Abstract
Kaposi sarcoma (KS) of the head and neck area is uncommon with limited published case series. Our routine and consultation files were reviewed for histologically and immunohistochemically proven KS affecting any cutaneous or mucosal head and neck site. Ten males and one female aged 42–78 years (median, 51 years; mean, 52 years) were retrieved. Eight patients were HIV-positive and three were HIV-negative. The affected sites were skin (n = 5), oral/oropharyngeal mucosa (n = 5), and lymph nodes (n = 3) in variable combination. The ear (pinna and external auditory canal) was affected in two cases; both were HIV-negative. Multifocal non-head and neck KS was reported in 50% of patients. At last follow-up (12–94 months; median, 46 months), most of patients were either KS-free (n = 8) or had ongoing remission under systemic maintenance therapy (n = 2). One patient was alive with KS (poor compliance). Histopathological evaluation showed classical features of KS. One case was predominantly sarcomatoid with prominent inflammation mimicking undifferentiated sarcoma. Immunohistochemistry showed consistent expression of CD31, CD34, ERG, D2-40 and HHV8 in all cases. This is one of the few series devoted to head and neck KS showing high prevalence of HIV-positivity, but also unusual presentations in HIV-negative patients with primary origin in the skin of the ear and the auditory canal. KS should be included in the differential diagnosis of difficult-to-classify spindle cell lesions at this uncommon location.
Keywords: Kaposi sarcoma, Ear canal, Differential diagnosis, Oral cavity, Head and neck, HIV, HHV8, Immunohistochemistry
Introduction
Kaposi sarcoma (KS) is an uncommon angioproliferative endothelial neoplasm with distinctive clinicopathological, epidemiological and immunophenotypic characteristics. Diagnosis relies on a combination of morphological and immunohistochemical findings in the appropriate clinicopathological settings [1]. Since its original description by the Hungarian dermatologist Moritz Kaposi in 1872 as “idiopathic multiple pigmented sarcoma,” [2], KS has been classified into five distinct clinical settings: (1) classical (idiopathic) KS; (2) African-endemic KS; (3) epidemic KS in patients with the acquired immunodeficiency syndrome (AIDS); (4) KS associated with immunosuppression (iatrogenic) in solid organ transplant recipients; and (5) KS in human immunodeficiency virus (HIV)-negative males who had anal receptive sexual intercourse with males [3, 4].
KS originating at cutaneous or mucosal head and neck sites is rare. Only a few studies have been devoted to this topic, all of which were published in clinical journals [5–7], without any focus on the histopathological findings. In this study, we describe our experience with 11 KS cases that presented as head and neck lesions, specifically highlighting their histopathological features and differential diagnosis together with other clinical and prognostic factors.
Materials and Methods
All tumors coded as Kaposi sarcoma and originating at any head and neck site including both cutaneous and mucosal tissues were retrieved from our routine and consultation files. Histological slides were reviewed to verify the diagnosis. Immunohistochemical analysis was performed on 4-µm sections cut from paraffin blocks using a fully automated system (Benchmark XT, Ventana Medical Systems Inc, Tucson, Arizona, USA) according to the manufacturer guidelines using the following antibodies: CD31 (clone JC/70A, 1:20, Dako), ERG (clone EPR3864, prediluted, Ventana Medical Systems), podoplanin (clone D2-40, 1:50, Zytomed), CD34 (clone QBEnd10, 1:500, Beckman-Coulter) and HHV8 (clone 13B10, 1:100, Novocastra). Positive and negative controls were used throughout.
Results
General Clinical and Demographic Features
Eleven patients with primary head and neck KS were identified (Table 1). There were 10 males and one female aged 42–78 years (median 51 years, mean 52 years). The HIV status was known in all cases: eight patients were HIV-positive and three were HIV-negative. The age range was 42–54 years (median: 47.9 years) for those who were HIV-positive and 54–78 years (median: 60 years) for the three HIV-negative patients (p = 0.009).
