Abstract
Papillary thyroid carcinoma (PTC) is defined by an invasive growth pattern and classic nuclear features: enlarged, grooved, overlapping nuclei with chromatin clearing and intranuclear cytoplasmic pseudoinclusions (INCP). True INCPs are characteristic of PTC, but may infrequently be seen in noninvasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTP). Nuclear abnormalities that mimic INCP (“pseudo-pseudoinclusions”) are common in a variety of thyroid lesions. H&E and ubiquitin-stained whole tissue sections of classic PTC (n = 25) and NIFTP (n = 35) were evaluated. On H&E, true INCPs were present in all (100%) PTCs and absent in all NIFTPs (0%). Pseudo-pseudoinclusions were present in 13 (37%) NIFTPs. In 24 (96%) PTCs, ubiquitin was strongly expressed within INCPs. In NIFTPs, optically clear nuclei or pseudo-pseudoinclusions did not express ubiquitin (0/35). Occasionally, nuclear vacuoles in NIFTP demonstrated a marginated staining pattern, in which strong ubiquitin expression was seen at the periphery of the nucleus, but the central pale area was negative. In addition, 2 NIFTPs demonstrated intrafollicular psammomatoid calcifications which were strongly ubiquitin-positive. Psammoma bodies in PTC were ubiquitin-negative in the majority of cases. We report a previously undescribed finding: strong ubiquitin expression in true INCPs in PTC, absence of true INCPs in NIFTP, and absence of ubiquitin expression in pseudo-pseudoinclusions in NIFTP. This finding supports the difference between true INCPs (found only in PTC) and pseudo-pseudoinclusions (found in NIFTP). Using strict histologic criteria and ubiquitin immunostaining, the presence of true pseudoinclusions may exclude a diagnosis of NIFTP. Caution should be exercised when interpreting nuclear vacuoles or pseudo-pseudoinclusions.
Keywords: Thyroid, NIFTP, Papillary thyroid carcinoma, Pseudoinclusions, Ubiquitin
Background
Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, representing approximately 80% of thyroid carcinomas [1]. Historically, nuclear features have superseded architectural features in thyroid neoplasms, and the presence of specific nuclear features equated to a diagnosis of PTC, regardless of the architectural pattern. These nuclear features include enlargement, overlapping, irregular contours or grooves, chromatin clearing or “Orphan Annie Eyes”, and intranuclear cytoplasmic pseudoinclusions [1]. With the recent introduction of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) to the diagnostic armamentarium, the presence of such nuclear features in the context of a circumscribed, follicular-patterned neoplasm no longer necessitates a diagnosis of carcinoma. If strict architectural and nuclear criteria are met, a NIFTP diagnosis can be rendered [2]. A comprehensive review of the diagnostic criteria for NIFTP is outside the scope of this study but has been well-described in recent publications [3, 4]. Although pseudoinclusions are allowed in NIFTP according to the consensus proposal, an abundance should incite a more rigorous search for features of classic PTC [3]. Currently, the quantity or frequency of pseudoinclusions allowed in NIFTP is unknown. It has been suggested by some that they are rare [3], and by others that their presence favors PTC over NIFTP [5, 6].
Furthermore, nuclear changes that mimic PTC-like nuclei can be seen in various non-neoplastic conditions, benign neoplasms, or as a result of tissue processing [1, 7, 8]. Inflammation or fibrosis in the context of thyroiditis can induce nuclear clearing [8]. Extreme temperature changes (including intraoperative freezing or overheating during processing) as well as inadequate fixation can result in fuzzy intranuclear vacuoles or bubbles (so-called pseudo-pseudoinclusions) [8–11]. A true intranuclear cytoplasmic pseudoinclusion is limited to one per nucleus, is round, is bound by a crisp nuclear membrane, contains cytoplasm that matches the cytoplasm peripheral to the nucleus, and is present in occasional cells [9]. Artefactual pseudo-pseudoinclusions are frequently multiple per nucleus, are pale, are poorly demarcated, and often occur in a high percentage of cells [9].
