Abstract
Sinonasal tract (SNT) leiomyosarcoma (LMS) is exceedingly rare with < 100 cases reported. Their relationship to retinoblastoma and other malignancies, along with previous irradiation has not been clarified. Routine and consultation cases were reviewed for histologically and immunohistochemically proven SNT LMS. The tumors were tested with antibodies against α-smooth muscle actin, desmin, h-caldesmon, HMB45, S100 protein, Rb1, MDM2, CDK4 and EBV (EBER-ISH). Nine tumors affecting 5 males and 4 females aged 26 to 77 years (median: 48 years) were identified in the maxillary sinus (n = 4), nasal cavity (n = 3) and combined SNT (n = 2). Three patients had previous irradiation (2 for retinoblastoma, 1 for fibrous dysplasia) and 1 patient had chemotherapy and stem cell transplantation for Hodgkin lymphoma. One patient had prostatic adenocarcinoma (prior) and rectal adenocarcinoma (post) to the LMS. All patients with follow-up developed either local recurrences and/or metastases, principally to lung (time to metastasis: 16–156 months, mean 62 months). Histologically, 6 tumors were conventional high-grade LMS, two had glycogen-rich clear cell (PEComa-like) morphology and one was spindle cell low-grade. The latter showed grade 2 in the recurrence and grade 3 in the lung metastases. Two cases showed dedifferentiation to anaplastic pleomorphic (inflammatory MFH-like) phenotype. Immunohistochemistry revealed diffuse expression of at least 2 smooth muscle markers in 8 and only actin in one case/s. All other markers were negative. RB1 loss was observed in 6/8 cases tested. Sinonasal tract leiomyosarcomas are rare aggressive sarcomas that frequently develop in a background of previous cancer therapy (4/9), most frequently irradiation. Their varied morphology underlines the wide differential diagnostic considerations. Long-term survival may be achieved with aggressive multimodal therapy.
Keywords: Leiomyosarcoma; Nasal cavity; Maxillary sinus; Sarcoma; Muscle, smooth; Immunohistochemistry; Retinoblastoma
Introduction
Leiomyosarcoma (LMS) is an aggressive mesenchymal neoplasm that shows smooth muscle differentiation by histological, immunophenotypic and ultrastructural examination [1]. LMSs are uncommon, comprising no more than 7% of all soft tissue sarcomas [1]. The main sites of origin are uterus, skin/soft tissue and retroperitoneum with only around 3% affecting the head and neck area [1].
Mesenchymal neoplasms of the sinonasal cavities are uncommon. They comprise < 5% of all tumors of the sinonasal tract. In a review of 256 mesenchymal tumors of the sinonasal and nasopharyngeal cavities by Fu and Perzin, smooth muscle neoplasms represented 3% of the total cohort [2]. In the same study, LMSs comprised 2.3% of all and 6% of malignant non-epithelial neoplasms [2]. Sinonasal tract (SNT) LMSs are exceptionally rare with no more than 100 cases reported in the English literature, with most as single case reports [3–7]. Thus, only limited data is available on their morphological spectrum and other clinicopathological and prognostic characteristics. In this study we analyzed histologically proven SNT LMS identified in our routine and consultation files.
Materials and Methods
All tumors coded as sinonasal tract leiomyosarcomas were identified in our routine surgical pathology and consultation files. Histological slides were reviewed to verify diagnosis and immunohistochemistry was performed on 4-µm sections cut from paraffin blocks using a fully automated system (Benchmark XT, Ventana Medical Systems Inc, Tucson, Arizona, USA) using the following antibodies: desmin (clone D33, 1:250, Dako), α-smooth muscle actin (clone 1A4, 1:200, Dako), h-caldesmon (clone h-CD, 1:100, Dako), S100 protein (polyclonal, 1:2500, Dako), HMB-45 (clone HMB45, 1:50, Enzo), p53 (clone DO-7, 1:50, Dako), MDM2 (clone IF1, 1:50, CalBiochem), CDK4 (clone DCS-156, 1:100, Zytomed) and RB1 (1:200, BD Biosciences, Heidelberg, Germany). Epstein Barr virus (EBV) in-situ hybridization (EBER 1/2 probes, ZytoVision, Bremerhaven, Germany) was performed according to the manufacturer guidelines. Positive and negative controls were used throughout.
