Abstract
The favorable features of high-risk human papillomavirus (HPV) in the head and neck are limited to those harboring transcriptionally-active HPV, which occur predominantly in the oropharynx (OP). Factors rendering the OP susceptible to HPV oncogenesis are largely unexplored. The role of cytokeratin 7 (CK7) in predisposition to HPV and cancer in the cervix has been evaluated. However, its significance in the H&N is unknown. CK7 immunohistochemistry was performed on a tissue microarray cohort of OP and non-oropharyngeal (NOP) squamous cell carcinomas (SCC) with known clinical follow-up and HPV E6/7 mRNA status. Expression was graded based on the distribution (1 ≤ 33%, 2 = 33–66%, 3 ≥ 66%) and intensity (1 = weak, 2 = strong) with combined score of ≥ 2 considered positive. Survival analysis was performed. Seventy-four NOPSCCs and 204 OPSCCs were studied. HPV was positive in 2.7% of NOPSCCs and 70.9% of OPSCCs. CK7 was positive in 23.0% of OPSCCs and 14.8% of NOPSCCs (p = 0.2), and in 24.1% of HPV positive versus 17.2% of negative patients (p = 0.2). There was no correlation with age, race, gender, HPV status, histologic type, tumor subsite, treatment, stage, or co-morbidities, and CK7 expression was not significantly associated with overall or disease specific survival. In our series, CK7 is positive in ~ 25% of H&N SCCs, although usually only focally. While CK7 has been suspected to be overexpressed selectively in HPV-related OPSCCs due to their origination from tonsillar crypt epithelium, we did not find any significant difference by anatomic H&N subsite, nor by HPV status, for its expression and found no association with patient survival.
Keywords: Oropharynx, Human papillomavirus, Cytokeratin 7, Squamous cell carcinoma, Head and neck, Tonsillar crypt epithelium
Introduction
Oropharyngeal squamous cell carcinoma (OPSCC) is now recognized as a distinct entity among the head and neck squamous cell carcinomas due to its frequent association with high-risk human papillomavirus (HPV). Studies have shown that HPV-positive OPSCC patients are generally younger and less frequently use alcohol and tobacco compared to those with HPV-negative SCC [1–4]. At the molecular level, HPV-positive SCCs less often harbor p53 mutations compared with HPV-negative SCCs [5], and they almost always overexpress p16 [1]. Finally, these tumors are associated with better patient survival despite higher rates of nodal metastases at presentation [6, 7].
HPV-positive SCCs occur in other anatomic subsites of the head and neck. However, the rates are much lower and despite showing transcriptionally-active HPV with associated p16 overexpression, the clinical significance of active viral oncogenesis in non-oropharyngeal subsites has not been established to date [8–10]. Factors influencing the selective susceptibility of the tonsillar crypt epithelium to HPV-induced oncogenesis are largely unknown/unexplored [11]. The prevailing explanation is that HPV needs access to basal cells in squamous epithelium for infection, and the tonsillar crypts provide this “easy access” due to their unique anatomy. Further, the microenvironment of tonsillar tissue, which is rich in immune cells, may play a role [11].
The uterine cervix is the most common non-head and neck site for HPV infection and subsequent neoplastic transformation. Although the oropharyngeal tonsillar crypt epithelium differs from the mucosal epithelium of the cervix in that it is intimately admixed with lymphoid tissue, the biological model of the cervix has nonetheless been used to try to identify mechanisms of HPV susceptibility and oncogenesis in the tonsils. Recently, Herfs et al. [12] suggested that HPV-related cervical SCCs are linked to a small, discrete cell population that localizes to the squamocolumnar junction of the cervix, expressing a number of specific proteins, including cytokeratin (CK) 7. The CK7 expression is not induced by HPV E6/E7 oncoproteins in squamous epithelial cells in vitro, and expression is obviously lost if the squamocolumnar junction is removed by cone biopsy or excision [13], which removes the risk of uterine cervical cancer altogether. This suggests that CK7 expressing cells may have a role in HPV oncogenesis and further, might be associated with HPV-related cervical (and oropharyngeal) SCC. Data on squamocolumnar junction markers in the head and neck, however, is very limited. A few studies have examined the expression of CK7 in cohorts of primary OPSCC patients and showed a significant association between CK7 expression and tumor HPV status [13–15]. In this study, we sought to investigate the expression of the CK7, as a squamocolumnar junction marker, in a large cohort of well-characterized OPSCC and non-OPSCC (NOPSCC) patients and to correlate the findings with tumor HPV status, clinico-pathologic features, and patient survival.
