Abstract
Metastases to the head and neck organs are uncommon, potentially representing the initial presentation of an occult malignancy. Single case reports and small series report metastases to the parathyroid gland, but there is no large review of the literature on secondary tumors involving the parathyroid glands. A review of the English literature between 1950 and 2017 was performed of all metastases or secondary involvement of the parathyroid glands. One hundred and twenty-seven cases of metastatic tumors were reported, although potentially significantly unrepresented in autopsy series (parathyroid glands are not routinely examined) and due to reporting bias. Women were affected more commonly than men (5.8:1; 99 vs. 17, respectively), with a mean age at presentation of 58.5 years, when reported. The most common primary sites of malignancies that metastasized to the parathyroid glands were breast carcinomas (66.9%, n = 85), melanoma (11.8%, n = 15), and lung carcinoma (5.5%, n = 7), with carcinomas representing 86.6% of metastases. Metastases were nearly always identified as part of widely metastatic disease, with only five (3.2%) cases reported as isolated metastases. Tumor-to-tumor metastases comprised 5.5% of all metastases to the parathyroid glands (metastases to parathyroid adenoma). A significant clinical finding of metastases to the parathyroid glands was the development of deranged calcium homeostasis, well beyond the 9 (7.2%) cases with primary parathyroid gland disease present. Although concurrent conditions (renal disease; bone metastases) may partially affect calcium metabolism, the onset of calcium derangement seemed to coincide with parathyroid gland metastases and not systemic disease. In summary, metastases to the parathyroid glands are uncommon, potentially under-recognized in patients who have otherwise widely metastatic tumors. Women are affected more often than men, with breast carcinomas (66.9%) and melanoma (11.8%) the most common primary tumors. Calcium homeostasis is affected, probably as a result of parathyroid gland parenchymal destruction.
Keywords: Parathyroid glands, Parathyroid neoplasms, Parathyroid hormone (PTH), Comorbidity, Calcium, Metastatic tumors, Secondary neoplasms, Breast neoplasms, Lung neoplasms, Melanoma
Introduction
Many tumors metastasize to the head and neck region. However, if lymph node metastases are excluded, organ metastases are uncommon. Specifically, metastases to the parathyroid glands are seldom reported, with only isolated single case reports, small series or autopsy reports that include parathyroid gland involvement in generalized disease. Parathyroid gland tissue is seldom systematically evaluated during a typical autopsy [1, 2]. Great care is taken to preserve parathyroid gland function during thyroid gland surgery [3]. Symptoms, if present at all, are likely to be nonspecific in nature, and thus antemortem manifestations are rare. As a result, the actual incidence and prevalence of cancers metastatic to the parathyroid glands is unknown. One of the few studies to highlight metastases to the parathyroid glands was a prospective study of 160 consecutive cancer death autopsies, identifying parathyroid gland involvement by metastatic tumor in 11.9% of patients [4]. In other series, parathyroid gland involvement was identified in the context of widely metastatic disease, just one of many organs affected [5], while isolated metastases to the parathyroid gland in surgical pathology material as the only metastatic site is exceptional [3, 6–8]. The most comprehensive literature review on the topic thus far was by Shifrin et al. [5], with this report expanding the review of 15 articles to 28 articles in an attempt to more thoroughly elucidate the nature of the metastatic phenomenon.
Methods
A review of English publications between 1950 and 2017 was performed to identify all reported cases of metastases to the parathyroid glands [1–28]. The review was conducted via searches of PubMed and Google Scholar databases using various combinations of search terms “parathyroid gland”, “parathyroid”, “metastasis”, “metastases”, “cancer”, “malignancy”, “endocrine organ”, “autopsy”, “post-mortem”, “neoplasm”, “surgical pathology,” “melanoma”, “sarcoma” and “tumor-to-tumor”. Individual case reports, case series, surgical pathology studies, and autopsy studies were all included. Particular attention was applied to series from the same institution or by the same authors to reduce duplication. All leukemias and lymphomas and direct extension/invasion into the parathyroid gland from adjacent malignancies were excluded, as they are not regarded as true metastases.
