Abstract
Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplastic proliferation with variable clinical behavior caused by the accumulation of CD1a+/CD207+ histiocytes, associated with a variable number of eosinophils, lymphocytes, plasma cells and multinucleated giant cells, most commonly observed in male children. LCH is uncommon in the head and neck region, occurring as ulcerated and reddened plaques or nodules that cause destruction of adjacent soft tissues and bone. The exact etiology of LCH is still unknown and controversial, with possible etiologic role of viruses, including Epstein–Barr virus (EBV). The aim of this study was to describe the clinicopathologic and immunohistochemical features of patients with LCH of the head and neck region. Clinical data from 19 patients with LCH were obtained from the archives of the Federal University of Rio de Janeiro and the Clinical Head and Neck Center of Guatemala. All cases were submitted to morphological, immunohistochemical analysis with CD1a, CD207, CD3, CD20, CD68, S-100 and Ki-67 and in situ hybridization for EBV. Ten cases were female and 9 male, with mean age of 11.5 years. Fourteen cases were located in the oral cavity, three cases in lymph nodes, and two cases in the scalp. In regard to the oral lesions, 13 cases were intra-osseous with six cases in anterior mandible, five cases in posterior mandible, and two cases in posterior maxilla while one case was located exclusively in the gingiva. The inflammatory pattern showed variation in the number of plasma cells, eosinophils and lymphocytes, while tumor cells were positive for CD1a, S-100 and CD68 in all cases, and positive for CD207 in 18 cases. In situ hybridization for EBV were negative in all cases.
Keywords: Langerhans cell histiocytosis, Immunohistochemistry, Head and neck, Oral cavity
Introduction
Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplastic proliferation with variable clinical behavior caused by the accumulation of CD1a+/CD207+ histiocytes in an inflammatory background [1–4]. It is most common in children from 1 to 3 years and the clinical presentation depends on the extension and location of the lesions, and therefore treatment may vary from conservative curettage for patients with single-bone lesions, to chemotherapy for patients with disseminated involvement of liver, bone marrow and/or spleen [4, 5].
LCH may affect the head and neck, particularly the oral and maxillofacial region, where is usually identified as an ill-defined unilocular radiolucency with tooth displacement or premature tooth loss [6–8]. Oral soft tissue lesions appear as a painful reddish swelling or ulcer in the gingiva and hard palate, which may be accompanied by enlargement of the lymph nodes, diabetes insipidus, skin lesions, and hepatomegaly [7, 9]. The diagnosis of LCH depends on the microscopic identification of histiocytes with abundant eosinophilic cytoplasm and lobulated or coffee bean-like nucleus containing delicate chromatin and inconspicuous nucleoli admixed with variable number of eosinophils, lymphocytes, neutrophils, plasma cells and multinucleated giant cells associated with immunohistochemical study [1, 10]. The histiocytes are consistently positive for CD1a and CD207, an immunophenotype shared by normal epidermal Langerhans cells [11]. Recent studies indicate the origin of LCH as a misguided differentiation of myeloid dendritic cell precursors through activation of ERK signaling pathway [4]. However, the exact etiology of LCH is still unknown and controversial, with possible etiologic roles of viruses, including Epstein–Barr virus (EBV) [12–21].
To the best of our knowledge there are only a few case series of head and neck LCH [22–27], most of them from developed countries, with limited immunohistochemical findings (Table 1). Thus, the aim of this study is to describe the clinicopathological features of 19 patients with LCH of the head and neck region from Latin America with emphasis on the oral cavity, including immunohistochemical findings and in situ hybridization for EBV.
Table 1.