Materials and Methods
After approval from the Washington University in St. Louis Human Research Protection Office, head and neck SCC cases from 1996 to 2007 were retrieved from surgical pathology databases. Clinical follow-up information, including survival data, smoking, drinking, and other clinical variables, were obtained from electronic medical records as well as hospital oncology data services. Staging information was based on American Joint Commission on Cancer (AJCC) 7th edition. Whole-tissue sections of all cases were reviewed by the study pathologist (JSL) and confirmed as SCC. Other pathologic features were obtained by pathology report review. Tumors were histologically typed as keratinizing SCC, nonkeratinizing SCC and nonkeratinizing with maturation SCC according to our established system [7]. All patients were treated either with primary surgery with or without postoperative radiation and chemotherapy or were treated definitively with radiation therapy with or without chemotherapy. According to the amount of available biopsied or resected tumor tissue, duplicate 2 mm punches (or if inadequate tumor tissue present, then 0.6 mm punches) were taken from each case. Patients had been treated without regard to their p16 status.
Immunohistochemistry
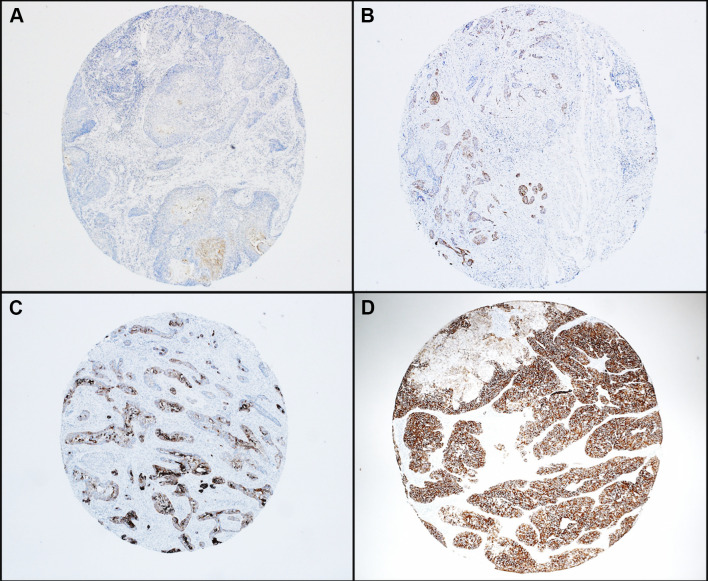
CK7 (clone RN7; Leica; predilute) immunohistochemistry (IHC) was performed on a Ventana Benchmark automated immunostainer (Ventana Medical Systems Inc., Tucson, AZ) according to standard protocols with appropriate controls including normal lung. Expression was assessed visually by one study pathologist (MM), and was graded in tumors based on the distribution of staining (1 ≤ 33%, 2 = 33–66%, 3 ≥ 66%) and intensity (0 = negative, 1 = weak, 2 = strong). A combined score of 2 or more was considered positive (Fig. 1). Immunohistochemistry was performed for p16 (clone E6H4; Ventana; predilute) on formalin-fixed, paraffin-embedded tissue sections on a Leica Bond automated instrument (Leica Biosystems, Inc.) with antigen retrieval consisting of 10 min in the ER1 proprietary antigen retrieval solution. The Bond Polymer Refine detection system was used for visualization. Slides were then dehydrated, cleared, and coverslipped. Cases for which there was < 10% tumor tissue across the two punches or for which the tumor on the tissue cores were folded or markedly distorted were excluded.
Fig. 1.
