Ovid Medline to conduct a comprehensive search of health science journals (published between February 2016 and 15 December 2017) and SCOPUS (published before 15 December 2017); hand-checking of the references of relevant articles was then carried out. The search team comprised of three clinicians, a statistician and a scientist. The search strategy involved a combination of the following two sets of keywords (1) ‘quick sequential organ failure assessment’, OR ‘quick SOFA’, OR ‘qSOFA’, OR ‘quick sepsis related organ failure assessment’ and; (2) ‘mortalit*’. This meta-analysis was registered in PROSPERO (CRD42017079364, http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017079364). The search strategies were shown in Supplementary Table S1.
Identification and Selection of Studies
Study selection was performed based on their titles or abstracts, and only studies which appeared to fulfil the eligibility criteria were selected for full-text review. To be included, studies must fulfil the following criteria: inclusion of adult patients (≥18 years old) presenting to outside of ICU (EDs and in wards); usage of Sepsis-3 definition with suspected infection; usage of qSOFA score for mortality prognostication; and written in English. Papers were excluded if they were: related to review articles; articles without complete texts; or animal studies.
Data Extraction and Study Appraisal
The selection of papers to be included into this review was completed in four phases. First, an initial search of the selected databases was performed using the pre-specified keywords to identify relevant keywords and index terms. Second, a thorough search was conducted in which papers that failed to meet the inclusion criteria based solely on their titles and abstracts were excluded. In the third phase, the remaining papers from the second phase were extensively reviewed, and papers that did not meet our inclusion criteria were excluded. Finally, all relevant data from the included papers was subjected to meta-analysis to determine conclusions regarding the proposed hypothesis.
After the initial screening of titles and abstracts by two independent reviewers who are clinicians, articles without full text were removed. The remaining papers were screened again by the two reviewers. To minimize errors, both reviewers were trained and standardized using QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies-2)13, with subsequent practice using several articles as calibration. Any discrepancies were resolved through discussion with a third reviewer who is an Emergency Physician. The QUADAS-2 criteria was also used to assess the quality of all selected articles. The risk of bias of each included study was summarized in Supplementary Tables S2 and S3. Data extraction was conducted independently in a standardized manner with a data collection form. Study data including author, publication year, type of study conducted, brief description of the study population/sample, methods used in the study and mortality outcome were extracted from the full text of each article and summarized in detail (Supplementary Tables S4 and S5). Short-term mortality was defined as ≤30 days or in-hospital mortality. Long-term mortality was defined as >30 days. This analysis was reported according to Transparent Reporting of Systematic Reviews and Meta-Analyses (PRISMA) guideline. A flow diagram of study identification and article selection for the meta-analysis can be found in Fig. 1.
Figure 1.

Identification and Selection of Articles for Meta-analysis. Flow chart shows process of article selection and exclusion throughout the study.
Statistical Analysis
All statistical analysis was performed using the Review Manager 5 (Version 5.3.5) software by Cochrane Community and the Comprehensive Meta-Analysis Software (CMA, Version 3) by Biostat (AnalystSoft Inc.). Based on this model, pooled sensitivity, specificity and odds ratio (OR) with 95% CI were determined. Random effects model was used to report short- and long-term mortality individually with estimates of sensitivity, specificity and ORs. The Cochran’s Q test and Higgin’s I2 statistics were calculated to determine the proportion of between-study variation caused by heterogeneity. Using Higgin’s I2 the suggested heterogeneity thresholds for low (25–49%), moderate (50–74%), and high (75%) values were used. The publication bias of included studies was assessed using effective sample-size funnel plot (OR values vs sample size of each study), Begg-adjusted rank correlation tests and the Egger regression asymmetry test for small study effects. We then performed subgroup analyses according to age group (younger age group at <65 years old and older age group at ≥65years old)14, geographical region (Africa, Asia, Central America, Europe and Oceania)15 and higher and middle/low income countries based on World Bank list of economies16, June 2018.
Results
The search identified relevant studies from MEDLINE via Ovid Medline (February 2016 to 15 December 2017) and SCOPUS databases (through 15 December 2017). The numbers of relevant records identified in MEDLINE and SCOPUS were 42 and 80, respectively, for a total of 122 references retrieved. Forty-two records were identified as duplicates and were removed from our selection. Subsequently, from the 80 references, 41 were excluded based on titles and abstracts: 22 did not meet the primary objective of our review, two did not meet our inclusion criteria, two studies were published in languages other than English, and 15 were other articles including review, consensus, perspective, commentary and editorial papers. The full texts of the remaining 39 studies were then successfully retrieved. Three papers were excluded due to incomplete data (Supplementary Tables S6 and S7). The authors of the three studies failed to be contacted via electronic mail. Finally, 36 studies fulfilled the inclusion criteria and were included. The characteristics of the included studies5–7,17–49 are summarized in Table 1.
Table 1.