Summarized data about clinicopathologic features of large case series of head and neck LCH published in the English-language literature
| Authors, year | Country | N | Gender (M/F) | Mean age (range) | Site | Immunoprofile | Follow-up |
|---|---|---|---|---|---|---|---|
| Dagenais et al., 1992 [28] | United States | 29 | 23/4 | 17.3 years (2–45 years) | 28 Mand, 1 Max | NR | NR |
| Eckardt et al., 2003 [26] | Germany | 10 | 8/2 | 26.2 years (13–42 years) | 9 Mand, 5 Max, 1 Parietal bone | NR | All patients alive |
| Mínguez et al., 2004 [8] | Spain | 10 | 6/4 | 1.7 years (4 m—3.2 years) | 6 Max, 2 Mand, 2 Unspecified | NR | 6 recurrences, 1DOD |
| Buchmann et al., 2006 [25] | United States | 14 | 12/2 | 3.2 years (8 m—11 years) | 6 Skin, 5 Calvarium, 4 Temporal bone, 3 Skull, 2 Max, 1 Mand | NR | 1 DOD |
| Nicollas et al., 2010 [29] | France | 31 | 14/17 | 3 years (4.7 m—5.3 years) | 17 Skull, 11 Orbit, 6 Paranasal sinuses | NR | All patients alive |
| Lewoczko et al., 2014 [9] | United States | 88 | 54/34 | 4.1 years | 40 Skull, 32 Skin, 11 Orbit, 5 Max, 5 Temporal bone, 3 Mand | NR | 8 DOD |
| Present study | Brazil & Guatemala | 19 | 9/10 | 11.5 years (1 m—51 years) | 11 Mand, 5 Max, 4 Lymph nodes, 4 Skin, 3 Skull, 2 Gingiva, 2 Hard palate, 2 Liver, 2 Scalp, 2 Lymph node, 1 Spleen, 1 Thymus | CD1a, CD207, CD3, CD20, CD68, S-100, Ki-67, EBER | 2 recurrences, 2 DOD |
LCH langerhans cell histiocytosis, NR not reported, M male, F female, Mand mandible, Max maxilla, DOD died of disease
Materials and Methods
Nineteen Langerhans cell histiocytosis’ patients with involvement of the head and neck region were retrieved from the files of 3 Latin American pathology diagnostic services for this retrospective cross-sectional study from 1984 to 2017 (13 cases from Federal University of Rio de Janeiro, Brazil [8 cases from oral pathology laboratory of School of Dentistry and 5 cases from pathology laboratory of School of Medicine], and 6 cases from Centro Clínico de Cabeza y Cuello, Guatemala City, Guatemala). Demographic, clinical, and radiological data were obtained from the laboratories’ records. All cases were analyzed under light microscopy using 3 μm sections on histologic slides stained with hematoxylin-eosin (HE). This study was carried out following the Helsinki Declaration for study involving human subjects, being approved by the local research ethics committee (HUCFF/UFRJ—CAAE 52652816.4.0000.5257).
Immunohistochemical analysis was performed in formalin-fixed paraffin-embedded tissues (FFPET) from diagnostic biopsies with antibodies directed to CD207 (dilution 1:500, polyclonal, Monosan, Uden, The Netherlands), CD1a (dilution 1:500, clone 010, Dako, Carpinteria, CA, USA), S-100 (dilution 1:5000, polyclonal, Dako, Carpinteria, CA, USA), CD3 (dilution 1:200, polyclonal, Dako, Carpinteria, CA, USA), CD20 (dilution 1:400, clone L26, Dako, Carpinteria, CA, USA), CD68 (dilution 1:300, clone KP-1, Dako, Carpinteria, CA, USA), and Ki-67 (dilution 1:200, clone MIB-1, Dako, Carpinteria, CA, USA). Paraffin sections (3 μm thick, on silane-coated histologic slides) from formalin-fixed material were dehydrated and deparaffinized according to standard procedures. Heat-induced antigen retrieval was then performed. The slides were incubated overnight with primary antibodies and secondary antibody used was EnVision®+Dual Link/Peroxidase (Dakocytomation®). Positive and negative controls were included in all reactions.
The Ki-67 staining was analyzed by visualizing and quantifying the cells in 3 high-power fields (×400) containing the highest number of positive cells (hot spot). An objective analyze were performed, where the images were observed and photographed in a microscope connected through a camcorder (Leica DMR, Leica Microsystems, Germany). The analysis of the photographed images was done with the help of the Image J program (Java, U.S.A.). Positive and negative cells were counted and the results expressed as percentage of positive cells per field. The images were captured in True Image Format File (TIFF) format. Expression of all other antibodies was considered negative (0–5% of positive cells), weak positive (between 5 and 50% of positive cells), and strong positive (> 50% of positive cells) based on the average percentage of positive cells in 10 high-power fields (×400) from each specimen analyzed under light microscopy, regardless of staining intensity.
For detection of Epstein–Barr virus’s RNA, PNA Probe/Fluorescein probe (code Y5200, Dako®) and PNA/ISH Detection Kit (code k5201, Dako®) were used for histological sections of tissue microarray (TMA), according to the manufacturer instructions. The EBER signal has to be detected in the nucleus of the tumor cells, which has to be dark brown, using the DAKO kit.
Results
Clinical Data
The clinical data of 19 cases of LCH are summarized in Table 2. Ten patients were female and 9 were male. The mean age was 11.5 years, ranging from one month to 51 years. Fourteen cases (73.6%) were located in the oral cavity, three cases (15.7%) in lymph nodes, and two cases (10.5%) in the scalp. Oral lesions were mainly intra-osseous (13 cases [92.8%]—six cases in anterior mandible, five cases in posterior mandible, and two cases in posterior maxilla) while one case was located exclusively in the gingiva (Figs. 1, 2). Ten cases were unifocal lesions and nine cases presented as multifocal lesions. Eight patients (42%) had pain and the size of lesions ranged from 0.3 to 4 cm. The main extra-oral clinical findings were facial swelling (4 cases) and cervical lymphadenopathy (4 cases). The most common intra-oral findings were ill-defined unilocular radiolucency (8 cases) with gingival involvement (7 cases) and tooth mobility (7 cases), followed by ulcerated lesion (4 cases), cortical bone expansion/destruction (2 cases), nodular lesion (1 case), and plaque lesion (1 case). Four cases had systemic disease, with the first clinical manifestation as cutaneous lesions followed by visceral compromise (gastrointestinal tract, liver, spleen, thymus or lymph nodes). Information about treatment was obtained in 5 cases. All patients received treatment with vinblastine and steroids according to Histiocyte Society’s LCH-III (3 cases) and LCH-II (2 cases). Two patients showed recurrence and two patients died.