Burgeoning research into classic PTC has illuminated a number of underlying pathogenetic mechanisms, among them mutations in proteins associated with ubiquitination [12–14]. Some mutations lead to an increase in cellular ubiquitin content [13]. Members of the ubiquitin family are frequently involved in post-translational protein modification [15, 16]. Following covalent bonding to the ubiquitin molecule, intracellular proteins are targeted for destruction or sub-cellular organelle localization. They can also be involved in signaling and serve to maintain the quality of protein products [15, 16]. Alterations in proteins in the ubiquitin pathway have been implicated in the development of a variety of malignancies including PTC [16], likely due to aberrantly-enhanced degradation of tumor-suppressing proteins or inappropriately hindered degradation of oncoproteins associated with cell proliferation and survival. While patterns of ubiquitin expression in PTC or follicular neoplasms have not been systematically studied, we have anecdotally observed that immunohistochemical (IHC) staining of classic PTCs with ubiquitin-targeting antibodies preferentially highlights the cytoplasm within nuclear pseudoinclusions in PTC. However, the significance and reproducibility of this staining pattern is unknown.
The aim of this study is to determine the prevalence of true intranuclear cytoplasmic pseudoinclusions in classic PTC versus NIFTP and to evaluate ubiquitin expression patterns in classic PTC versus NIFTP, with a specific emphasis on nuclear expression.
Methods
With Institutional Review Board Approval, classic PTCs and NIFTPs were identified in the diagnostic pathology archives (2010–2017). Twenty-five PTCs were selected based on the presence of classic architectural and nuclear features: an invasive growth pattern with the presence of well-developed branching papillae, usually with dense eosinophilic cytoplasm, and well-developed nuclear features of PTC. The presence of nuclear pseudoinclusions in PTC was not specifically used as an inclusion criterion. Thirty-five NIFTPs were selected based on adequate nuclear atypia consistent with a diagnosis of NIFTP and abundant tumor volume. The presence of nuclear pseudoinclusions or nuclear pseudo-pseudoinclusions in NIFTP was not specifically used as an inclusion criterion. All slides from all cases were reviewed and a block with abundant tumor volume was selected from each case. Given the relatively recent definition of NIFTP, cases that pre-dated the NIFTP classification were reviewed and diagnosis confirmed for the purposes of this investigation.
From each case, one block most representative of the tumor morphology was selected, and whole tissue sections 3 microns in thickness were cut. One slide was stained with Hematoxylin & Eosin. A second slide was stained for ubiquitin: After deparaffinization and rehydration, tissue sections were treated with 80% formic acid for 60 min at room temperature. Anti-ubiquitin antibody (1:200) was applied on tissue sections for a 1-h incubation at room temperature in a humidity chamber. Following TBS wash, the antigen–antibody binding was detected with Envision+ anti-mouse-HRP system (DAKO, K4001) and DAB + chromogen (DAKO, K3468) (Agilent, Santa Clara, CA). Tissue sections were briefly immersed in hematoxylin for counterstaining and were covered with cover glasses.
H&E sections were reviewed from all cases for the presence of pseudoinclusions and pseudo-pseudoinclusions. True intranuclear cytoplasmic pseudoinclusions were considered present if they met the following criteria: one per nucleus; present in scattered cells and not the majority of cells; had a crisp border resembling nuclear membrane; contained material with tinctorial and textural similarity to the peripheral cytoplasm (usually eosinophilic and dense). IHC-stained sections were reviewed for the presence of cytoplasmic and nuclear ubiquitin expression patterns.
When available, next generation sequencing data was gathered on the NIFTPs. Sequencing was performed for clinical or research purposes, as previously described [17]. In brief, using a clinically validated assay, formalin-fixed, paraffin-embedded DNA was amplified for somatic mutations located within mutational hotspot regions of 50 cancer-related genes including BRAF, HRAS, NRAS, and KRAS.
