Abstract
Solitary fibrous tumors (SFTs) are well recognized in the head and neck region, but rarely arise in the sinonasal tract (SNT). Six primary SNT SFTs were identified in the files of Southern California Permanente Medical Group between 2006 and 2017. The patients included five males and one female ranging in age from 33 to 72 years (mean 52 years), most of whom presented clinically with nasal obstruction. Three tumors involved the nasal cavity alone, one involved the paranasal sinuses, and two involved both the nasal cavity and paranasal sinuses. Histologically, the tumors were characterized by a variably cellular proliferation of cytologically bland spindle cells within a collagenous stroma with prominent interspersed branching vessels. Mitotic activity was low (range 0–2 per 10 high power fields) and there was no evidence of pleomorphism or tumor necrosis. Surface ulceration was noted. By immunohistochemistry, the lesional cells were positive for CD34, STAT6 and bcl-2. Clinical follow up information was available for all patients (range 32–102 months; mean 72 months). There were no recurrences or metastases and all were alive with no evidence of disease at last follow-up. SFTs rarely affect the SNT, but should be considered in the differential diagnosis of SNT mesenchymal lesions. Immunohistochemical expression of STAT6 can aid in diagnosis and separation of SFT from other spindle cell lesions occurring at this anatomic site. In combination with cases reported in the literature, primary SNT SFT behave in an indolent manner with conservative treatment.
Keywords: Solitary fibrous tumors, Nasal cavity, Paranasal sinuses, Immunohistochemistry, Differential diagnosis, STAT6 protein, Human
Introduction
Solitary fibrous tumor (SFT) is an uncommon fusion gene-associated neoplasm composed of spindled fibroblastic cells set within a branching vasculature. The tumor may occur in any anatomic site, with approximately 5–27% of SFTs arising in the head and neck region [1–9]. Within this anatomic region, preferred sites of involvement include the oral cavity and orbit [2, 10, 11]. In contrast, SFT infrequently affects the sinonasal tract (SNT). Due to its relative rarity and variable morphologic appearance, SNT SFT may be difficult to distinguish from other mesenchymal lesions that are more commonly recognized at this site. While the majority of SFTs behave in a benign fashion a small subset will recur or metastasize [12–14]. Whether SFTs of the SNT exhibit a similarly wide spectrum of biologic behavior remains unclear. A limited number of SNT SFTs have been reported, comprised mostly of isolated case reports, small series, and partial descriptions of small numbers of cases included among larger series of SFTs involving the head and neck or encompassing all anatomic sites [1, 2, 10, 11, 15–66]. In this study, six new cases of SFT of the SNT were evaluated in conjunction with a comprehensive literature review in order to further characterize the clinicopathologic features of this uncommon sinonasal neoplasm.
Materials and Methods
Six cases of SFT involving the nasal cavity and/or paranasal sinuses were identified from the files of the departments of pathology within Southern California Permanente Medical Group between 2007 and 2017, representing 0.021% of the 28,026 SNT specimens submitted during this period. Hematoxylin and eosin stained slides from all cases were reviewed, with a range of 1–9 slides (mean 4 slides) per case available for analysis. In addition, previously performed immunohistochemical stains from each case were reviewed, which included CD34 (n = 6), pan-cytokeratin (n = 4), desmin (n = 2), smooth muscle actin (SMA) (n = 6), bcl-2 (n = 3), muscle specific actin (MSA) (n = 3), CD31 (n = 2), and S-100 protein (n = 3). Additional immunohistochemical studies were performed on all cases using a monoclonal antibody directed against STAT6 (clone EP325; Cell Marque; Rocklin, California) utilizing standard techniques.
Clinical data, treatment, and follow up information were obtained from electronic medical records augmented by the surgical pathology reports. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of an Internal Review Board authorization (#5968) performed under the direction of Southern California Permanente Medical Group.
A review of the English literature based on a MEDLINE search from 1966 to 2017 was performed and all cases of SNT SFT were reviewed, with specific attention to the clinical series and those which included immunohistochemistry information.
Results
The patients included five males and one female who ranged in age from 33 to 72 years, with a mean age at presentation of 52 years (Table 1). Patients most frequently presented with nasal obstruction. Other symptoms included nasal discharge and epistaxis. The duration of symptoms ranged from 0.25 to 24 months, with an average of 9.9 months.
Table 1.
Clinicopathologic features of six cases of sinonasal tract solitary fibrous tumor
| Case no. | Age (years) | Sex | Symptom duration (months) | Symptoms | Laterality | Site | Size (cm) | Treatment | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M | 9 | Discharge | Left | Ethmoid sinus, sphenoid sinus | 0.4 | FESS | ANED (102) |
| 2 | 33 | M | 0.25 | Epistaxis | Left | Nasal cavity | 4.0 | FESS | ANED (32) |
| 3 | 36 | M | 12 | Obstruction | Right | Nasal cavity | 6.2 | FESS | ANED (84) |
| 4 | 53 | M | 12 | Obstruction | Left | Nasal cavity, sphenoid sinus | 4.5 | FESS | ANED (57) |
| 5 | 73 | M | 2 | Obstruction | Right | Nasal cavity | 4.0 | FESS | ANED (67) |
| 6 | 52 | F | 24 | Obstruction | Left | Nasal cavity, ethmoid sinus | 3.5 | FESS | ANED (90) |
M male, F female, FESS functional endoscopic sinus surgery, ANED alive with no evidence of disease
The tumors ranged from 0.4 up to 6.2 cm in greatest dimension with an average size of 3.8 cm. Three tumors involved the nasal cavity alone, one tumor was confined to the paranasal sinuses (ethmoid and sphenoid), and two involved both the nasal cavity and paranasal sinuses. Four tumors were centered on the left side and two on the right side. The tumors were submitted as multiple irregular fragments of tissue, described as white to pale tan with a firm consistency.
The tumors were situated beneath an intact surface respiratory epithelium and/or metaplastic squamous mucosa. Surface ulceration was noted in four cases, perhaps due to the size of the polypoid mass as it was subjected to trauma within the SNT. The tumors showed a moderately cellular proliferation of spindle cells dispersed within a collagenous stroma (Fig. 1). Entrapment of minor mucoserous glands was observed in three cases (Fig. 2). All tumors were characterized by variable cellularity with hypocellular areas admixed with areas of increased cellularity (Fig. 3). Paucicellular areas of the tumor were dominated by sclerotic to hyalinized stroma with rare interspersed spindle cells (Fig. 4). In the more cellular regions of the tumors, the spindle cells had a vaguely storiform or fascicular arrangement or were randomly distributed with no consistent architectural pattern of growth (Fig. 5). The neoplastic spindle cells were cytologically bland, with uniform fusiform to ovoid nuclei with fine chromatin, inconspicuous nucleoli and scant eosinophilic cytoplasm (Fig. 6). The spindle cells lacked atypical features and no pleomorphic cells were observed. All tumors were characterized at least focally, by the presence of a prominent vascular component ranging from small to medium sized thin-walled, dilated vessels to larger, thick-walled vessels with perivascular hyalinization (Fig. 7). Areas of cellular dyshesion, resulting in a pseudovascular appearance, similar to what has been described in giant cell angiofibroma, was observed in one case, though no stromal giant cells were identified (Fig. 8). Mitotic figures were present, ranging from 0 to 2 with a mean of 1.2 per 10 high power fields. No atypical mitotic figures were identified. Tumor necrosis was absent in all cases, although if surface ulceration was present, associated degenerative changes were noted.
