Abstract
Sclerosing epithelioid fibrosarcoma (SEF) is an uncommon variant of fibrosarcoma that is characterized by a distinct morphology. It most frequently presents in the deep soft tissues of the lower extremities, often in intimate association with fascia and periosteum, although reports of the head and neck involvement have been reported. A minority of cases show morphological, immunohistochemical and molecular overlap with low grade fibromyxoid sarcoma (LG-FMS). Herein, we describe a case of a bland spindle cell neoplasm presenting in the jaw that was initially incompletely excised. Over the course of 20 years the tumor subsequently recurred with a SEF morphology. Molecular testing performed on both specimens subsequently confirmed the presence of an EWSR1-CREB3L1 gene fusion. This report highlights the diagnostic difficulty with LG-FMS, particularly in unusual anatomic locations; reiterates the potential for the uncommon EWSR1-CREB3L1 fusion product in LG-FMS; and, reaffirms the potential for progression and/or overlap between LG-FMS to SEF over time.
Keywords: EWSR1-CREB3L1, Sclerosing epithelioid fibrosarcoma, Low-grade fibromyxoid sarcoma, Jaw, Mandible, Sarcoma, Case report
Introduction
Sclerosing epithelioid fibrosarcoma (SEF) is a rare form of fibrosarcoma originally described by Meis-Kindblom et al. [1]. Tumors occur predominantly in young to middle aged adults; however, a broad age range of involvement has been reported [1, 2]. There is a marked predilection for the deep soft tissue of lower extremities. The oral and maxillofacial areas are considered extremely uncommon [3]. Tumors tend to be large and deep, with involvement of skeletal muscle, fascia, periosteum and very rarely bone [4]. SEF is an aggressive sarcoma, with a high propensity towards local recurrence—despite attempts at wide resection and adjuvant radiotherapy—and frequently late metastases [2]. Sites of metastases include lung and bone, as well as soft tissue, lymph nodes and brain [1].
Histologically, SEF is characterized by cords, nests and/or sheets of epithelioid cells set within dense hyalinized stroma. The cytoplasm is generally clear-eosinophilic and scant. The nuclei are round to ovoid with minimal atypia, although pleomorphism may occasionally be encountered; mitotic activity is usually paradoxically low. The characteristic stroma separating groups of tumor cells may be absent in a minority of cases [5]. Rarely SEF has been reported to progress from a low-grade fibromyxoid (LG-FMS); indeed, a subset of cases also contains hybrid features of both entities.
Herein we report the case of a 55-year-old woman with an intraosseous SEF of the mandible, characterized by an EWSR1-CREB3L1 fusion product. On re-review of a tumor previously excised from the same anatomic location 20 years earlier, a morphology consistent with LG-FMS was identified.
Case Report
A 55-year-old woman presented to our institution with swelling in the posterior body of the right mandible. The mass was initially asymptomatic, but over several months became progressively more painful on palpation. Her past medical history was significant for excision of a lesion, from the same anatomic location, 20 years earlier.
Diagnostic imaging revealed a 4.9 cm multi-loculated radiolucent mandibular lesion at the angle and posterior body, extending into the ramus. The cortex was thinned with multiple foci of disruption, including soft tissue extension. While non-specific, the imaging findings raised the possibility of ameloblastoma. The patient underwent a segmental resection of the posterior mandible with immediate reconstruction with a non-vascularized bicortical iliac crest graft.
On gross examination the tumor was tan-gray, lobulated and centered in bone. Histologically, it was characterized by epithelioid cells with a fibrotic background (Fig. 1). Immunohistochemistry was positive for vimentin and MUC4; it was negative for S100, CD34, desmin, smooth muscle actin, epithelial membrane antigen and keratins (AE1/AE3, LMWK, HMWK). The prior excision, performed 20 years earlier, was requested for comparison. It had originally been diagnosed as desmoplastic fibroma; however, on re-review, it showed a bland spindle-stellate cell neoplasm with fibro-myxoid stroma, arcades of thin-walled vessels and immunoreactivity for MUC4, in keeping with low-grade fibromyxoid sarcoma (Fig. 2). Fluorescence in situ hybridization revealed EWSR1 rearrangement, and FUS was normal, in both samples (Vysis, Abbott Molecular). Subsequent testing of both cases by next generation sequencing demonstrated an EWSR1-CREB3L1 fusion product (Trusight RNA Fusion Panel, Illumina). Overall, the findings were consistent with progression of a low-grade fibromyxoid sarcoma to a SEF.