Results
General Clinical and Demographic Features
Nine patients with primary SNT LMS were identified (Table 1). One patient had a sinonasal metastasis from a primary soft tissue LMS (of the hand) diagnosed > 20 years ago with recurrent metastases at diverse sites over two decades. This patient (the only case with sinonasal metastasis from a soft tissue LMS in our records) was excluded from further analysis. In one of our institutions, there were 3 SNT LMSs (0.01%) out of 28,026 SNT specimens submitted in the period 2007–2017. During this same period, 6 SNT leiomyomas were encountered, thus suggesting benign leiomyomas are more common than LMSs. Finally, the 3 SNT LMSs represented 0.35% of all 861 LMSs from all body sites during the same period.
Table 1.
Clinicopathological features of sinonasal leiomyosarcomas (n = 9)
| No. | Age sex | Site | Treatment | Associated tumors | Previous irradiation or chemotherapy? | Family history | Follow up and outcome |
|---|---|---|---|---|---|---|---|
| 1 | 77 M | Right nasal cavity + maxillary sinus | Excision and radiotherapy |
Prostatic carcinoma at age 73 Sinonasal leiomyosarcoma at age 77 Rectal carcinoma at age 81 |
Anti-androgen therapy for prostate cancer | No |
55 months later, there was resection of multiple lung metastases of both rectal cancer and leiomyosarcoma Currently, alive with disease at 88 months |
| 2 | 48 F | Left nasal cavity + sinuses | Excision and adjuvant radiochemo-therapy | Tubulovillous rectal adenoma, 13 years later | No | No |
Local recurrence middle turbinate (26 months) Two metastases to the right lower lung lobe (13 years) Alive with no evidence of disease at 17 years |
| 3 | 43 F | Right nasal cavity | Excision and radiochemo-therapy | Retinoblastoma of right eye at age 3 | Radiotherapy for retinoblastoma | No |
At 16 months, imaging showed solitary lung metastasis, cerebral metastasis and local recurrence (not verified histologically) Lost to follow-up thereafter |
| 4 | 61 M | Nasal cavity | Surgery | Not known | Not known | Not known | Not known |
| 5 | 28 M | Maxillary sinus, infiltrating dura | Surgery | Not known | Not known | Not known | Multiple recurrences over 16 months, then lost to follow-up |
| 6 | 26 F | Maxillary sinus | Radical maxillect-omy |
Hodgkin Lymphoma (Stage IIIB in 3/2003) Allogenic stem cell transplantation after recurrent Hodgkin (2011) Bad GVHD |
Chemotherapy then stem cell transplant in 2004 | No |
Lung metastases at 20 months, treated with surgery Alive without evidence of disease at 8.2 years |
| 7 | 27 M | Maxillary sinus | Wide excision |
Retinoblastoma (bilateral) at 18 months (bilateral blindness) Macrocytosis Intellectual disability with epilepsy/seizures |
Yes: for retinoblastoma; 2 times: at initial and then at age 2 after second eye developed retinoblastoma | Mother had retinobla-stoma and rhabdo-myosarcoma |
Local recurrence at 2 years which resulted in brain invasion Dead of disease at 2.4 years |
| 8 | 66 F | Nasal cavity | Wide excision | Grade 1, Stage 1A uterine endometrioid adenocarcinoma (1999) | No | No | Alive without evidence of disease at 6.6 years |
| 9 | 66 M | Maxillary sinus | Wide excision | Polyostotic fibrous dysplasia | Yes: irradiation for fibrous dysplasia at a younger age | No | Brain extension resulted in death from disease at 0.5 years |
M male, F female, GVHD graft-versus-host-disease
The nine primary SNT LMSs affected 5 males and 4 females aged 26 to 77 years (median 48 years, mean 49 years). Tumor affected the maxillary sinus (n = 4), nasal cavity (n = 3) and combined nasal cavity and paranasal sinuses (n = 2). Three patients had a history of previous irradiation (2 for retinoblastoma and one for fibrous dysplasia). A fourth patient (the youngest in this series) had a history of chemotherapy and stem cell transplantation for Hodgkin lymphoma performed five years prior. The retinoblastomas were diagnosed at age 3 and 1.5 years, respectively. The interval between the retinoblastoma and/or irradiation and LMS diagnosis was 40 and 25 years, respectively. Of the two patients with retinoblastoma, one had bilateral disease with a positive family history indicating hereditary retinoblastoma syndrome. The other patient had unilateral disease and a negative family history. Her LMS was ipsilateral to the retinoblastoma and irradiation side. One patient (the oldest in this series) had prostatic adenocarcinoma at age 73, SNT LMS at age 77 and rectal adenocarcinoma at age 81. Notably, this patient developed multiple lung nodules, which proved after resection to represent an admixture of metastases from both his LMS as well as his rectal adenocarcinoma. Analysis of the mismatch repair proteins in this patient revealed intact expression making a hereditary non-polyposis colorectal cancer (HNPCC) syndrome unlikely. A final patient had a history of endometrioid endometrial adenocarcinoma.
