Abstract
Human papillomavirus (HPV)-related multiphenotypic sinonasal carcinoma is a peculiar sinonasal tract tumor that demonstrates features of both a surface-derived and salivary gland carcinoma. Implicit in its name, this tumor has a consistent association with high-risk HPV, particularly type 33. It was first described in 2013 under the designation of HPV-related carcinoma with adenoid cystic carcinoma-like features. However, since its initial description additional cases have emerged which demonstrate a wide morphologic spectrum and relatively indolent clinical behavior. Herein we report our experience with a case of HPV-related multiphenotypic sinonasal carcinoma that was initially classified as adenoid cystic carcinoma in the 1980s. The patient recurred after a 30-year disease free interval. RNA in situ hybridization confirmed the presence of high-risk HPV in both her recurrence and her initial tumor in the 1980s, which allowed for reclassification as HPV-related multiphenotypic sinonasal carcinoma. Our case adds to the literature of this relatively newly described entity and supports the indolent clinical behavior of this neoplasm but also demonstrates a potential for very late local recurrence.
Keywords: Human papillomavirus, Multiphenotypic sinonasal carcinoma, Adenoid cystic carcinoma, Sinonasal carcinoma, Carcinoma with adenoid cystic-like features
Introduction
The sinonasal tract is one of the so-called anatomic “hot spots” for human papillomavirus (HPV)-related carcinomas [1]. While most of these tumors are characterized by a nonkeratinizing squamous morphology [1], in 2013 Bishop et al. [2] described a unique HPV-related carcinoma with features of both a surface-derived and salivary gland carcinoma which they termed HPV-related carcinoma with adenoid cystic carcinoma-like features, given its propensity to mimic adenoid cystic carcinoma. Since this initial description, additional cases have been reported which have expanded the morphologic spectrum of the salivary gland component to include tumors that do not resemble adenoid cystic carcinoma, including those with a basal cell adenocarcinoma-like and epithelial-myoepithelial carcinoma like phenotype [3–5]. Still this tumor has been shown to have a consistent association with high-risk HPV, particularly type 33 [2, 5]. Because of its wide morphologic spectrum and to avoid confusion with true adenoid cystic carcinoma, the tumor has been redesignated as HPV-related multiphenotypic sinonasal carcinoma (HMSC) [5]. Currently, HMSC is a provisional entity in the most recent WHO classification of head and neck tumors [6].
To date there are 50 cases of HMSC in the literature; about half of these cases presented at advanced clinical stage and almost all have a high-grade histologic appearance, with high mitotic rates and frequent bone invasion and tumor necrosis [3–5]. Despite these high-grade features HMSC paradoxically appears to behave in a relatively indolent fashion with frequent local recurrences but only rare metastases and no reported deaths [5]. Here we provide documentation of another HMSC and discuss the patient’s unique oncologic presentation and history.
Case Report
The patient is a 69-year-old woman who carried a history of adenoid cystic carcinoma involving the palate and nasal cavity. Her oncologic history began in 1986, when she first noted a small mass on the left side of her hard palate. CT imaging studies at that time demonstrated no identifiable tumor, however, a biopsy of her palate was performed and was interpreted as adenoid cystic carcinoma. While the original pathology reports were not available, the clinical notes from her paper chart documented that the biopsy mentioned the presence of respiratory epithelium suggesting that there was also tumor in the nasal cavity. The patient underwent a left subtotal maxillectomy through a midface degloving approach and was subsequently fitted with an obturator prosthesis. The margins of her resection were negative. Two years later she was noted to have a recurrence along the remnant of her nasal septum and floor of nose. She underwent a partial palatectomy and partial septectomy with negative margins. Her obturator prosthesis was modified and she did well until 2017 when she began experiencing right palatal soreness beneath her prosthesis. Physical examination revealed a 3.5 cm raised, tender erythematous lesion along the hard palate. CT imaging studies demonstrated no identifiable tumor. A punch biopsy was performed and was interpreted as squamous cell carcinoma in situ with basaloid features and small focus suspicious for invasion. A completion palatectomy and right subtotal maxillectomy were performed.