Results
Ubiquitin Immunostaining in PTC
Of the 25 included cases of classic PTC, true intranuclear cytoplasmic pseudoinclusions were identified on H&E stained sections in all 25 (100%). Ubiquitin showed patchy, weak-moderate nuclear and/or cytoplasmic expression in both the tumor and surrounding normal thyroid tissue in 23 (92%). In 24 (96%), ubiquitin was strongly expressed within intranuclear cytoplasmic pseudoinclusions, and was darker than the background nuclear and/or cytoplasmic staining (Fig. 1). Psammoma bodies were present in 12 (48%) cases: the psammoma bodies in 4 (33%) cases had moderate to strong ubiquitin staining; the remaining 8 (67%) cases were negative (Fig. 2).
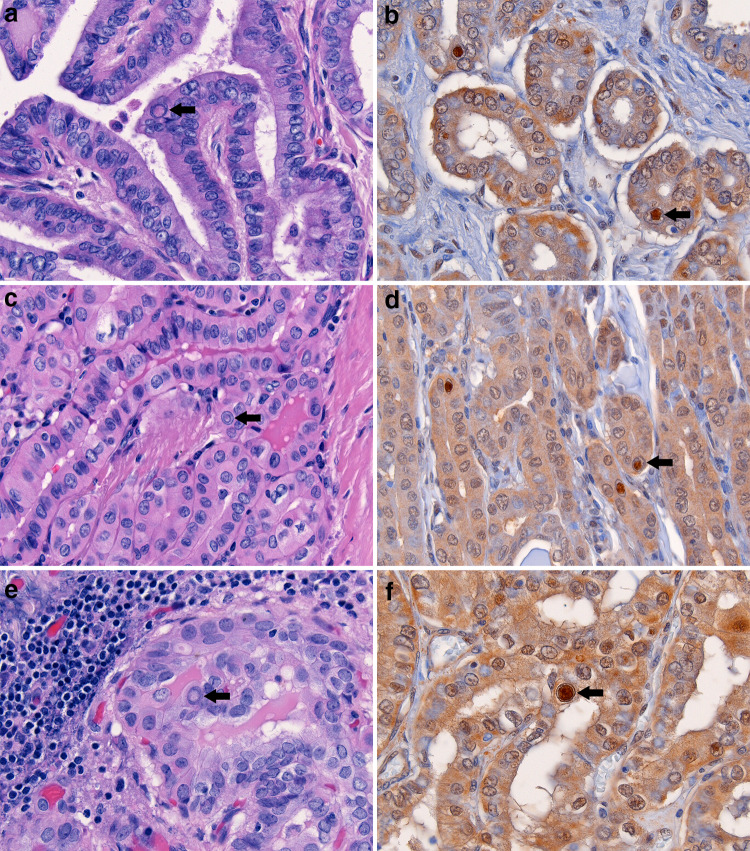
Fig. 1.
Three cases of classic PTC with characteristic nuclear features including numerous intranuclear cytoplasmic pseudoinclusions (arrows; a, c, and e) bound by a crisp inner membrane and containing dense eosinophilic cytoplasm. Corresponding ubiquitin immunostains strongly highlight the pseudoinclusions in each case (arrows; b, d, and f). Weak background nuclear and cytoplasmic ubiquitin staining is appreciable in the carcinoma cells
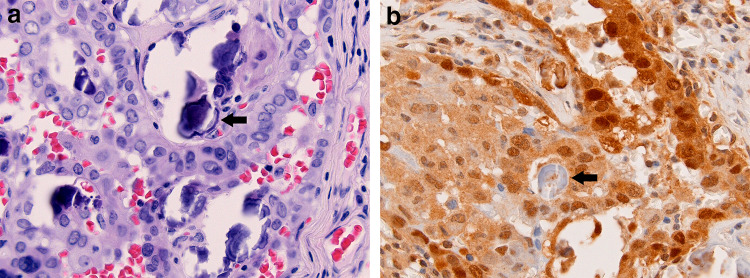
Fig. 2.