Summary of Characteristics of Included Studies.
| Source | No. of Participants | Mean Age, y | Men, No. (%) | Main Inclusion Criteria | Outcome |
|---|---|---|---|---|---|
| Short-term mortality | |||||
| April17 | 214 | 68 | 126 (59%) | ED patients admitted to any ICU with suspected or proven infection | In-hospital mortality |
| Askim18 | 1535 | 61a | 813 (53%) | New onset of suspected or confirmed infection according to the ESS47 | 30-day mortality |
| Brabrand19 | 3824 | 65a | 2426 (63%) | Patients presenting or discharged with suspected infection | In-hospital mortality and/or ICU stay >3days |
| Chen20 | 1641 | 73a | 968 (59%) | Patients with CAP or healthcare-associated pneumonia | 28-day mortality |
| Churpek5 | 30677 | 58 | 14561 (47%) | Patients with suspicion of infection in wards or ED | 28-day mortality |
| Churpek21 | 53849 | 57 | 24719 (46%) | Patients meeting suspicion of infection in ED or wards | In-hospital mortality |
| deGroot22 | 2280 | 61 | 1315 (58%) | ED patients with suspected infection | In-hospital mortality |
| Donnelly23 | 2593 | NA | NA | Admitted patients who meet SIRS criteria, SOFA and qSOFA criteria | 28-day mortality |
| Finkelsztein24 | 152 | 64a | 83 (55%) | Patients with suspicion of infection admitted to the medical ICU from emergency department or hospital wards | In-hospital mortality |
| Forward25 | 161 | 70 | 89 (55%) | Non-ICU inpatients who triggered the hospital SK pathway with acute deterioration and suspected or proven infection | In-hospital mortality |
| Freund26 | 879 | 67a | 465 (53%) | Patients admitted to ED with clinical suspicion of infection | In-hospital mortality |
| Giamarellos27 | 3436 | NA | NA | Patients with signs of infection | 28-day mortality |
| Gonzalez28 | 1071 | 84 | 544 (51%) | Patients ≥75 years old clinically diagnosed with acute infection in ED | 30-day mortality |
| Haydar29 | 199 | 71a | 109 (55%) | ED patients treated for suspected sepsis | In-hospital mortality |
| Henning30 | 7637 | 60 | 3799 (50%) | ED patients admitted to the hospital with an infection-related diagnosis | In-hospital mortality |
| Huson31 | 329 | 34a | 125 (38%) | Patients with suspected infection with ≥2 SIRS criteria | In-hospital mortality |
| Huson32 | 458 | 35a | 243 (53%) | Patients admitted to the adult medical ward with suspected infection | In-hospital mortality |
| Hwang33 | 1395 | 65a | 787 (56%) | Patients who received a diagnosis of severe sepsis or septic shock during ED stay | 28-day mortality |
| Kim34 | 615 | 54 | 204 (33%) | Patients with fever and chemotherapy-induced neutropenia | 28-day mortality |
| Kim35 | 125 | 76 | 78 (62%) | Patients admitted to ED with discharge diagnosis of CAP | 28-day mortality |
| Kolditz36 | 9327 | 63a | 5249 (56%) | Patients with CAP | 30-day mortality |
| LeGuen37 | 182 | 72a | 88 (48%) | Patients reviewed by the RRT | 30-day mortality |
| Moskowitz38 | 24164 | 64 | 12299 (51%) | Patients with suspected infection presented to ED | In-hospital mortality |
| Patidar39 | 124 | 57 | NA | Cirrhotic patients hospitalized non-electively for infectious etiologies | 30-day mortality |
| Quinten40 | 193 | 60 | 108 (56%) | Non-trauma patients in ED with suspected infection or sepsis | 28-day mortality |
| Ranzani41 | 6874 | 66 | 4259 (62%) | Patients with clinical diagnosis of CAP | 30-day mortality |
| Rothman42 | 3926 | NA | NA | Patients admitted to hospital with sepsis | In-hospital mortality |
| Seymour7 | 66522 | 60 | 27446 (41%) | Patients with suspected infection | In-hospital mortality |
| Shetty43 | 12555 | 50a | 6585 (52%) | Patients with suspected infection, suspected or confirmed sepsis | Mortality and/or prolonged ICU stay ≥72 hours |
| Singer44 | 22530 | 54 | 10589 (47%) | ED patients whom qSOFA score could be calculated according to simultaneous reporting of vital signs and a MEWS score | In-hospital mortality |
| Szakmany45 | 380 | 74a | 180 (47%) | Patients with high degree of clinical suspicion of infection | 30-day mortality |
| Tusgul46 | 886 | 80 | 462 (52%) | Patients with suspected infection without alternative diagnosis, or microbiologically proven infection found in the ED workup | In-hospital mortality |
| Umemura47 | 387 | 74a | 232 (60%) | ED patients admitted to ICU with diagnosis of severe sepsis | In-hospital mortality |
| Wang48 | 477 | 73a | 295 (62%) | Patients treated at ED with clinically diagnosed infection | 28-day mortality |
| Williams6 | 8871 | 49a | 4453 (50%) | ED patients admitted with a diagnosis indicating presumed or potential infection | 30-day mortality |
| Long-term mortality | |||||
| Donnelly23 | 2593 | NA | NA | Admitted patients who meet the SIRS criteria, SOFA and qSOFA criteria | 1-year mortality |
| Quinten40 | 193 | 60 | 108 (56%) | Non-trauma patients in ED with suspected infection or sepsis | 6-month mortality |
| Rannikko49 | 497 | 68a | 262 (53%) | Adult patients admitted to the ED who had blood culture-positive sepsis | 90-day mortality |
Abbreviations: ED, emergency department; ICU, intensive care unit; ESS47, Emergency Symptoms and Signs algorithm for infection; CAP, community acquired pneumonia; NA, not available; SIRS, systemic inflammatory response syndrome; SOFA, Sequential organ failure assessment; qSOFA, quick sequential organ failure assessment; SK, “Sepsis Kills”; RRT, Rapid Response Team; MEWS, Modified Early Warning System.
aMedian.