Fig. 1.
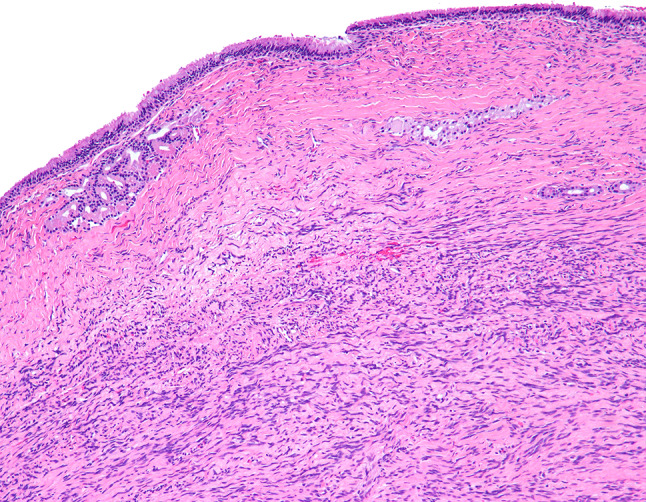
SNT SFT below an intact respiratory epithelium
Fig. 2.
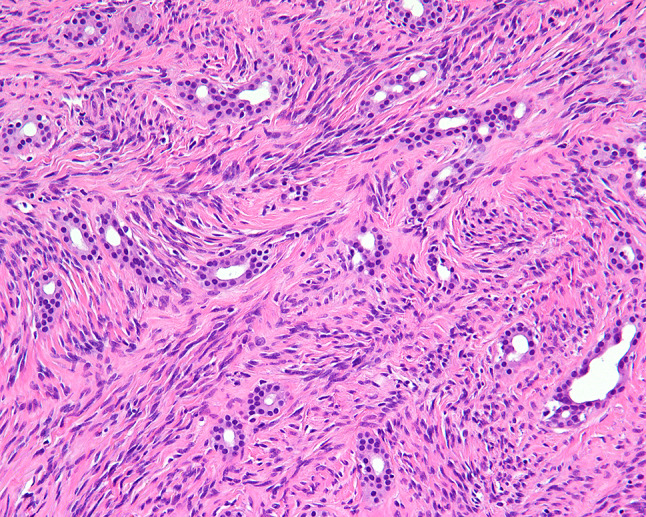
Entrapment of normal mucoserous glands at the edge of the tumor
Fig. 3.
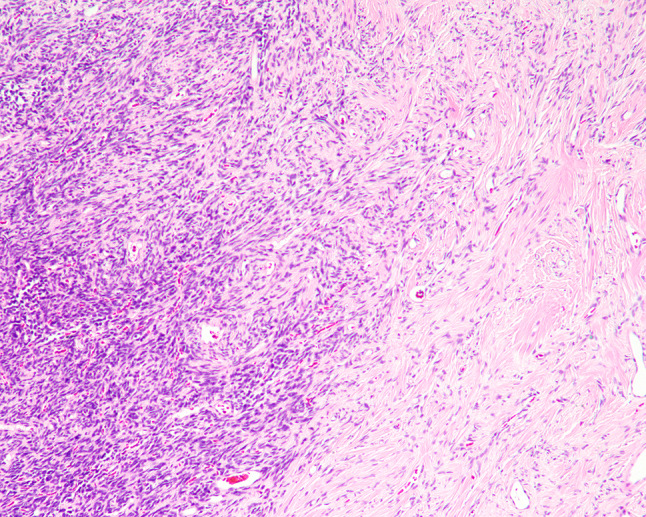
Intratumoral variation in cellularity with transition between hyper- and hypo-cellular areas
Fig. 4.
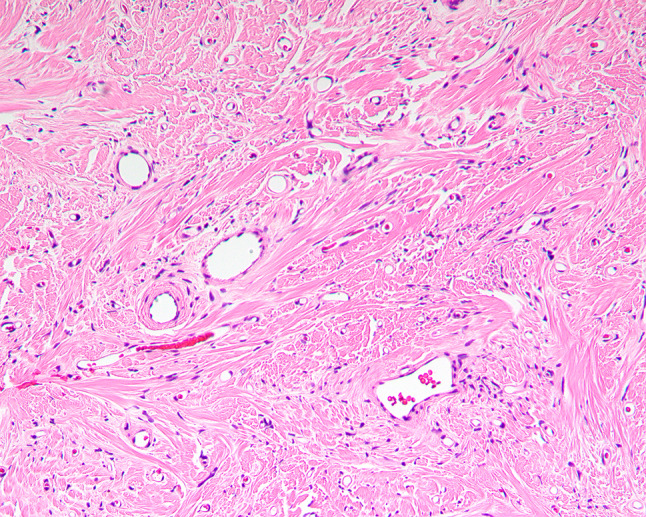
Paucicellular area of SFT with few scattered spindle cells in a collagen rich stroma
Fig. 5.
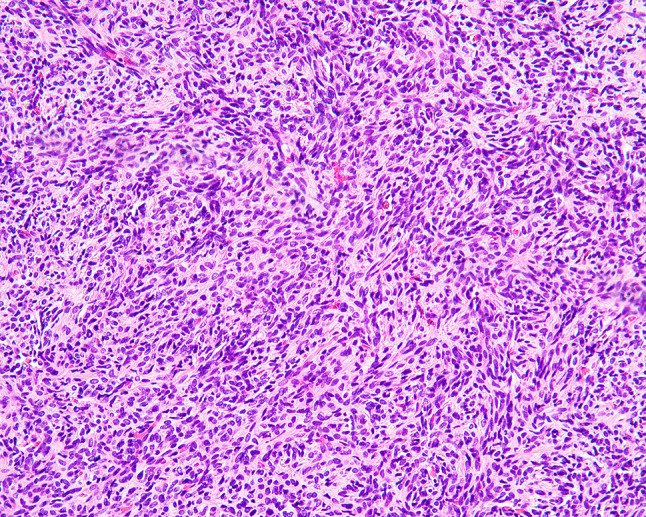
More cellular focus in SFT
Fig. 6.
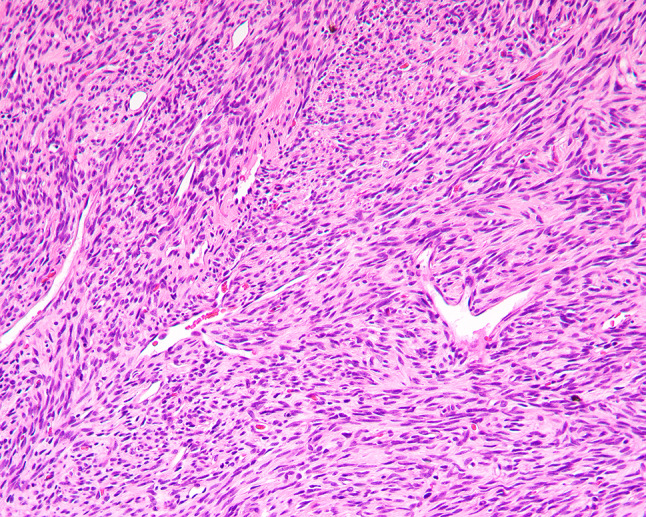
Cytologically bland spindle cells lacking nuclear atypia
Fig. 7.