Table 1.
Clinicopathological features of head and neck Kaposi sarcomas (n = 11)
| No | Age (years)/sex | Site/duration since HIV diagnosis | Size (cm) | Histological pattern | HIV-status | Other KS manifestations/duration since HIV diagnosis | Treatment | Outcome/last follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | 45 M | Hard palate & tongue (12 mo), lymph node level II left (15 mo) | 1.0 | Classical | Positive | Cecum (15 mo), cardia (28 mo) | Excision, valganciclovir (for 2 mo), antiretroviral therapy | ANED (78 mo) |
| 2 | 46 M | Hard palate (0 mo), intra-parotid lymph node (18 mo) | 1.2 | Classical | Positive | Skin both thighs (0 mo), left lower leg, both feet, left lung, back, penis (5 mo) | Excision, antiretroviral therapy | AWD due to poor compliance (12 mo) |
| 3 | 48 M | Posterior pharyngeal wall, uvula, right cheek, left nose (0 mo) | 2.2 | Classical | Positive | Multiple skin lesions on trunk, thighs and arms (0) | Valganciclovir (3 mo), Interferon-alpha 2a therapy (5 mo), antiretroviral therapy | Full remission on interferon-alpha, ANED (22 mo) |
| 4 | 54 M | Gingiva close to an infected tooth (> 141 mo) | 0.5 | Classical | Positive | No | Emergence of KS 6 weeks after start of antiretroviral therapy, regression on therapy with valganciclovir combined with antiretroviral therapy | ANED (13 mo) |
| 5 | 42 M | Palatine tonsil (right) (11 mo) | 1.0 | Classical | Positive | No | Tonsillectomy, antiretroviral therapy | ANED (94 mo) |
| 6 | 51 M | Lymph node (submental mass) (67 mo) | 1.2 | Classical | Positive | No | Lymph node excision, antiretroviral therapy | ANED (75 mo) |
| 7 | 43 M | Right lower eyelid (9 mo) | 0.6 | Classical | Positive | No | Excision, antiretroviral therapy | ANED (12 mo) |
| 8 | 54 M | Skin, right angle of the jaw (34 mo) | 1.1 | Classical | Positive | No | Excision, antiretroviral therapy | ANED (76 mo) |
| 9 | 60 F | Skin ear (pinna) | 0.6 | Sarcomatous | Negative | No | Excision | ANED (46 mo) |
| 10 | 78 M | External auditory canal | 1.0 | Classical | Negative | Disseminated KS on all extremities 1 mo after excision of ear lesion | Excision, 5 cycles liposomal doxorubicin for disseminated disease | Alive, ongoing remission (18 mo) |
| 11 | 54 M | Posterior neck | 1.2 | Classical | Negative | Skin of thigh, forearm and calf (2 mo, 22 mo, and 43 mo) after head and neck KS | Excision | ANED (86 mo) |
ANED alive with no evidence of disease, AWD alive with disease, KS Kaposi sarcoma, mo months
At time of diagnosis, the head and neck KS was identified in several cutaneous sites (n = 5), oral/oropharyngeal mucosal sites (n = 5), cervical lymph nodes (n = 3) or different combination thereof. The skin of the ear (pinna and external auditory canal) was affected in two patients; both were HIV-negative. In the HIV-positive group, head and neck KS was diagnosed at the same time as HIV infection (n = 2) or subsequently (9 months to > 141 months).
In five patients, other multifocal manifestations of KS were diagnosed either concurrently or upon follow-up, including cutaneous manifestations and/or visceral organs. Both patients who developed visceral disease (gastrointestinal tract, lung) were HIV-positive.
Follow-up was available for all patients, ranging from 12 to 94 months (median, 46 months). Eight patients (6 HIV-positive, 2 HIV-negative) have remained disease-free to last follow-up. Two other patients had ongoing disease remission under systemic maintenance therapy. One patient was alive with ongoing KS (due to poor compliance).