Representative photomicrographs of CK7 immunohistochemical staining in head and neck squamous cell carcinomas: a Positive score of 2 (strength: 1, distribution 1). b Positive score of 3 (strength: 1, distribution 2). c Positive score of 4 (strength: 2, distribution 2). d Positive score of 5 (strength: 2, distribution 3). ×4 magnification
HPV RNA In Situ Hybridization (ISH)
In situ hybridization for high risk HPV E6/E7 mRNA had been performed and interpreted as previously described [16] and using the RNAscope HPV kit (Advanced Cell Diagnostics, Inc., Hayward, CA) according to the manufacturer’s instructions. Probes covered HPV types 16, 18, 31, 33, 35, 52, and 58. Slides were read by one study pathologist (JSL) without knowledge of patient outcomes. Positive staining was identified as brown, punctate dots present in the nucleus and/or cytoplasm. Control probes for the bacterial gene DapB (negative control) and for the housekeeping gene ubiquitin C (positive control evidence of adequate RNA) were also included on each case. Cases in which there was < 10% surface area consisting of tumor were excluded. The array (and corresponding control) slides and were classified in a binary manner as either positive or negative. Positive cases had to have granular cytoplasmic and/or nuclear brown staining that was higher than the signal on the DapB negative control slide.
Statistical Analysis
Categorical data were presented as frequency and percentage, whereas continuous variables were described with mean and SD. The Pearson χ2 test was used to evaluate the association between dichotomous variables. Overall survival was defined as the time from date of commencement of treatment (either surgical resection or beginning of radiation or chemotherapy) to the date of last follow-up or of death. Disease-specific survival was calculated as the time from date of commencement of treatment to date of death in patients with known persistent or recurrent tumor at that time. This is denoted death with recurrence in Fig. 2. Survival differences between groups for an individual risk factor were examined by the log rank test. All tests were 2-sided with p values ≤ 0.05 considered statistically significant.
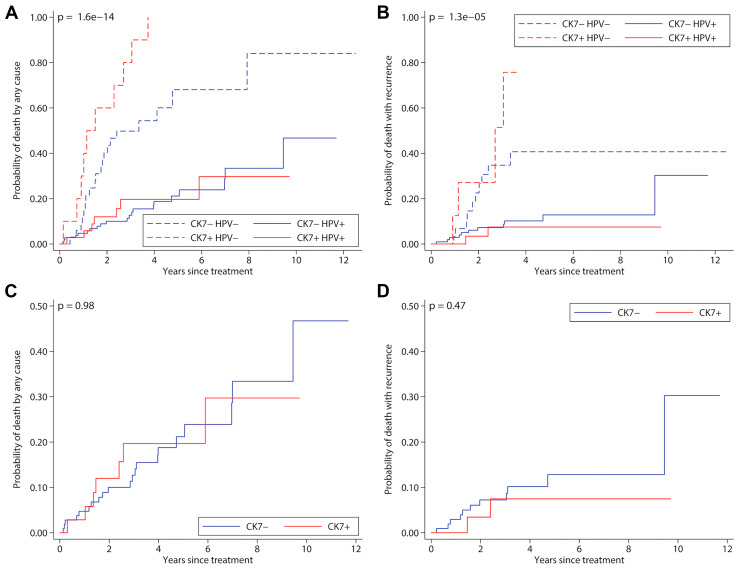
Fig. 2.
Cumulative mortality plots with oropharyngeal squamous cell carcinoma. Panel A and C give all-cause mortality while Panels B and C give mortality with recurrent cancer. Panels A and B show the interaction between HPV and CK7 positivity while panels C and D show the effects of CK7 positivity. CK7 positivity is based on the distribution and intensity of immunohistochemical staining, while HPV positivity is based on RNA in situ hybridization
Results
The clinical and pathologic features of the patients are presented in Tables 1 and 2. A total of 74 NOPSCC and 204 OPSCC patients were studied, most of whom were men (84%) of Caucasian race (87%) and current or former smokers (77%). Among the NOPSCC cases, 40/74 (54.1%) were from the oral cavity, 24/74 (32.4%) from the larynx and 10/74 (13.5%) from the hypopharynx. For the OPSCC cases, 47/204 (23.0%) had keratinizing morphology, 113/204 (55.3%) non-keratinizing, and 44/204 (21.5%) non-keratinizing SCC with maturation.