The prognostic accuracy of qSOFA was evaluated in different countries, with most studies conducted in the United States of America and Europe, followed by Asia, Africa, New Zealand and Australia. The cut-off values of the Glasgow Coma Scale (GCS) used in all these studies to determine altered mentation in the qSOFA included GCS less than 15, 14 and 13, except nine which only stated altered mentation20,24,25,29,30,36,38,40,41. Thirty-three studies reported on short-5–7,17–22,24–39,41–48, one reported on long-49, while two studies23,40 reported on both short- and long-term mortality, respectively.
qSOFA for short- and long- term mortality prognostication
In this meta-analysis, 35 studies with 269,544 patients reported on the prognostic accuracy of the qSOFA and short-term mortality. Twenty-seven were retrospective studies5–7,17,19,20,22–25,27,29,31,33–36,38,41–44,46–49, while 8 studies were prospective studies18,21,26,28,30,32,37,39,45. Due to the heterogeneity of the inclusion criteria, a random-effects model was used to calculate the pooled sensitivity and specificity of the included studies. The forest plot for the sensitivity and specificity of the qSOFA predicting short-term mortality is shown in Fig. 2. The pooled sensitivity was 48% and the specificity was 86%. The pooled odds ratio (OR) was 5.6 (95% CI: 4.6–6.8), indicating that an elevated qSOFA score was associated with increased short-term mortality. The forest plot for the OR is shown in Fig. 3. We detected significant heterogeneity according to the heterogeneity tests (Cochran’s Q Test P < 0.01, Higgins’s I2 = 94%). Publication bias was not detected as shown in the funnel plot (Supplementary Fig. S1). Egger’s regression and Begg’s test revealed no statistical significance with p = 0.84 (2-tailed) and p = 0.46 respectively, indicating no publication bias (Supplementary Table S8).
Figure 2.
Sensitivity and Specificity of quick Sepsis-Related Organ Failure Assessment (qSOFA) in Predicting Short-term and Long-term Mortality. Studies included into the meta-analysis and their corresponding sensitivity and specificity of quick Sepsis-Related Organ Failure Assessment (qSOFA) values in predicting short- and long-term mortality is shown using a forest plot.
Figure 3.
Odds Ratio of quick Sepsis-Related Organ Failure Assessment (qSOFA) in Predicting Short-term and Long-term Mortality. Odds of each study is shown in the forest plot. All studies found odds ratio of >1 for quick Sepsis-Related Organ Failure Assessment (qSOFA) in predicting short- and long-term mortality.
Only three studies with a total of 3,076 patients reported on the prognostic accuracy of the qSOFA and long-term mortality. Among these studies, two were retrospective23,49 and one was a prospective study40. The forest plot for the sensitivity and specificity of the qSOFA for predicting long-term mortality is shown in Fig. 2. The pooled sensitivity and specificity were calculated using a random-effects model, which yielded a pooled sensitivity of 32% and a pooled specificity of 92%. The three studies reported distinct mortality intervals: 90-day mortality (sensitivity = 56%, specificity = 79%)49, 6-month mortality (sensitivity = 33%, specificity = 85%)40 and 12-month mortality (sensitivity = 21%, specificity = 95%)23. The forest plot for the odds ratio is shown in Fig. 3. The pooled OR was 4.7 (95% CI: 3.5–6.1), and the studies were homogenous (Cochran’s Q Test P = 0.52, Higgins’s I2 = 0%). However, publication bias was not assessed due to the small number of studies included in the long-term mortality analysis.
Performing further analysis for these two groups, we found that qSOFA was able to significantly predict both short- and long-term mortality with the OR of 5.5 (95% CI: 4.6–6.6). Both groups were homogenous and there was no evidence of interaction between short- and long-term mortality (Cochran’s Q Test P = 0.28, Higgins’s I2 = 14.9%).
Subgroup analyses for qSOFA short-term mortality prognostication
Age group
Three studies were excluded from this analysis due to missing information for age23,27,42. The test for subgroup differences indicates that there is no statistically significant subgroup effect (p = 0.27), suggesting that age group does not modify the effect of short-term mortality in comparison to survival. Our subgroup analysis indicated that patients that younger than 65 years old with elevated qSOFA had almost 6.0 times significantly higher risk for short-term mortality, while those in the with older age group of ≥65 years old with elevated qSOFA had almost 4.6 times significantly higher risk for short-term mortality (Fig. 4). There was substantial heterogeneity within each of these subgroups (age group <65, Cochran’s Q Test, P < 0.01, Higgins’s I2 = 95%; and age group ≥65, Cochran’s Q Test, P < 0.01, Higgins’s I2 = 82%). The age subgroup analysis was homogenous (Cochran’s Q Test, P = 0.21, Higgins’s I2 = 36.3%) indicated that there was no evidence of subgroup effect between the age groups.
Figure 4.
Age group sub-analysis: Odds Ratio of quick Sepsis-Related Organ Failure Assessment (qSOFA) in Predicting Short-term Mortality. Both groups showed significance difference and heterogeneity. However, there is no evidence of interaction between the subgroups.
Geographical region
There was nominal statistically significant subgroup effect (p = 0.05) between geographical regions and short-term mortality (Fig. 5). Geographical region subgroup analysis showed that African patients with elevated qSOFA scores had the highest risk (OR:8.4; 95% CI: 2.5–27.9) of short-term mortality, followed by patients from Central America (OR: 6.9; 95% CI: 4.7–10.2), Europe (OR: 5.4; 95% CI: 4.3–6.9), Oceania (OR: 4.7; 95% CI: 1.6–14.1) and Asia (OR: 3.5; 95% CI: 2.6–4.7). All studies showed heterogeneity with I2 ranging from 53–98%, an indication that the results in all subgroup studies were inconsistent.
Figure 5.