Materials and Methods
Immunohistochemical studies were performed on 5-µm thick formalin-fixed paraffin embedded tissue using fully automated systems on the BenchMark ULTRA instrument (Ventana Medical Systems Inc., Tucson, AZ) and Bond-III instrument (Leica Biosystems, Buffalo Grove, IL). The following antibodies were utilized: cytokeratin AE1/AE3 (AE1/AE3, 1:200, Millipore, Temecula, CA), CK 7 (OV-TL, 1:40, Dako, Carpinteria, CA), CK5/6 (D5/16B4, 1:150, Millipore), SMA (1A-4, 1:50, Dako), SMMS-1 (SMMS-1, predilute, Ventana, Tucson, AZ), SOX-10 (BC34, predilute, Biocare, Concord, CA), S-100 (polyclonal, 1:800, Dako), CD117 (polyclonal, 1:500, Dako), p40 (BC28, 1:100, Biocare) and p16 (E6H4, predilute, Ventana).
HPV testing was performed by RNA in situ hybridization using the RNAscope method (Advanced Cell Diagnostics, Hayward, CA), as previously detailed [7]. 5-µm thick formalin-fixed paraffin embedded tissue were evaluated with the HPV-HR18 Probe which detects 18 high-risk HPV genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 59, 66, 68, 73 and 82) as well as the HPV type 33-specific probe (Advanced Cell Diagnostics, Hayward, CA).
A break-apart fluorescence in situ hybridization (FISH) assay for MYB (Empire Genomics, Buffalo, NY) was also performed according to the manufacturer’s protocol using the HYBrite platform (Abbott Molecular, Des Plaines, IL), as previously described [2].
Results
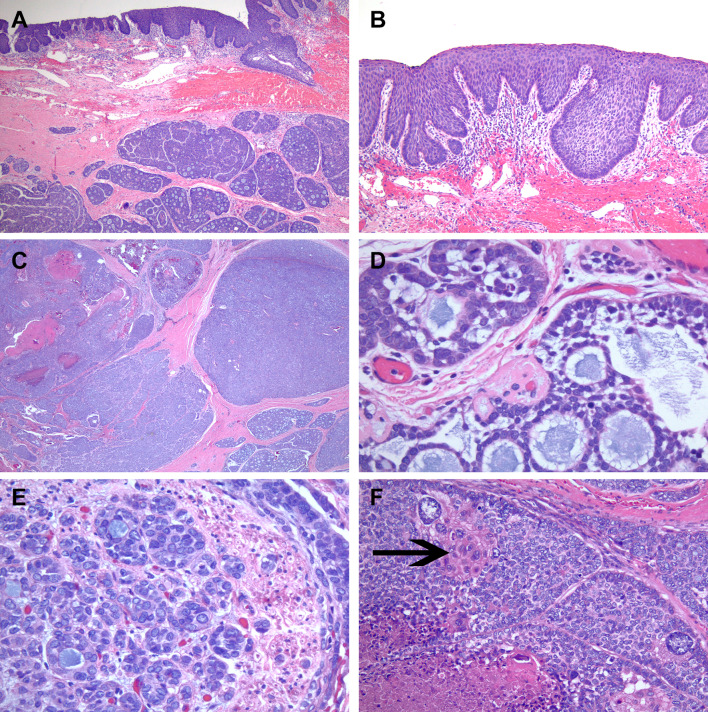
Histologic examination of her completion palatectomy demonstrated a tumor with an unusual morphologic appearance and immunohistochemical profile. The palatal mucosa indeed contained a basaloid type squamous dysplasia which extended into the underlying seromucinous ducts (Fig. 1a, b). However, within the submucosa which was not sampled in the preceding biopsy, was an expansile high-grade neoplasm with a salivary tumor-like appearance (Fig. 1a, c). The tumor was mostly composed of large nests, some solid, some papillary and others had a cribriform architecture wherein the tumor cells surrounded microcystic spaces filled with mucoid material (Fig. 1c, d). The majority of tumor cells had indistinct cell borders and contained medium to large irregular nuclei with minimal eosinophilic cytoplasm, imparting an overall basaloid appearance to the tumor. The nuclear chromatin quality in many of the cells was vesicular and pale with peripherally placed nucleoli. These nuclear features coupled with the variable architectural patterns were reminiscent of polymorphous adenocarcinoma/cribriform adenocarcinoma of minor salivary glands (Fig. 1e). There were scattered anaplastic cells and within some nests, the cytoplasm of the cells had brighter eosinophilic appearance imparting a squamoid morphology (Fig. 1f). Necrosis and high mitotic activity (49 per 10 consecutive high power fields) were present. There were rare scattered cribriform and tubular nests of cells between the larger solid nests and at the tumor periphery in which the tumor cells had a low-grade cytologic appearance with hyperchromatic small nuclei and cleared cytoplasm resembling adenoid cystic carcinoma, however, definitive ducts were not identified in these areas. Perineural invasion was present (Fig. 1e) but no angiolymphatic invasion was identified.