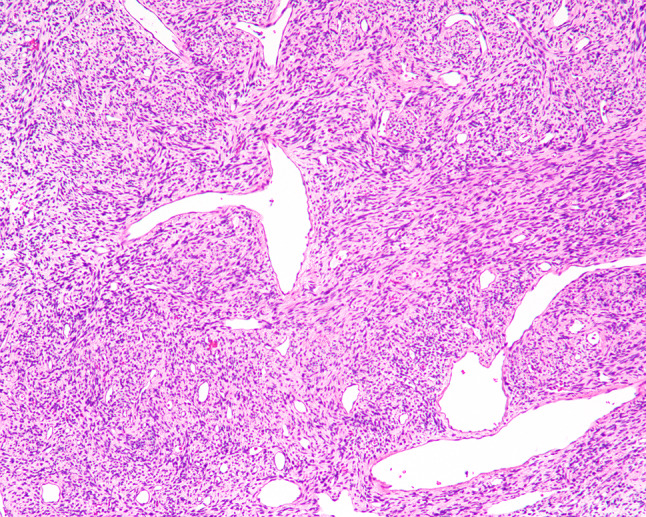
SFT with prominent branching vessels
Fig. 8.
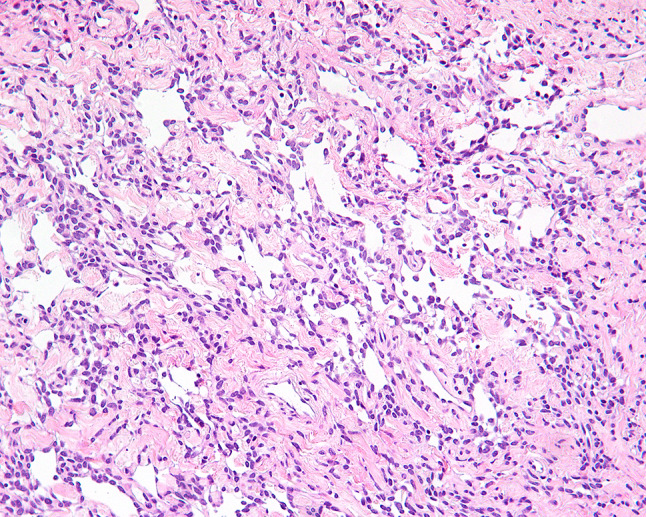
Pseudovascular spaces in SFT
All six SNT SFTs showed strong and diffuse nuclear expression of STAT6 (Fig. 9). CD34 had been performed at the time of initial diagnosis and was also positive in all cases. All three cases tested were positive for bcl-2. The tumors were negative for SMA (0/6), MSA (0/2), desmin (0/2), CD31 (0/2), pan-cytokeratin (0/4), and S-100 protein (0/3).
Fig. 9.
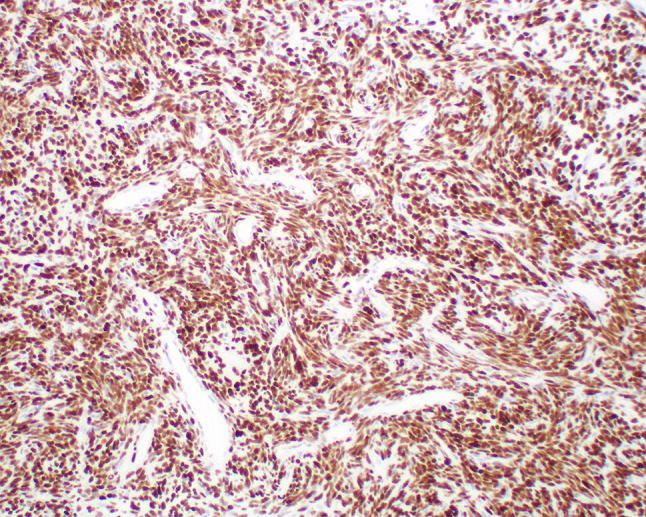
Strong, diffuse, nuclear STAT6 expression in SFT
All of the tumors were removed by endoscopic surgical excision only without additional treatment. Clinical follow up was available for all patients with a mean duration of 72 months (range 32–102 months). None of the patients developed local recurrence or metastasis and all were alive with no evidence of disease at last follow-up.
Discussion
Clinical Presentation
SFTs are well described in the head and neck region, but rarely involve the nasal cavity and paranasal sinuses. Review of the English literature yielded 86 cases of SFT arising in the SNT, most of which were represented by single case reports [1, 2, 10, 11, 15–66]. Table 2 summarizes the published clinicopathologic characteristics of SNT SFTs combined with this clinical series. While the present series of SNT SFTs was comprised predominantly of men, overall, males and females are affected equally, similar to the sex distribution observed when SFTs of all anatomic sites are considered [13]. There is a wide age range at presentation (18–79 years) with a mean of 49.0 years. Most patients presented clinically with nasal obstruction and/or epistaxis, with symptoms present for an average of 18.5 months. A minority of patients reported proptosis, epiphora, and visual field changes, which correlated with orbital involvement by the tumor [17, 27, 42, 51, 54, 62]. The majority of tumors were unilateral with a mean size of 4.7 cm, and involved the nasal cavity alone followed by combined involvement of the nasal cavity and paranasal sinuses. Rarely, tumors were observed to extend beyond the confines of the SNT at presentation, involving the skull base, orbit, or nasopharynx [17, 20, 27, 37, 39, 42, 44, 49–51, 54–56, 59, 62].
Table 2.
Literature summary and current cases of sinonasal tract solitary fibrous tumor
| Characteristicsa | Number (n = 92) |
|---|---|
| Sex | |
| Female | 48 |
| Male | 44 |
| Age (in years) | |
| Range | 18–79 |
| Mean | 49.0 |
| Symptom duration (in months) | |
| Range | 0.3–240 |
| Mean | 18.5 |
| Clinical presentation | |
| Obstructive symptoms | 55 |
| Epistaxis | 22 |
| Nasal discharge | 14 |
| Visual symptoms (blurred vision, epiphora, proptosis) | 12 |
| Headache | 9 |
| Anatomic site | |
| Nasal cavity alone | 45 |
| Paranasal sinus alone | 12 |
| Nasal cavity and paranasal sinuses | 35 |
| Skull base involvement | 7 |
| Orbital involvement | 6 |
| Laterality | |
| Right | 32 |
| Left | 25 |
| Bilateral | 2 |
| Tumor size (cm) | |
| Range | 0.4–9.0 |
| Mean | 4.7 |
| Patients with follow up (72) | |
| Alive, no evidence of disease | 70 |
| Alive, with disease | 2 |
| Patients with recurrence | 5 |
| Follow up (months) | |
| Range | 1–288 |
| Mean | 38.2 |
aNot stated in all cases
Pathologic Features
SNT SFTs display histologic features identical to those occurring in other anatomic sites. The tumor is characteristically composed of cytologically bland spindle cells set in a collagenous stroma. Variation in cellularity is a typical finding, with both hyper- and hypo-cellular areas identified. The spindle cells may be haphazardly arranged or exhibit a fascicular or storiform pattern of growth. The presence of a prominent vascular network is another characteristic feature, with dilated, branching, stellate, or staghorn shaped vessels. Histologic variants include mature adipose tissue within SFT (previously designated as lipomatous hemangiopericytoma) or pseudovascular spaces lined with multinucleated stromal giant cells (previously designated as giant cell angiofibroma). While well recognized at other soft tissue sites, only rare examples of fat-containing or giant cell rich SFTs affecting the SNT have been reported [2, 47].