Table 2.
Summarized data about clinicopathologic features of 19 cases of head and neck LCH from Brazil and Guatemala
| N, Country | Age (years) | Gender | Unifocal site | Multifocal sites | Pain | GI | Tooth mobility | Eosinophils | Giant cells | Necrosis | Ki-67 index |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Guatemala | 1 | M | – | Mand, anta; Max, post; Skull | No | Yes | Yes | < 5% | Yes | No | 33% |
| 2 Guatemala | 2 | M | LN, cervicala | – | No | No | No | < 5% | Yes | No | 26% |
| 3 Guatemala | 2 | M | Gingivaa | – | Yes | Yes | No | < 5% | No | No | 19% |
| 4 Guatemala | 2 | F | Max, posta | – | No | Yes | Yes | > 50% | No | Yes | 40% |
| 5 Guatemala | 9 | F | Mand, posta | – | No | No | Yes | < 5% | Yes | Yes | 48% |
| 6 Guatemala | NR | F | Max, posta | – | No | No | Yes | 5–50% | No | No | 32% |
| 7 Brazilc | 1 m | M | – | Scalpa; Skin; Liver, Spleen, Thymus | Yes | No | No | < 5% | No | No | 37% |
| 8 Brazil | 1 | F | – | Scalpa; Skin; Hard palate; LN, cervical; GIT | Yes | No | No | zero | No | No | 5% |
| 9 Brazil | 1 | F | LN, cervicala | – | No | No | No | 5–50% | No | No | 36% |
| 10 Brazilc | 4 | M | – | LN, cervicala; Skull; Skin; Liver | Yes | No | No | 5–50% | Yes | No | 23% |
| 11 Brazil | 4 | M | – | Mand anta; Skin | No | Yes | Yes | > 50% | No | No | 25% |
| 12 Brazil | 9 | F | Mand, anta | – | No | Yes | Yes | < 5% | No | No | 6% |
| 13 Brazil | 10 | M | Mand, posta | – | Yes | No | No | 5–50% | No | No | 33% |
| 14 Brazil | 12 | F | Mand, posta | – | Yes | No | No | 5–50% | No | No | 23% |
| 15 Brazil | 18 | F | – | Mand, posta; Max post; Skull | No | No | No | > 50% | No | Yes | 48% |
| 16 Brazil | 23 | M | – | Mand, anta; Bilateral Max, post | No | No | Yes | > 50% | No | Yes | 5% |
| 17 Brazilb | 24 | F | – | Mand, anta; upper gingiva, ant | No | Yes | No | < 5% | No | No | 26% |
| 18 Brazil | 34 | M | – | Mand, anta; Hard palate | Yes | No | No | 5–50% | No | Yes | 21% |
| 19 Brazilb | 51 | F | Mand, posta | – | Yes | Yes | No | > 50% | No | No | 29% |
| Total |
11.5 (1 m—51) |
F—10 cases M—9 cases |
10 cases | 9 cases |
Yes—8 No—11 |
Yes—7 No—12 |
Yes—7 No—12 |
< 5%—7 cases 5–50%—6 cases > 50%—5 cases |
Yes—4 No—15 |
Yes—5 No—14 |
0–20%—4 cases 21–40%—13 cases 41–50%—2 cases |
LCH Langerhans cell histiocytosis, GI gingival involvement, NR not reported, M male, F female, Mand mandible, Max maxilla, ant anterior, post posterior, LN Lymph node, GIT gastrointestinal tract
aSite of the biopsied lesion
bPatient with recurrence
cPatient died of the disease
Fig. 1.

Radiographic features of Langerhans cell histiocytosis of the head and neck: panoramic radiograph revealed an ill-defined radiolucency in the left posterior mandible with loss of the alveolar bone and the characteristic aspect of “floating tooth”
Fig. 2.
Clinical and radiographic features of Langerhans cell histiocytosis of the head and neck: a Child with LCH showing a large painless swelling involving the left midface. b Axial section of magnetic resonance image shows an intraosseous lesion in the left ascending ramus of the mandible
Immunohistochemical Findings
The histopathological and immunohistochemical features of 19 cases of LCH are summarized in Tables 2 and 3. All cases exhibited the characteristic histiocytic cell of the LCH, which were positive for S-100, CD68, CD1a, and CD207, with one negative case for CD207 (Figs. 3, 4). The inflammatory reaction was heterogeneous with a large variation in the number of plasma cells, eosinophils and lymphocytes. Epithelial surface was present in 8 cases whereas 10 cases showed dense connective tissue and 4 cases showed loose connective tissue. Necrosis was present in 5 cases (27.8%), multinucleated giant cells in 4 cases (22.2%), atypical mitotic figures in 4 cases (22.2%), bacterial colonies in 3 cases (16.7%), and apoptotic bodies in 3 cases (16.7%). Four cases (21%) showed Ki-67 proliferation index lower than 20%, 13 cases (68.4%) between 21 and 40%, and two cases (10.5%) between 41 and 50%. In situ hybridization for EBV was negative in all the cases studied.
