Abstract
Adenoid cystic carcinoma in the oral cavity is an uncommon salivary gland malignancy that has a propensity for perineural spread. A high-grade variant is evidenced by an abundance of pleomorphic cells, loss of the classic biphasic epithelial-myoepithelial growth pattern, and comedonecrosis, as well as elevated Ki-67. CT and MRI can both be useful for demonstrating the extent of invasion in oral cavity-associated adenoid cystic carcinoma, which can attain the inferior alveolar nerve for perineural spread by direct invasion through the mandible. Reflecting the aggressive nature of this high-grade malignancy, 18FDG-PET can demonstrate hypermetabolism and can be useful for staging. These features are exemplified in this sine qua non radiology–pathology correlation article.
Keywords: Adenoid cystic carcinoma, Pathology, Radiology
History
A 59-year-old male with a 25 pack-year smoking history presented with a lump under the left tongue, dysphagia, and left chin numbness. The patient had undergone recent dental extractions, and it was initially believed by his dentist to be an abscess. The patient was subsequently referred to otolaryngology at our institution when the lesion started bleeding 3 months later, during which time the patient experienced 30 pounds of weight loss. The patient did not have additional significant past medical history.
Radiological Features
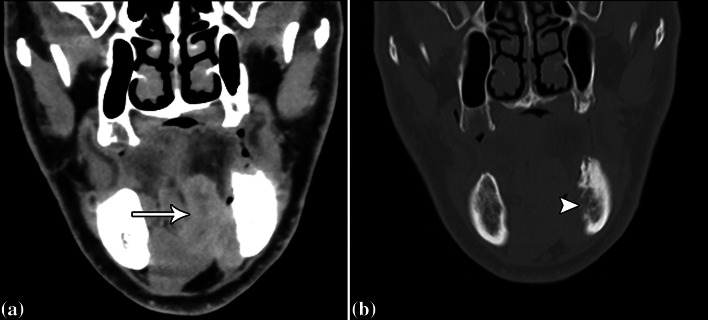
CT with contrast was performed, which showed an infiltrative mass centered in the left sublingual space (arrow) with invasion into the left mandibular body (Fig. 1). The degree of mandibular bone marrow invasion was more conspicuous on MRI, which was obtained shortly after the CT (Fig. 2). Furthermore, the mass was markedly hypermetabolic on 18FDG-PET/CT, which otherwise did not demonstrate metastases. The differential considerations based on the imaging include salivary gland malignancy, squamous cell carcinoma, and sarcoma.
Fig. 1.
Coronal soft tissue (a) and bone (b) window post-contrast CT images show an infiltrative mass centered in the left sublingual space (arrow) with direct invasion into the left mandibular body (arrowhead), with obliteration of the inferior alveolar canal, which attests to the aggressive behavior of a malignant neoplasm
Fig. 2.