Molecular Genetics and Immunohistochemistry
A recurrent NAB2–STAT6 gene fusion resulting from a paracentric inversion in chromosome 12q13 has been consistently and specifically associated with SFT irrespective of anatomic site [67–69]. Several different fusion variants have been identified, but in the SNT SFTs evaluated, the majority have harbored a NAB2–STAT6 gene fusion with the breakpoint between NAB2 exon 4 and STAT6 exon 2, which is the most common fusion variant detected when tumors of all sites are considered [2, 65].
By immunohistochemistry, SFTs consistently express CD34, as well as bcl-2 and CD99 [12–14, 70]. None of these markers, however, are entirely specific for SFT, as immunoreactivity can be seen in a variety of other mesenchymal neoplasms. SFTs are generally negative for cytokeratins, actins, desmin, and S-100 protein [70]. Recently, STAT6 has emerged as a more reliable marker for establishing a diagnosis of SFT. Nuclear expression of STAT6 protein is thought to reflect the presence of a NAB2–STAT6 gene fusion characteristic of these tumors. The sensitivity of STAT6 for a diagnosis of SFT exceeds 95% when strong and diffuse nuclear expression is considered positive [1, 2, 71–76]. STAT6 nuclear expression seems highly specific for SFT with only occasional (~ 2%) non SFT mesenchymal neoplasms (such as fibrous histiocytoma, desmoid tumor, and dedifferentiated liposarcoma) exhibiting both nuclear and cytoplasmic positivity [72–76], rather than just nuclear reactivity seen in SFT.
Differential Diagnosis
The histologic differential diagnosis for SFT in the SNT is quite broad, as many mesenchymal lesions affecting this region have a similar appearance characterized by spindled-shaped cells accompanied by a variably prominent background vascular network.
Sinonasal glomangiopericytoma, similar to SFT, is composed of uniform, cytologically bland spindle cells separated by numerous, ectatic, branching vessels [77]. Glomangiopericytoma, however, lacks the variable cellularity and dense hyalinized collagen, typical of SFT. The characteristic peritheliomatous hyalinization, extravasated erythrocytes and mast cells are not features of SFT. By immunohistochemistry, unlike SFT, glomangiopericytoma exhibits a myoid phenotype with diffuse positivity for smooth muscle and muscle specific actins, and lacks expression of CD34 and STAT6 [65, 72, 76, 77]. While nuclear accumulation of β-catenin is consistently present in glomangiopericytoma secondary to the presence of CTNNB1 mutations [78], it should be noted that this finding is not unique to glomangiopericytoma, as nuclear β-catenin expression has also been frequently observed in SNT SFT [79].
Nasopharyngeal angiofibroma has a distinctive clinical presentation, affecting only males, while SNT SFT typically occur in middle age adults. Nasopharyngeal angiofibroma is characterized by a fibrocollagenous stroma containing numerous variably sized, irregularly shaped vessels imparting an appearance that may resemble SFT. These neoplasms are, however, overall less cellular than SFTs, with the constituent stromal cells lacking CD34 and STAT6 immunoreactivity [2, 72, 76, 80]. The presence of androgen receptor positivity also helps to support a diagnosis of nasopharyngeal angiofibroma, as SFTs are typically negative for this marker [81].
Leiomyoma and angioleiomyoma are rare sinonasal tumors composed of intersecting fascicles of spindled tumor cells, the latter also associated with a prominent vascular component [82, 83]. Unlike SFT, the constituent spindle cells of angioleiomyoma are intimately associated with the vascular component of the tumor, encasing or merging with thick walled vascular channels. Immunohistochemically, in contrast with SFT, the spindle cells of leiomyoma and angioleiomyoma are positive for SMA, MSA, h-caldesmon and desmin, while negative for CD34 and STAT6 [2, 72, 74, 82].
Nerve sheath tumors such as schwannoma and neurofibroma occasionally involve the SNT and can be potentially confused with SFT [84, 85]. Schwannoma in particular shares several features with SFT including hypercellular and hypocellular areas as well as the presence of hyalinized blood vessels. Neural tumors can be readily distinguished from SFT by strong expression of S-100 protein and SOX10. Although neurofibromas contain some CD34 immunoreactive cells, both schwannoma and neurofibroma lack STAT6 positivity [2, 71–76, 84].
Synovial sarcoma, similar to SFT, is composed of uniform spindle cells and can exhibit collagen bands or a branching vascular pattern in a subset of cases [86, 87]. Synovial sarcoma typically has a more densely cellular appearance with alternating light and dark-staining areas (marbling) and unlike SFT, shows variable keratin and EMA expression, but is negative for both CD34 and STAT6 [2, 71–76]. TLE1 is often used as a biomarker for distinguishing synovial sarcoma from other soft tissue neoplasms, however weak nuclear expression has been observed in a minority of SFTs [88].
Although commonly regarded as a small round cell neoplasm, mesenchymal chondrosarcoma is also included among the differential diagnostic considerations of SNT SFT due to the occasional spindle shaped morphology of the nuclei and characteristic peritheliomatous arrangement of the lesional cells around sinusoidal vascular spaces [89]. Mesenchymal chondrosarcoma is readily distinguished from SFT by the presence of foci of cartilaginous tissue. In addition, these tumors do not express CD34 or STAT6, but are CD99 and Sox9 reactive [71, 74, 76].
Biphenotypic sinonasal sarcoma is a low grade spindle cell neoplasm unique to the SNT, which bears some morphologic resemblance to SFT [90]. Although biphenotypic sinonasal sarcoma is comprised of cytologically low grade spindle cells and frequently displays branching staghorn type vessels, the tumor is uniformly hypercellular and lacks the collagenous stroma of SFT. The presence of entrapped invaginations of hyperplastic surface respiratory epithelium is another distinguishing feature. The immunohistochemical features of biphenotypic sinonasal sarcoma are distinctive, with tumors exhibiting immunoreactivity for S-100 protein and actins indicative of neural and myogenic differentiation. Furthermore, frequent nuclear β-catenin expression, although often focal and of weak to moderate intensity, along with a negative SOX10 are characteristic of this tumor [91]. Although CD34 expression may occur in a subset of biphenotypic sinonasal sarcomas, it is typically focal in nature, in contrast with the diffuse positivity typical of SFT. In addition, biphenotypic sinonasal sarcomas have been shown to lack STAT6 expression [2].
Treatment and Prognosis
Surgery is the preferred treatment for SNT SFTs. Complete surgical removal of the tumor is optimal, however pathologic evaluation of margins is often difficult as endoscopic surgical approaches are frequently employed in this anatomic region, with tumors resected in a piecemeal fashion. It is thus often left to the surgeon to determine whether adequate excision of the tumor has been performed. Additional therapy beyond surgical resection is generally not required for the majority of SNT SFTs, although radiation and chemotherapy have been employed to manage locally advanced or recurrent disease in individual cases [11, 48, 51].