Two articles included in this study, namely the Bumpers et al. and Cifuentes et al. articles presented cases from the same institution over the years 1959–1974 [14] and 1971–1990 [22]. Given a 4-year overlap, there may potentially be a small number of repeated cases. Further, the Viadana et al. article includes only breast carcinoma metastatic to endocrine organs, but does not separate out parathyroid glands specifically, but no doubt also has potential overlap [29]. Similar to the Viadana et al. paper, the Patel et al. article contained cases of metastases to the parathyroid gland amongst a larger analysis of autopsy or surgical pathology specimens, but did not provide details as to how many parathyroid metastasis cases were included, and thus were excluded from this analysis [29, 30].
Results
Table 1 includes a listing of all 127 cases of metastasis to the parathyroid glands reported and incorporated into this literature review [1–28]. The vast majority of the cases developed in female patients (n = 99), no doubt accounted for by the high number of breast carcinoma cases (n = 85), with the overall average age when reported, of 58.5 years. Women outnumbered men by 5.8:1 (85.3 vs. 14.7%, respectively). Carcinomas were the most common tumor type (86.6%, n = 110), followed by melanoma (11.8%, n = 15), and sarcoma (1.6%; n = 2). Within the carcinoma category, breast carcinoma (66.9%, n = 85) were most common (Fig. 1) [4, 6, 8, 9, 12, 14, 17–20, 22, 23, 29], with the majority ductal carcinoma, followed by lobular carcinoma, although in many cases the exact tumor type was unspecified (Table 2). Some suggest that lobular carcinomas are more likely to metastasize, but this finding was not identified by others. Similarly, lung and thymic carcinoma were often not further broken down, although neuroendocrine carcinomas (n = 3) were noted. Melanomas were identified from primarily skin primaries (n = 12), although anal and palate (mucosal) sites were also documented, along with an ocular tumor [1, 4, 16]. A soft tissue sarcoma and a rhabdomyosarcoma each metastasized to the parathyroid glands [4]. Thyroid neoplasms most commonly showed direct extension [31], but hematogenous metastases were noted in three cases (Fig. 2). Nearly all of the cases (n = 122) were part of widely disseminated disease at the time of the identification of the parathyroid gland involvement, with only 5 (3.2%) cases reported as isolated metastases [3, 6–8, 28].
Table 1.
Literature review of 127 reported metastases to parathyroid glands (listed in chronological publication order) [1–28]
| Author | Date | Sex | Primary | Tumor type | Tumor-to-tumor metastasisa |
|---|---|---|---|---|---|
| Woolner et al. [9] | 1958 | F | Breast | Lobular carcinoma | Yes |
| Drickman et al. [10] | 1961 | M | Rectum | Adenocarcinoma | |
| F | Rectum | Adenocarcinoma | |||
| King et al. [11] | 1964 | F | Breast | Lobular carcinoma | |
| Margolis et al. [12] | 1969 | F | Breast | Lobular carcinoma | Yes |
| Horwitz et al. [4] | 1972 | M | Lung | nr | |
| M | Stomach | nr | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Skin | Melanoma | |||
| F | Breast | nr | |||
| F | Thymus | Thymoma | |||
| F | Breast | nr | |||
| F | Skin | Melanoma | |||
| M | Thigh | Spindle cell sarcoma | |||
| F | Skin | Melanoma | |||
| F | Salivary gland | Mucoepidermoid carcinoma | |||