Table 1.
Demographic and pathologic variables in both oropharyngeal and nonoropharyngeal groups
| Characteristics | Oropharyngeal SCC, n: 204 (%) | Nonoropharyngeal SCC, n: 74 (%) |
|---|---|---|
| Male | 177 (86.7) | 58 (78.4) |
| Female | 27 (13.2) | 16 (21.6) |
| Caucasian | 182 (89.2) | 60 (81.0) |
| Mean and median age in years | 56.01, 54.9 | 69.4, 69 |
| Smoker (current or former) | 150 (73.5) | 60 (81.0) |
| HPV RNA ISH positive | 143 (70.9) | 2 (2.7) |
| Surgical treatment | 162 (79.4) | 64 (91.4) |
| Non-surgical treatment | 42 (20.6) | 6 (8.6) |
| Cytokeratin 7 (+) | 47 (23.0) | 11 (14.8) |
| AJCC 7th edition stagea | ||
| I | 6 (2.9) | 4 (6.1) |
| II | 12 (5.9) | 9 (13.9) |
| III | 31 (15.4) | 13 (20) |
| IV | 153 (75.8) | 39 (60) |
SCC squamous cell carcinoma
aData on staging was not available on all cases
Table 2.
Clinicopathologic features by cytokeratin 7 expression
| Characteristics | Oropharyngeal SCC, n: 204 (%) | Nonoropharyngeal SCC, n: 74 (%) | ||
|---|---|---|---|---|
| Cytokeratin 7 positive, n: 47 (%) | Cytokeratin 7 negative, n: 157 (%) | Cytokeratin 7 positive, n: 11 (%) | Cytokeratin 7 negative, n: 63 (%) | |
| Sex | ||||
| Male | 41 (89.3) | 136 (86.6) | 8 (72.7) | 50 (79.3) |
| Female | 6 (12.7) | 21 (13.4) | 3 (27.3) | 13 (20.7) |
| Race | ||||
| Caucasian | 44 (93.6) | 138 (87.9) | 9 (81.2) | 51 (80.9) |
| Non-Caucasian | 3 (6.4%) | 19 (12.1) | 2 (18.2) | 12 (19.0) |
| Mean and median age in years | 55.1, 54 | 56.2, 55 | 67.1, 64 | 69.9, 71 |
| Smoking status | ||||
| Smoker (current or former) | 32 (68.0) | 118 (75.1) | 1 (9.1) | 59 (93.6) |
| Non-smoker | 15 (32.0) | 39 (24.8) | 10 (90.9) | 4 (6.4) |
| HPV status | ||||
| HPV RNA ISH positive | 35 (74.4) | 108 (68.8) | 0 | 2 (3.1) |
| HPV RNA ISH negative | 12 (25.6) | 49 (31.2) | 11 (100) | 61 (96.9) |
| Morphology | ||||
| Keratinizing | 8 (17.0) | 39 (24.8) | 11 (100) | 63 (100) |
| Non-keratinizing with maturation | 8 (17.0) | 36 (22.9) | 0 | 0 |
| Non-keratinizing | 31 (66.0) | 82 (52.2) | 0 | 0 |
| AJCC stagea | ||||
| I | 2 (4.2) | 4 (2.5) | 1 (9.1) | 3 (4.7) |
| II | 2 (4.2) | 10 (6.3) | 0 | 9 (14.3) |
| III | 2 (4.2) | 29 (18.4) | 3 (27.3) | 10 (15.8) |
| IV | 40 (85.1) | 113 (71.9) | 7 (63.6) | 32 (50.8) |
SCC squamous cell carcinoma
aData on staging was not available on all cases
Patients were followed from 0.7 to 150.2 months (mean 43.2, median 37.1 months). Of the NOPSCC patients, 35 (55.5%) had cervical lymph node metastasis while 175 (85.7%) of the oropharyngeal SCC patients had nodal metastasis. The rate of distant metastasis was identical in both groups at 9.8%.