When all anatomic sites are considered, most SFTs follow an indolent clinical course, with disease progression in the form of local recurrence or metastasis only observed in a small minority of cases [3, 6–9]. Histologic criteria for malignancy are not well defined, though the World Health Organization categorizes SFT as malignant based on the presence of hypercellularity, increased mitoses (> 4 mitoses per 10 high power fields), cytological atypia, tumor necrosis, and/or infiltrative margins [92]. Many of these parameters have been shown to be significantly associated with a higher risk of recurrence and decreased survival [3, 6–9]. SFTs exhibiting a high grade sarcomatous component (dedifferentiated SFT) are also considered malignant [93]. Histology is an imperfect predictor of clinical behavior, however, as occasional tumors classified as malignant based on morphologic parameters will behave in a benign manner [6, 94, 95], while conversely, recurrence and metastases are well documented in SFTs lacking atypical histologic features [5, 6, 8, 95, 96]. In addition to histology, patient age (≥ 55 years), tumor size (> 10 or ≥ 15 cm), and margin status have also demonstrated utility in predicting clinical outcome [6, 7, 9]. Various risk stratification models based on combinations of patient age, tumor size, and mitotic index [7], or mitotic rate, cellularity, and pleomorphism [3] have been proposed as a method of more accurately predicting behavior of SFTs.
SNT SFTs also appear to show variable biologic behavior, though overall prognosis is considered favorable. Among 72 reported cases of SNT SFT with available follow-up data, there were no instances of metastasis or death due to disease. Five patients developed local recurrence at 3–69 months after resection [43, 51–54]. None of these tumors exhibited any atypical morphologic features at the time of presentation, though an increased mitotic rate was observed in the recurrences of two cases [51, 54]. Among all anatomic sites, the incidence rate of histologically malignant SFT is approximately 10–20% [6, 9, 95, 97]. Malignant examples of SFT of the SNT are rare, with eight cases reported to date [1, 2, 11, 48, 50, 62, 63]. Seven were classified as such based on the presence of increased mitotic rate (> 4 mitoses per 10 high power fields) with or without hypercellularity, nuclear atypia, and necrosis [1, 2, 11, 48, 62, 63], while the remaining tumor was a histologically dedifferentiated SFT [50]. Follow up of a relatively short duration was reported in six of the cases; four patients were alive with no evidence of disease at 10, 12, 16 and 23 months, respectively [2, 11, 63], one patient died from postoperative complications related to colon cancer 5 weeks after resection of the SFT [62], and one patient was alive with disease at 54 months [48]. As with other anatomic sites, it is difficult to reliably predict prognosis of SNT SFTs. Although most will behave in a benign fashion, it should be recognized that a small potential for recurrence exists, even for those tumors lacking worrisome histologic features. While evidence from other anatomic regions has shown the presence of atypical morphologic features in SFTs to usually correlate with adverse outcomes [5–8, 95] the low number of malignant cases and relatively short reported follow up duration precludes meaningful analysis of the prognostic impact of malignant histology in SNT SFTs.
Conclusion
SFTs rarely originate within the SNT. Patients are typically middle aged with males and females equally affected. The most common presenting symptoms are nasal obstruction and epistaxis. The variable histologic appearance of SFT may lead to difficulties in identifying these neoplasms based on morphologic features alone, though nuclear expression of STAT6 allows distinction from other sinonasal spindle cell tumors in the differential diagnosis. The tumors are managed by complete surgical excision. The present series combined with prior reported cases suggests that SNT SFTs are indolent neoplasms associated with a good prognosis. Clinically malignant behavior is rare, and is not necessarily predicted by morphologic features. There is a low potential for disease recurrence, necessitating close clinical surveillance.
Acknowledgements
The views expressed are those of the authors solely and do not represent endorsement from Southern California Permanente Medical Group.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB #5968), which did not require informed consent.
References
- 1.Han Y, Zhang Q, Yu X, Han X, Wang H, Xu Y, et al. Immunohistochemical detection of STAT6, CD34, CD99 and BCL-2 for diagnosing solitary fibrous tumors/hemangiopericytomas. Int J Clin Exp Pathol. 2015;8(10):13166–13175. [PMC free article] [PubMed] [Google Scholar]
- 2.Kao YC, Lin PC, Yen SL, Huang SC, Tsai JW, Li CF, et al. Clinicopathological and genetic heterogeneity of the head and neck solitary fibrous tumours: a comparative histological, immunohistochemical and molecular study of 36 cases. Histopathology. 2016;68(4):492–501. doi: 10.1111/his.12772. [DOI] [PubMed] [Google Scholar]
- 3.Pasquali S, Gronchi A, Strauss D, Bonvalot S, Jeys L, Stacchiotti S, et al. Resectable extra-pleural and extra-meningeal solitary fibrous tumours: a multi-centre prognostic study. Eur J Surg Oncol. 2016;42(7):1064–1070. doi: 10.1016/j.ejso.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Wushou A, Jiang YZ, Liu YR, Shao ZM. The demographic features, clinicopathologic characteristics, treatment outcome and disease-specific prognostic factors of solitary fibrous tumor: a population-based analysis. Oncotarget. 2015;6(39):41875–41883. doi: 10.18632/oncotarget.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeVito N, Henderson E, Han G, Reed D, Bui MM, Lavey R, et al. Clinical characteristics and outcomes for solitary fibrous tumor (SFT): a single center experience. PLoS ONE. 2015;10(10):e0140362. doi: 10.1371/journal.pone.0140362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94(4):1057–1068. doi: 10.1002/cncr.10328. [DOI] [PubMed] [Google Scholar]
- 7.Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25(9):1298–1306. doi: 10.1038/modpathol.2012.83. [DOI] [PubMed] [Google Scholar]
- 8.Wilky BA, Montgomery EA, Guzzetta AA, Ahuja N, Meyer CF. Extrathoracic location and “borderline” histology are associated with recurrence of solitary fibrous tumors after surgical resection. Ann Surg Oncol. 2013;20(13):4080–4089. doi: 10.1245/s10434-013-3241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Houdt WJ, Westerveld CM, Vrijenhoek JE, van Gorp J, van Coevorden F, Verhoef C, et al. Prognosis of solitary fibrous tumors: a multicenter study. Ann Surg Oncol. 2013;20(13):4090–4095. doi: 10.1245/s10434-013-3242-9. [DOI] [PubMed] [Google Scholar]