| M | Lung | nr | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Tongue | nr | |||
| M | Skin | Melanoma | |||
| F | Breast | nr | |||
| M | Skin | Melanoma | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| F | Breast | Lobular carcinoma | |||
| F | Skin | Melanoma | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| M | nr (Testis) | Carcinoma (? Seminoma) | |||
| F | Skin | Melanoma | |||
| M | Lung | nr | |||
| M | Prostate | nr | |||
| F | Breast | nr | |||
| F | Anus | Melanoma | |||
| F | Thigh | Rhabdomyosarcoma | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| M | Kidney | Clear cell renal cell carcinoma | |||
| F | Breast | nr | |||
| F | Breast | nr | |||
| Borden et al. [13] | 1976 | M | Skin | Melanoma | |
| Cifuentes et al. [14] | 1979 | F | Breast | Ductal adenocarcinoma | |
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| Schwartz et al. [15] | 1982 | F | Kidney | Clear cell renal carcinoma | Yes |
| de la Monte et al. [16] | 1983 | nr | nr | Melanoma | |
| nr | nr | Melanoma | |||
| nr | nr | Melanoma | |||
| nr | nr | Melanoma | |||
| nr | nr | Melanoma | |||
| Watanabe et al. [17] | F | Breast | Ductal adenocarcinoma | ||
| de la Monte et al. [18] | 1984 | F | Breast | Ductal adenocarcinoma | |
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Lobular carcinoma | |||
| Inoshita et al. [1] | 1985 | M | Hard palate | Melanoma | Yes |
| Gattuso et al. [2] | 1988 | nr | Lung | Small cell carcinoma | |
| nr | Lung | Large cell carcinoma | |||
| Hermus et al. [19] | F | Breast | Mammary carcinoma | ||
| Goddard et al. [20] | 1990 | F | Breast | Lobular carcinoma | |
| Benisovich et al. [21] | 1991 | F | Lung | Adenocarcinoma | Yes |
| Bumpers et al. [22] | 1993 | F | Breast | Ductal adenocarcinoma | |
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Ductal adenocarcinoma | |||
| F | Breast | Lobular carcinoma | |||
| F | Breast | Lobular carcinoma | |||
| F | Breast | Lobular carcinoma | |||
| F | Breast | Lobular carcinoma | |||
| F | Breast | Lobular carcinoma | |||
| F | Breast | Lobular carcinoma | |||
| F | Breast | Lobular carcinoma | |||
| F | Breast | Lobular carcinoma | |||
| Mariette et al. [23] | F | Breast | nr | ||
| Tang et al. [24] | 2002 | nr | Thyroid | Papillary thyroid carcinoma | |
| nr | Thyroid | Papillary thyroid carcinoma | |||
| Pazaitou-Panayiotou et al. [25] | 2005 | n/r | Thyroid | Differentiated thyroid carcinoma | |
| Venkatraman et al. [26] | 2006 | F | Lung | Adenocarcinoma | PH |
| Fulciniti et al. [6] | 2010 | F | Breast | Ductal adenocarcinoma | Yes |
| Lee, HE et al. [7] | 2011 | M | Liver | Hepatocellular carcinoma | Yes |
| Chrisoulidou et al. [3] | 2012 | nr | Thyroid | Follicular thyroid carcinoma | |
| Lee et al. [8] | 2013 | F | Breast | Ductal adenocarcinoma | PH |
| Shifrin et al. [5] | M | Thymus | Well differentiated neuroendocrine carcinoma | PH | |
| Ofo et al. [27] | 2014 | M | Kidney | Clear cell renal cell carcinoma | |
| Torregrossa et al. [28] | 2016 | M | Kidney | Clear cell renal cell carcinoma (intrathyroidal parathyroid gland) |
aPH parathyroid gland hyperplasia, nr not reported
Fig. 1.
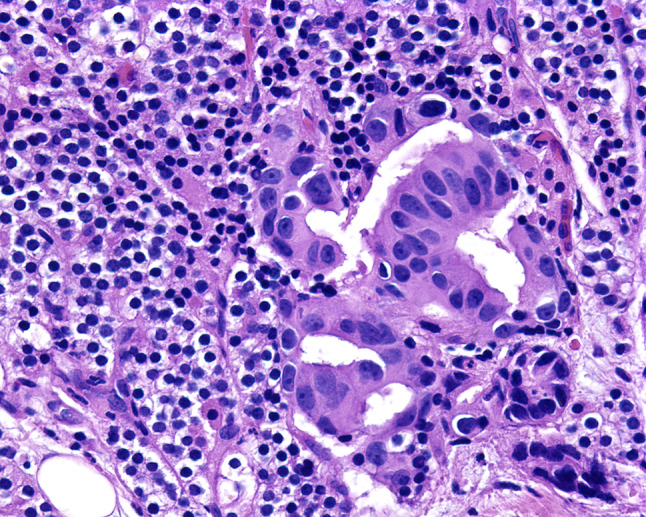
Pleomorphic ductal cells are arranged in a glandular formation, representing metastatic breast ductal adenocarcinoma to the parathyroid gland parenchyma
Table 2.