Fig. 1.
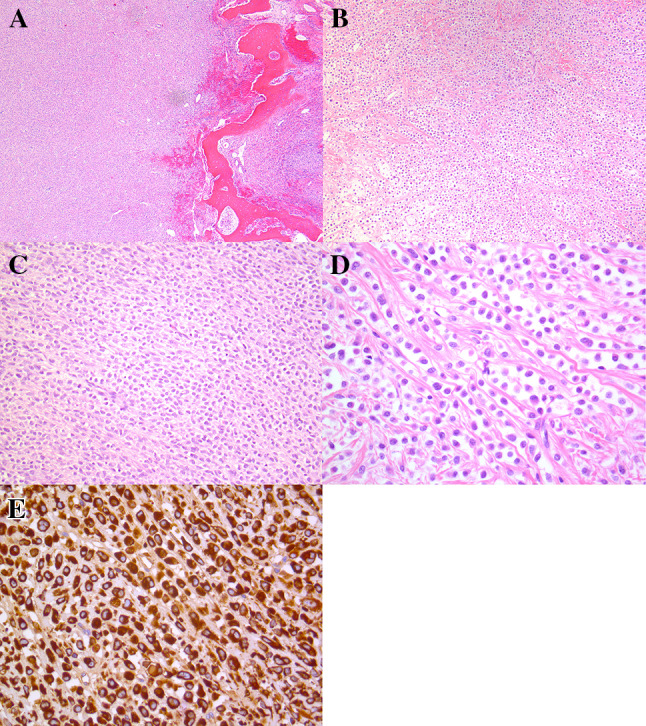
Current, recurrent neoplasm. a Low-power photomicrograph showing a lobulated neoplasm within bone. b Intermediate magnification showing cords of epithelioid cells set within delicate collagen bands. c Intermediate magnification showing an area of increased cellularity lacking collagenous stroma. d High-power magnification showing monomorphic epithelioid cells without conspicuous mitotic activity. e Immunohistochemistry for MUC4
Fig. 2.
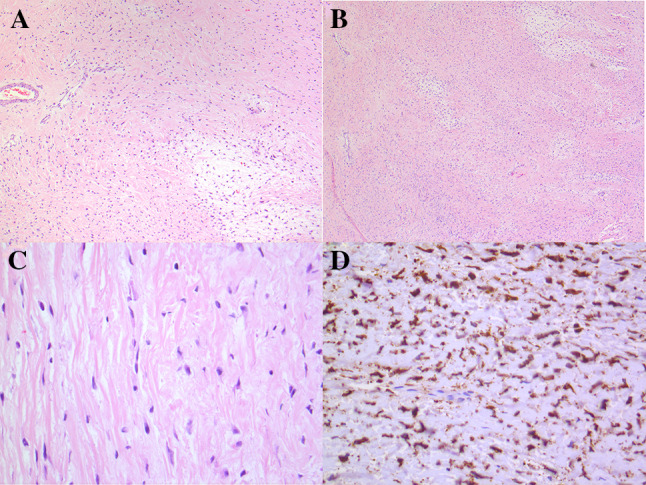
Original specimen, resected 20 years earlier. a Low-power photomicrograph showing a hypocellular spindle cell proliferation with areas of collagenous and myxoid stroma, and occasional arcades of thin-wallled vessels. b Intermediate magnification showing bland cytomorphology. c High-power photomicrograph showing monomorphic nuclei. d Immunohistochemistry for MUC4
Discussion
Largely encountered in the deep soft tissues of lower extremities, there are rare reports of SEF occurring in the head and neck. Even less common, however, is the presence of oral and maxillofacial involvement; [3] to our knowledge, this represents only the second reported case of an intraosseous maxillofacial SEF. Moreover, this case is remarkable for showing progression to SEF from an initial presentation of LG-FMS some 20 years earlier. Given the rarity and subtle morphologic findings of both SEF and LG-FMS, this case illustrates the need for an awareness and low threshold for consultation and/or molecular interrogation in such neoplasms.