- 10.Cox DP, Daniels T, Jordan RC. Solitary fibrous tumor of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(1):79–84. doi: 10.1016/j.tripleo.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Ganly I, Patel SG, Stambuk HE, Coleman M, Ghossein R, Carlson D, et al. Solitary fibrous tumors of the head and neck: a clinicopathologic and radiologic review. Arch Otolaryngol Head Neck Surg. 2006;132(5):517–525. doi: 10.1001/archotol.132.5.517. [DOI] [PubMed] [Google Scholar]
- 12.Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. 2006;48(1):63–74. doi: 10.1111/j.1365-2559.2005.02290.x. [DOI] [PubMed] [Google Scholar]
- 13.Thway K, Ng W, Noujaim J, Jones RL, Fisher C. The current status of solitary fibrous tumor: diagnostic features, variants, and genetics. Int J Surg Pathol. 2016;24(4):281–292. doi: 10.1177/1066896915627485. [DOI] [PubMed] [Google Scholar]
- 14.Chan JK. Solitary fibrous tumour—everywhere, and a diagnosis in vogue. Histopathology. 1997;31(6):568–576. doi: 10.1046/j.1365-2559.1997.2400897.x. [DOI] [PubMed] [Google Scholar]
- 15.Varghese L, Mehan R, Lukka V, Mukhopadhyay S. Solitary fibrous tumor: an unusual nasal cavity tumor. J Clin Case Rep. 2016;6(6):824. doi: 10.4172/2165-7920.1000824. [DOI] [Google Scholar]
- 16.Agale SV, Arole VC, Jadhav DB, Juvekar JV. Solitary fibrous tumor of nasal cavity: a rare case report with review of literature. Int J Res Med Sci. 2015;3(10):2877–2879. doi: 10.18203/2320-6012.ijrms20150847. [DOI] [Google Scholar]
- 17.Namon A. Solitary fibrous tumor of the sinonasal tract: case report and review of the literature. Am J Rhinol. 1992;6(4):135–137. doi: 10.2500/105065892781874658. [DOI] [Google Scholar]
- 18.Dnyaneshwar A, Smita N, Jagade M, Agarwal S, Joshi S, Kashide R, et al. Solitary fibrous tumor in the maxillary sinus treated by caldwell luc surgery. Open Access Sci Rep. 2013;2(2):631. [Google Scholar]
- 19.Janjua A, Sklar M, Macmillan C, Vescan A, Witterick IJ. Endoscopic resection of solitary fibrous tumors of the nose and paranasal sinuses. Skull Base. 2011;21(2):129–134. doi: 10.1055/s-0031-1275259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turkoglu E, Dolgun H, Kazanci B, Yilmaz ER, Kahveci R, Sekerci Z. Solitary fibrous tumour of the ethmoid sinuses and anterior fossa. BMJ Case Rep. 2009. [DOI] [PMC free article] [PubMed]
- 21.Takasaki K, Watanabe T, Hayashi T, Kinoshita N, Kumagami H, Takahashi H. Solitary fibrous tumor arising from the sphenoid sinus. Case Rep Med. 2009;2009:316042. doi: 10.1155/2009/316042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stringfellow HF, Khan IA, Sissons MC. Solitary fibrous tumour arising in the nasal cavity: report of a case. J Laryngol Otol. 1996;110(5):468–470. doi: 10.1017/S0022215100134000. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinidis I, Triaridis S, Triaridis A, Pantzaki A. A rare case of solitary fibrous tumor of the nasal cavity. Auris Nasus Larynx. 2003;30(3):303–305. doi: 10.1016/S0385-8146(03)00064-6. [DOI] [PubMed] [Google Scholar]
- 24.Rajan KV, Santhi T. A case of solitary fibrous tumour of the nose and paranasal sinuses. Indian J Otolaryngol Head Neck Surg. 2006;58(3):316–318. doi: 10.1007/BF03050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jurado-Ramos A, Ropero Romero F, Cantillo Banos E, Salas Molina J. Minimally invasive endoscopic techniques for treating large, benign processes of the nose, paranasal sinus, and pterygomaxillary and infratemporal fossae: solitary fibrous tumour. J Laryngol Otol. 2009;123(4):457–461. doi: 10.1017/S0022215108002132. [DOI] [PubMed] [Google Scholar]
- 26.Rizzo S, Giunta AA, Pennacchi A. Sinonasal and rhinopharyngeal solitary fibrous tumour: a case report and review of the literature. Acta Otorhinolaryngol Ital. 2015;35(6):455–458. doi: 10.14639/0392-100X-163813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith LM, Osborne RF. Solitary fibrous tumor of the maxillary sinus. Ear Nose Throat J. 2007;86(7):382–383. doi: 10.1177/014556130708600709. [DOI] [PubMed] [Google Scholar]
- 28.Kessler A, Lapinsky J, Berenholz L, Sarfaty S, Segal S. Solitary fibrous tumor of the nasal cavity. Otolaryngol Head Neck Surg. 1999;121(6):826–828. doi: 10.1053/hn.1999.v121.a95230. [DOI] [PubMed] [Google Scholar]
- 29.Kodama S, Fujita K, Suzuki M. Solitary fibrous tumor in the maxillary sinus treated by endoscopic medial maxillectomy. Auris Nasus Larynx. 2009;36(1):100–103. doi: 10.1016/j.anl.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Mathew GA, Ashish G, Tyagi AK, Chandrashekharan R, Paul RR. Solitary fibrous tumor of nasal cavity: a case report. Iran J Otorhinolaryngol. 2015;27(81):307–312. [PMC free article] [PubMed] [Google Scholar]
- 31.Pasquini E, Cantaroni C, Salfi N, Tamburini G, Marchi C, Sciarretta V. Endoscopic treatment of an ethmoidal solitary fibrous tumour. J Laryngol Otol. 2003;117(11):889–891. doi: 10.1258/002221503322542926. [DOI] [PubMed] [Google Scholar]
- 32.Chauhan SS, Krishnan J, Heffner DK. Solitary fibrous tumor of nasal cavity in patient with long-standing history of cocaine inhalation. Arch Pathol Lab Med. 2004;128(1):e1–e4. doi: 10.5858/2004-128-e1-SFTONC. [DOI] [PubMed] [Google Scholar]
- 33.Corina L, Volante M, Carconi M, Contucci AM. An unusual solitary fibrous tumor after sphenoethmoidectomy. Otolaryngol Head Neck Surg. 2006;134(6):1063–1065. doi: 10.1016/j.otohns.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 34.Vermeulen S, Ketels P, Salgado R, Creytens D, Vanderveken OM, Claes J. Solitary fibrous tumour of the nasal cavity: a case report and literature review. B-ENT. 2012;8(3):219–223. [PubMed] [Google Scholar]
- 35.Alobid I, Alos L, Blanch JL, Benitez P, Bernal-Sprekelsen M, Mullol J. Solitary fibrous tumour of the nasal cavity and paranasal sinuses. Acta Otolaryngol. 2003;123(1):71–74. doi: 10.1080/003655402000028052. [DOI] [PubMed] [Google Scholar]
- 36.Nai GA, Ramalho Neto GC. Solitary fibrous tumor of the nasal cavity. Braz J Otorhinolaryngol. 2009;75(5):769. doi: 10.1016/S1808-8694(15)30535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohmura T, Nakashima T, Hasegawa Y, Matsuura H. Solitary fibrous tumor of the paranasal sinuses. Eur Arch Otorhinolaryngol. 1999;256(5):233–236. doi: 10.1007/s004050050148. [DOI] [PubMed] [Google Scholar]
- 38.Zielinska-Kazmierska B, Grodecka J, Szyszkowski A. Solitary fibrous tumor of the nasal cavity and paranasal sinuses: a case report. J Oral Biol Craniofac Res. 2015;5(2):112–116. doi: 10.1016/j.jobcr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eloy PH, Nollevaux MC, Watelet JB, Van Damme JP, Collet ST, Bertrand B. Endonasal endoscopic resection of an ethmoidal solitary fibrous tumor. Eur Arch Otorhinolaryngol. 2006;263(9):833–837. doi: 10.1007/s00405-006-0073-3. [DOI] [PubMed] [Google Scholar]
- 40.Fujikura T, Ishida M, Sekine K, Aoki H, Okubo K. Solitary fibrous tumor arising from the superior nasal turbinate: a case report. J Nippon Med Sch. 2012;79(5):373–376. doi: 10.1272/jnms.79.373. [DOI] [PubMed] [Google Scholar]