One HIV-negative patient (the oldest in this series) presented with KS in the external auditory canal followed by disseminated KS manifestation on all extremities soon thereafter. The clinical impression was that the ear lesion was the primary tumor with the metachronous lesions interpreted to be metastatic, although this postulation is impossible to verify. Interestingly, the ear lesion was the initial manifestation while also the largest clinically. This patient remained in ongoing disease remission 18 months after 5 cycles of liposomal doxorubicin therapy.
Pathological Findings
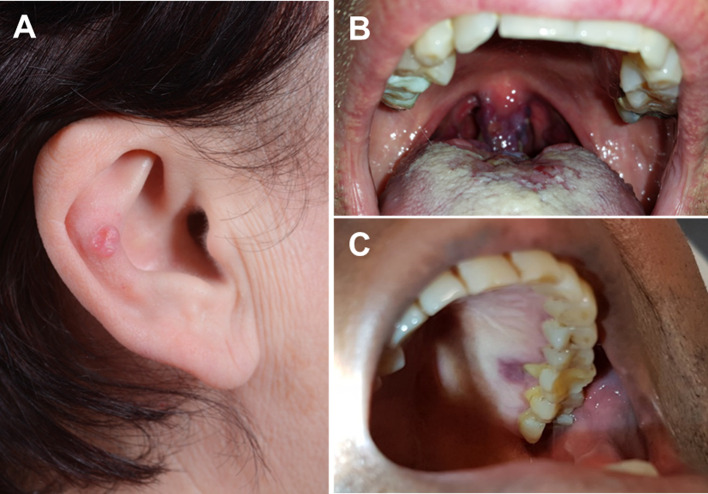
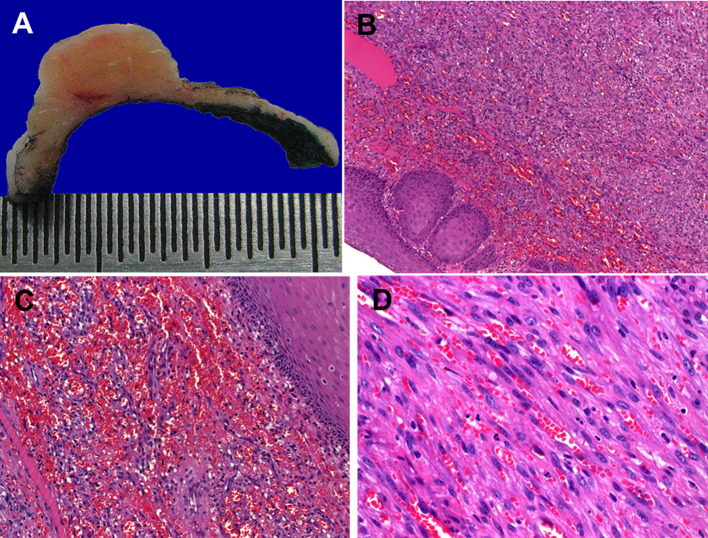
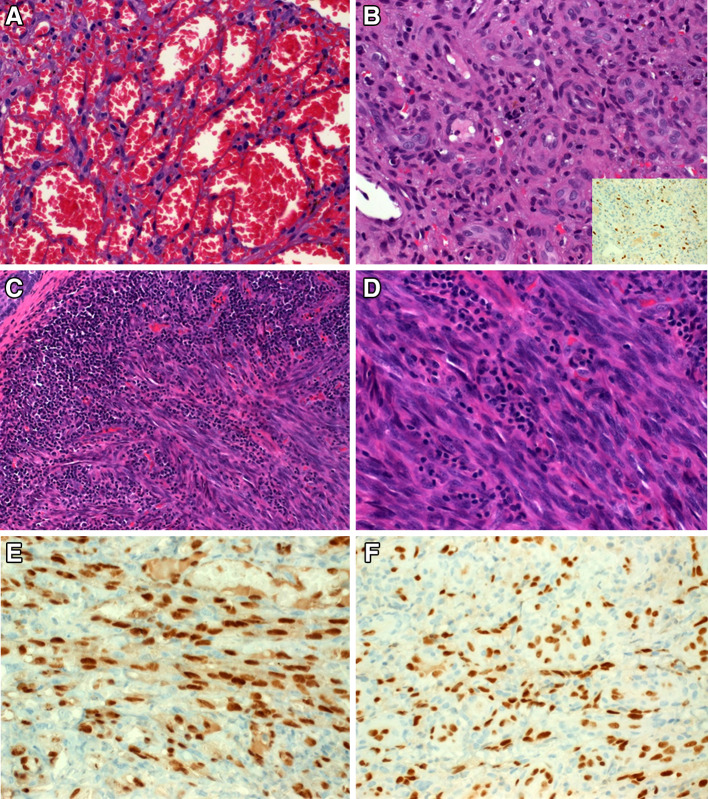
Clinically, all lesions presented as nodular skin lesions (Fig. 1a) or plaque-like, violaceous or erythematous mucosal lesions (Fig. 1b). One lesion was closely associated with an inflamed tooth (Fig. 1c). Their sizes ranged from 5 to 22 mm (median, 10 mm; mean, 9.6 mm). The cut-surface varied from reddish in classical cases to fleshy in the sarcomatoid case (Fig. 2a). Histopathological evaluation revealed the classical histology of KS in 10 tumors. The tumors were composed of compact fascicles of monomorphic spindle cells with elongated, tapered nuclei, pale-eosinophilic cytoplasm with frequent glassy-hyaline cytoplasmic or