Follow-up was available for 8 patients, ranging from 6 months to 17 years. Two patients died of local disease complications (extension into brain from primary tumor or local recurrence) at 6 and 30 months. Local recurrence developed in four of eight patients at 16–28 months. In addition, distant metastases affected four of eight patients (all had lung metastases, while one also developed brain metastases). Metastases developed at 16, 20, 55 and 156 months. Thus, two developed local recurrence only, two developed distant metastases only and two patients had both. At last follow-up, three patients were alive without evidence of disease 6–17 years. Notably, two of the survivors had surgically resected lung metastases several years earlier. Only one patient was alive without metastases at last follow-up. Thus, all patients with follow-up developed either local recurrences and/or metastases, the latter primarily to lung between 16 and 156 months (mean 62 months).
Pathological Findings
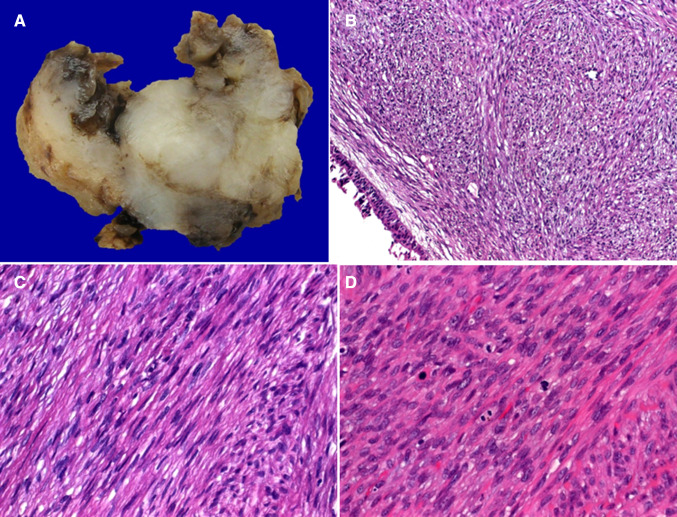
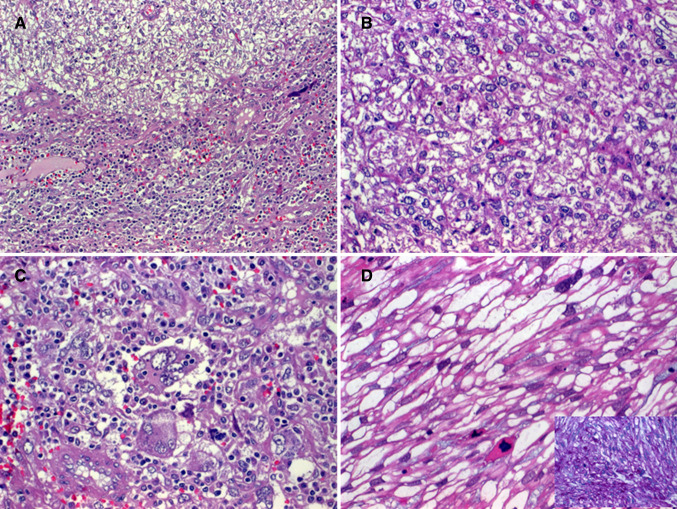
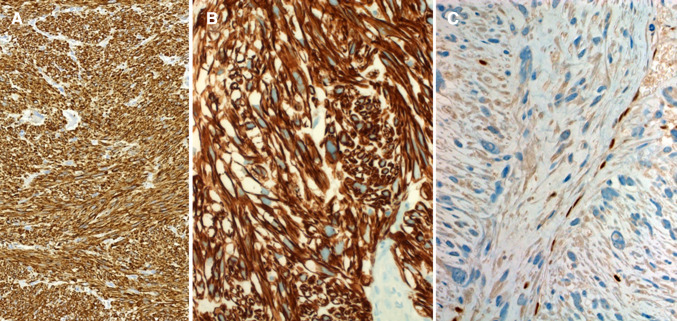
Grossly, the tumors were described as fleshy with whorled whitish cut-surface, firm to soft, with variable regions of hemorrhagic necrosis (Fig. 1a). Histologically, all tumors showed cytological and architectural features of smooth muscle neoplasms with elongated, blunt-ended, vesicular nuclei and brightly eosinophilic fibrillary cytoplasm arranged into compact intersecting fascicles (Fig. 1b–d). Perinuclear vacuoles were characteristic. Six tumors were conventional high-grade neoplasms, showing brisk mitotic activity [defined as > 10 mitoses/10 high power fields (Fig. 1d)] and areas of tumor necrosis, two tumors had variable epithelioid glycogen-rich clear cell (PEComa-like) morphology but with high-grade nuclear features (Fig. 2a–d) and one was spindled cell, but low-grade (Fig. 1c), showing only 2 mitoses/10 high power fields and lacking necrosis (Table 2). The latter showed grade 2 LMS in the recurrence and grade 3 LMS in the lung metastases. Two cases showed dedifferentiation with abrupt transition to anaplastic pleomorphic (inflammatory MFH-like; Fig. 2a, c) or epithelioid pleomorphic phenotype in the primary tumor or in the recurrence. Immunohistochemistry revealed diffuse expression of 2 or more smooth muscle markers in eight cases and only actin in one case (Fig. 3a, b). All other markers were negative. RB1 loss was observed in 6/8 cases (Fig. 3c).