Fig. 1.
Overlying basaloid type of surface squamous dysplasia involving the hard palate mucosa with extension in seromucinous duct and submucosal high-grade salivary carcinoma component arranged in large nests (a). Higher power image of basaloid type of surface dysplasia (b). Salivary carcinoma component with large solid and cribriform nests containing necrosis (c). High power image of cribriform area with microcystic spaces filled with mucoid material, in lower half of image the tumor cells have more of angulated hyperchromatic nuclei. However, in the upper half the tumor cells are larger in size with vesicular chromatin and some eosinophilic cytoplasm (d). Perineural invasion and the nuclear features of the tumor cells with open, vesicular chromatin and eosinophilic cytoplasm are illustrated (e). Anaplastic tumor cells, tumor cells with a squamoid morphology (arrows), necrosis and mitotic activity is shown (f)
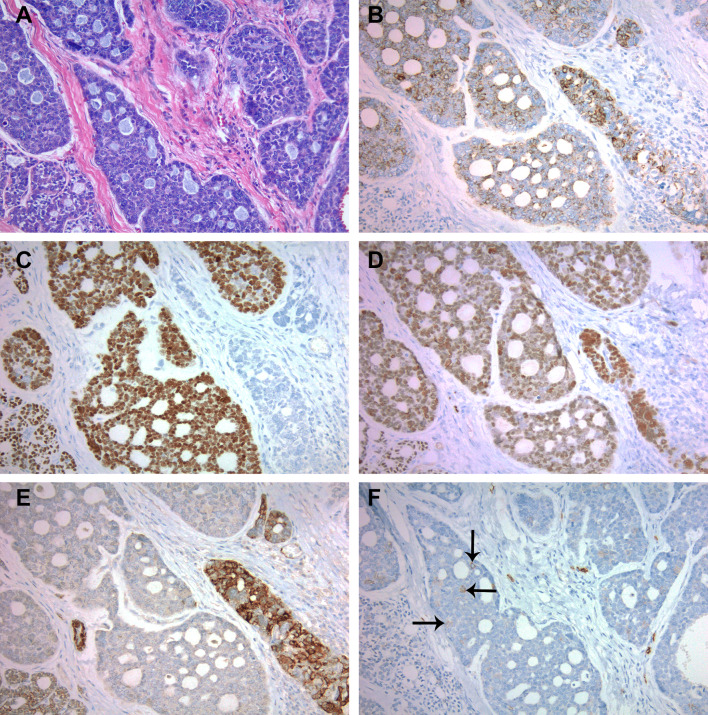
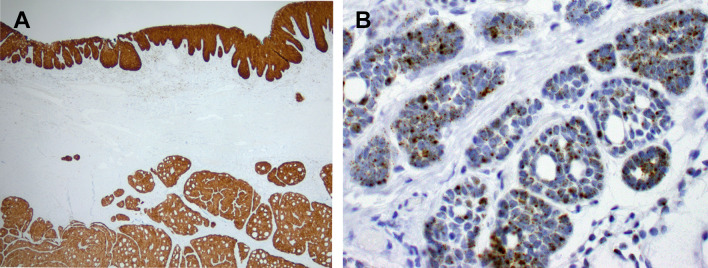
A battery of immunohistochemical stains were performed: cytokeratin AE1/AE3, CK 7, CK 5/6, SMA, SMMS-1, SOX-10, S-100, CD117, p40 and p16. The surface palatal mucosa with basaloid type squamous dysplasia expressed cytokeratin AE1/AE3, CK5/6, p40 and strong and diffuse p16 and was negative for all other markers. The majority of tumor cells in the salivary type neoplasm demonstrated expression of cytokeratin AE1/AE3, CK7, SOX-10, p40 and a subset demonstrated expression of SMA, CK5/6, SMMS-1 and S-100 in a non-patterned distribution (Fig. 2a–e). There were rare weakly staining CD117 positive cells (Fig. 2f). All of the submucosal salivary type tumor cells were strongly and diffusely positive for p16 (Fig. 3a). RNA in situ hybridization using both the high-risk HPV probe set (Fig. 3b) and HPV type 33-specific probe were positive in both the surface dysplasia and salivary gland components. FISH for MYB was intact.
Fig. 2.