interstitial globules and slit-like vascular spaces, which frequently contained erythrocytes (Fig. 2b–d). Mucosal lesions were frequently associated with florid granulation tissue-type reactions beneath the mucosa, which might have obscured the lesions in some areas (Fig. 3a, b). One case showed prominent associated inflammation, high cellularity (Fig. 3c) and, in most of the tumor areas, lacked the characteristic kaposiform pattern resulting in initial diagnosis of undifferentiated sarcoma at a referral hospital (Fig. 3d). Immunohistochemistry showed consistent coexpression of CD31, ERG (Fig. 3e), CD34, D2-40 and HHV8 (Fig. 3f) in all cases.
Fig. 1.
Clinical appearance of head and neck Kaposi sarcoma. a Case of non-AIDS Kaposi sarcoma arising from the ear pinna. b A violaceous-reddish lesion in the uvula from an HIV-positive patient. c Another HIV-associated lesion closely associated with an inflamed tooth
Fig. 2.
Gross and histological appearance of classical KS in the head and neck. a Fleshy cut-surface of the ear lesion shown in Fig. 1a. b Fascicles of uniform spindle cells are covered by reactive non-dysplastic mucosa. c Prominent hemorrhagic changes in the most superficial areas may closely resemble angiosarcoma. d Fascicles of uniform spindle cells entrapping slit-like vascular spaces containing erythrocytes
Fig. 3.