Fig. 1.
Sinonasal leiomyosarcomas present as non-encapsulated irregular fleshy masses with areas of necrosis (a) covered by respiratory mucosa (b). They range from low-grade (c) to high-grade (d) with variable mitotic activity
Fig. 2.
a Transition from epithelioid clear cell (upper field in a; b high power of clear cell epithelioid areas) to inflammatory “MFH”-like pattern (lower field in a; c high power). d another case showed spindled glycogen-rich clear cells with high-grade features (inset: PAS)
Table 2.
Histological and immunohistochemical features of sinonasal leiomyosarcomas (n = 9)
| No. | Histology | Grade | SMA | Desmin | h-caldesmon | HMB45 | S100 | EBER ISH | Rb1 | TP53 | MDM2 | CDK4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Conventional | G3 | +++ | +++ | +++ | − | − | − | Lost | − | − | − |
| 2 | Conventional low-grade | Initially G1; recurrence G2; Metastasis G3 | +++ | +++ | +++ | − | − | − | Lost | − | − | − |
| 3 | Conventional | G3 | + | +++ | +++ | − | − | − | Lost | − | − | − |
| 4 | clear cell, glycogen-rich | G3 | +++ | ++ | +++ | − | − | np | np | np | − | np |
| 5 | Conventional low-grade with dedifferentiation | G1 + G3 | +++ | − | − | − | − | − | Lost | − | − | − |
| 6 | Conventional | G3 | +++ | +++ | − | − | − | − | Intact | +++ | − | − |
| 7 | Conventional, focal myxoid | G2 | +++ | +++ | +++ | − | − | − | Lost | − | − | − |
| 8 | Conventional with clear cell and dedifferentiated (inflammatory MFH-like) | G1 + G3 | +++ | +++ | +/− | − | − | − | Lost | − | − | − |
| 9 | Conventional, focal epithelioid | G3 | +++ | − | ++ | − | − | − | Intact | − | − | − |
ISH in-situ hybridization, np not performed, SMA smooth muscle actin, EBER ISH Epstein Barr virus in-situ hybridization
Fig. 3.
Sinonasal leiomyosarcomas with strong expression of smooth muscle actin (a) and h-caldesmon (b). Loss of Rb1 (c) was observed in 6 of 8 tumors
Discussion
SNT LMSs are rare aggressive sarcomas that represent only 2–3% of non-epithelial tumors of the SNT and nasopharynx [2]. They frequently develop in a background of previous cancer therapy or irradiation for retinoblastoma [8–10], other malignancies such as lymphomas or even for benign conditions as exemplified by fibrous dysplasia in one of these cases. 4 of 9 cases in our current study fall into this group of LMS developing after previous cancer therapy applied to the general anatomic region.