Geographical region sub-analysis: Odds Ratio of quick Sepsis-Related Organ Failure Assessment (qSOFA) in Predicting Short-term Mortality. All studies showed heterogeneity except studies from Africa and Asia. Both Cochran’s Q Test P = 0.05, Higgins’s I2 = 58.9% showed nominal significant interaction between all geographical regions in short-term mortality prediction.
Country Income
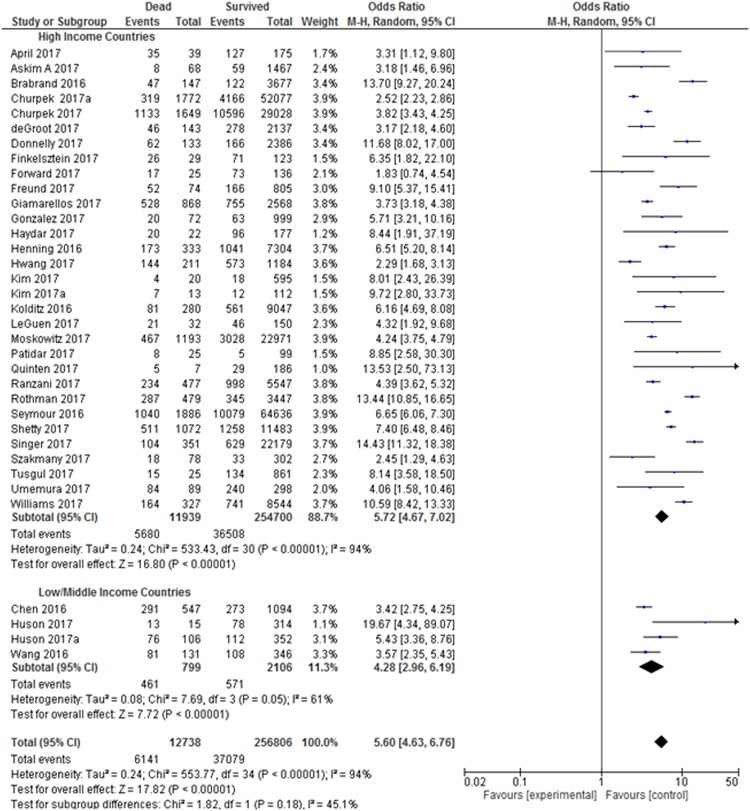
Analysis on countries’ income (high versus low/middle income) revealed that patients from high income countries with elevated qSOFA scores had almost 6 times significantly higher risk for short-term mortality, while those from low and middle income countries with elevated qSOFA scores had almost 5 times significantly higher risk for short-term mortality (Fig. 6). All studies indicated heterogeneity, showing variability in the results of the associated studies. However, there is no evidence of subgroup effect between the low/middle subgroup with high income countries subgroup in terms of short-term mortality (Cochran’s Q Test P = 0.18, Higgins’s I2 = 45.1%).
Figure 6.
Country Income sub-analysis: Odds Ratio of quick Sepsis-Related Organ Failure Assessment (qSOFA) in Predicting Short-term Mortality. Low and middle income countries showed homogeneity while high income countries indicated heterogeneity. However, there is no evidence of interaction between the subgroups with short-term mortality (Cochran’s Q Test P = 0.18, Higgins’s I2 = 45.1%).
Sensitivity Analysis
We further performed sensitivity analysis with fixed effect model (Supplementary Fig. S2). The pooled OR for short-term mortality was 4.9 (95% CI: 4.7–5.1) and long-term mortality was 4.6 (95% CI: 3.5–6.1). However, there was no evidence of subgroup effect between the short- and long-term mortality (Cochran’s Q Test P = 0.73, Higgins’s I2 = 0%). This finding is similar to random effect analysis in Fig. 3. We conclude that the random effect analysis is conclusive and robust.
Discussion
Most of the studies included into this systematic review and meta-analysis suggested that a qSOFA score of ≥2 was able to predict short and long-term mortality. A total of 36 studies were reviewed, and the quality of the studies varied. Most of the studies had good quality according to QUADAS-2. Seven studies showed evidence of bias. These seven studies had excluded many missing data and missing data analysis were not mentioned.
Our analysis revealed that qSOFA score exhibited fair sensitivity and specificity in predicting mortality. The pooled specificity of qSOFA in this study was higher compared to SIRS (66%)50. According to our analysis, qSOFA can predict sepsis mortality, with the odds of 5.6 for short-term mortality and 4.7 for long-term mortality. Nevertheless, test for subgroup analysis showed no differences in qSOFA prediction of short- and long-term mortality in sepsis. Although long-term mortality analysis showed homogeneity, only three studies were analysed – the number of studies was too small to be conclusive.
All 35 papers reporting on short-term mortality showed clinical, methodological and statistical heterogeneity. Factors that may have contributed to the high heterogeneity included mean age (ranging from 54 to 84 years old), variation in clinical settings, variation in the timing of qSOFA scoring, and broad range of clinical diagnosis and criteria. This heterogeneity contributed to a lower pooled sensitivity of the qSOFA that may not represent the actual accuracy of the qSOFA for mortality prognostication. However, this finding was expected as the study populations were diverse and multiple confounding factors were present. All studies showed positive direction in the forest plot reflecting a high pooled OR. The funnel plot revealed no publication bias for the studies investigating qSOFA in predicting short-term mortality. Recently, three new publications reported on qSOFA short-term mortality prediction with similar findings to our meta-analysis51–53. Nevertheless, these studies did not perform further analysis on qSOFA long-term mortality prediction nor compared its prognostic accuracy with short-term mortality.