a Prominent ectatic vessels can be mistaken for reactive lesion or benign hemangioma if KS is not considered. b Florid granulation tissue on top of the mucosal lesions is a frequent feature, which may obscure the underlying tumor (the few scattered tumor cells are highlighted by HHV8 immunostain in the inset). c This HIV-unrelated lesion from the ear showed prominent inflammatory reaction adjacent to and within the lesion. d Fibrosarcoma-like histology from same tumor (note absence of kaposiform features). e Uniform strong nuclear reactivity for ERG. f HHV8 was expressed in all cases
Discussion
Kaposi sarcoma (KS) of the head and neck is uncommon. However, due to the rarity of head and neck sarcomas in general, it represents 20–25% of all head and neck sarcomas in large epidemiological studies [8, 9]. Oral, craniofacial and cutaneous manifestations of AIDS-related KS are relatively common, affecting 95% of patients with AIDS-related KS. However, head and neck KS is rare in non-AIDS cases of KS, accounting for < 5% of KS cases [5, 6]. This significant association with an HIV-infection is reflected in the very low number of HIV-negative patients who present with oral KS (only 5% of oral KS cases were in HIV-negative patients) [5, 6]. Taken together, however, mucosal KS is uncommon and represents about 5% of all KS cases [10]. It is remarkable that two-thirds of mucosal KS affect head and neck sites [10]. An extensive literature review published in 2009 identified 251 published cases of head and neck mucosal KS and pointed to the hard palate and the oropharynx as the most frequently affected sites [6]. Other sites include the tongue, gingiva, buccal mucosa, major salivary glands and jaw bones. In one-fifth of patients, oral lesions preceded other KS manifestations [6].
The pathogenesis of KS is likely multifactorial but infection of the neoplastic cells with the KS-associated herpes virus (KSHV) or the human herpes virus 8 (HHV8) seems to represent a consistent etiological factor, detectable in nearly all cases irrespective of the clinicopathological setting of the disease [3]. However, rarely, KS of the head and neck has been reported to be associated with other potential causative agents/conditions including irradiation [11] and corticosteroid therapy for bullous pemphigoid [12], thought to be related to impaired local immunosurveillance and release of pro-inflammatory cytokines [11].
In this series, we report two cases of ear skin KS (pinna and external auditory canal). A literature search disclosed an additional 8 cases, highlighting the rarity and distinctiveness of this presentation [12–15]. Other rarely reported, but in our series not represented head and neck sites, include the sinonasal mucosa [16, 17]. The salivary glands represent another rarely reported head and neck site [18], represented in our current study by a single case with involvement of intra-parotid lymph nodes.
While the classical morphology of KS is straight forward, a variety of histological variants and unusual morphological patterns have been reported, underscoring the complexity of the differential diagnosis. Among the reported variants are lymphangiomatous, telangiectatic, lymphedematous, hyperkeratotic, keloidal, micronodular, pseudogranulomatous, lobular hemangioma-like, tufted, pyogenic granuloma-like, ecchymotic, intravascular, inflammatory and sarcomatous/anaplastic variants [1, 16, 19, 20]. Furthermore, the diagnosis is complicated by the remarkable diversity seen in relation to the stage of the disease, usually most difficult in the initial stage [1, 20].
In the differential diagnosis, a variety of other head and neck malignancies seen among HIV-positive patients and organ transplant recipients on immunosuppression should be considered [21–23]. Fortunately, most of the HIV-associated head and neck neoplasms are carcinomas, which are distinctive histologically and generally do not enter the differential diagnosis of KS, except for rare variants with spindled morphology, and specifically on limited biopsy material. However, uncommon infectious lesions characterized by vascular proliferations might be encountered in the same patient cohort including in particular, bacillary angiomatosis [24]. Bacillary angiomatosis is a rare but known AIDS-associated vasoproliferative lesion that may mimic KS. However, careful analysis of the cytological and architectural features should help to distinguish this lesion from KS. Demonstration of the causative microorganism, Bartonella henselae, by Warthin–Starry stain, immunohistochemistry or molecular testing is confirmatory. Other vascular neoplasms that need be considered include the rare occurrence of kaposiform hemangioendothelioma of the head and neck [25]. This especially rare intermediate grade neoplasm occurs predominantly in children, is not associated with immunodeficiency of any type, and lacks HHV8 infection. Sarcomatous and atypical variants of KS may be mistaken for aggressive angiosarcoma on the basis of endothelial marker reactivity. However, angiosarcomas are usually frankly malignant, large destructive neoplasms with high-grade nuclear features, lacking HHV8 immunoreactivity [26].