A history of retinoblastoma was recorded in 24% of reported cases [8–10]. Moreover, a history of head and neck irradiation and of systemic chemotherapy was recorded in 18/63 (28%) and 3/63 (4.8%) reviewed cases, respectively [6–10]. It is well known that second primary neoplasms occur more frequently in survivors of hereditary retinoblastoma (14%) than in those with presumably non-heritable retinoblastoma (1.8%) [8–10]. In one large study from the United Kingdom, LMS was the main malignancy in the hereditary subcohort followed by osteosarcoma and skin melanoma [9]. In that study, head and neck sarcomas were more frequent in the hereditary versus non-hereditary subgroups and within the hereditary subgroup, the head and neck involvement was more frequent than other body sites, suggesting a multifactorial or synergistic (combined) effect for the presence of germline Rb1 deficiency and irradiation in the sarcomagenesis.
In contrast to earlier assumptions that SNT LMS have a lower rate of distant metastasis (8%), our series showed a distant metastasis rate of 50%. This discrepancy is likely a reflection of the extent and completeness of follow-up. Distant metastases in our series occurred 16 months to 13 years (mean, 62 months) after diagnosis and therapy of the primary tumors and 2 of 4 cases developed metastases > 4.5 years after initial therapy, well beyond the median follow-up of 38 months in a review of 63 previously reported cases [6]. Accordingly, our series highlights the necessity for a careful and extended follow-up for patients with SNT LMS due to the high frequency of late distant metastases, developing as late as 13 years after primary treatment. We also observed a high local recurrence rate (4/8), with patient death due to local complications.
Another important finding highlighted by the current series, is the possibility of long-term survival in spite of metastatic disease. Thus, it seems justified or reasonable to consider surgery for oligometastatic disease. This feature seems to be unique from LMS of soft tissue and other organs, where disease progression is usually rapid and fatal after development of systemic metastases from high-grade tumors.
Another interesting finding is the histological and immunophenotypic heterogeneity of SNT LMS with 2 cases showing focal dedifferentiation while 2 other cases showing glycogen-rich clear cell histology, mimicking perivascular epithelioid cell tumors (PEComas). However, unlike monotypic angiomyolipoma (PEComa) [11], none expressed the melanocytic markers. Instead, both tumors showed high-grade nuclear features consistent with leiomyosarcoma. From our data, dedifferentiation (observed in two tumors) does not seem to be associated with or explained by aberrant expression of TP53, MDM2 or CDK4. The frequent loss of nuclear RB1 in SNT LMS parallels their soft tissue counterparts and is not indicative of a genetic or heritable basis.
Distinguishing SNT LMS from benign smooth muscle tumors is usually straight forward given that the majority are high-grade tumors. Sinonasal leiomyoma (angioleiomyoma) is the second most commonly reported smooth muscle tumor at this site [12]. These usually small polypoid lesions are identical to their cutaneous counterparts and lack atypia and mitotic activity. A variable mature fatty component is observed in several lesions [12]. The latter feature is absent in SNT LMS. Likewise, thick-walled blood vessels are not a feature of SNT LMS, although a vascular smooth muscle origin has been proposed by some authors [5]. However, a rare subset of SNT LMSs shows intermediate features between benign leiomyoma (no mitoses) and leiomyosarcoma with brisk mitotic activity (defined as > 10 mitoses/10 high power fields). Analogous to soft tissue counterparts, the term smooth muscle tumor of unknown malignant potential has been used by some authors [5]. In our experience however, this variant of sinonasal smooth muscle tumors is exceptionally rare. We encountered none in our combined files. Likewise, SNT smooth muscle tumors with symplastic or bizarre nuclear features are exceptionally rare [13].