Primary sites of 127 reported malignancies metastatic to the parathyroid glands
| Primary site | Number of cases |
|---|---|
| Breast (adenocarcinoma) | 85 |
| Skin melanoma | 13 |
| Lung (adenocarcinoma and neuroendocrine carcinomas) | 7 |
| Unknown | 6 |
| Thyroid carcinoma (differentiated) | 4 |
| Kidney clear cell renal cell carcinoma | 4 |
| Thymic tumors | 2 |
| Soft tissue sarcoma (thigh) | 2 |
| Rectum adenocarcinoma | 2 |
| Liver hepatocellular carcinoma | 1 |
| Hard palate melanoma | 1 |
| Stomach adenocarcinoma | 1 |
| Salivary gland (mucoepidermoid carcinoma) | 1 |
| Tongue | 1 |
| Prostate adenocarcinoma | 1 |
| Anus melanoma | 1 |
Fig. 2.
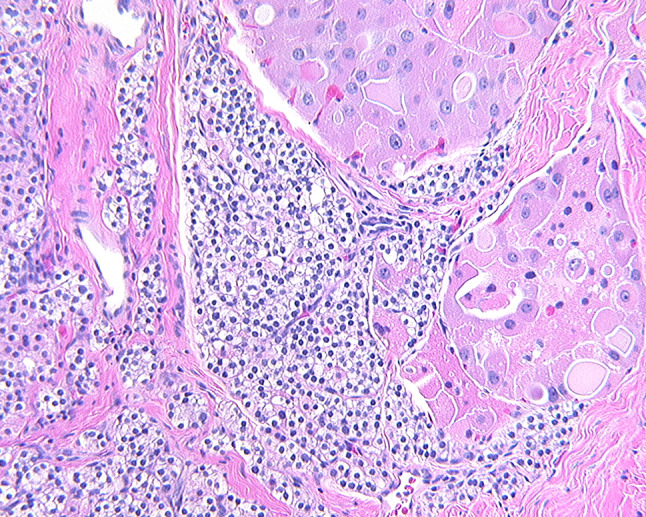
Tumor deposits of an oncocytic follicular thyroid carcinoma identified within parathyroid gland tissue
Tumor-to-tumor metastasis is uncommon, defined as one tumor metastasizing to another tumor. In this review, 5.5% of the total reported cases of metastases to the parathyroid glands were shown to represent metastases to parathyroid gland adenomas (n = 7; Table 3). An additional three cases had underlying parathyroid hyperplasia affected by metastases [5, 8, 26].
Table 3.
| Article | Primary malignancy | Primary tumor site | Secondary tumor |
|---|---|---|---|
| Woolner et al. [9] | Carcinoma | Breast | Parathyroid adenoma |
| Margolis et al. [12] | Poorly differentiated adenocarcinoma | Breast | Parathyroid adenoma |
| Schwartz et al. [15] | Clear cell carcinoma | Kidney | Parathyroid adenoma |
| Inoshita et al. [1] | Melanoma | Hard palate | Parathyroid adenoma |
| Benisovich et al. [21] | Adenocarcinoma | Lung | Parathyroid adenoma |
| Fulciniti et al. [6] | Infiltrating ductal carcinoma | Breast | Parathyroid adenoma |
| Lee et al. [7] | Hepatocellular carcinoma | Liver | Parathyroid adenoma |
Serum calcium levels were reported in only 58 patients. Of these patients, 23 (39.7%) experienced hypercalcemia while 17 patients (29.3%) experienced hypocalcemia, and the remaining patients were eucalcemic. Serum parathyroid hormone levels were reported in 12 patients, with 9 patients (75%) experiencing elevated levels, and only 1 patient (8.3%) reported with a reduced level. Reporting of numbers of parathyroid glands evaluated, number of glands affected and the percent of gland replaced by tumor was inconsistent. However, all eight patients reported to have nearly complete replacement of the parathyroid gland tissue by the metastatic deposit, had hypocalcemia [4, 11, 17, 19, 20, 23]. Four of these eight patients had parathyroid hormone (PTH) values measured, three had elevated PTH levels and one had a low PTH value. Thus, in the patients with elevated PTH levels, it would seem that the remaining parathyroid gland tissue was responding appropriately to the hypocalcemia from a different cause, and resulting in elevated PTH levels.