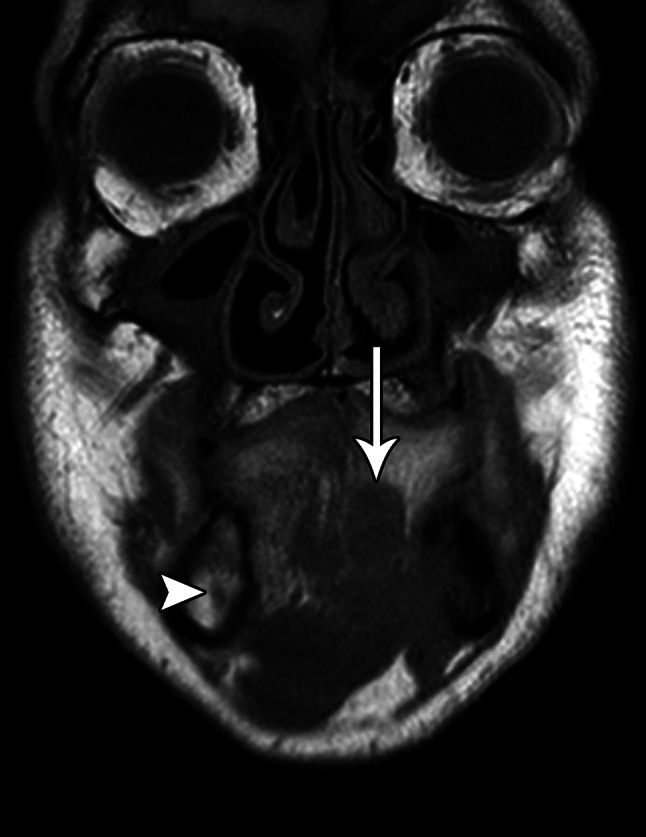
Coronal T1-weighted MRI shows an infiltrative mass (arrow) in the left oral cavity with invasion of the surrounding musculature and extension into the left mandibular bone marrow, where it envelops the left inferior alveolar nerve, which is consequently inseparable from the tumor. By comparison, the intact right inferior alveolar nerve is visible in cross-section (arrowhead)
Diagnosis and Treatment
The patient underwent left hemi-mandibulectomy, hemi-glossectomy, and floor of mouth resection with bilateral neck dissection. The gross resection specimen showed an unencapsulated, ill-defined, firm, tan-white mass measuring 5.7 cm in greatest dimension, centered in the floor of mouth with infiltration into adjacent mandible, lingual skeletal muscle, and fibroadipose tissue (Fig. 3). There was also direct extension of the tumor into an adjacent lymph node.
Fig. 3.
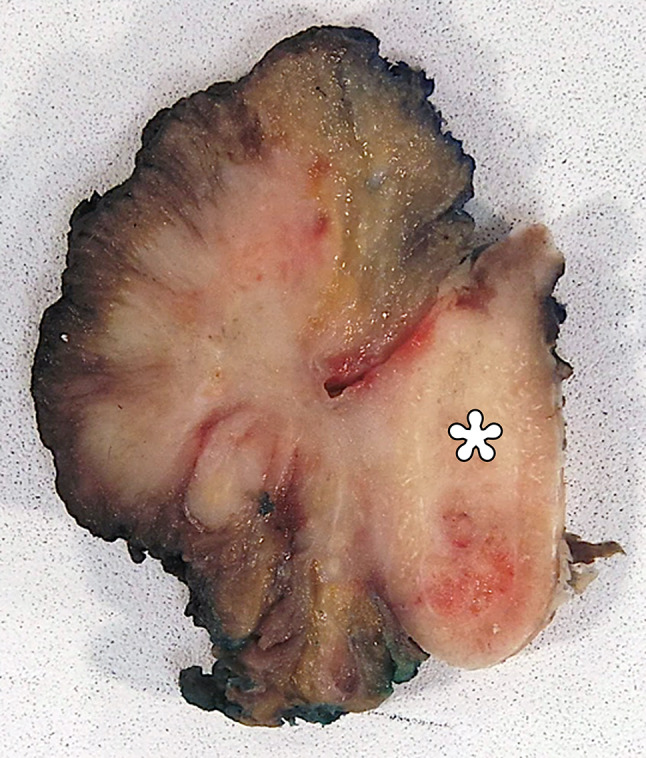
The gross specimen reveals an ill-defined, firm, tan-white mass centered on the floor of mouth with invasion of lingual skeletal muscle and the mandible (asterisk), without distinguishable residual normal sublingual gland
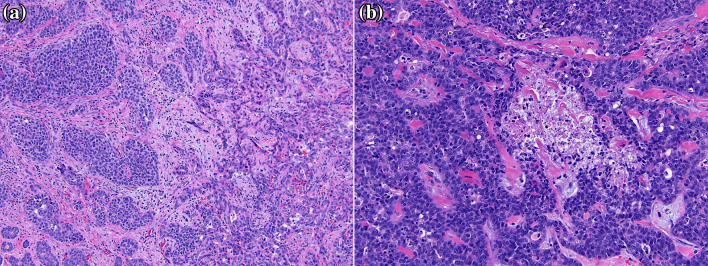
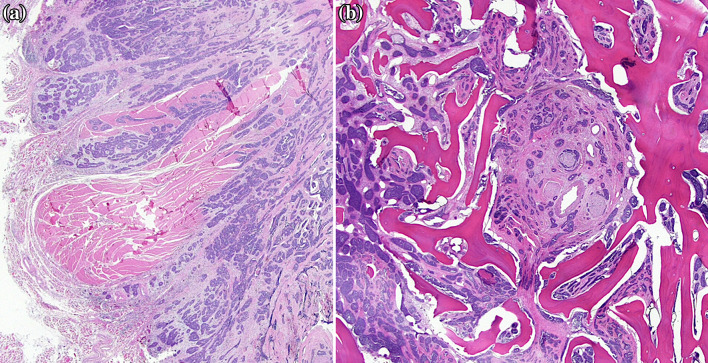
Hematoxylin and eosin-stained sections revealed two distinct areas in the tumor: an area of nested, duct-like growth containing cells with high nuclear:cytoplasmic ratios, oval to angulated nuclei with coarse chromatin, and variably prominent nucleoli; and an adjacent area of solid sheet-like growth with pleomorphic nuclei and increased mitoses (Fig. 4). In addition, variably-sized cords and nests of cells infiltrated into skeletal muscle and bone with perineural invasion (Fig. 5). Immunostains demonstrated p40, p63, and CK5/6 positive myoepithelial cells at the periphery of the nests and c-kit (CD117) positive epithelial cells at the center of the nests. Overall, these features were consistent with a high-grade variant of adenoid cystic carcinoma.
Fig. 4.
Hematoxylin and eosin-stained sections show two distinct areas: a relatively well-differentiated carcinoma with nested, duct-like growth containing cells with high nuclear:cytoplasmic ratios, oval nuclei with coarse chromatin, and variably prominent nucleoli (a, left side); and a more poorly-differentiated carcinoma with solid, sheet-like growth with pleomorphic nuclei and increased mitoses (a, right side). Occasional tumor necrosis was present in areas of solid-to-nested growth (b)