HPV RNA in situ hybridization was positive in 2 (2.7%) of NOPSCC and 143 (70.9%) of OPSCC. CK7 was positive in 47/204 (23.0%) of OPSCC and 11/74 (14.8%) of NOPSCC (p = 0.2). CK7 was positive in 35/143 (24.4%) of HPV RNA ISH positive OPSCCs versus neither of the 2 HPV RNA ISH positive NOPSCC patients. Overall, CK7 was positive in 35/145 (24.1%) of total HPV RNA ISH positive patients versus 23/133 (17.2%) of HPV RNA ISH negative patients (p = 0.2) (Table 2). Thus, oropharyngeal SCCs were 55% more likely, and HPV RNA ISH positive OPSCCs 40% more likely, than non-oropharyngeal SCCs to be CK7 positive.
Among NOPSCC cases, CK7 expression was strongly associated with better differentiation status, i.e. well or moderately versus poorly differentiated (p = 0.001). There was no correlation between CK7 expression and age, race, gender, HPV RNA status, histologic type, tumor subsite, chemotherapy treatment, T- stage, N-stage, or co-morbidities including smoking and drinking in either cohort.
Univariate survival analysis results are shown in Table 3. CK7 positivity was not significantly associated with overall (p = 0.75) or disease specific survival (p = 0.75) in the overall patient cohort, nor in separately studied OPSCC and NOPSCC cohorts. Kaplan Meier plots are shown in Fig. 2. HPV RNA status remained as the single feature associated with improved prognosis, independent of CK7 expression (Fig. 2a, b).
Table 3.
Univariate survival analysis by cytokeratin 7 status
| Entire cohort | Overall survival | Disease specific survival |
|---|---|---|
| Cytokeratin 7 (positive versus negative) | p = 0.7 | p = 0.7 |
| Nonoropharyngeal SCC | Overall survival | Disease specific survival |
|---|---|---|
| Cytokeratin 7 (positive versus negative) | p = 0.1 | p = 0.8 |
| Oropharyngeal SCC | Overall survival | Disease specific survival |
|---|---|---|
| Cytokeratin 7 (positive versus negative) | p = 0.4 | p = 0.8 |
SCC squamous cell carcinoma
Discussion
HPV-positive OPSCCs are biologically and clinically unique, and their incidence in the United States and worldwide is on the rise [17, 18]. The explanation as to why the prevalence of HPV is higher in OPSCC compared with other head and neck SCCs is far from clear, but several suggestions are emerging as to why the tissues of the oropharynx (and specifically the tonsillar tissues of the palatine tonsils and base of tongue) are at risk.
The oropharyngeal epithelium has a distinctive anatomy in that it is juxtaposed to organized lymphoid tissue, playing a key role in defense mechanisms against ingested or inhaled foreign pathogens. As such, it differs from the remainder of mucosal epithelium in the head and neck as well as other sites commonly infected by high-risk HPV such as the genital tract and the anus. It also has a distinct anatomy as the reticulated crypt epithelium is irregular, has a discontinuous basement membrane, and has intraepithelial capillaries [19]. This is presumed to be functional in nature, allowing easy interplay between ingested or inhaled antigens and the epithelium and underlying immune system. Lymphocytes are sprinkled throughout the reticulated epithelium. Hence, it is important to better understand the relationship between HPV and the tumor microenvironment (both epithelium and inflammatory cells) in OPSCC for prognostic, therapeutic, and potentially even preventative purposes.
Cytokeratins are major intermediate filaments of epithelial cells and make up the central part of the cytoskeleton. CK7 is a type II keratin expressed in breast, lung, and ovarian epithelium and in urothelium. It is not usually expressed in normal squamous epithelium [13, 20]. However, it does appear to be specifically expressed in junctional/transformational zones, including in the uterine cervical, anorectal, and gastroesophageal junctions of humans and mice. Studies have also shown it to be strongly expressed by the oropharyngeal reticulated tonsillar crypt epithelium, but not by the tonsillar surface squamous epithelium [13], so it may have a role in cancer development in these locations [12, 21, 22]. Furthermore, CK7 is among the proteins that the HPV-16 viral oncoprotein E7 directly interacts with [23] and it is expressed in up to 87% of cervical SCCs and 27% of head and neck SCCs across anatomic subsites [24]. It has also been reported that CK7 expression can be used as a predictive factor of response to concommitant radiochemotherapy for locally advanced cervical cancer [25]. Recently, Lee et al. [26] evaluated the expression of CK7 by IHC and HPV infection by DNA ISH in 25 cases of high grade cervical intraepithelial neoplasia (CIN3) and in 30 cases of cervical SCC. They found CK7 expression in all CIN3 and in 2/3 of SCC cases. Their results suggested that CK7 may be related with viral episomal replication and therefore, is a predictive marker for HPV infection and CIN3 progress, however, it was unclear why CK7 expression was absent in 1/3 of SCC cases. The lack of CK7 expression among all invasive cervical SCCs is in concordance with our study findings.