Table 3.
Inflammatory cells and their immunoprofile in 19 cases of head and neck LCH from Latin America
| Lymphocytes | Plasma cells | Eosinophils (%) | Histiocytes | |||||
|---|---|---|---|---|---|---|---|---|
| CD3 | CD20 | CD68 | S100 | CD1a | CD207 | |||
| 0% | None | None | None | 5.9 | None | None | None | 5.2% |
| < 5% | 58% | 52.6% | 83.3% | 29.4 | None | None | None | None |
| 5–50% | 31.5% | 36.9 | 16.7% | 35.3 | 100% | 15.3% | 5.6% | None |
| > 50% | 10.5% | 10.5% | None | 29.4 | None | 84.2% | 94.4% | 94.8% |
Fig. 3.
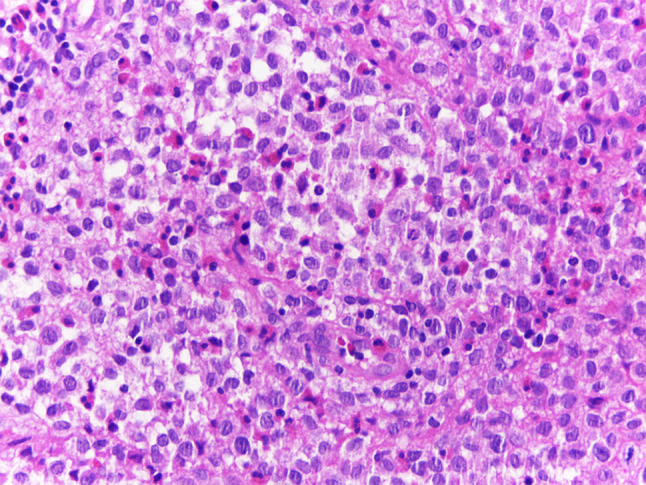
Microscopical features of Langerhans cell histiocytosis of the head and neck: many oval to round LCH cells with abundant cytoplasm and twisted or lobulated nucleus containing delicate chromatin and inconspicuous nucleoli, resembling “coffee bean”, is observed admixed with eosinophils and lymphocytes (HE, ×400)
Fig. 4.
Immunohistochemical profile of Langerhans cell histiocytosis of the head and neck: LCH cells were positive for a S-100 protein, b CD1a, c CD207 and d CD68 (Immunoperoxidase, A-D ×400)
Discussion
Langerhans cell histiocytosis (LCH) is the most common type of histiocytosis affecting children, with involvement of the head and neck region in up to 90% of cases, particularly the skull and jaws. The peak age range is between 1 and 4 years old, and patients with focal lesions are generally older (0.1–15.1 years) than those with multisystemic disease (0.09–14.8 years) [30]. In the present study, 74% of the patients were children, and the mean age of patients with multisystemic disease was 1.7 years. Despite the male predilection of LCH patients in the literature, our study showed no difference between genders [10, 28, 31–34].
Considering LCH involving the oral cavity, most patients are younger than 40 years of age, with a mean age of about 19 years [2]. Unifocal single system disease represents about 50% of oral LCH, with multifocal single system disease and multifocal multisystem disease equally distributed in the remaining 50% of cases, as also observed in our cases [2, 35]. The jaws are involved twice as frequently as the oral soft tissues. The mandible is preferentially affected over the maxilla, particularly the posterior regions [2–28, 30–35]. Gingiva and hard palate are common oral soft tissues affected by LCH, as painful or bleeding ulcerated nodules [2]. In our study, six cases were located in anterior mandible, five cases in posterior mandible, and two cases in posterior maxilla, while one case was located exclusively in the gingiva. Jaw lesions tend to present as unilocular radiolucencies with well-demarcated or poorly defined borders, which may involve adjacent teeth with consequent dental displacement, dental pain and mobility with “floating teeth” appearance, findings also observed in seven of our cases [2–28, 30–32, 35].