As exemplified by one of our cases, unusual looking KS of the head and neck, especially in HIV-negative patients, may represent a diagnostic challenge if not considered in the differential diagnosis. Such lesions may be mistaken for undifferentiated spindle cell sarcoma, and especially when tested for anti-endothelial markers, misclassified as angiosarcoma, with potential for overtreatment. The presence of florid granulation tissue associated with mucosal ulceration may also represent a pitfall, resulting in a non-neoplastic diagnosis. HHV8 immunohistochemistry provides an excellent marker to detect minor foci of KS in limited biopsy material, while also identifying early lesions.
In summary, we herein describe our experience with 11 cases of Kaposi sarcoma presenting primarily as head and neck lesions, highlighting their frequent association with HIV infection, while also illustrating unusual occurrence in sites such as the ear, the latter often in HIV-negative patients. Thus, KS should be considered in any uniform looking spindled, vascularized lesion and upon diagnosis of head and neck KS, additional evaluation of the patient’s HIV status should be suggested.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board, which did not require informed consent.
References
- 1.Mentzel T, Knuutila S, Lamovec J. Kaposi sarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. World Health Organisation classification of tumours of soft tissue and bone. 4. Lyon: IARC Press; 2013. pp. 151–153. [Google Scholar]
- 2.Kaposi M. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syphilol. 1872;4:265–273. doi: 10.1007/BF01830024. [DOI] [Google Scholar]
- 3.Szajerka T, Jablecki J. Kaposi’s sarcoma revisited. AIDS Rev. 2007;9:230–236. [PubMed] [Google Scholar]
- 4.Lanternier F, Lebbé C, Schartz N, Farhi D, Marcelin AG, Kérob D, Agbalika F, Vérola O, Gorin I, Janier M, Avril MF, Dupin N. Kaposi’s sarcoma in HIV-negative men having sex with men. AIDS. 2008;22:1163–1168. doi: 10.1097/QAD.0b013e3283031a8a. [DOI] [PubMed] [Google Scholar]
- 5.Patrikidou A, Vahtsevanos K, Charalambidou M, Valeri RM, Xirou P, Antoniades K. Non-AIDS Kaposi’s sarcoma in the head and neck area. Head Neck. 2009;31:260–268. doi: 10.1002/hed.20945. [DOI] [PubMed] [Google Scholar]
- 6.Ramírez-Amador V, Anaya-Saavedra G, Martínez-Mata G. Kaposi’s sarcoma of the head and neck: a review. Oral Oncol. 2010;46:135–145. doi: 10.1016/j.oraloncology.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Choussy O, Van Haverbeke C, Babin E, Francois A, Duval-Modeste AB, Dehesdin D. Unusual presentation of oropharyngeal Kaposi’s sarcoma. Head Neck. 2008;30:411–415. doi: 10.1002/hed.20691. [DOI] [PubMed] [Google Scholar]
- 8.Peng KA, Grogan T, Wang MB. Head and neck sarcomas: analysis of the SEER database. Otolaryngol Head Neck Surg. 2014;151:627–633. doi: 10.1177/0194599814545747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stavrakas M, Nixon I, Andi K, Oakley R, Jeannon JP, Lyons A, McGurk M, Urbano TG, Thavaraj S, Simo R. Head and neck sarcomas: clinical and histopathological presentation, treatment modalities, and outcomes. J Laryngol Otol. 2016;130:850–859. doi: 10.1017/S0022215116008604. [DOI] [PubMed] [Google Scholar]