In this study, we evaluated the expression of CK7 in a large cohort of oropharyngeal and non-oropharyngeal SCCs. CK7 was positive in nearly a quarter (23.0%) of OPSCC and in approximately 15% of NOPSCC. This trend also was seen when comparing CK7 expression by HPV RNA ISH status. CK7 expression was present in 24.1% of HPV RNA ISH positive patients (24.4% of HPV positive OPSCCs) versus 17.2% of HPV RNA ISH negative patients. While there is a trend towards more CK7 expression in HPV-positive SCCs, these differences were not statistically significant.
This is somewhat different than results in a recently published study by Woods et al. 13, in which they studied 226 OPSCC patients. In their study, 50.8% of the HPV-related OPSCCs (defined as HPV DNA PCR and p16 immunohistochemistry positive) were CK7 expressing versus 15.7% of the HPV-negative OPSCCs, a difference (3.2 times higher in HPV positive patients) that was statistically significant. Our study shows that 35/143 (24.4%) of HPV-positive OPSCCs had CK7 expression versus 12/61 (19.6%) of the HPV-negative OPSCCs, or 1.2 times higher in HPV-positive tumors. Although the difference might be postulated to be due to criteria for positivity, our study used a very low cutoff to define patients as positive. A more reasonable consideration is that the difference is due to heterogeneity in CK7 expression within different areas of tumor and thus a limitation in tumor representation on our tissue microarrays. As these are mostly large array punches and in duplicate (2 mm each) and as many other studies on this tissue microarray cohort have shown results to both match with whole tissue findings and to be consistent with other study findings and clinicopathologic features of OPSCC patients, we think this may not be an actual limitation. Another possibility is that CK7 may be associated with the presence of viral DNA but not with transcriptionally-active HPV with associated oncoprotein expression and driving of tumor growth. It is well documented that the presence of high risk HPV DNA in SCC may be contributory to carcinogenesis but not necessarily to active promotion of tumor growth and progression [27, 28]. Similar to our study, Woods et al. did not find any significant correlation between CK7 and patient demographics [13].
We also attempted to determine the relationship between CK7 expression and patient outcomes. Amongst all patients, CK7 expression was not prognostic, and more specifically to address the HPV question, HPV positive OPSCC patients with CK7 positivity had no significant differences in overall or disease specific survival compared to CK7 negative patients. This was again similar to the results reported by Woods et al. [13].
In summary, this study shows slightly greater rates of CK7 expression in HPV positive OPSCC patients, although not statistically significant. This, along with prior literature, suggests that tonsillar crypt epithelium, unlike normal surface squamous epithelium, expresses CK7 and that CK7 interacts with high risk HPV proteins. As a result, tonsillar tumors may more frequently express CK7. While it doesn’t argue for or against the concept that CK7 is important for HPV-mediated oncogenesis, it does argue that there is no clinical significance to CK7 expression in established HPV-positive SCCs.
Funding
No funding was obtained for this project.
Compliance with Ethical Standards
Conflict of interest
Mitra Mehrad, William D. Dupont, W Dale Plummer Jr, and James S. Lewis, Jr declare that they have no conflict of interest.