SEF is frequently characterized by nests and cords of epithelioid cells set within a dense hyaline stroma. The multifaceted appearance of SEF has been acknowledged since its initial inception. Tumors may have areas with fibrous, myxoid and chondro-osseous differentiation, respectively resembling low-grade fibromyxoid sarcoma, cellular myxoma and osteosarcoma [1]. Indeed, SEF may at times mimic sclerosing carcinoma and myoepithelioma, amongst a host of other considerations [3, 6]. The potential for morphologic overlap between SEF and LG-FMS has been known for some time [1, 2, 7–9]. Further, both recurrent and metastatic LG-FMS are known to rarely progress to a morphology that is partial or completely reminiscent of SEF [6, 10, 11].
Initial reports on the molecular pathogenesis of LG-FMS identified a characteristic FUS-CREB3L2 gene fusion, with a smaller numbers of cases reported to show FUS-CREB3L1 [12, 13]. A minority of cases of LG-FMS was subsequently found to harbor EWSR1-CREB3L1 fusions genes [14]. Relatively recently, the molecular pathogenesis of SEF was identified, demonstrating FUS-CREB3L2 [10], EWSR1-CREB3L1 [6, 15], and EWSR1-CREB3L2 [15] to be the disease-defining gene fusions. Hybrid lesions have been reported, thus far, to have FUS-CREB3L2 [6, 15] and EWSR1-CREB3L1 fusion genes [10]. There are relatively few molecularly interrogated cases of LG-FMS relapsing as SEF; in contrast to the current case showing EWSR1-CREB3L1, prior cases have only been reported to show FUS-CREB3L2 [6]. It is conceivable that all four of the aforementioned fusion combinations, if not others, will ultimately be identified amongst the spectrum LG-FMS, SEF and hybrid lesions. Molecular demonstration of a disease-defining translocation remains the ‘gold standard’ in the diagnosis of LG-FMS and SEF; nevertheless, it is worth noting that MUC4 represents a sensitive marker in the diagnosis of these tumors [10, 14].
SEF usually presents indolently in the form of a slowly growing mass that has often been present for months or years before instigating investigation. Headache and tooth mobility have been reported in cases occurring in the oral and maxillofacial regions [3], and bone lesions are often associated with pain [16–18]. Similar to our patient, a previous report of intraosseous maxillofacial SEF was also initially misdiagnosed as desmoplastic fibroma [3]. Complete surgical resection, with or without adjuvant radiotherapy, remains the mainstay of therapy; there is a limited response to chemotherapy and/or radiotherapy as sole treatment modalities. Ongoing follow-up is indicated given a very high rate of both local and distal recurrence.
There are multiple reports of low-grade fibromyxoid sarcoma arising in the jaw [19, 20]. However, to our knowledge, this is only the second reported case of SEF occurring within the jaw, and the first to transform from an initial presentation of LG-FMS. This case highlights the diagnostic challenges, and potential pitfalls, that can be associated with mesenchymal tumours of the jaw. It further underscores the benefit of subspecialty-based consultation in these rare tumours, and the value of advanced molecular diagnostics in their definitive classification.
Acknowledgements
The authors are extremely grateful for exemplary technologic support provided by Mr. Anthony Wing and Ms. Jasmine Wong (Immunohistochemistry), Ms. Martha Wood (molecular cytogenetics), and Mr. David Swanson (next generation sequencing).
Compliance with Ethical Standards
Conflict of interest
None of the authors have a conflict of interest to disclose.
Ethical Approval
This study was performed following institutional REB approval.
Informed Consent
Informed consent was obtained from the patient reported in this study.