Cutaneous involvement of LCH may present as an early manifestation of disseminated disease [36–42], as observed in four cases of the present study. One patient was born with skin lesions on the scalp and chest, but the disease progressed and reached the liver, spleen and thymus, leading to death of the patient 1 year after treatment [36–42]. The involvement of cervical lymph nodes appears either in the isolated form or as part of a multisystemic disease [43]. In our study, two cases exhibited only cervical lymph node involvement, and one case was part of the multisystem disease. The thymus plays an important role in the development of the immune system and is composed of dendritic cells, but its involvement in LCH is rare. Thymus involvement is observed in younger patients with multisystem disease and a higher mortality rate [44, 45]. In our study, a 1-month-old boy presented aggressive and systemic form of LCH with involvement of the thymus and progression to death.
In the morphological analysis, we observed a great heterogeneity of the inflammatory reaction, with no differences between proportions of T lymphocytes (CD3) and B lymphocytes (CD20), variable proportion of eosinophils, and small quantity of plasma cells in most cases. Multinucleated giant cells were observed only in four cases of the present study, two cases in the oral cavity and two cases in cervical lymph nodes. Necrosis, apoptosis and atypical mitotic figures, often found in malignant neoplasms, were observed in 17, 22, and 28% respectively. Necrosis was mainly observed in cases with high proliferative index. The Ki-67 antigen is expressed during several phases of the cell cycle (i.e., G1, S, G2, and M) and can be correlated with mitotic counts and aggressiveness of disease [46, 47]. In our study, patients with systemic disease had Ki-67 higher than 40%, and still showed foci of necrosis in the histopathological analysis.
In correlation with clinical and morphological features, CD1a antigen expression provides the basis for the diagnosis of LCH in most instances [10]. However, CD1a expression is not specific for LCH, since it may also observed in other histiocytic proliferations such as juvenile xanthogranuloma, sinus histiocytosis with massive lymphadenopathy, and histiocytic sarcoma [48]. Langerin (CD207) is a relatively new immunohistochemical marker that may serve as a surrogate indicator for the presence of Birbeck granules in LCH histiocytes [48]. When directly compared with CD1a in the present study, expression of Langerin exhibited similar sensitivity, but slightly lower specificity, since one case was negative. Therefore, CD207 immunoreactivity seems to be a reliable alternative to CD1a antigen expression as evidence for a definitive diagnosis of LCH.
The etiology of LCH is widely discussed, but today it is known that Langerhans cell histiocytosis is a disease caused by somatic driver mutations at critical stages of myeloid differentiation that result in cellular transformation [4]. Mutations in the MAPK pathway have been identified in approximately 75% of patients with LCH. Additionally, immunohistochemical studies were able to identify the expression of the common BRAFV600E mutation in CD207+ [49, 50]. An important implication of the new understanding of LCH biology, suggesting a new definition as an inflammatory myeloid neoplasia [4]. A series of studies, have reported the association of Epstein–Barr virus (EBV) infection with several malignancies and LCH, representing a possible etiologic role or contribution to its pathophysiology [12–17]. Our study was conducted in two Latin American countries, where EBV infection is very prevalent. We decided to evaluate it by two methodologies (IHQ and HIS), and all cases were negative for EBV, as also showed by McClain et al. in 56 LCH cases [19]. Therefore, the evidence of EBV as an etiologic factor for LCH is weak and its expression could be just coincidental in some cases, which was not observed in these Latin American patients.
Currently, investigation into LCH treatment is continuing with the Histiocyte Society’s ongoing LCH-III trial. LCH-I and LCH-II have been completed and demonstrated the effectiveness of chemotherapy with vinblastine and steroids, and now LCH-III is prospectively studying treatment of high risk patients with methotrexate or 6-mercaptopurine and adjusting treatment length and intensity based on response [9, 51, 52]. Also, intralesional steroid injections have been proposed for the bone lesions and radiation was proposed by some authors but should be given only in low-doses (6–10 Gy) to reduce the risk of secondary tumors. This treatment is considered suitable for individual clinical use if treatment is important to conserve function and if intralesional therapy is not feasible. In relation to prognosis, the two main poor prognostic factors are the involvement of a high-risk-organ (liver, spleen, lung and bone marrow) and the lack of efficacy of the treatment [9, 51, 52].
In summary, this is the first case series of LCH of the head and neck in a Latin American population, with immunohistochemical findings. LCH is uncommon in the head and neck region, mainly presenting as unifocal or multifocal disease in the posterior regions of the mandible of young patients, which may present gingival bleeding and premature loss of teeth. The identification of lymphoplasmacytic infiltrate with predominance of eosinophils and histiocytes positive for CD1a and CD207 is required to confirm the diagnosis of LCH.
Author Contributions
NRB contributed to the clinical and microscopic analyses of the cases studied, final data analyses, and manuscript writing, RC and BABdA contributed to the clinical and microscopic analyses of the cases studied, final data analyses, and manuscript editing. APSB contributed with information about treatment modalities and manuscript editing. MJR and CBM contributed to the study design and manuscript writing. All authors gave final approval and agreed to be accountable for all aspects of the work.
Compliance with Ethical Standards
Conflict of interest
All of authors have indicated they have no potential conflicts of interest and no financial relationships relevant to this article to disclose.