- 41.Abe T, Murakami A, Inoue T, Ohde S, Yamaguchi T, Watanabe K. Solitary fibrous tumor arising in the sphenoethmoidal recess: a case report and review of the literature. Auris Nasus Larynx. 2005;32(3):285–289. doi: 10.1016/j.anl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Furze AD, Peng Y, Myers LL. Pathology case quiz 2. Solitary fibrous tumor of the nasal cavity and ethmoid sinus with intracranial extension. Arch Otolaryngol Head Neck Surg. 2008;134(3):334, 336–7. doi: 10.1001/archotol.134.3.334. [DOI] [PubMed] [Google Scholar]
- 43.Morales-Cadena M, Zubiaur FM, Alvarez R, Madrigal J, Zarate-Osorno A. Solitary fibrous tumor of the nasal cavity and paranasal sinuses. Otolaryngol Head Neck Surg. 2006;135(6):980–982. doi: 10.1016/j.otohns.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 44.Kim TA, Brunberg JA, Pearson JP, Ross DA. Solitary fibrous tumor of the paranasal sinuses: CT and MR appearance. AJNR Am J Neuroradiol. 1996;17(9):1767–1772. [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HJ, Lee HK, Seo JJ, Shin JH, Jeong AK, Lee JH, et al. MR imaging of solitary fibrous tumors in the head and neck. Korean J Radiol. 2005;6(3):136–142. doi: 10.3348/kjr.2005.6.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morimitsu Y, Nakajima M, Hisaoka M, Hashimoto H. Extrapleural solitary fibrous tumor: clinicopathologic study of 17 cases and molecular analysis of the p53 pathway. Apmis. 2000;108(9):617–625. doi: 10.1034/j.1600-0463.2000.d01-105.x. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen GP, Dickersin GR, Provenzal JM, Rosenberg AE. Lipomatous hemangiopericytoma. A histologic, ultrastructural and immunohistochemical study of a unique variant of hemangiopericytoma. Am J Surg Pathol. 1995;19(7):748–756. doi: 10.1097/00000478-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Xue Y, Chai G, Xiao F, Wang N, Mu Y, Wang Y, et al. Post-operative radiotherapy for the treatment of malignant solitary fibrous tumor of the nasal and paranasal area. Jpn J Clin Oncol. 2014;44(10):926–931. doi: 10.1093/jjco/hyu100. [DOI] [PubMed] [Google Scholar]
- 49.Hicks DL, Moe KS. Nasal solitary fibrous tumor arising from the anterior cranial fossa. Skull Base. 2004;14(4):203–207. doi: 10.1055/s-2004-860951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramaniam MM, Lim XY, Venkateswaran K, Shuen CS, Soong R, Petersson F. Dedifferentiated solitary fibrous tumour of the nasal cavity: the first case reported with molecular characterization of a TP53 mutation. Histopathology. 2011;59(6):1269–1274. doi: 10.1111/j.1365-2559.2011.03997.x. [DOI] [PubMed] [Google Scholar]
- 51.Roy S, Mallick S, Kakkar A, Jana M, Julka PK. Recurrent malignant sino-nasal solitary fibrous tumor: eliminate the enemy at the first instance. J Cancer Res Ther. 2015;11:650. doi: 10.4103/0973-1482.138045. [DOI] [PubMed] [Google Scholar]
- 52.Insabato L, Siano M, Somma A, Gentile R, Santangelo M, Pettinato G. Extrapleural solitary fibrous tumor: a clinicopathologic study of 19 cases. Int J Surg Pathol. 2009;17(3):250–254. doi: 10.1177/1066896909333779. [DOI] [PubMed] [Google Scholar]
- 53.Bowe SN, Wakely PE, Jr, Ozer E. Head and neck solitary fibrous tumors: diagnostic and therapeutic challenges. Laryngoscope. 2012;122(8):1748–1755. doi: 10.1002/lary.23350. [DOI] [PubMed] [Google Scholar]
- 54.Kunzel J, Hainz M, Ziebart T, Pitz S, Ihler F, Strieth S, et al. Head and neck solitary fibrous tumors: a rare and challenging entity. Eur Arch Otorhinolaryngol. 2016;273(6):1589–1598. doi: 10.1007/s00405-015-3670-1. [DOI] [PubMed] [Google Scholar]
- 55.Witkin GB, Rosai J. Solitary fibrous tumor of the upper respiratory tract. A report of six cases. Am J Surg Pathol. 1991;15(9):842–848. doi: 10.1097/00000478-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Cassarino DS, Auerbach A, Rushing EJ. Widely invasive solitary fibrous tumor of the sphenoid sinus, cavernous sinus, and pituitary fossa. Ann Diagn Pathol. 2003;7(3):169–173. doi: 10.1016/S1092-9134(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 57.Zukerberg LR, Rosenberg AE, Randolph G, Pilch BZ, Goodman ML. Solitary fibrous tumor of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1991;15(2):126–130. doi: 10.1097/00000478-199102000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Ge W, Yu DC, Chen G, Ding YT. Clinical analysis of 47 cases of solitary fibrous tumor. Oncol Lett. 2016;12(4):2475–2480. doi: 10.3892/ol.2016.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fukunaga M, Ushigome S, Nomura K, Ishikawa E. Solitary fibrous tumor of the nasal cavity and orbit. Pathol Int. 1995;45(12):952–957. doi: 10.1111/j.1440-1827.1995.tb03421.x. [DOI] [PubMed] [Google Scholar]
- 60.Mentzel T, Bainbridge TC, Katenkamp D. Solitary fibrous tumour: clinicopathological, immunohistochemical, and ultrastructural analysis of 12 cases arising in soft tissues, nasal cavity and nasopharynx, urinary bladder and prostate. Virchows Arch. 1997;430(6):445–453. doi: 10.1007/s004280050054. [DOI] [PubMed] [Google Scholar]
- 61.Brunnemann RB, Ro JY, Ordonez NG, Mooney J, El-Naggar AK, Ayala AG. Extrapleural solitary fibrous tumor: a clinicopathologic study of 24 cases. Mod Pathol. 1999;12(11):1034–1042. [PubMed] [Google Scholar]
- 62.Zeitler DM, Kanowitz SJ, Har-El G. Malignant solitary fibrous tumor of the nasal cavity. Skull Base. 2007;17(4):239–246. doi: 10.1055/s-2007-984489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papadakis I, Koudounarakis E, Haniotis V, Karatzanis A, Velegrakis G. Atypical solitary fibrous tumor of the nose and maxillary sinus. Head Neck. 2013;35(3):E77–E79. doi: 10.1002/hed.21909. [DOI] [PubMed] [Google Scholar]
- 64.Fukunaga M, Naganuma H, Nikaido T, Harada T, Ushigome S. Extrapleural solitary fibrous tumor: a report of seven cases. Mod Pathol. 1997;10(5):443–450. [PubMed] [Google Scholar]
- 65.Agaimy A, Barthelmess S, Geddert H, Boltze C, Moskalev EA, Koch M, et al. Phenotypical and molecular distinctness of sinonasal haemangiopericytoma compared to solitary fibrous tumour of the sinonasal tract. Histopathology. 2014;65(5):667–673. doi: 10.1111/his.12452. [DOI] [PubMed] [Google Scholar]
- 66.Yang BT, Song ZL, Wang YZ, Dong JY, Wang ZC. Solitary fibrous tumor of the sinonasal cavity: CT and MR imaging findings. AJNR Am J Neuroradiol. 2013;34(6):1248–1251. doi: 10.3174/ajnr.A3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chmielecki J, Crago AM, Rosenberg M, O’Connor R, Walker SR, Ambrogio L, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45(2):131–132. doi: 10.1038/ng.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45(2):180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohajeri A, Tayebwa J, Collin A, Nilsson J, Magnusson L, von Steyern FV, et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosom Cancer. 2013;52(10):873–886. doi: 10.1002/gcc.22083. [DOI] [PubMed] [Google Scholar]