Psamomma bodies are present in approximately half of PTCs (arrow; a). In most cases, psammoma bodies are negative for ubiquitin (arrow; b). Variable background nuclear and cytoplasmic staining is present in the carcinoma cells
Ubiquitin Immunostaining in NIFTP
Of the 35 included cases of NIFTP, none (0%) had true intranuclear cytoplasmic pseudoinclusions on H&E stained sections. However, pseudo-pseudoinclusions were present in 13 (37%). Ubiquitin showed patchy, weak-moderate nuclear and/or cytoplasmic expression in both the tumor and surrounding normal thyroid tissue in 26 (74%). Ubiquitin was not strongly expressed in optically clear nuclei, blurry intranuclear vacuoles, or intranuclear pseudo-pseudoinclusions (0; 0%). In 4 (11%) cases, nuclear vacuoles demonstrated a marginated staining pattern, where moderate-strong ubiquitin staining was present inside the nucleus at the chromatin-rich periphery, but the central vacuole was negative (Fig. 3). Two (6%) cases contained intrafollicular psammomatoid calcifications of colloid which strongly stained for ubiquitin (Fig. 4).
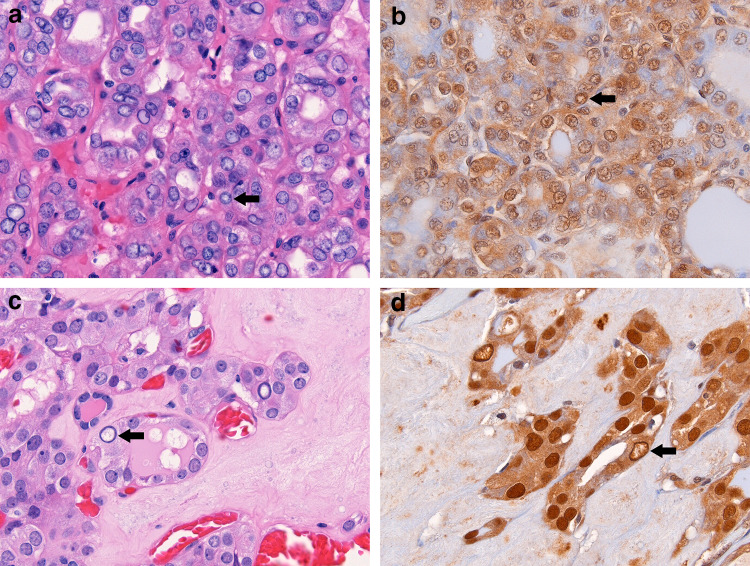
Fig. 3.
Two cases of NIFTP showing mimics of true pseudoinclusions. Nuclear pseudo-pseudoinclusions (arrow; a) are present in the context of mild nuclear enlargement and membrane irregularity. Correspondingly, ubiquitin is not preferentially expressed in these pseudo-pseudoinclusions (arrow; b). Occasional optically clear nuclei with chromatin margination are present (arrow; c). These nuclei demonstrate a marginated staining pattern, in which ubiquitin is strongly expressed at the periphery of the nucleus, but the central pale area has weak to absent staining (arrow; d). True pseudoinclusions are absent. Both pictured NIFTPs harbor KRAS mutations (G12V and Q61R)
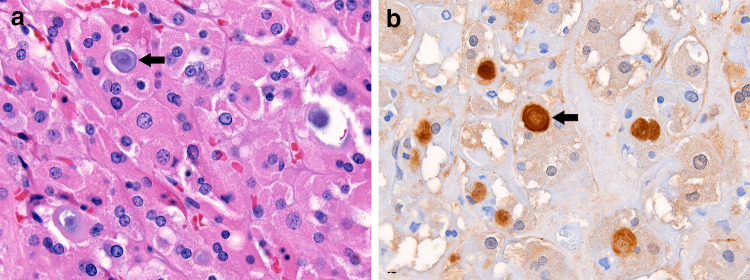
Fig. 4.