Discussion
This English literature review from 1950 to 2017 selected 127 reported cases of metastatic disease, which secondarily affected the parathyroid gland tissue. It is likely that clinically significant disease identified during life is uncommon, but that when there is widely disseminated disease, parathyroid gland involvement by metastatic tumors is probably significantly underreported. Horwitz et al. [4] reported a prospective study of 160 cancer patient autopsies, with 11.9% of patients having at least one parathyroid gland with metastatic involvement. de la Monte et al. [16] identified parathyroid gland metastases in 28% of patients with melanoma. In another study, de la Monte et al. [18] reported 6% of 187 patients with breast cancer showed parathyroid gland metastases, while Bumpers et al. [22] reported 24% of patients who died of breast cancer had parathyroid gland involvement. Further, Cifuentes et al. [14] reported that when a parathyroid gland was affected by metastatic breast cancer, an average of 18 other sites throughout the body also contained metastases. Horwitz et al. [4] also reported that parathyroid gland involvement was part of widespread metastases (bone, liver, lymph nodes, lungs, spleen, kidney, heart, gastrointestinal tract, genital system, brain and skin) in addition to other endocrine organs affected (adrenal glands, pituitary gland, thyroid gland and pancreas). Bumpers et al. [22] reported 35% of patients who died of breast cancer had multiple endocrine organs involved with metastatic breast cancer. Thus, it can be extrapolated, in the vast majority of cases, parathyroid gland metastases present as part of widely disseminated disease. Thus, the identification of only 127 cases reported in the literature is probably due to a selection and publication bias, with parathyroid gland involvement in metastatic disease much more common than suggested.
If one considers the incidence of new cancers diagnosed each year, with breast, lung, prostate, colorectal and melanoma listed in descending order of frequency (Cancer Facts & Figures 2017, American Cancer Society, Atlanta, Georgia, accessed 07/10/2017), it is perhaps not surprising that breast (66.9%), melanoma (11.8%) and lung (5.5%) account for the most common tumors that metastasize to the parathyroid glands. However, disease burden, lymphovascular supply, and perhaps embryologic considerations must also be taken into consideration, with reporting bias of tumor types that cause the most disease-related death also contributing. Metastatic pathways may be related, with thyroid, parathyroid, thymus and anterior pituitary derived from branchial arches, via the foregut ultimately. Thus, metastases to these organs are also documented in other foregut derived organs like stomach and pancreas [16]. Functional relationships as suggested by calcium regulation, which include bone, kidney, thyroid and parathyroid glands, may also account for patterns of organ involvement, while several endocrine organs are also commonly simultaneously affected (adrenal, pituitary, parathyroid, thyroid and pancreas) [4, 14, 16, 18, 22]. Thus, the single case reports of isolated metastatic disease to the parathyroid glands alone, as the only metastatic finding in living patients are exceptional [3, 6–8], with widespread disease the more likely scenario. It is of note that metastatic renal cell carcinoma (clear cell carcinoma specifically) to parathyroid glands is very infrequently reported [4, 15, 27, 28], even though late and widespread metastases from renal cell carcinoma are well known [32]. Importantly, the differential diagnostic consideration of a metastatic clear cell carcinoma within the clear cell parathyroid gland parenchyma may prove challenging since both tumors could be PAX8 and RCC immunoreactive. However, a positive reaction with CAIX and CD10 and a negative reaction with chromogranin and parathyroid hormone studies would help confirm a renal cell carcinoma.