Hematoxylin and eosin stained sectioned corresponding to the immunohistochemical stains of the salivary gland component (a). Cytokeratin AE1/AE3 (b), p40 (c), and SOX10 (d) stains the majority of tumor cells with variable intensity but not in a patterned distribution. SMMS-1 stains some of the tumor cells also in a non-patterned distribution (e). CD117 only weakly stains rare tumor cells (arrows indicate positive staining) (f)
Fig. 3.
Immunohistochemistry for p16 was strong and diffuse in the surface squamous and salivary gland components (a) RNA in situ hybridization with a high-risk HPV probe set was positive and demonstrated a predominantly punctate nuclear signal pattern with rare diffuse signals in both the surface squamous and salivary gland components, the salivary gland component is shown here (b)
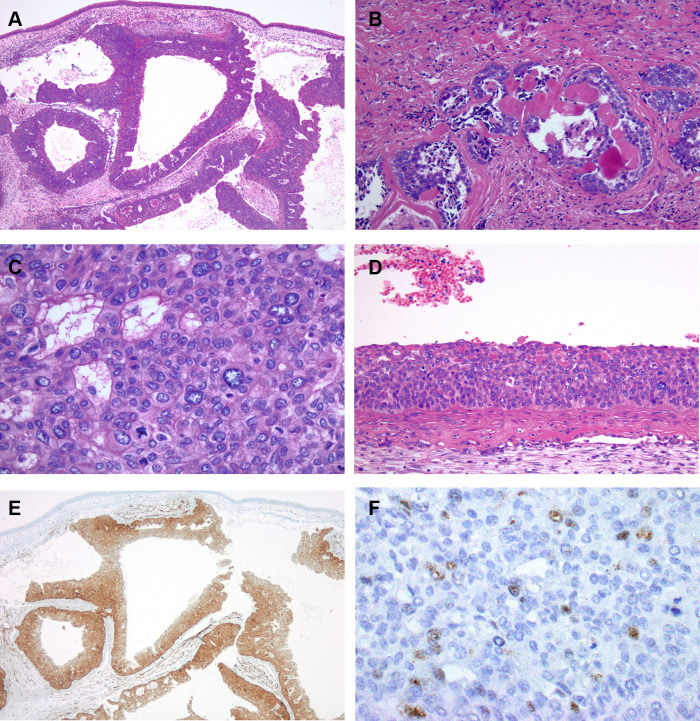
The patient’s carcinoma from the 1980s was reviewed and compared to her current carcinoma. The carcinoma from the 1980s had a greater proportion of papillary, tubular and cribriform architectural patterns with less solid nests (Fig. 4a, b). The nuclear features were similar between both tumors, however some of the tumor cells demonstrated distinct cell borders with occasional clearing of the cytoplasm (Fig. 4b, c). The cytologic difference may be in part due to difference in fixation techniques. Surface dysplasia of the respiratory sinonasal mucosa, scattered anaplastic tumor cells, brisk mitotic activity and necrosis were also present (Fig. 4c, d). No perineural or angiolymphatic invasion was seen. The prior tumor was subsequently found to be diffusely positive for p16 (Fig. 4e) and was also positive for high-risk HPV using the high-risk HPV probe set (Fig. 4f) as well as the HPV type 33-specific probe.
Fig. 4.
The salivary carcinoma component from 1988 also consisted of large expansile nests. Papillary areas with fibrovascular cores are shown lining the nests. Unremarkable sinonasal mucosa lines the surface in this image (a). Cribriform growth pattern was also present (b). The tumor cells had open chromatin, eosinophilic cytoplasm and distinct membranes. Mitotic activity is also apparent (c). Basaloid type of surface dysplasia involving the sinonasal mucosa was focally present (d). Immunohistochemistry for p16 was strongly and diffusely positive (e). RNA in situ hybridization with a high-risk HPV probe set was positive and demonstrated both punctate and diffuse nuclear signals (f)
Discussion
HMSC is relatively new and distinct sinonasal carcinoma which exhibits features of both a surface-derived and salivary gland carcinoma and has a consistent association with HPV [2–5]. In this case report we present the clinical and pathologic findings of another HMSC which further substantiates this as a distinct tumor type with a varied morphologic appearance.