The three studies which reported on qSOFA prognostication for long-term mortality showed clinical and methodological heterogeneity, but they were statistically homogenous. The performance of the qSOFA in long-term mortality prediction was more specific but less sensitive compared to its performance in short-term mortality. Further studies will be important to provide insight into this intriguing finding.
Subgroup analyses based on age group, geographical region and country income for short-term mortality were performed. The sub-analyses showed that only geographical region has nominal significant influence on qSOFA short-term mortality prediction. Although it is not conclusive, this observation is new and interesting, we suspect it could be related to cultures and lifestyles specific to certain geographical areas. Our sub-analysis showed that qSOFA risk prognostication for short-term mortality were highest in studies from the African region, followed by Central America, Europe, the Oceania region and Asia. For both studies from Gabon, Africa31,32. where HIV is endemic, one fifth of the study cohort were HIV positive. This pre-existing co-morbidity may have contributed to higher risk of short-term mortality. In addition, Moss et al. found that both African Americans and other non-whites had similar elevated risk of sepsis, compared with whites54. Dombrovskiy et al. found that blacks had higher hospitalization rates and mortality for sepsis than in whites55. It is interesting to observe that Asians have the lowest qSOFA risk prediction for short-term sepsis mortality. This could be linked to the nature of health-conscious lifestyle in Asian countries like Japan. Marmot et al. found that differences in diet, living environment and work contributed to reduced mortality rates in the Japanese56.
Sepsis was redefined in 2016 and the qSOFA was introduced as a parsimonious model to SOFA score for sepsis prognostication. The advantage of the qSOFA is that it can be repeatedly performed over time without laboratory investigations, which can be time-consuming34. Since sepsis can deteriorate in a short period of time, a simple screening tool for early detection is warranted. The SIRS criteria introduced in previous sepsis definitions3,57 was found to be overly sensitive relative to its specificity5. It has high sensitivity and poor specificity and could lead to an excessive number of false positives, causing unnecessary diagnostic or therapeutic procedures. Over-diagnosing patients poses a significant economic impact and further increases patients’ medical burden. In addition to qSOFA scoring, several publications have suggested lactate level could be avaluable biomarker when added to the original qSOFA score and may improve its prognostic value30,38,43. These studies provide insight into modification of the qSOFA which may improve its sensitivity and efficacy in detecting patients with sepsis. Efforts to modify the qSOFA could consider combining the present scoring criteria with other sepsis biomarkers such as C-reactive protein (CRP), lactate58–61, serum secretory phospholipase A2-IIa (SPLA2-IIA)62–65 and procalcitonin (PCT)66.
Although the qSOFA exhibited high specificity and low sensitivity in most of the studies included in our meta-analysis, seven papers showed contradictory results. The studies reported that qSOFA was highly sensitive but had poor specificity. On further analysis, four of the studies had sample populations comprised of patients who were directly admitted from the ED to the ICU17,24,33,47, and two other studies included high numbers of HIV carriers31,32. The remaining paper had a distinct study population including elderly and disabled patients, in whom assessment of altered mental status was regarded as challenging29. The population included in these studies were more specific and likely to present to the ED with greater illness severity. Due to the specificity of these study populations, patients in these studies tended to be screened as positive as reflected by the identification of more true-positive patients compared to the other studies’ populations, resulting in heightened sensitivity of the qSOFA.
Limitations
In this meta-analysis, we successfully retrieved all full-texts and a standardized tool was used to examine the quality of the included papers. One limitation of our analysis was the small numbers of articles available on long-term mortality. Secondly, we discovered that the study populations were substantially diverse, as some studies included specific infection groups of patients31,32. However, all of the included patients fulfilled our inclusion criterion of patients with suspected infection. Since random sampling was not performed in most of the included studies, a sampling bias is likely. Some studies had combined outcomes of mortality and/or ICU admission, thus complicating precise categorization of outcomes19,43. We classified in-hospital mortality as short-term mortality. Since in-hospital mortality may be longer than 30 days, this assumption may lead to a misclassification bias and mask the true predictive ability of the qSOFA. Most of the included studies were retrospective studies, posing a certain disadvantage as these studies relied on available medical records. Therefore, missing records or data may have influenced the results and the predictive accuracy of qSOFA in the current analysis. In addition, most of the studies were single-centered with variability across methods and study designs, which contributed to heterogeneity. Multiple confounders were likely to coexist, which may have jeopardized the validity of these studies. Future research should consider prospective randomization in sampling methods to minimize sampling bias. More studies exploring the qSOFA for long-term mortality prediction should be conducted in the near future.
Conclusion
This meta-analysis revealed that the qSOFA score had a poor sensitivity but moderate specificity for both short and long-term mortality prediction in patients with suspected infection. Geographical region had nominal significant influence on qSOFA short-term mortality prediction. Further research on modification of qSOFA may improve its sensitivity in detecting sepsis patients for prompt intervention.
Electronic supplementary material
Acknowledgements
The authors wish to thank Universiti Kebangsaan Malaysia for funding of the manuscript. This study was funded by grant Fundamental Research Grant Scheme (FRGS/1/2014/SKK01/UKM/03/3), Prototype Research Grant Scheme (PRGS/1/2017/STG05/UKM/03/1) from the Ministry of Higher Education, Malaysia and Fundamental Fund, FF-2018-015 from Faculty of Medicine, Universiti Kebangsaan Malaysia.