- 70.Geramizadeh B, Marzban M, Churg A. Role of immunohistochemistry in the diagnosis of solitary fibrous tumor, a review. Iran J Pathol. 2016;11(3):195–203. [PMC free article] [PubMed] [Google Scholar]
- 71.Cheah AL, Billings SD, Goldblum JR, Carver P, Tanas MZ, Rubin BP. STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology. 2014;46(5):389–395. doi: 10.1097/PAT.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 72.Demicco EG, Harms PW, Patel RM, Smith SC, Ingram D, Torres K, et al. Extensive survey of STAT6 expression in a large series of mesenchymal tumors. Am J Clin Pathol. 2015;143(5):672–682. doi: 10.1309/AJCPN25NJTOUNPNF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27(3):390–395. doi: 10.1038/modpathol.2013.164. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38(4):552–559. doi: 10.1097/PAS.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 75.Koelsche C, Schweizer L, Renner M, Warth A, Jones DT, Sahm F, et al. Nuclear relocation of STAT6 reliably predicts NAB2-STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology. 2014;65(5):613–622. doi: 10.1111/his.12431. [DOI] [PubMed] [Google Scholar]
- 76.Tai HC, Chuang IC, Chen TC, Li CF, Huang SC, Kao YC, et al. NAB2-STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol. 2015;28(10):1324–1335. doi: 10.1038/modpathol.2015.90. [DOI] [PubMed] [Google Scholar]
- 77.Thompson LD, Miettinen M, Wenig BM. Sinonasal-type hemangiopericytoma: a clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Am J Surg Pathol. 2003;27(6):737–749. doi: 10.1097/00000478-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 78.Lasota J, Felisiak-Golabek A, Aly FZ, Wang ZF, Thompson LD, Miettinen M. Nuclear expression and gain-of-function β-catenin mutation in glomangiopericytoma (sinonasal-type hemangiopericytoma): insight into pathogenesis and a diagnostic marker. Mod Pathol. 2015;28(5):715–720. doi: 10.1038/modpathol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jo VY, Fletcher CDM. Nuclear β-catenin expression is frequent in sinonasal hemangiopericytoma and its mimics. Head Neck Pathol. 2017;11(2):119–123. doi: 10.1007/s12105-016-0737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sánchez-Romero C, Carlos R, Díaz Molina JP, Thompson LDR, de Almeida OP, Rumayor Piña A. Nasopharyngeal angiofibroma: a clinical, histopathological and immunohistochemical study of 42 cases with emphasis on stromal features. Head Neck Pathol. 2017 doi: 10.1007/s12105-017-0824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bongiovanni M, Viberti L, Pecchioni C, Papotti M, Thonhofer R, Hans Popper H, Sapino A. Steroid hormone receptor in pleural solitary fibrous tumours and CD34 + progenitor stromal cells. J Pathol. 2002;198(2):252–257. doi: 10.1002/path.1195. [DOI] [PubMed] [Google Scholar]
- 82.Agaimy A, Michal M, Thompson LD. Angioleiomyoma of the sinonasal tract: analysis of 16 cases and review of the literature. Head Neck Pathol. 2015;9(4):463–473. doi: 10.1007/s12105-015-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang HY, Antonescu CR. Sinonasal smooth muscle cell tumors: a clinicopathologic and immunohistochemical analysis of 12 cases with emphasis on the low-grade end of the spectrum. Arch Pathol Lab Med. 2003;127(3):297–304. doi: 10.5858/2003-127-0297-SSMCT. [DOI] [PubMed] [Google Scholar]
- 84.Azani AB, Bishop JA, Thompson LD. Sinonasal tract neurofibroma: a clinicopathologic series of 12 cases with a review of the literature. Head Neck Pathol. 2015;9(3):323–333. doi: 10.1007/s12105-014-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buob D, Wacrenier A, Chevalier D, Aubert S, Quinchon JF, Gosselin B, et al. Schwannoma of the sinonasal tract: a clinicopathologic and immunohistochemical study of 5 cases. Arch Pathol Lab Med. 2003;127(9):1196–1199. doi: 10.5858/2003-127-1196-SOTSTA. [DOI] [PubMed] [Google Scholar]
- 86.Subramaniam MM, Shuen CS, Petersson F. Poorly differentiated synovial sarcoma of the sphenoid sinus: report of the first case and review of synovial sarcomas of the sinonasal tract. Histopathology. 2012;61(6):1232–1237. doi: 10.1111/j.1365-2559.2012.04340.x. [DOI] [PubMed] [Google Scholar]
- 87.Thway K, Fisher C. Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol. 2014;18(6):369–380. doi: 10.1016/j.anndiagpath.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Foo WC, Cruise MW, Wick MR, Hornick JL. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol. 2011;135(6):839–844. doi: 10.1309/AJCP45SSNAOPXYXU. [DOI] [PubMed] [Google Scholar]
- 89.Knott PD, Gannon FH, Thompson LD. Mesenchymal chondrosarcoma of the sinonasal tract: a clinicopathological study of 13 cases with a review of the literature. Laryngoscope. 2003;113(5):783–790. doi: 10.1097/00005537-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Lewis JT, Oliveira AM, Nascimento AG, Schembri-Wismayer D, Moore EA, Olsen KD, et al. Low-grade sinonasal sarcoma with neural and myogenic features: a clinicopathologic analysis of 28 cases. Am J Surg Pathol. 2012;36(4):517–525. doi: 10.1097/PAS.0b013e3182426886. [DOI] [PubMed] [Google Scholar]
- 91.Rooper LM, Huang SC, Antonescu CR, Westra WH, Bishop JA. Biphenotypic sinonasal sarcoma: an expanded immunoprofile including consistent nuclear β-catenin positivity and absence of SOX10 expression. Hum Pathol. 2016;55:44–50. doi: 10.1016/j.humpath.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fletcher CDM, Bridge JA, Lee J-C. Extrapleural solitary fibrous tumour. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO Classification of tumours of soft tissue and bone. Lyon: IARC; 2013. pp. 80–82. [Google Scholar]
- 93.Mosquera JM, Fletcher CD. Expanding the spectrum of malignant progression in solitary fibrous tumors: a study of 8 cases with a discrete anaplastic component–is this dedifferentiated SFT? Am J Surg Pathol. 2009;33(9):1314–1321. doi: 10.1097/PAS.0b013e3181a6cd33. [DOI] [PubMed] [Google Scholar]
- 94.Nielsen GP, O’Connell JX, Dickersin GR, Rosenberg AE. Solitary fibrous tumor of soft tissue: a report of 15 cases, including 5 malignant examples with light microscopic, immunohistochemical, and ultrastructural data. Mod Pathol. 1997;10(10):1028–1037. [PubMed] [Google Scholar]
- 95.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22(12):1501–1511. doi: 10.1097/00000478-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Hasegawa T, Matsuno Y, Shimoda T, Hasegawa F, Sano T, Hirohashi S. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol. 1999;30(12):1464–1473. doi: 10.1016/S0046-8177(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 97.Barthelmeß S, Geddert H, Boltze C, Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A, Agaimy A, Haller F. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol. 2014;184(4):1209–1218. doi: 10.1016/j.ajpath.2013.12.016. [DOI] [PubMed] [Google Scholar]