Spindle cell carcinoma, which can also occur in the post-radiation setting and may show some immunopositivity for smooth muscle markers, represents an important differential diagnosis of SNT LMS. While a subset of spindle cell SCCs are keratin negative, they lack histological features of smooth muscle differentiation (cigar-shaped blunt-ended nuclei, brightly eosinophilic cytoplasm, perinuclear halos/vacuoles and uniform fascicular growth pattern), are usually prominently polypoid, and when SMA is positive, it is usually only focal and weak. Most importantly, they are not desmin-positive, except those with rhabdomyoblastic differentiation, which are rare [14]. The presence of surface dysplasia or foci of carcinoma in situ are strong clues that aid in diagnosing spindle cell squamous cell carcinoma.
It is most important for therapeutic and prognostic reasons to recognize biphenotypic sinonasal sarcomas (BSS), which shows combined myoneural features and is separate from genuine LMS. BSS can be recognized on routine H&E stained material as a non-LMS given their basophilic (fibrosarcoma-like) morphology [15, 16]. However, IHC can be misleading as this rare and newly defined sarcoma type frequently expresses smooth muscle markers such as actins and less frequently desmin, thus suggesting a smooth muscle tumor if one is not aware of the entity or if other stains have not been concurrently performed (S100 protein and ß-catenin) [16]. In our series, none of the 9 LMSs expressed S100 protein, which is in contrast to the frequent co-expression of this marker in BSS. Furthermore, BSS is characterized by a distinctive gene fusion involving the MAML3 and PAX3 gene loci in majority of cases [17]. Indeed, inclusion of some BSS cases in older series/case reports of SNT LMS might explain the reported lower rate of distant metastases in the literature. EBV has been suggested as a possible etiologic agent for some smooth muscle tumors, especially in children and the immunocompromised [18], but we were not able to confirm EBV association (by EBER) in this series.
An important distinction, albeit rare, is to exclude sinonasal metastasis from LMS of other sites. We excluded from this study the one case of sinonasal metastasis from a LMS of the soft tissue of the hand. A few reports in the literature showed similarly rare metastases of uterine and soft tissue LMS to the head and neck area, including the sinonasal tract, suggesting a need for careful staging procedures to exclude another primary tumor [19, 20].
In summary, we herein described our experience with 9 sinonasal tract leiomyosarcomas highlighting their frequent, in part yet unexplained, association with previous cancer therapy and/or other synchronous/metachronous malignancies, their high propensity for local and occasionally fatal disease relapse, their high (50%) late distant metastatic rate, and occasional long survival irrespective of systemic metastasis, possibly as a result of improved multimodality therapy. These findings underscore the need for optimized therapeutic approaches and long-term follow-up with careful consideration of local therapeutic strategies for oligometastatic disease for each patient.
Funding
No external funding was obtained for this study.
Compliance with Ethical Standards
Conflict of interest
All authors declare that he/she has no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board, which did not require informed consent.
References
- 1.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. World Health Organisation classification of tumours of soft tissue and bone. 4. Lyon: IARC Press; 2013. [Google Scholar]
- 2.Fu YS, Perzin KH. Nonepithelial tumors of the nasal cavity, paranasal sinuses, and nasopharynx: a clinicopathologic study. IV. Smooth muscle tumors (leiomyoma, leiomyosarcoma) Cancer. 1975;35:1300–1308. doi: 10.1002/1097-0142(197505)35:5<1300::AID-CNCR2820350508>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Flucke U, Franchi A. Leiomyosarcoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. Lyon: IARC Press; 2017. pp. 35–36. [Google Scholar]
- 4.Kuruvilla A, Wenig BM, Humphrey DM, Heffner DK. Leiomyosarcoma of the sinonasal tract. A clinicopathologic study of nine cases. Arch Otolaryngol Head Neck Surg. 1990;116:1278–1286. doi: 10.1001/archotol.1990.01870110050005. [DOI] [PubMed] [Google Scholar]
