Abstract
Neuroendocrine carcinomas of the head and neck are rare and are classified as well differentiated, moderately differentiated, and poorly differentiated carcinomas with the latter category being subdivided into small cell and large cell neuroendocrine carcinoma (LCNEC). While most carcinomas in the nasopharynx are associated with Epstein-Barr virus (EBV), there has been only one previous report demonstrating a link between EBV and LCNEC of the nasopharynx. In this report we describe a second case of EBV-positive LCNEC arising in the nasopharynx with bilateral cervical metastases. The patient was treated with a combination of radiation and chemotherapy which resulted in a complete clinical and radiological response. The patient is still disease free 3 years after presentation. The results of this case suggest that EBV-positive LCNEC is sensitive to chemoradiotherapy and as a result may have better prognosis than EBV-negative LCNEC arising in the nasopharynx or other sites.
Keywords: Epstein-Barr virus, Large cell neuroendocrine carcinoma, Nasopharynx, Radiation therapy
Introduction
Neuroendocrine carcinomas (NEC) of the head and neck are rare and the majority of tumours arise in the larynx. The 2016 WHO Classification of Head and Neck Tumours includes three categories of NEC: well-differentiated, moderately differentiated, and poorly differentiated [1]. The well differentiated category is synonymous with the term “carcinoid” while the poorly differentiated category includes both small cell neuroendocrine carcinoma (SCNEC) and large cell neuroendocrine carcinoma (LCNEC). Etiologic agents described include smoking and HPV, which largely mirror the agents known to be associated with squamous cell carcinoma in the oral cavity and oropharynx, respectively [2, 3]. In contrast, most non-neuroendocrine tumours that arise in the nasopharynx are associated with Epstein-Barr virus (EBV) although very little is known about the role of EBV in the development NEC in this site [4]. Moreover, poorly differentiated NEC of the head and neck are associated with poor prognosis and it is unclear if the presence of EBV alters the outcome for these patients [5]. The current report expands on our knowledge by describing a case of LCNEC associated with EBV arising in the nasopharynx which demonstrated complete clinical and radiological response after combined chemoradiation therapy.
Case Report
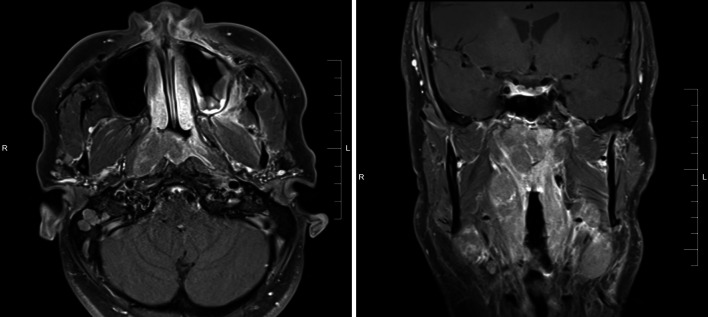
The patient was a 40-year-old male never-smoker who presented with right ear fullness, epistaxis, and bilateral cervical and supraclavicular lymphadenopathy. His past medical history included tonsillectomy and adenoidectomy at age 20, as well as tuberous sclerosis (TSC). Nasopharyngoscopy identified a right-sided mass involving the roof and lateral wall of the nasopharynx and magnetic resonance imaging (MRI) confirmed the presence of a 3.7 cm × 2.9 cm nasopharyngeal mass which extended into the oropharynx and expanded the fossa of Rosenmuller, but did not involve the nasal cavity (Fig. 1). Staging studies including computed tomography (CT) and fluorodeoxyglucose positron emission tomography (FDG-PET) demonstrated bilateral enlargement of level II through V lymph nodes in the neck but no evidence of distant metastatic disease.
Fig. 1.