Fig. 5.
Low power hematoxylin and eosin-stained sections show variably-sized cords and nests of cells that infiltrate into skeletal muscle (a) and bone with perineural invasion of the inferior alveolar nerve (b)
The patient received seven cycles of TFHX (paclitaxel, infusional 5-fluorouracil, hydroxyurea, and twice-daily radiation therapy administered every other week) with no evidence of recurrence at 6 months after resection.
Discussion
Adenoid cystic carcinoma is a malignant neoplasm that predominantly originates from the submandibular, sublingual, and minor salivary glands and is comprised of epithelial and myoepithelial cells organized in tubular, cribriform, solid, or combined architectural forms [1]. It is a relatively uncommon neoplasm, representing about 1% of all malignant tumors in the head and neck region and is the fourth most common malignant salivary gland tumor accounting for 10% of all such tumors [2, 3]. Adenoid cystic carcinoma is slightly more prevalent in females than males and occurs most commonly during the fifth and sixth decades of life [4, 5].
Salivary gland tumors are usually best depicted on MRI. In particular, involvement of cranial nerves and tumoral infiltration around the nerves and osseous structures is optimally assessed via non-contrast T1-weighted and contrast-enhanced, fat-suppressed T1-weighted MR sequences [6]. Perineural spread typically appears as enlargement and abnormal enhancement of the affected nerve and widening or obliteration of the nerve canal. Since adenoid cystic carcinoma of the oral cavity can attain, engulf, and infiltrate the inferior alveolar nerve by first eroding through the mandibular cortex and infiltrating through the bone marrow, CT can be complementary to MRI, as demonstrated in this case.
In general, 18FDG-PET is considered complementary and sometimes superior to anatomical imaging modalities for staging and restaging of salivary gland malignancies and PET often has a positive impact on clinical management for such cases [7]. High-grade salivary gland malignancies tend to be more hypermetabolic than low- and intermediate-grade malignancies, as exemplified in this case of adenoid cystic carcinoma [8]. However, adenoid cystic carcinomas often do not have significant activity on 18FDG-PET, perhaps due to their slow growth [9]. The aggressive nature of the adenoid cystic carcinoma in this case may account for the hypermetabolism on 18FDG-PET.