References
- 1.Meis-Kindblom JM, Kindblom LG, Enzinger FM. Sclerosing epithelioid fibrosarcoma. A variant of fibrosarcoma simulating carcinoma. Am J Surg Pathol. 1995;19:979–993. doi: 10.1097/00000478-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Antonescu CR, Rosenblum MK, Pereira P, et al. Sclerosing epithelioid fibrosarcoma: a study of 16 cases and confirmation of a clinicopathologically distinct tumor. Am J Surg Pathol. 2001;25:699–709. doi: 10.1097/00000478-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Folk GS, Williams SB, Foss RB, et al. Oral and maxillofacial sclerosing epithelioid fibrosarcoma: report of five cases. [Erratum appears in Head Neck Pathol. 2013 Mar;7(1):103] Head Neck Pathol. 2007;1:13–20. doi: 10.1007/s12105-007-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojcik JB, Bellizzi AM, Dal Cin P, et al. Primary sclerosing epithelioid fibrosarcoma of bone: analysis of a series. Am J Surg Pathol. 2014;38:1538–1544. doi: 10.1097/PAS.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 5.Puls F, Magnusson L, Niblett A, et al. Non-fibrosing sclerosing epithelioid fibrosarcoma: an unusual variant. Histopathology. 2016;68:760–763. doi: 10.1111/his.12791. [DOI] [PubMed] [Google Scholar]
- 6.Arbajian E, Puls F, Magnusson L, et al. Recurrent EWSR1-CREB3L1 gene fusions in sclerosing epithelioid fibrosarcoma. Am J Surg Pathol. 2014;38:801–808. doi: 10.1097/PAS.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 7.Evans HL. Low-grade fibromyxoid sarcoma. A report of 12 cases. Am J Surg Pathol. 1993;17:595–600. doi: 10.1097/00000478-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Folpe AL, Lane KL, Paull G, et al. Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol. 2000;24:1353–1360. doi: 10.1097/00000478-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Guillou L, Benhattar J, Gengler C, et al. Translocation-positive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French Sarcoma Group. Am J Surg Pathol. 2007;31:1387–1402. doi: 10.1097/PAS.0b013e3180321959. [DOI] [PubMed] [Google Scholar]
- 10.Doyle LA, Wang W-L, Dal Cin P, et al. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol. 2012;36:1444–1451. doi: 10.1097/PAS.0b013e3182562bf8. [DOI] [PubMed] [Google Scholar]
- 11.Evans HL. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol. 2011;35:1450–1462. doi: 10.1097/PAS.0b013e31822b3687. [DOI] [PubMed] [Google Scholar]
- 12.Mertens F, Fletcher CD, Antonescu CR, et al. Clinicopathologic and molecular genetic characterization of low-grade fibromyxoid sarcoma, and cloning of a novel FUS/CREB3L1 fusion gene. Lab Invest. 2005;85:408–415. doi: 10.1038/labinvest.3700230. [DOI] [PubMed] [Google Scholar]
- 13.Doyle LA, Möller E, Dal Cin P, et al. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am J Surg Pathol. 2011;35(5):733–741. doi: 10.1097/PAS.0b013e318210c268. [DOI] [PubMed] [Google Scholar]
- 14.Lau PP, Lui PC, Lau GT, et al. EWSR1-CREB3L1 gene fusion: a novel alternative molecular aberration of low-grade fibromyxoid sarcoma. Am J Surg Pathol. 2013;37:734–738. doi: 10.1097/PAS.0b013e31827560f8. [DOI] [PubMed] [Google Scholar]
- 15.Prieto-Granada C, Zhang L, Chen HW, et al. A genetic dichotomy between pure sclerosing epithelioid fibrosarcoma (SEF) and hybrid SEF/low-grade fibromyxoid sarcoma: a pathologic and molecular study of 18 cases. Genes Chromosomes Cancer. 2015;54:28–38. doi: 10.1002/gcc.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunewald TGP, von Luettichau I, Weirich G, et al. Sclerosing epithelioid fibrosarcoma of the bone: a case report of high resistance to chemotherapy and a survey of the literature. Sarcoma. 2010;2010:1–5. doi: 10.1155/2010/431627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow LT, Lui YH, Kumta SM, et al. Primary sclerosing epithelioid fibrosarcoma of the sacrum: a case report and review of the literature. J Clin Pathol. 2004;57:90–94. doi: 10.1136/jcp.57.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Eyden B. A primary sclerosing epithelioid fibrosarcoma of the pubic bone, with evidence of divergent epithelial differentiation. Ultrastruct Pathol. 2010;34:99–104. doi: 10.3109/01913121003605576. [DOI] [PubMed] [Google Scholar]
- 19.Cowan ML, Thompson LD, Leon ME, Bishop JA. Low-grade fibromyxoid sarcoma of the head and neck: a clinicopathologic series and review of the literature. Head Neck Pathol. 2016;10(2):161–166. doi: 10.1007/s12105-015-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rekhi B, Deshmukh M, Jambhekar NA. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 18 cases, including histopathologic relationship with sclerosing epithelioid fibrosarcoma in a subset of cases. Ann Diagn Pathol. 2011;15(5):303–311. doi: 10.1016/j.anndiagpath.2011.02.005. [DOI] [PubMed] [Google Scholar]