Author Contributions
Dr. Toh Leong Tan has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. He contributed in study concept and design, acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; and obtained funding for the study. Dr. Ying Jing Tang was responsible for acquisition of data; analysis and interpretation of data; drafting of the manuscript and statistical analysis. Dr. Ling Jing Ching was responsible for acquisition of data; analysis and interpretation of data; drafting of the manuscriptand statistical analysis. Dr. Noraidatulakma Abdullah is the statistician who analysed and interpreted the data; performed statistical analysis and drafted the manuscript. Associate Professor Dr. Hui-min Neoh analysed and interpreted the data; drafted and was involved in revision of the manuscript.
Data Availability
All data supporting the findings of this study are available within the paper and its Supplementary Information File.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35144-6.
References
- 1.Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. American journal of respiratory and critical care medicine. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Levy MM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Medicine. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 4.Churpek MM, Zadravecz FJ, Winslow C, Howell MD, Edelson DP. Incidence and Prognostic Value of the Systemic Inflammatory Response Syndrome and Organ Dysfunctions in Ward Patients. American journal of respiratory and critical care medicine. 2015;192:958–964. doi: 10.1164/rccm.201502-0275OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Churpek MM, et al. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients outside the Intensive Care Unit. American Journal of Respiratory & Critical Care Medicine. 2017;195:906–911. doi: 10.1164/rccm.201604-0854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams JM, et al. Systemic Inflammatory Response Syndrome, Quick Sequential Organ Function Assessment, and Organ Dysfunction: Insights From a Prospective Database of ED Patients With Infection. Chest. 2017;151:586–596. doi: 10.1016/j.chest.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 7.Seymour CW, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Erratum appears in JAMA. 2016 May 24–31; 315(20): 2237; PMID: 27218643] JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angus DC, Wax RS. Epidemiology of sepsis: an update. Critical care medicine. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 9.Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. Journal of leukocyte biology. 2004;75:408–412. doi: 10.1189/jlb.0503214. [DOI] [PubMed] [Google Scholar]
- 10.Lemay AC, Anzueto A, Restrepo MI, Mortensen EM. Predictors of long-term mortality after severe sepsis in the elderly. The American journal of the medical sciences. 2014;347:282–288. doi: 10.1097/MAJ.0b013e318295a147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Critical care medicine. 2003;31:2316–2323. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson S, Ruokonen E, Varpula T, Ala-Kokko TI, Pettilä V. Long-term outcome and quality-adjusted life years after severe sepsis. Critical care medicine. 2009;37:1268–1274. doi: 10.1097/CCM.0b013e31819c13ac. [DOI] [PubMed] [Google Scholar]
- 13.Whiting PF, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Pirozzi, N. et al. Sepsis: epidemiology, pathophysiology, classification, biomarkers and management. J Emerg Med Trauma Surg Care3, 14 (2016).
- 15.United Nation. Methodology - Standard country or area codes for statistical use (M49), https://unstats.un.org/unsd/methodology/m49/ (2018).
- 16.The World Bank. World Bank Country and Lending Groups, https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (2018).
- 17.April MD, et al. Sepsis Clinical Criteria in Emergency Department Patients Admitted to an Intensive Care Unit: An External Validation Study of Quick Sequential Organ Failure Assessment. Journal of Emergency Medicine. 2017;52:622–631. doi: 10.1016/j.jemermed.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Askim, Å. et al. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality - a prospective study of patients admitted with infection to the emergency department. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine25, 56 (2017). [DOI] [PMC free article] [PubMed]
- 19.Brabrand M, Havshoj U, Graham CA. Validation of the qSOFA score for identification of septic patients: A retrospective study. European Journal of Internal Medicine. 2016;36:e35–e36. doi: 10.1016/j.ejim.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Chen YX, Wang JY, Guo SB. Use of CRB-65 and quick Sepsis-related Organ Failure Assessment to predict site of care and mortality in pneumonia patients in the emergency department: a retrospective study. Critical Care (London, England) 2016;20:167. doi: 10.1186/s13054-016-1351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Churpek Matthew M., Snyder Ashley, Sokol Sarah, Pettit Natasha N., Edelson Dana P. Investigating the Impact of Different Suspicion of Infection Criteria on the Accuracy of Quick Sepsis-Related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores*. Critical Care Medicine. 2017;45(11):1805–1812. doi: 10.1097/CCM.0000000000002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Groot B, et al. The most commonly used disease severity scores are inappropriate for risk stratification of older emergency department sepsis patients: an observational multi-centre study. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 2017;25:91. doi: 10.1186/s13049-017-0436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly JP, Safford MM, Shapiro NI, Baddley JW, Wang HE. Application of the Third International Consensus Definitions for Sepsis (Sepsis-3) Classification: a retrospective population-based cohort study. The Lancet Infectious Diseases. 2017;17:661–670. doi: 10.1016/S1473-3099(17)30117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkelsztein, E. J. et al. Comparison of qSOFA and SIRS for predicting adverse outcomes of patients with suspicion of sepsis outside the intensive care unit. Critical Care21, 73 (2017). [DOI] [PMC free article] [PubMed]