Grossly, the adenoid cystic carcinoma tends to be poorly circumscribed, unencapsulated, and firm with a white to gray-white cut surface and significant variability in size [5]. Hemorrhage and necrosis are not common gross features, but if observed, should raise concern for a high-grade variant [5]. Histologically, the tumor is heterogeneous with varying combinations of the three distinct architectural patterns [1, 5]. The tumor cells are usually quite uniform in appearance with scant clear to slightly eosinophilic cytoplasm and bland, oval to sharply angulated basophilic nuclei with coarse chromatin. The tubular pattern is the least common type and lowest grade with small nests of ductal cells surrounded completely by a second myoepithelial cell layer in a background of eosinophilic hyalinized stroma [5]. The cribriform pattern is most common and is comprised of invasive islands of basaloid cells with many cystlike spaces, referred to as pseudocysts, forming a “Swiss-cheese” or “sieve-like” pattern [1, 2, 5]. The solid pattern demonstrates large islands and cords of carcinoma with areas of necrosis, increased mitotic activity, and perineural spread [5]. A high-grade variant must be identified in the setting of conventional adenoid cystic carcinoma and is evidenced by increasingly pleomorphic cells, loss of the classic biphasic epithelial-myoepithelial growth pattern, and comedonecrosis [5].
Immunohistochemical stains for smooth muscle actin, S100, p40, p63 demonstrate positivity in myoepithelial cells of adenoid cystic carcinoma [5, 10]. Adenoid cystic carcinoma also demonstrates strong positive staining for c-kit (CD117) in tumor cells regardless of pattern or grade [10–12]. Increased cellular proliferation as assessed by Ki-67 has also been described in high-grade solid-type adenoid cystic carcinoma and been shown to correlate with a worse prognosis [13]. The histologic differential diagnosis includes polymorphous adenocarcinoma, carcinoma ex pleomorphic adenoma, and basal cell adenocarcinoma.
Many adenoid cystic carcinomas are characterized by recently described recurrent chromosomal rearrangements, including t(6;9) [14, 15]. Persson et al. revealed that this translocation resulted in the fusion of two transcription factors, MYB-NFIB, which subsequently spurred studies on the role of MYB (myeloblastosis) overexpression in the pathogenesis of ACC [14, 16]. While a significant proportion of adenoid cystic carcinomas do not harbor this specific chromosomal aberration, the identification of the MYBL1-NFIB fusion product resulting from t(8;9) translocations suggested that overexpression of transcription factors from the family of MYB genes may play a key role in adenoid cystic carcinoma tumorigenesis [17, 18]. The exact biological sequence of events that drives the growth of adenoid cystic carcinoma still remains unclear. However, the emerging model suggests transformation of a low-grade neoplasm resulting from altered regulation of the MYB locus leads to subsequent growth and acquisition of additional genetic hits [15]. Novel therapies targeting the MYB protein may show more promise as our understanding of this sequence improves.
The likelihood of lymph node metastases increases by 5–10 fold in patients with high-grade variants of adenoid cystic carcinoma, occurring in 43–57% of patients with tumors showing these histologic features [19]. Thus, standard neck dissection is considered mandatory in patients with such tumors.