Research involving Human and Animal Participants
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Lewis JS, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, DC. Head Neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 3.Shiboski CH, Schmidt BL, Jordan RCK. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Kumar B, Cordell KG, Lee JS, Titer HPV, et al. EGFR, p16, Bcl-xL and p53, Sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakhry C, Westra WH, Li S, et al. Improved Survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 7.Chernock RD, El-Mofty SK, Thorstad WL, et al. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meshman J, Wang P-C, Chin R, et al. Prognostic significance of p16 in squamous cell carcinoma of the larynx and hypopharynx. Am J Otolaryngol. 2017;38:31–37. doi: 10.1016/j.amjoto.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Isayeva T, Xu J, Ragin C, et al. The protective effect of p16(INK4a) in oral cavity carcinomas: p16(Ink4A) dampens tumor invasion-integrated analysis of expression and kinomics pathways. Mod Pathol. 2015;28:631–653. doi: 10.1038/modpathol.2014.149. [DOI] [PubMed] [Google Scholar]
- 10.Combes J-D, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol. 2014;50:370–379. doi: 10.1016/j.oraloncology.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Kim S-H, Koo B-S, Kang S, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120:1418–1425. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 12.Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci USA. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods RSR, Keegan H, White C, et al. Cytokeratin 7 in oropharyngeal squamous cell carcinoma: a junctional biomarker for human papillomavirus-related tumors. Cancer Epidemiol Biomarkers Prev. 2017;26:702–710. doi: 10.1158/1055-9965.EPI-16-0619. [DOI] [PubMed] [Google Scholar]
- 14.Morbini P, Capello GL, Alberizzi P, et al. Markers of squamocolumnar junction cells in normal tonsils and oropharyngeal cancer with and without HPV infection. Histol Histopathol. 2015;30:833–839. doi: 10.14670/HH-11-590. [DOI] [PubMed] [Google Scholar]
- 15.Regauer S, Beham A, Mannweiler S. CK7 expression in carcinomas of the Waldeyer’s ring area. Hum Pathol. 2000;31:1096–1101. doi: 10.1053/hupa.2000.6279. [DOI] [PubMed] [Google Scholar]
- 16.Ukpo OC, Flanagan JJ, Ma X-J, et al. High-Risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380–386. doi: 10.1016/j.oraloncology.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry ME. The specialised structure of crypt epithelium in the human palatine tonsil and its functional significance. J Anat. 1994;185(Pt 1):111–127. [PMC free article] [PubMed] [Google Scholar]
- 20.Hosoya A, Kwak S, Kim E-J, et al. Immunohistochemical localization of cytokeratins in the junctional region of ectoderm and endoderm. Anat Rec. 2010;293:1864–1872. doi: 10.1002/ar.21233. [DOI] [PubMed] [Google Scholar]
- 21.Fritsch H, Zehm S, Illig R, et al. New insights into the development and differentiation of the human anorectal epithelia. Are there clinical consequences? Int J Colorectal Dis. 2010;25:1231–1242. doi: 10.1007/s00384-010-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanduc D. Translational regulation of human papillomavirus type 16 E7 mRNA by the peptide SEQIKA, shared by rabbit alpha(1)-globin and human cytokeratin 7. J Virol. 2002;76:7040–7048. doi: 10.1128/jvi.76.14.7040-7048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]
- 25.Lambaudie E, Chereau E, Pouget N, et al. Cytokeratin 7 as a predictive factor for response to concommitant radiochemotherapy for locally advanced cervical cancer: a preliminary study. Anticancer Res. 2014;34:177–181. [PubMed] [Google Scholar]
- 26.Lee H, Lee H, Cho YK. Cytokeratin7 and cytokeratin19 expression in high grade cervical intraepithelial neoplasm and squamous cell carcinoma and their possible association in cervical carcinogenesis. Diagn Pathol. 2017;12:18. doi: 10.1186/s13000-017-0609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rautava J, Syrjänen S. Biology of human papillomavirus infections in head and neck carcinogenesis. Head Neck Pathol. 2012;6:3–15. doi: 10.1007/s12105-012-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop JA, Lewis JS, Rocco JW, et al. HPV-related squamous cell carcinoma of the head and neck: an update on testing in routine pathology practice. Semin Diagn Pathol. 2015 doi: 10.1053/j.semdp.2015.02.013. [DOI] [PubMed] [Google Scholar]