Ethical Approval
This study was carried out following the Helsinki Declaration for study involving human subjects, being approved by the local research ethics committee (HUCFF/UFRJ—CAAE 52652816.4.0000.5257).
Informed Consent
This was a descriptive, retrospective study using only stock material that does not correspond to a biobank or a bio repository and was collected for assistance purposes. Many of the study participants either died or had outdated contact information. Therefore, the exemption of the informed consent term was requested and acquired.
Contributor Information
Natália Rocha Bedran, Email: nataliabedran@hotmail.com.
Román Carlos, Email: monchorcb@yahoo.com.
Bruno Augusto Benevenuto de Andrade, Email: augustodelima33@hotmail.com.
Ana Paula Silva Bueno, Email: apbueno65@gmail.com.
Mário José Romañach, Email: marioromanach@ufrj.br, Email: marioromanach@yahoo.com.br.
Cristiane Bedran Milito, Email: crismilito@gmail.com.
References
- 1.Pileri SA, Feldman AL, Cheuk W, Slater L. Langerhans cell histiocytosis. In: El-Nagar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. 4. Lyon: IARC; 2017. pp. 130–131. [Google Scholar]
- 2.Hicks J, Flaitz Langerhans cell histiocytosis: current insights in a molecular age with emphasis on clinical oral and maxillofacial pathology practice. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:S42–S66. doi: 10.1016/j.tripleo.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Madrigal-Martínez-Pereda C, Guerrero-Rodríguez V, Guisado-Moya B, Meniz-García C. Langerhans cell histiocytosis: literature review and descriptive analysis of oral manifestations. Med Oral Patol Oral Cir Bucal. 2009;14(5):E222–E228. [PubMed] [Google Scholar]
- 4.Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of Langerhans cell histiocytosis: back to Histiocytosis X? Br J Haematol. 2015;169:3–13. doi: 10.1111/bjh.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28:9–14. doi: 10.1002/(SICI)1096-911X(199701)28:1<9::AID-MPO3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.David R, Oria RA, Kumar R, Singleton EB, Lindell MM, Shirkhoda A, Madewell JE. Radiologic features of eosinophilic granuloma of bone. MR Am J Roentgenol. 1989;153:1021. doi: 10.2214/ajr.153.5.1021. [DOI] [PubMed] [Google Scholar]
- 7.Hernández-Juyol M, Boj-Quesada JR, Gallego Melcon S. Oral manifestations of Langerhans cell histiocytosis. Case study of a two year-old boy. Med Oral. 2003;8:19–25. [PubMed] [Google Scholar]
- 8.Mínguez I, Mínguez JM, Bonet J, Peñarrocha M, Sanchis JM. Oral manifestations of chronic disseminated histiocytosis. A report of 10 cases. Med Oral. 2004;9:149–154. [PubMed] [Google Scholar]
- 9.Lewoczko KB, Rohman GT, LeSueur JR, Stocks RM, Thompson JW. Head and neck manifestations of langerhan’s cell histiocytosis in children: a 46-year experience. Int J Pediatr Otorhinolaryngol. 2014;78:1874–1876. doi: 10.1016/j.ijporl.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO classification of tumors of hematopoietic and lymphoid tissues. 2017; p. 359.
- 11.Querings K, Starz H, Balda BR. Clinical spectrum of cutaneous Langerhans’ cell histiocytosis mimicking various diseases. Acta Derm Venereol. 2006;86:39–43. doi: 10.2340/00015555-0003. [DOI] [PubMed] [Google Scholar]
- 12.Chen CJ, Ho TY, Lu JJ, Sheu LF, Lee SY, Tien CH, et al. Identical twin brothers concordant for Langerhans’ cell histiocytosis and discordant for Epstein–Barr virus-associated hemophagocytic syndrome. Eur J Pediatr. 2004;163:536–539. doi: 10.1007/s00431-004-1493-y. [DOI] [PubMed] [Google Scholar]
- 13.Sakata N, Toguchi N, Kimura M, Nakayama M, Kawa K, Takemura T. Development of Langerhans cell histiocytosis associated with chronic active Epstein–Barr virus infection. Pediatr Blood Cancer. 2008;50:924–927. doi: 10.1002/pbc.21249. [DOI] [PubMed] [Google Scholar]
- 14.Ashraf MJ, Makarempour A, Monabati A, Azarpira N, Khademi B, Hakimzadeh A. Comparison between presence of epstein barr virus in nodal and extra nodal diffuse large B cell lymphoma of head and neck, an Iranian experience. Iran Red Crescent Med J. 2012;14:764–770. doi: 10.5812/ircmj.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimakage M, Sasagawa T, Kimura M, Shimakage T, Seto S, Kodama K, et al. Expression of Epstein–Barr virus in Langerhans’ cell histiocytosis. Hum Pathol. 2004;35:862–868. doi: 10.1016/j.humpath.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Purtilo DT. Epstein–Barr-virus-induced oncogenesis in immunedeficient individuals. Lancet. 1980;1:300–303. doi: 10.1016/S0140-6736(80)90792-8. [DOI] [PubMed] [Google Scholar]