Tumor-to-tumor metastases are a well-documented, albeit uncommon, finding. Endocrine organs, kidney, lung, and liver all have a significant blood flow, expressly related to functional requirements of the organ. Blood flow is disrupted by the development of a neoplasm or in the setting of hyperplasia. Thus, the disrupted blood supply or change in blood flow results in entrapment of tumor emboli within the tumor, and tumor-to-tumor metastasis may be seen [1, 6, 7, 9, 12, 21, 32, 33]. All of the tumor-to-tumor metastases in the reported cases were to parathyroid gland adenoma (Table 3). However, several cases also developed in parathyroid gland hyperplasia, another potential source of abnormal architecture and blood flow.
The parathyroid glands function in calcium homeostasis, with PTH secretion integrally related to the interplay of calcium metabolism by bone, kidney, skin and gastrointestinal functions. As such, deranged calcium metabolism reflected through hyper- or hypocalcemia, would be an expected finding. While the calcium levels were not reported in many of the patients, when it was reported, 68.4% of patients experienced either hypercalcemia (38.6%) or hypocalcemia (29.8%) during evaluation. If the patients with parathyroid gland adenoma and hyperplasia are removed from further consideration (n = 9), a significant cohort of patients still manifested calcium abnormalities. Serum parathyroid hormone levels were elevated in 75% of patients tested, while reduced in only 8.3%. These findings may suggest that at least part of the serum calcium abnormalities may be accounted for by destruction or replacement of the parathyroid gland parenchyma by the neoplastic infiltrate, while other factors in cancer patients (low albumin due to cachexia; gut absorption; renal function; bone metastases releasing calcium; paraneoplastic syndromes related to parathyroid hormone related peptide or malignant hyperthermia) contribute to calcium and parathyroid hormone alterations [4, 5, 11, 13, 17, 19–21, 23]. The inability of the glands to produce parathyroid hormone could lead to clinical hypocalcemia, while destruction of the gland by a rapidly growing tumor could also lead to release of stored PTH, flooding PTH into the peripheral circulation and causing abnormal serum calcium levels [5].
In summary, metastatic malignancies to the parathyroid glands usually present in middle aged to older women, who have widely disseminated disease. Breast carcinomas represent the majority of tumors (66.9%), while melanomas are also identified quite commonly (11.8%). Tumor-to-tumor metastases are uncommon (5.5%). Calcium homeostasis is frequently affected, more disproportionately than disseminated disease would suggest, probably as a result of parathyroid gland parenchymal destruction. Thus, parathyroid gland involvement should be considered in patients who have metastatic disease and calcium alterations.
Acknowledgements
A special thanks to Ms. Hannah B. Herrera for her research assistance. The views expressed are those of the authors solely and do not represent endorsement from Southern California Permanente Medical Group.
Funding
No external funding was obtained for this study.
Compliance with Ethical Standards
Conflict of interest
All authors declare that he/she has not conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB #5968), which did not require informed consent.
Contributor Information
Justin L. Bauer, Email: jlb@dartmouth.edu
Sherwin Toluie, Email: sherwintoluie@yahoo.com.