Acknowledgements
We are grateful for support received from the University of Chicago Office of Faculty Affairs through the Faculty Initiatives Fund for our Head and Neck Radiology–Pathology Trainee Conference, during which this case was presented.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Azumi N, Battifora H. The cellular composition of adenoid cystic carcinoma. An immunohistochemical study. Cancer. 1987;60:1589–1598. doi: 10.1002/1097-0142(19871001)60:7<1589::AID-CNCR2820600729>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg. 1974;128:512–520. doi: 10.1016/0002-9610(74)90265-7. [DOI] [PubMed] [Google Scholar]
- 3.Dillon PM, Chakraborty S, Moskaluk CA, et al. Adenoid cystic carcinoma: a review of recent advances, molecular targets, and clinical trials. Head Neck. 2016;38:620–627. doi: 10.1002/hed.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li N, Xu L, Zhao H, et al. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer. 2012;118:3945–3953. doi: 10.1002/cncr.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaso J, Malhotra R. Adenoid cystic carcinoma. Arch Pathol Lab Med. 2011;135:511–515. doi: 10.5858/2009-0527-RS.1. [DOI] [PubMed] [Google Scholar]
- 6.Yousem DM, Kraut MA, Chalian AA. Major salivary gland imaging. Radiology. 2000;216:19–29. doi: 10.1148/radiology.216.1.r00jl4519. [DOI] [PubMed] [Google Scholar]
- 7.Bertagna F, Nicolai P, Maroldi R, Mattavelli D, Bertoli M, Giubbini R, Lombardi D, Treglia G. Diagnostic role of (18)F-FDG-PET or PET/CT in salivary gland tumors: a systematic review. Rev Esp Med Nucl Imagen Mol. 2015;34:295–302. [PubMed] [Google Scholar]
- 8.Roh JL, Ryu CH, Choi SH, et al. Clinical utility of 18 F-FDG PET for patients with salivary gland malignancies. J Nucl Med. 2007;48:240–246. [PubMed] [Google Scholar]
- 9.Purohit BS, Ailianou A, Dulguerov N, Becker CD, Ratib O, Becker M. FDG-PET/CT pitfalls in oncological head and neck imaging. Insights Imaging. 2014;5:585–602. doi: 10.1007/s13244-014-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rooper L, Sharma R, Bishop JA. Polymorphous low grade adenocarcinoma has a consistent p63+/p40- immunophenotype that helps distinguish it from adenoid cystic carcinoma and cellular pleomorphic adenoma. Head Neck. 2015;9:79–84. doi: 10.1007/s12105-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penner CR, Folpe AL, Budnick SD. C-kit expression distinguishes salivary gland adenoid cystic carcinoma from polymorphous low-grade adenocarcinoma. Mod Pathol. 2002;15:687–691. doi: 10.1097/01.MP.0000018973.17736.F8. [DOI] [PubMed] [Google Scholar]
- 12.Vila L, Liu H, Al-Quran S, et al. Identification of c-kit gene mutations in primary adenoid cystic carcinoma of the salivary gland. Mod Pathol. 2009;22:1296–1302. doi: 10.1038/modpathol.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spaak LN, Dardick I, Ledin T. Adenoid cystic carcinoma: use of cell proliferation, bcl-2 expression histologic grade, and clinical stage as predictors of clinical outcome. Head Neck. 2000;22:489–497. doi: 10.1002/1097-0347(200008)22:5<489::AID-HED8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Wysocki PT, Izumchenko E, Meir J, et al. Adenoid cystic carcinoma: emerging role of translocations and gene fusions. Oncotarget. 2016;7:66239–66254. doi: 10.18632/oncotarget.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskaluk CA. Adenoid cystic carcinoma: clinical and molecular features. Head Neck. 2013;7:17–22. doi: 10.1007/s12105-013-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persson M, Andren Y, Mark J, et al. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA. 2009;106:18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitani Y, Liu B, Rao P, et al. Novel MYBL1 gene rearrangements with recurrent MYBL1-NFIB fusions in salivary adenoid cystic carcinomas lacking t(6;9) translocations. Clin Cancer Res. 2015. [DOI] [PMC free article] [PubMed]
- 18.Brayer KJ, Frerich CA, Kang H, et al. Recurrent fusions in MYB and MYBL1 define a common transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov. 2015. [DOI] [PMC free article] [PubMed]
- 19.Hellquist H, Skálová A, Barnes L, et al. Cervical lymph node metastasis in high-grade transformation of head and neck adenoid cystic carcinoma: a collective international review. Adv Ther. 2016;33:357–368. doi: 10.1007/s12325-016-0298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]