- 17.Young LS, Rickinson AB. Epstein–Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 18.Jeziorski E, Senechal B, Molina TJ, Devez F, Leruez-Ville M, Morand P. Herpes-virus infection in patients with Langerhans cell histiocytosis: a case-controlled sero-epidemiological study, and in situ analysis. PLoS ONE. 2008;3:e3262. doi: 10.1371/journal.pone.0003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClain K, Jin H, Gresik V, Favara B. Langerhans cell histiocytosis: lack of a viral etiology. Am J Hematol. 1994;47:16–20. doi: 10.1002/ajh.2830470104. [DOI] [PubMed] [Google Scholar]
- 20.Schenka AA, De Angelo Andrade LA, Amstalden EM, Cintra ML, Vassallo J, Cardinalli IA, et al. Langerhans cell histiocytosis and its relationship with Epstein–Barr virus. Hum Pathol. 2006;37:1508–1509. doi: 10.1016/j.humpath.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Brousset P. Epstein–Barr virus and Langerhans cell histiocytosis. Hum Pathol. 2004;35:1573–1574. doi: 10.1016/j.humpath.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Panis V, Nikitakis N, Deskalopoulos A, Maragkou T, Tsiklakis K, Skavouvou A. Langerhans cell histiocytosis mimicking aggressive periodontitis: challenges in diagnosis and management. Quintessence Int. 2016;47:731–738. doi: 10.3290/j.qi.a36568. [DOI] [PubMed] [Google Scholar]
- 23.Facciolo MT, Riva F, Gallenzi P, Patini R, Gaglioti D. A rare case of oral multisystem Langerhans cell histiocytosis. J Clin Exp Dent. 2017;9:e820–e824. doi: 10.4317/jced.53774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guimarães LF, Dias PFBPD, Janini ME, Souza IPR. Langerhans cell histiocytosis: impact on the permanent dentition after an 8-year follow-up. J Dent Child. 2008;75:64–68. [PubMed] [Google Scholar]
- 25.Buchmann L, Emami A, Wei JL. Primary head and neck Langerhans cell histiocytosis in children. Otolaryngology. 2006;135:312–317. doi: 10.1016/j.otohns.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt A, Schultze A. Maxillofacial manifestations of Langerhans cell histiocytosis: a clinical and therapeutic analysis of 10 patients. Oral Oncol. 2003;39:687–694. doi: 10.1016/S1368-8375(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 27.Murray M, Dean J, Slater L. Multifocal oral langerhans cell histiocytosis. J Oral Maxillofac Surg. 2011;69:2585–2591. doi: 10.1016/j.joms.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Dagenais M, Pharoah MJ, Sikorski PA. The radiographic characteristics of histiocytosis X. A study of 29 cases that involve the jaws. Oral Surg Oral Med Oral Pathol. 1992;74:230–236. doi: 10.1016/0030-4220(92)90388-7. [DOI] [PubMed] [Google Scholar]
- 29.Nicollas R, Rome A, Belaïch H, Roman S, Volk M, Gentet JC, Michel G, Triglia JM. Head and neck manifestation and prognosis of Langerhans’ cell histiocytosis in children. Int J Pediatr Otorhinolaryngol. 2010;74:669–673. doi: 10.1016/j.ijporl.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Saliba I, Sidani K, El Fata F, Arcand P, Quintal MC, Abela A. Langerhans’ cell histiocytosis of the temporal bone in children. Int J Pediatr Otorhinolaryngol. 2008;72:775–786. doi: 10.1016/j.ijporl.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Yashoda-Devi B, Rakesh N, Agarwal M. Langerhans cell histiocytosis with oral manifestations: a rare and unusual case report. J Clin Exp Dent. 2012;4:e252–e255. doi: 10.4317/jced.50728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacino GA, Serrat A, Redondo LM, Verrier A. Langerhans cell histiocytosis: clinical diagnostic features and current concepts. Med Oral. 1999;4:607–618. [PubMed] [Google Scholar]
- 33.Duncan WK, Post AC, McCoy BP. Eosinophilic granuloma. Oral Surg Oral Med Oral Pathol. 1988;65:736–741. doi: 10.1016/0030-4220(88)90020-5. [DOI] [PubMed] [Google Scholar]
- 34.Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9–14. doi: 10.1002/(SICI)1096-911X(199701)28:1<9::AID-MPO3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Stålemark H, Laurencikas E, Karis J, Gavhed D, Fadeel B, Henter J. Incidence of Langerhans cell histiocytosis in children: a population-based study. Pediatr Blood Cancer. 2008;51:76e81. doi: 10.1002/pbc.21504. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto K, Pritzker MS. Electron microscopic study of reticulohistiocytoma: an unusual case of congenital, self-healing reticulohistiocytosis. Arch Dermatol. 1973;107:263–270. doi: 10.1001/archderm.1973.01620170071020. [DOI] [PubMed] [Google Scholar]