Lester D. R. Thompson, Phone: 818-719-2613, Email: Lester.D.Thompson@kp.org
References
- 1.Inoshita T, Laurain AR. Tumor-to-tumor metastasis: malignant melanoma metastatic to parathyroid adenoma. Mil Med. 1985;150:323–325. doi: 10.1093/milmed/150.6.323. [DOI] [PubMed] [Google Scholar]
- 2.Gattuso P, Khan NA, Jablokow VR, Kathuria S. Neoplasms metastatic to parathyroid glands. South Med J. 1988;81:1467. doi: 10.1097/00007611-198811000-00039. [DOI] [PubMed] [Google Scholar]
- 3.Chrisoulidou A, Mandanas S, Mitsakis P, et al. Parathyroid involvement in thyroid cancer: an unforeseen event. World J Surg Oncol. 2012;10:121. doi: 10.1186/1477-7819-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz CA, Myers WP, Foote FW., Jr Secondary malignant tumors of the parathyroid glands. Report of two cases with associated hypoparathyroidism. Am J Med. 1972;52:797–808. doi: 10.1016/0002-9343(72)90086-1. [DOI] [PubMed] [Google Scholar]
- 5.Shifrin AL, LiVolsi VA, Zheng M, et al. Neuroendocrine thymic carcinoma metastatic to the parathyroid gland that was reimplanted into the forearm in patient with multiple endocrine neoplasia type 1 syndrome: a challenging management dilemma. Endocr Pract. 2013;19:e163–e167. doi: 10.4158/EP13267.CR. [DOI] [PubMed] [Google Scholar]
- 6.Fulciniti F, Pezzullo L, Chiofalo MG, et al. Metastatic breast carcinoma to parathyroid adenoma on fine needle cytology sample: report of a case. Diagn Cytopathol. 2011;39:681–685. doi: 10.1002/dc.21528. [DOI] [PubMed] [Google Scholar]
- 7.Lee HE, Kim DH, Cho YH, Kim K, Chae SW, Sohn JH. Tumor-to-tumor metastasis: hepatocellular carcinoma metastatic to parathyroid adenoma. Pathol Int. 2011;61:593–597. doi: 10.1111/j.1440-1827.2011.02707.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Kim BH, Bae MJ, et al. Concurrence of primary hyperparathyroidism and metastatic breast carcinoma affected a parathyroid gland. J Clin Endocrinol Metab. 2013;98:3127–3130. doi: 10.1210/jc.2013-1227. [DOI] [PubMed] [Google Scholar]
- 9.Woolner LB, Keating FR, Jr, Black BM. Primary hyperparathyroidism and metastatic breast carcinoma: a case in which breast carcinoma metastasized to a parathyroid adenoma. Cancer. 1958;11:975–979. doi: 10.1002/1097-0142(195809/10)11:5<975::AID-CNCR2820110518>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Drickman A. Metastatic carcinoma involving the parathyroid glands. Report of two cases and a short review of the literature. Arch Surg. 1961;82:576–578. doi: 10.1001/archsurg.1961.01300100090009. [DOI] [PubMed] [Google Scholar]
- 11.King LR, Goldsmith RE. Atypical hypoparathyroidism. Acta Endocrinol. 1964;47:121–132. doi: 10.1530/acta.0.0470121. [DOI] [PubMed] [Google Scholar]
- 12.Margolis CI, Goldenberg VE. Breast carcinoma metastatic to parathyroid adenoma. N Y State J Med. 1969;69:702–703. [PubMed] [Google Scholar]
- 13.Borden H, Hummer GJ, Landon CW, Paris J. The use of procaine in acquired malignant hyperthermia in a patient with malignant melanoma metastatic to the parathyroid gland: a case report. Can Anaesth Soc J. 1976;23:616–623. doi: 10.1007/BF03006744. [DOI] [PubMed] [Google Scholar]
- 14.Cifuentes N, Pickren JW. Metastases from carcinoma of mammary gland: an autopsy study. J Surg Oncol. 1979;11:193–205. doi: 10.1002/jso.2930110303. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz IS, Feld HJ, Imberman MM, Schwartz AE. Hypernephroma metastatic to a parathyroid adenoma eighteen years after nephrectomy. Mt Sinai J Med. 1982;49:499–503. [PubMed] [Google Scholar]
- 16.de la Monte SM, Moore GW, Hutchins GM. Patterned distribution of metastases from malignant melanoma in humans. Cancer Res. 1983;43:3427–3433. [PubMed] [Google Scholar]