Magnetic resonance imaging (MRI) demonstrate the presence of a 3.7 cm x 2.9 cm mass arising in the nasopharynx extending into the oropharynx. No involvement of the nasal cavity was identified
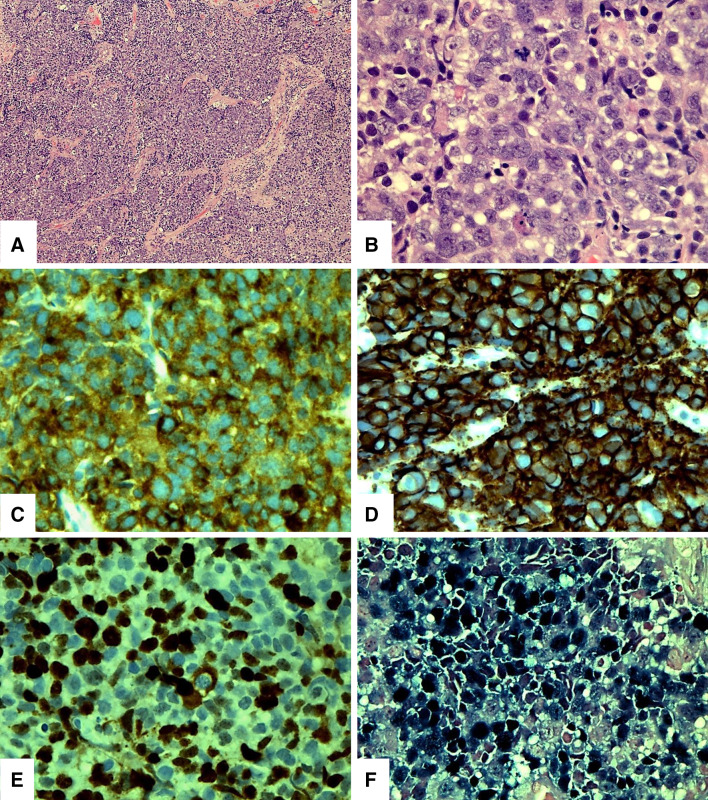
A biopsy of the nasopharyngeal mass demonstrated a tumor composed of medium to large cells arranged in confluent nests and sheets (Fig. 2a). The neoplastic cells exhibited a high nuclear to cytoplasmic ratio with a moderate amount of pale eosinophilic or amphophilic cytoplasm, indistinct cell membranes, irregular nuclear contours, vesicular chromatin, and small to medium sized nucleoli (Fig. 2b). The mitotic rate was 12 mitotic figures per 10 high-power fields with numerous atypical forms. Foci of tumor necrosis, numerous apoptotic cells and hyperchromatic “crushed” cells were also identified.
Fig. 2.
The biopsy of the nasopharyngeal mass demonstrated a tumor composed of medium to large cells arranged in nests and sheets (a). The neoplastic cells exhibited a high nuclear to cytoplasmic ratio with a moderate amount of pale eosinophilic or amphophilic cytoplasm, indistinct cell membranes, irregular nuclear contours, vesicular chromatin, and small to medium sized nucleoli (b). The tumor cells are positive for synaptophysin (c), and CD56 (d). The proliferation index as measured by MIB-1 was approximately 50% (e). In-situ hybridization for EBV-RNA (EBER) was strongly reactive in the neoplastic population (f)
Immunohistochemical analysis found the neoplastic population to be positive for low-molecular weight keratin (CAM5.2), synaptophysin (Fig. 2c), CD56 (Fig. 2d), and p16. The neoplastic cells did not demonstrate any reactivity for for high-molecular weight keratin (34BE12), pan-keratin (AE1:AE3), p63, keratin 7 (CK7), chromogranin, S100 protein, thyroid-transcription factor-1 (TTF-1) and MART-1. The proliferation index as measured by MIB-1 was approximately 50% (Fig. 2e). In-situ hybridization for EBV-RNA (EBER) was strongly reactive in the neoplastic population (Fig. 2f).
The patient’s serum EBV copy number was evaluated using real time quantitative PCR and was found to be elevated at 16508.59 copies/mL (normal < 2000 copies/mL). The patient was treated with external beam radiotherapy (70 Gy in 35 fractions), and 3 cycles of high-dose cisplatin (later changed to carboplatin) along with etoposide. He remains disease free to date more than 3 years after treatment.
Discussion
Large cell neuroendocrine carcinoma is a well established entity in the lung although it was only recently added to the 4th edition of the WHO Classification of Head and Neck Tumours [1]. The criteria for diagnosing LCNEC in the head and neck largely mimic the histologic and immunohistochemical features described in the lung: large cells with a relatively low nuclear to cytoplasmic ratio, coarse chromatin, frequent nucleoli, a mitotic count greater than 10 per 10 HPFs, tumour necrosis; and reactivity for synaptophysin or chromogranin, and CD56 [6]. While LCNEC and SCNEC are related tumours, they differ in that the neoplastic cells in SCNEC have a very high nuclear to cytoplasmic ratio, evenly dispersed chromatin, and lack nucleoli. The presence of significant crush artefact, normally associated with SCNEC, can also be seen in LCNEC, and better preserved areas of the tumour should be used to identify the defining cytologic features.