- 37.Longaker MA, Frieden IJ, LeBoit PE, Sherertz EF. Congenital ‘‘self-healing’’ Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31:910–916. doi: 10.1016/S0190-9622(94)70258-6. [DOI] [PubMed] [Google Scholar]
- 38.Larralde M, Rositto A, Giardelli M, Gatti CF, Munoz AS. Congenital self-healing histiocytosis (Hashimoto-Pritzker) Int J Dermatol. 1999;38:693–696. doi: 10.1046/j.1365-4362.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- 39.Jang KA, Ahn SJ, Choi JH, Sung KJ, Moon KC, Koh JK. Histiocytic disorders with pontaneous regression in infancy. Pediatr Dermatol. 2000;17:364–368. doi: 10.1046/j.1525-1470.2000.017005364.x. [DOI] [PubMed] [Google Scholar]
- 40.Geissmann F, Emile JF, Andry R, Thomas C, Fraitag S, De Prost Y, et al. Lack of expression of E-cadherin is associated with dissemination of Langerhans cell histiocytosis and poor outcome. J Pathol. 1997;181:301–304. doi: 10.1002/(SICI)1096-9896(199703)181:3<301::AID-PATH779>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Stein SL, Paller AS, Haut PR, Mancini AJ. Langerhans cell histiocytosis presenting in the neonatal period: a retrospective case series. Arch Pediatr Adolesc Med. 2001;155:778–783. doi: 10.1001/archpedi.155.7.778. [DOI] [PubMed] [Google Scholar]
- 42.Merglová V, Hrusák D, Boudová L, Mukensnabl P, Valentová E, Hosticka L. Langerhans cell histiocytosis in childhood—review, symptoms in the oral cavity, differential diagnosis and report of two cases. J Craniomaxillofac Surg. 2014;42:93–100. doi: 10.1016/j.jcms.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Haupt R, Minkov M, Astigarraga I, Schäfer E, Nanduri V, Jubran R, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175–184. doi: 10.1002/pbc.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672–2681. doi: 10.1182/blood-2016-01-690636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ducassou S, Seyrig F, Thomas C, Lambilliotte A, Marec-Berard P, Berger C, et al. Thymus and mediastinal node involvement in childhood Langerhans ceel histiocytosis: long-term follow-up from the French national cohort. Pediatr Blood Cancer. 2013;60:1759e65. doi: 10.1002/pbc.24603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hage C, Willman CL, Favara BE, Isaacson PG. Langerhans’ cell histiocytosis (histiocytosis): immunophenotype and growth fraction. Hum Pathol. 1993;24:840–845. doi: 10.1016/0046-8177(93)90133-2. [DOI] [PubMed] [Google Scholar]
- 47.Bank MI, Rengtved P, Carstensen H, Petersen BL. Langerhans cell histiocytosis: an evaluation of histopathological parameters, demonstration of proliferation by Ki-67 and mitotic bodies. APMIS. 2003;111:300–308. doi: 10.1034/j.1600-0463.2003.1110202.x. [DOI] [PubMed] [Google Scholar]
- 48.Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of Langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615–619. doi: 10.1097/PAS.0b013e31815b212b. [DOI] [PubMed] [Google Scholar]
- 49.Sahm F, Capper D, Preusser M, Meyer J, Stenzinger A, Lasitschka F, Berghoff AS, Habel A, Schneider M, Kulozik A, Anagnostopoulos I, Mullauer L, Mechtersheimer G, von Deimling A. BRAFV600E mutant protein is expressed in cells of variable maturation in Langerhans cell histiocytosis. Blood. 2012;120:e28–e34. doi: 10.1182/blood-2012-06-429597. [DOI] [PubMed] [Google Scholar]
- 50.Varga E, Korom I, Polyánka H, Szabó K, Széll M, Baltás E, Bata-Csörgő Z, Kemény L, Oláh J. BRAFV600E mutation in cutaneous lesions of patients with adult Langerhans cell histiocytosis. J Eur Acad Dermatol Venereol. 2015;29:1205–1211. doi: 10.1111/jdv.12792. [DOI] [PubMed] [Google Scholar]
- 51.Kilpatrick SE, Wenger DE, Gilchrist GS, et al. Langerhans’ cell histiocytosis (histiocytosis X) of bone. A clinicopathologic analysis of 263 pediatric and adult cases. Cancer. 1995;76(12):2471–2484. doi: 10.1002/1097-0142(19951215)76:12<2471::AID-CNCR2820761211>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 52.Monsereenusorn C, Rodriguez-Galindo C. Clinical characteristics and treatment of langerhans cell histiocytosis. Hematol Oncol Clin N Am. 2015;29:853–873. doi: 10.1016/j.hoc.2015.06.005. [DOI] [PubMed] [Google Scholar]