- 17.Watanabe T, Adachi I, Kimura S, et al. A case of advanced breast cancer associated with hypocalcemia. Jpn J Clin Oncol. 1983;13:441–448. [PubMed] [Google Scholar]
- 18.de la Monte SM, Hutchins GM, Moore GW. Endocrine organ metastases from breast carcinoma. Am J Pathol. 1984;114:131–136. [PMC free article] [PubMed] [Google Scholar]
- 19.Hermus A, Beex L, van Liessum P, et al. Hypocalcemia due to osteoblastic metastases and diminished parathyroid reserve in a patient with advanced breast cancer. Klin Wochenschr. 1988;66:643–646. doi: 10.1007/BF01728807. [DOI] [PubMed] [Google Scholar]
- 20.Goddard CJ, Mbewu A, Evanson JM. Symptomatic hypocalcaemia associated with metastatic invasion of the parathyroid glands. Br J Hosp Med. 1990;43:72. [PubMed] [Google Scholar]
- 21.Benisovich VI, Rybak BJ, Ross FA. A case of adenocarcinoma of the lung associated with a neck mass and hypercalcemia. Cancer. 1991;68:1106–1108. doi: 10.1002/1097-0142(19910901)68:5<1106::AID-CNCR2820680534>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Bumpers HL, Hassett JM, Jr, Penetrante RB, Hoover EL, Holyoke ED. Endocrine organ metastases in subjects with lobular carcinoma of the breast. Arch Surg. 1993;128:1344–1347. doi: 10.1001/archsurg.1993.01420240052009. [DOI] [PubMed] [Google Scholar]
- 23.Mariette X, Khalifa P, Boissonnas A, Sereni D, Cremer G. Hypocalcaemia due to parathyroid metastases. Eur J Med. 1993;2:242–244. [PubMed] [Google Scholar]
- 24.Tang W, Kakudo K, Nakamura MY, et al. Parathyroid gland involvement by papillary carcinoma of the thyroid gland. Arch Pathol Lab Med. 2002;126:1511–1514. doi: 10.5858/2002-126-1511-PGIBPC. [DOI] [PubMed] [Google Scholar]
- 25.Pazaitou-Panayiotou K, Kaprara A, Boudina M, et al. Thyroid carcinoma in children and adolescents: presentation, clinical course, and outcome of therapy in 23 children and adolescents in Northern Greece. Hormones. 2005;4:213–220. doi: 10.14310/horm.2002.11160. [DOI] [PubMed] [Google Scholar]
- 26.Venkatraman L, Kalangutkar A, Russell CF. Primary hyperparathyroidism and metastatic carcinoma within parathyroid gland. J Clin Pathol. 2007;60:1058–1060. doi: 10.1136/jcp.2005.035352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ofo E, Mandavia R, Jeannon JP, Odell E, Simo R. Renal cell carcinoma metastasis to the parathyroid gland: a very rare occurrence. Int J Surg Case Rep. 2014;5:378–380. doi: 10.1016/j.ijscr.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torregrossa L, Rotondo MI, Insilla AC, et al. Metastasis of renal cell carcinoma to the parathyroid gland 16 years after radical nephrectomy: a case report. Oncol Lett. 2016;12:3224–3228. doi: 10.3892/ol.2016.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viadana E, Cotter R, Pickren JW, Bross ID. An autopsy study of metastatic sites of breast cancer. Cancer Res. 1973;33:179–181. [PubMed] [Google Scholar]
- 30.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-X. [DOI] [PubMed] [Google Scholar]
- 31.Ito Y, Kakudo K, Hirokawa M, et al. Clinical significance of extrathyroid extension to the parathyroid gland of papillary thyroid carcinoma. Endocr J. 2009;56:251–255. doi: 10.1507/endocrj.K08E-297. [DOI] [PubMed] [Google Scholar]
- 32.Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002;95:1869–1878. doi: 10.1002/cncr.10901. [DOI] [PubMed] [Google Scholar]
- 33.Thompson LDR, Heffess CS. Renal cell carcinoma to the pancreas in surgical pathology material: a clinicopathologic study of 21 cases with a review of the literature. Cancer. 2000;89:1076–1088. doi: 10.1002/1097-0142(20000901)89:5<1076::AID-CNCR17>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]