LCNEC have been reported at a variety of head and neck sites although the majority have been described in the larynx. To the best of our knowledge, the current case is only the fourth report of a LCNEC primarily involving the nasopharynx [7–9]. In two of these previously reported cases, the patients did not have nodal disease but developed early and widespread metastatic disease. The third case is similar to our in that the patient presented with bulky bilateral lymphadenopathy but no distant metastatic disease. Thus the data available to date suggests that LCNEC is an aggressive malignancy that presents with advanced stage disease. While three out of the four patients were males in their 30s or early 40s, the case described by Dumars et al. is unique in that their patient was only 9 years old at diagnosis [9].
The vast majority of malignant neoplasms that arise in the nasopharynx are nasopharyngeal carcinoma (NPC), a type of squamous cell carcinoma that is divided into keratinizing, non-keratinizing, and basaloid variants. The non-keratinizing variant is almost always associated with EBV and has a very high incidence in among some ethnic groups including Inuit, Northern Africans, and Chinese from south-eastern Asia [10]. Of the four LCNECs reported in the nasopharynx, two (including our case) were associated with EBV as demonstrated by reactivity for EBER. Unfortunately, the two other reports did not mention whether in situ hybridization for EBER was performed so we cannot definitely say those cases were unrelated to EBV. Regardless, the presence of EBV in both nasopharyngeal carcinoma and LCNEC suggests that the virus has a unique tropism for the nasopharynx and is capable of infecting cells that can give rise to histologically and immunophenotypically distinct entities. Moreover, the limited reports available to date suggest that EBV-associated LCNEC also has a tendency to disseminate early to cervical lymph nodes and patients often present with bilateral neck masses.
The prognosis for patients with LCNEC is poor regardless of body site. In the lung the 5 year survival has been reported to range from 15 to 57% for all stages [11]. Adjuvant chemotherapy appears to improve outcome but the results vary greatly by stage. Iyoda et al. reported 5 year survival of 89% after adjuvant chemotherapy however most of the patients in this study had stage 1 disease [12]. A larger study also found a trend towards improved survival after neoadjuvant or adjuvant chemotherapy although no significant difference in overall 5 year survival [13]. To date there have been very few large studies that have examined the prognosis of LCNEC in the head and neck. Kao et al. performed a retrospective analysis of all NEC involving the head and neck and identified seven cases of LCNEC: 4 involving the larynx and 3 involving the sinonasal tract [5]. They found that all patients presented with advanced disease and reported a 5 year rate of survival of 21%. It is worth noting, however, that none of the tumours in this study were assessed for EBV so it is unclear what if ay role EBV may have played in the pathogenesis of these tumours. Similarly, Laan et al. performed a retrospective analysis of all NEC involving the larynx and reported a 5 year rate of survival of 14% [14].
In contrast, the outcome for patients with EBV-positive NPC is significantly better. Most patients diagnosed with NPC receive intensity-modulated radiation with concordant chemotherapy regardless of stage [15]. This regimen results in a local control rate of > 90% for patients with T1–T3 disease. Patients with T4 disease fare slightly worse with a local control rate ranging between 74–80%. NPC has a high propensity for cervical node metastases although the nodal disease is controlled with radiotherapy in 86–97% of all cases. The 5 year survival rate regardless of stage is 79% and most patients who recur do so within 5 years [15]. The radiosensitivity of EBV-positive NPC is well known and a recent paper by Gao et al. found that this sensitivity is related to the expression of the EBV-encoded microRNA BART7 which upregulates the production of TGFβ1 in neoplastic cells [16]. It is possible that these same mechanisms are active in EBV-positive LCNEC and they may explain the dramatic response observed in our case.
The patient described in this report had previously been diagnosed with TSC, an autosomal dominant disorder that is associated with the development of multiple hamartomas in various organ systems including the skin, kidneys, central nervous system, and lungs. The clinical manifestations of TSC are variable and some authors have suggested that neuroendocrine tumours may be a complication of the disease. However, in a systematic review, Dworakowska and Grossman [17] found that there were only isolated reports of neuroendocrine tumours in patients with TSC and that the only islet tumours appeared to occur more frequently in patients with TSC. Notably, this review did not find any association between TSC and NEC.