- 10.Thariat J, Kirova Y, Sio T, Choussy O, Vees H, Schick U, Poissonnet G, Saada E, Thyss A, Miller RC. Mucosal Kaposi sarcoma, a rare cancer network study. Rare Tumors. 2012;4:e49. doi: 10.4081/rt.2012.e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Pasquale R, Nasca MR, Micali G. Postirradiation primary Kaposi’s sarcoma of the head and neck. J Am Acad Dermatol. 1999;40:312–314. doi: 10.1016/S0190-9622(99)70473-1. [DOI] [PubMed] [Google Scholar]
- 12.Rachadi H, Zemmez Y, Znati K, Ismaili N, Hassam B. External ear nodule revealing a disseminated Kaposi disease. Dermatol Online J. 2016;22(8). https://escholarship.org/uc/item/1z53b1x5. [PubMed]
- 13.Delbrouck C, Kampouridis S, Chantrain G. An unusual localisation of Kaposi’s sarcoma: the external auditory canal. Acta Otorhinolaryngol Belg. 1998;52:29–36. [PubMed] [Google Scholar]
- 14.Nervi SJ, Benson B, Gounder S, Jyung R. Pathology quiz case 2. Kaposi sarcoma (KS) of the pinna and external auditory canal. Arch Otolaryngol Head Neck Surg. 2006;132:555, 557–8. [DOI] [PubMed]
- 15.Kumarasamy N, Venkatesh KK, Devaleenol B, Poongulali S, Ahilasamy N. Regression of Kaposi’s sarcoma lesions following highly active antiretroviral therapy in an HIV-infected patient. Int J STD AIDS. 2008;19:786–788. doi: 10.1258/ijsa.2008.008016. [DOI] [PubMed] [Google Scholar]
- 16.Wyatt ME, Finlayson CJ, Moore-Gillon V. Kaposi’s sarcoma masquerading as pyogenic granuloma of the nasal mucosa. J Laryngol Otol. 1998;112:280–282. doi: 10.1017/S0022215100158359. [DOI] [PubMed] [Google Scholar]
- 17.Mouden K, Khmou M, Loughmari S, Semmar A, El Kacemi H, El Khannoussi B, Kebdani T, Elmajjaoui S, Benjaafar N. Primary Kaposi’s sarcoma of the nasal cavity: a case report and review of the literature. Clin Sarcoma Res. 2016;6:4. doi: 10.1186/s13569-016-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castle JT, Thompson LD. Kaposi sarcoma of major salivary gland origin: a clinicopathologic series of six cases. Cancer. 2000;88:15–23. doi: 10.1002/(SICI)1097-0142(20000101)88:1<15::AID-CNCR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Cossu S, Satta R, Cottoni F, Massarelli G. Lymphangioma-like variant of Kaposi’s sarcoma: clinicopathologic study of seven cases with review of the literature. Am J Dermatopathol. 1997;19:16–22. doi: 10.1097/00000372-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31. doi: 10.1186/1746-1596-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purgina B, Pantanowitz L, Seethala RR. A review of carcinomas arising in the head and neck region in HIV-positive patients. Pathol Res Int. 2011;2011:469150. doi: 10.4061/2011/469150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunn BK, van Heerden WF. HIV/AIDS associated malignancies of the head and neck. SADJ. 2012;67:590–592. [PubMed] [Google Scholar]
- 23.Gourin CG, Terris DJ. Head and neck cancer in transplant recipients. Curr Opin Otolaryngol Head Neck Surg. 2004;12:122–126. doi: 10.1097/00020840-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Batsakis JG, Ro JY, Frauenhoffer EE. Bacillary angiomatosis. Ann Otol Rhinol Laryngol. 1995;104:668–672. doi: 10.1177/000348949510400815. [DOI] [PubMed] [Google Scholar]
- 25.Wong BL, Lee VN, Tikka T, Kim D, Dwivedi RC. Kaposiform haemangioendothelioma of the head and neck. Crit Rev Oncol Hematol. 2016;104:156–168. doi: 10.1016/j.critrevonc.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Nelson BL, Thompson LD. Sinonasal tract angiosarcoma: a clinicopathologic and immunophenotypic study of 10 cases with a review of the literature. Head Neck Pathol. 2007;1:1–12. doi: 10.1007/s12105-007-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]