- 5.Huang HY, Antonescu CR. Sinonasal smooth muscle cell tumors: a clinicopathologic and immunohistochemical analysis of 12 cases with emphasis on the low-grade end of the spectrum. Arch Pathol Lab Med. 2003;127:297–304. doi: 10.5858/2003-127-0297-SSMCT. [DOI] [PubMed] [Google Scholar]
- 6.Ulrich CT, Feiz-Erfan I, Spetzler RF, Isaacs JD, Hott JS, Nakaji P, Coons SW, Joganic EJ, Kresl JJ, Milligan JM, Lettieri SC. Sinonasal leiomyosarcoma: review of literature and case report. Laryngoscope. 2005;115:2242–2248. doi: 10.1097/01.mlg.0000183767.97518.09. [DOI] [PubMed] [Google Scholar]
- 7.Papoian V, Yarlagadda BB, Devaiah AK. Multifocal, recurrent sinonasal leiomyosarcoma: case report and review of literature. Am J Otolaryngol. 2013 doi: 10.1016/j.amjoto.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Rodjan F, de Graaf P, Brisse HJ, et al. Second cranio-facial malignancies in hereditary retinoblastoma survivors previously treated with radiation therapy: clinic and radiologic characteristics and survival outcomes. Eur J Cancer. 2013;49:1939–1947. doi: 10.1016/j.ejca.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 9.MacCarthy A, Bayne AM, Brownbill PA, et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951–2004. Br J Cancer. 2013;108:2455–2463. doi: 10.1038/bjc.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong JR, Morton LM, Tucker MA, Abramson DH, Seddon JM, Sampson JN, Kleinerman RA. Risk of subsequent malignant neoplasms in long-term hereditary retinoblastoma survivors after chemotherapy and radiotherapy. J Clin Oncol. 2014;32:3284–3290. doi: 10.1200/JCO.2013.54.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee SS, Eyden B, Trenholm PW, Sheikh MY, Wakamatsu K, Ancans J, Rosai J. Monotypic angiomyolipoma of the nasal cavity: a heretofore undescribed occurrence. Int J Surg Pathol. 2001;9:309–315. doi: 10.1177/106689690100900410. [DOI] [PubMed] [Google Scholar]
- 12.Agaimy A, Michal M, Thompson LDR, Michal M. Angioleiomyoma of the sinonasal tract. Analysis of 16 cases and review of the literature. Head Neck Pathol. 2015;9:463–473. doi: 10.1007/s12105-015-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincenzi A, Rossi G, Monzani D, Longo L, Rivasi F. Atypical (bizarre) leiomyoma of the nasal cavity with prominent myxoid change. J Clin Pathol. 2002;55:872–875. doi: 10.1136/jcp.55.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop JA, Thompson LD, Cardesa A, et al. Rhabdomyoblastic differentiation in head and neck malignancies other than rhabdomyosarcoma. Head Neck Pathol. 2015;9:507–518. doi: 10.1007/s12105-015-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis JT, Oliveira AM, Nascimento AG, Schembri-Wismayer D, Moore EA, Olsen KD, Garcia JG, Lonzo ML, Lewis JE. Low-grade sinonasal sarcoma with neural and myogenic features: a clinicopathologic analysis of 28 cases. Am J Surg Pathol. 2012;36:517–525. doi: 10.1097/PAS.0b013e3182426886. [DOI] [PubMed] [Google Scholar]
- 16.Rooper LM, Huang SC, Antonescu CR, Westra WH, Bishop JA. Biphenotypic sinonasal sarcoma: an expanded immunoprofile including consistent nuclear β-catenin positivity and absence of SOX10 expression. Hum Pathol. 2016;55:44–50. doi: 10.1016/j.humpath.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritchie KJ, Jin L, Wang X, et al. Fusion gene profile of biphenotypic sinonasal sarcoma: an analysis of 44 cases. Histopathology. 2016;69:930–936. doi: 10.1111/his.13045. [DOI] [PubMed] [Google Scholar]
- 18.Hussein K, Rath B, Ludewig B, Kreipe H, Jonigk D. Clinico-pathological characteristics of different types of immunodeficiency-associated smooth muscle tumours. Eur J Cancer. 2014;50:2417–2424. doi: 10.1016/j.ejca.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Sandruck J, Escobar P, Lurain J, Fishman D. Uterine leiomyosarcoma metastatic to the sphenoid sinus: a case report and review of the literature. Gynecol Oncol. 2004;92:701–704. doi: 10.1016/j.ygyno.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 20.Schütz A, Smeets R, Driemel O, Hakim SG, Kosmehl H, Hanken H, Kolk A. Primary and secondary leiomyosarcoma of the oral and perioral region–clinicopathological and immunohistochemical analysis of a rare entity with a review of the literature. J Oral Maxillofac Surg. 2013;71:1132–1142. doi: 10.1016/j.joms.2012.12.011. [DOI] [PubMed] [Google Scholar]