- 25.Forward E, et al. Predictive validity of the qSOFA criteria for sepsis in non-ICU inpatients. Intensive Care Medicine. 2017;43:945–946. doi: 10.1007/s00134-017-4776-2. [DOI] [PubMed] [Google Scholar]
- 26.Freund Y, et al. Prognostic Accuracy of Sepsis-3 Criteria for In-Hospital Mortality Among Patients With Suspected Infection Presenting to the Emergency Department. JAMA. 2017;317:301–308. doi: 10.1001/jama.2016.20329. [DOI] [PubMed] [Google Scholar]
- 27.Giamarellos-Bourboulis EJ, et al. Validation of the new Sepsis-3 definitions: proposal for improvement in early risk identification. Clinical Microbiology and Infection. 2017;23:104–109. doi: 10.1016/j.cmi.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 28.González Del Castillo, J. et al. Prognostic accuracy of SIRS criteria, qSOFA score and GYM score for 30-day-mortality in older non-severely dependent infected patients attended in the emergency department. European Journal of Clinical Microbiology and Infectious Diseases 36, 2361–2369 (2017). [DOI] [PubMed]
- 29.Haydar Samir, Spanier Matthew, Weems Patricia, Wood Samantha, Strout Tania. Comparison of QSOFA score and SIRS criteria as screening mechanisms for emergency department sepsis. The American Journal of Emergency Medicine. 2017;35(11):1730–1733. doi: 10.1016/j.ajem.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Henning Daniel J., Puskarich Michael A., Self Wesley H., Howell Michael D., Donnino Michael W., Yealy Donald M., Jones Alan E., Shapiro Nathan I. An Emergency Department Validation of the SEP-3 Sepsis and Septic Shock Definitions and Comparison With 1992 Consensus Definitions. Annals of Emergency Medicine. 2017;70(4):544-552.e5. doi: 10.1016/j.annemergmed.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huson MAM, Kalkman R, Grobusch MP, van der Poll T. Predictive value of the qSOFA score in patients with suspected infection in a resource limited setting in Gabon. Travel Medicine and Infectious Disease. 2017;15:76–77. doi: 10.1016/j.tmaid.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Huson Michaëla A. M., Katete Chawezi, Chunda Lilian, Ngoma Jonathan, Wallrauch Claudia, Heller Tom, van der Poll Tom, Grobusch Martin P. Application of the qSOFA score to predict mortality in patients with suspected infection in a resource-limited setting in Malawi. Infection. 2017;45(6):893–896. doi: 10.1007/s15010-017-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang Sung Yeon, Jo Ik Joon, Lee Se Uk, Lee Tae Rim, Yoon Hee, Cha Won Chul, Sim Min Seob, Shin Tae Gun. Low Accuracy of Positive qSOFA Criteria for Predicting 28-Day Mortality in Critically Ill Septic Patients During the Early Period After Emergency Department Presentation. Annals of Emergency Medicine. 2018;71(1):1-9.e2. doi: 10.1016/j.annemergmed.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Kim M, et al. Predictive performance of the quick Sequential Organ Failure Assessment score as a screening tool for sepsis, mortality, and intensive care unit admission in patients with febrile neutropenia. Supportive Care in Cancer. 2017;25:1557–1562. doi: 10.1007/s00520-016-3567-6. [DOI] [PubMed] [Google Scholar]
- 35.Kim MW, Lim JY, Oh SH. Mortality prediction using serum biomarkers and various clinical risk scales in community-acquired pneumonia. Scandinavian Journal of Clinical and Laboratory Investigation. 2017;77:486–492. doi: 10.1080/00365513.2017.1344298. [DOI] [PubMed] [Google Scholar]
- 36.Kolditz M, et al. Vergleich der qSOFA- und CRB-Kriterien zur Risikoprädiktion bei Patienten mit CAP: erste multizentrische Validierung des qSOFA bei CAP. Pneumologie (Stuttgart, Germany) 2016;70:826–830. doi: 10.1055/s-0036-1596072. [DOI] [PubMed] [Google Scholar]
- 37.LeGuen M, et al. Frequency and significance of qSOFA criteria during adult rapid response team reviews: A prospective cohort study. Resuscitation. 2018;122:13–18. doi: 10.1016/j.resuscitation.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Moskowitz A, et al. Quick Sequential Organ Failure Assessment and Systemic Inflammatory Response Syndrome Criteria as Predictors of Critical Care Intervention Among Patients With Suspected Infection. Critical care medicine. 2017;45:1813–1819. doi: 10.1097/CCM.0000000000002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patidar KR, et al. No Association Between Quick Sequential Organ Failure Assessment and Outcomes of Patients With Cirrhosis and Infections. Clinical Gastroenterology and Hepatology. 2017;15:1803–1804. doi: 10.1016/j.cgh.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 40.Quinten Vincent M., van Meurs Matijs, Wolffensperger Anna E., ter Maaten Jan C., Ligtenberg Jack J.M. Sepsis patients in the emergency department. European Journal of Emergency Medicine. 2018;25(5):328–334. doi: 10.1097/MEJ.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranzani OT, et al. New Sepsis Definition (Sepsis-3) and Community-acquired Pneumonia Mortality. A Validation and Clinical Decision-Making Study. American journal of respiratory and critical care medicine. 2017;196:1287–1297. doi: 10.1164/rccm.201611-2262OC. [DOI] [PubMed] [Google Scholar]
- 42.Rothman M, et al. Sepsis as 2 problems: Identifying sepsis at admission and predicting onset in the hospital using an electronic medical record–based acuity score. Journal of Critical Care. 2017;38:237–244. doi: 10.1016/j.jcrc.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 43.Shetty A, et al. Lactate ≥2 mmol/L plus qSOFA improves utility over qSOFA alone in emergency department patients presenting with suspected sepsis. EMA - Emergency Medicine Australasia. 2017;29:626–634. doi: 10.1111/1742-6723.12894. [DOI] [PubMed] [Google Scholar]