As suggested by the prior literature, the clue to the diagnosis of HMSC is the presence of overlying surface dysplasia and a high-grade salivary malignancy which does not fit a specific named salivary gland entity [5]. Indeed this was the hallmark of our case. The high-grade salivary component of our tumor did not have consistent morphologic or immunophenotypic evidence to support classification as a biphasic salivary tumor, i.e., there were no distinct ducts surrounded by basal or myoepithelial cells. Instead the tumor demonstrated variable architectural patterns and nuclear features which were somewhat reminiscent of polymorphous adenocarcinoma/cribriform adenocarcinoma of minor salivary glands. However, the immunophenotypic profile with diffuse expression of p40 would not be compatible with polymorphous adenocarcinoma/cribriform adenocarcinoma of minor salivary glands. Rather the immunoprofile of p40 and SOX10 with some smooth muscle marker expression was more suggestive of a myoepithelial phenotype. The presence of strong and diffuse p16 expression in the salivary gland carcinoma and overlying squamous dysplasia suggested that this could represent a HMSC. In-situ hybridization for high-risk HPV was performed on this tumor and was positive in both the surface and salivary components allowing classification as a HMSC. Additionally, this tumor also was positive for HPV type 33 which has been the most common HPV genotype associated with HMSC.
There are several instructive aspects of this case. Firstly, our patient’s tumor was previously classified as an adenoid cystic carcinoma back in 1986 and when it recurred in 1988. This initial classification lends credence to the original designation of HMSC as “HPV-related carcinoma with adenoid cystic carcinoma-like features.” However, as previously mentioned our tumor did not have a distinct biphasic profile as is the case with adenoid cystic carcinoma. Additionally, her tumor from the 1980s and current tumor both had areas with high-grade cytologic and architectural features which would be unusual for a conventional adenoid cystic carcinoma and would suggest dedifferentiation or an alternative diagnosis. The patient’s long term survival is not in keeping with dedifferentiation and the immunophenotype with retention of SMA and diffuse p40 expression would also argue against a dedifferentiated adenoid cystic carcinoma as these myoepithelial markers are often lost with dedifferentiation [8].
Secondly, the patient’s tumor manifestation as a palatal mass in both in 1986 and 2017 is an unusual presentation for HMSC. The detailed pathologic reports from the 1980s were unavailable, however, the clinical notes regarding her initial 1986 biopsy states that the biopsy mentioned the presence of respiratory epithelium suggesting that there was tumor in the nasal cavity. This was confirmed by our review of the remaining slides from the patient’s prior 1986 resection. Additionally, the recurrence in 1988 was in the nasal septum and nasal floor. Our overall impression was that the patient’s tumor originated in the nasal cavity and there was extension and secondary involvement of the palatal mucosa.
Thirdly, this case represents the longest follow-up of any published HMSC. The patient’s clinical course with a 30-year interval between her tumor recurrences provides evidence that despite the high-grade morphology, HMSC’s behavior is indeed indolent. However, while indolent, there is a real potential for very late recurrences, and lifelong clinical surveillance may be warranted.
Contributor Information
Akeesha A. Shah, Phone: 216-636-9407, Email: shaha8@ccf.org
Eric D. Lamarre, Email: lamarre@ccf.org
Justin A. Bishop, Email: justin.bishop@utsouthwestern.edu
References
- 1.Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop JA, Ogawa T, Stelow EB, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37:836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasen S, Bishop JA, Hansen TV, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features of the sinonasal tract: clinical and morphological characterization of six new cases. Histopathology. 2017;70:880–888. doi: 10.1111/his.13162. [DOI] [PubMed] [Google Scholar]
- 4.Hwang SJ, Ok S, Lee HM, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features of the inferior turbinate: a case report. Auris Nasus Larynx. 2015;42:53–55. doi: 10.1016/j.anl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Bishop JA, Andreasen S, Hang JF, et al. HPV-related multiphenotypic sinonasal carcinoma: an expanded series of 49 cases of the tumor formerly known as HPV-related carcinoma with adenoid cystic carcinoma-like features. Am J Surg Pathol. 2017;41:1690–1701. doi: 10.1097/PAS.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Naggar AK, Chen JCK, Grandis JR, Takata T, Slootweg PJ, editors. World Health Organization classification of head and neck tumours. 4. Lyon: IARC Press; 2017. [Google Scholar]
- 7.Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seethala RR, Hunt JL, Baloch ZW, et al. Adenoid cystic carcinoma with high-grade transformation: a report of 11 cases and a review of the literature. Am J Surg Pathol. 2007;31:1683–1694. doi: 10.1097/PAS.0b013e3180dc928c. [DOI] [PubMed] [Google Scholar]