In summary, LCNEC is a rare tumour in the head and neck and the etiology appears to depend on the anatomical site involved. Tumours arising in the nasopharynx should be tested for EBV because EBV-positive tumours may respond very favorably to local radiotherapy even in patients who present with extensive nodal disease. Further study is needed to elucidate the mechanisms responsible for the development of EBV-positive NEC.
Compliance with Ethical Standards
Conflict of interest
None of the authors have any conflicts of interest to declare.
Informed Consent
Informed consent was received from the patient described in this case in compliance with institutional standards prior to initiating this study.
References
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. Lyon: IARC; 2017. [Google Scholar]
- 2.Kraft S, Faquin WC, Krane JF. HPV-associated Neuroendocrine Carcinoma of the Oropharynx. Am J Surg Pathol. 2012;36:321–330. doi: 10.1097/PAS.0b013e31823f2f17. [DOI] [PubMed] [Google Scholar]
- 3.Bates T, Mcqueen A, Iqbal MS, Kelly C, Robinson M. Small cell neuroendocrine carcinoma of the oropharynx harbouring oncogenic HPV-infection. Head Neck Pathol. 2013;8:127–131. doi: 10.1007/s12105-013-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 5.Kao H-L, Chang W-C, Li W-Y, Li AC-H, Li AF-Y. Head and neck large cell neuroendocrine carcinoma should be separated from atypical carcinoid on the basis of different clinical features, overall survival, and pathogenesis. Am J Surg Pathol. 2012;36:185–192. doi: 10.1097/PAS.0b013e318236d822. [DOI] [PubMed] [Google Scholar]
- 6.Travis WD. Pathology and diagnosis of neuroendocrine tumors. Thorac Surg Clin. 2014;24:257–266. doi: 10.1016/j.thorsurg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Elloumi F, Fourati N, Siala W, Ghorbell L, Jlidi R, Ghorbel A, et al. Tumeur neuroendocrine à grandes cellules du nasopharynx: à propos d’un cas. Cancer/Radiothérapie. 2014;18:208–210. doi: 10.1016/j.canrad.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Sturgis CD, Burkey BB, Momin S, Hoschar AP. High grade (large cell) neuroendocrine carcinoma of the nasopharynx: novel case report with touch preparation cytology and positive EBV encoded early RNA. Case Rep Pathol. 2015;2015:1–4. doi: 10.1155/2015/231070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumars C, Thebaud E, Joubert M, Renaudin K, Cariou-Patron G, Heymann M-F. Large cell neuroendocrine carcinoma of the nasopharynx. J Pediatric Hematol/Oncol. 2015;37:474–476. doi: 10.1097/MPH.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 10.Petersson F. Nasopharyngeal carcinoma: a review. Semin Diagn Pathol. 2015;32:54–73. doi: 10.1053/j.semdp.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Iyoda A, Makino T, Koezuka S, Otsuka H, Hata Y. Treatment options for patients with large cell neuroendocrine carcinoma of the lung. General Thorac Cardiovasc Surg. 2014;62:351–356. doi: 10.1007/s11748-014-0379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyoda A, Hiroshima K, Moriya Y, Takiguchi Y, Sekine Y, Shibuya K, et al. Prospective Study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82:1802–1807. doi: 10.1016/j.athoracsur.2006.05.109. [DOI] [PubMed] [Google Scholar]
- 13.Sarkaria IS, Iyoda A, Roh MS, Sica G, Kuk D, Sima CS, et al. Neoadjuvant and adjuvant chemotherapy in resected pulmonary large cell neuroendocrine carcinomas: a single institution experience. Ann Thorac Surg. 2011;92:1180–1187. doi: 10.1016/j.athoracsur.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Laan TPVD, Plaat BEC, Laan BFVD, Halmos GB. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: a meta-analysis of 436 reported cases. Head Neck. 2014;37:707–715. doi: 10.1002/hed.23666. [DOI] [PubMed] [Google Scholar]
- 15.Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356–3364. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 16.Gao W, Li Z-H, Chen S, Chan JY-W, Yin M, Zhang M-J, et al. Epstein-Barr virus encoded microRNA BART7 regulates radiation sensitivity of nasopharyngeal carcinoma. Oncotarget. 2017;8:20297. doi: 10.18632/oncotarget.15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dworakowska D, Grossman AB. Are neuroendocrine tumours a feature of tuberous sclerosis? A systematic review. Endocr Relat Cancer. 2009;16:45–58. doi: 10.1677/ERC-08-0142. [DOI] [PubMed] [Google Scholar]