- 44.Singer AJ, Ng J, Thode HC, Jr., Spiegel R, Weingart S. Quick SOFA Scores Predict Mortality in Adult Emergency Department Patients With and Without Suspected Infection. Annals of Emergency Medicine. 2017;69:475–479. doi: 10.1016/j.annemergmed.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Szakmany, T. et al. Defining sepsis on the wards: Results of a multi-centre point-prevalence study comparing two sepsis definitions. Anaesthesia73, 195–204 (2017). [DOI] [PubMed]
- 46.Tusgul, S., Carron, P. N., Yersin, B., Calandra, T. & Dami, F. Low sensitivity of qSOFA, SIRS criteria and sepsis definition to identify infected patients at risk of complication in the prehospital setting and at the emergency department triage. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine25, 108 (2017). [DOI] [PMC free article] [PubMed]
- 47.Umemura Yutaka, Ogura Hiroshi, Gando Satoshi, Kushimoto Shigeki, Saitoh Daizoh, Mayumi Toshihiko, Fujishima Seitaro, Abe Toshikazu, Ikeda Hiroto, Kotani Joji, Miki Yasuo, Shiraishi Shin-ichiro, Shiraishi Atsushi, Suzuki Koichiro, Suzuki Yasushi, Takeyama Naoshi, Takuma Kiyotsugu, Tsuruta Ryosuke, Yamaguchi Yoshihiro, Yamashita Norio, Aikawa Naoki. Assessment of mortality by qSOFA in patients with sepsis outside ICU: A post hoc subgroup analysis by the Japanese Association for Acute Medicine Sepsis Registry Study Group. Journal of Infection and Chemotherapy. 2017;23(11):757–762. doi: 10.1016/j.jiac.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Wang JY, Chen YX, Guo SB, Mei X, Yang P. Predictive performance of quick Sepsis-related Organ Failure Assessment for mortality and ICU admission in patients with infection at the ED. American Journal of Emergency Medicine. 2016;34:1788–1793. doi: 10.1016/j.ajem.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Rannikko J, Syrjänen J, Seiskari T, Aittoniemi J, Huttunen R. Sepsis-related mortality in 497 cases with blood culture-positive sepsis in an emergency department. International Journal of Infectious Diseases. 2017;58:52–57. doi: 10.1016/j.ijid.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2015;21:474–481. doi: 10.1016/j.cmi.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 51.Fernando SM, et al. Prognostic Accuracy of the Quick Sequential Organ Failure Assessment for Mortality in Patients With Suspected Infection. Ann Intern Med. 2018;168:266–275. doi: 10.7326/M17-2820. [DOI] [PubMed] [Google Scholar]
- 52.Song J-U, Sin CK, Park HK, Shim SR, Lee J. Performance of the quick Sequential (sepsis-related) Organ Failure Assessment score as a prognostic tool in infected patients outside the intensive care unit: a systematic review and meta-analysis. Critical Care. 2018;22:28. doi: 10.1186/s13054-018-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maitra S., Som A., Bhattacharjee S. Accuracy of quick Sequential Organ Failure Assessment (qSOFA) score and systemic inflammatory response syndrome (SIRS) criteria for predicting mortality in hospitalized patients with suspected infection: a meta-analysis of observational studies. Clinical Microbiology and Infection. 2018;24(11):1123–1129. doi: 10.1016/j.cmi.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 54.Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clinical infectious diseases. 2005;41:S490–S497. doi: 10.1086/432003. [DOI] [PubMed] [Google Scholar]
- 55.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Critical care medicine. 2007;35:763–768. doi: 10.1097/01.CCM.0000256726.80998.BF. [DOI] [PubMed] [Google Scholar]
- 56.Marmot MG, Smith GD. Why are the Japanese living longer? BMJ: British Medical Journal. 1989;299:1547. doi: 10.1136/bmj.299.6715.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bone RC, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 58.Gutierrez G, Wulf M. Lactic acidosis in sepsis: a commentary. Intensive care medicine. 1996;22:6–16. doi: 10.1007/BF01728325. [DOI] [PubMed] [Google Scholar]
- 59.Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent J-L. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. The American journal of surgery. 1996;171:221–226. doi: 10.1016/S0002-9610(97)89552-9. [DOI] [PubMed] [Google Scholar]
- 60.Fuller BM, Dellinger RP. Lactate as a hemodynamic marker in the critically ill. Current opinion in critical care. 2012;18:267. doi: 10.1097/MCC.0b013e3283532b8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan TL, et al. The 28-day mortality prediction in sepsis patients using static lactate concentration and early lactate clearance: an observational study. Med & Health. 2014;2:124–133. [Google Scholar]
- 62.Vijayan AL, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. Journal of Intensive Care. 2017;5:51. doi: 10.1186/s40560-017-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan TL, Goh YY. The role of group IIA secretory phospholipase A2 (sPLA2-IIA) as a biomarker for the diagnosis of sepsis and bacterial infection in adults—A systematic review. Plos One. 2017;12:e0180554. doi: 10.1371/journal.pone.0180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faix JD. Biomarkers of sepsis. Critical Reviews in Clinical Laboratory Sciences. 2013;50:23–36. doi: 10.3109/10408363.2013.764490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan Toh Leong, Ahmad Nurul Saadah, Nasuruddin Dian Nasriana, Ithnin Azlin, Tajul Arifin Khaizurin, Zaini Ida Zarina, Wan Ngah Wan Zurinah. CD64 and Group II Secretory Phospholipase A2 (sPLA2-IIA) as Biomarkers for Distinguishing Adult Sepsis and Bacterial Infections in the Emergency Department. PLOS ONE. 2016;11(3):e0152065. doi: 10.1371/journal.pone.0152065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bador KM, Intan S, Hussin S, Gafor AHA. Serum procalcitonin has negative predictive value for bacterial infection in active systemic lupus erythematosus. Lupus. 2012;21(11):1172–1177. doi: 10.1177/0961203312450085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information File.