Lamellated microcalcifications are occasionally identified in the follicular lumina of oncocytic neoplasms (arrow; a). Ubiquitin strongly highlights these calcifications (arrow; b) while background cytoplasm and nuclei show weak staining
Pathogenic Mutations in NIFTP
Next generation sequencing data was available in 23 (66%) of NIFTP cases. Pathogenic mutations were present in 16 (70%) of tested cases: 15 had a mutation in one of the three RAS isoforms (8 NRAS, 5 HRAS, 2 KRAS) and 1 had BRAF K601E mutation. This BRAF mutation is distinct from V600E, is predominantly present in follicular neoplasms, and is phenotypically considered to be RAS-like [18]. Pathogenic mutations were not detected in the remaining 7 (30%) cases.
Discussion/Conclusion
In this study, true intranuclear cytoplasmic pseudoinclusions were absent in NIFTP, and ubiquitin-expression patterns were different in true pseudoinclusions in PTC compared to pseudo-pseudoinclusions in NIFTP. While background weak-moderate nuclear or cytoplasmic ubiquitin expression was present in most cases of PTC and NIFTP, intense dark staining was only seen within true nuclear pseudoinclusions of PTC and was present in 96% of classic PTC cases. No case of NIFTP had true pseudoinclusions by H&E or by ubiquitin staining. However, nuclear pseudo-pseudoinclusions were present on H&E sections in 37% of NIFTP cases. Strong staining was not present in pseudoinclusion mimics, i.e. nuclei with chromatin clearing or with vacuoles (pseudo-pseudoinclusions). Instead, pseudo-pseudoinclusions had a marginated pattern, where ubiquitin highlighted the chromatin-rich periphery of the nucleus and the pseudo-pseudoinclusion or central vacuole was negative. This pattern was present in 11% of NIFTP cases. These patterns of ubiquitin expression have not previously been described. These findings support the different origin of true pseudoinclusions (cytoplasmic invaginations) compared to artefactual mimics. The diagnosis of NIFTP was supported with molecular studies in 23 cases, of which 16 (70%) had pathogenic RAS or RAS-like mutations [18].
The pathophysiologic events leading to the finding of strong ubiquitin expression in true pseudoinclusions are unknown, however, this staining pattern may be useful in diagnosis of classic PTCs showing follicular growth rather than adenomatous nodules with atypia or NIFTPs. According to recent consensus agreement, pseudoinclusions are allowed in NIFTP [3, 4], however, true pseudoinclusions meeting our strict definition were not present in this series of NIFTP. Previous studies have closely examined nuclear irregularities in PTC in order to discern the nature of their formation. Electron microscopy studies have suggested that nuclear grooves and nuclear pseudoinclusions are manifestations of a continuum of nuclear changes resulting from redundancy of the nuclear membrane. Namely, nuclear pseudoinclusions develop from expansion or deep intussusception of nuclear grooves [19]. Similarly, nuclear pseudoinclusions contain cytoplasmic organelles and are lined by a lipid bilayer, further supporting that nuclear pseudoinclusions are invaginations of cytoplasm [19]. Studies of major nuclear structural proteins including lamin A, lamin B1, lamin C, lamin B1 receptor, lamina-associated polypeptide 2, and emerin have indicated that alterations in nuclear structural proteins are not the source of nuclear irregularities in PTC [20]. Rather, nuclear irregularities may be the result of alterations in differential mechanical forces between the chromatin within the nuclear matrix and cytoskeletal extranuclear proteins [20]. Studies evaluating emerin immunoreactivity have served to eliminate ambiguity in differentiation of nuclear atypia in PTC from artefactual nuclear aberrations in follicular neoplasms [21, 22]. Specifically, emerin labels the nuclear membrane and clearly highlights both outer and inner nuclear envelopes in pseudoinclusions. It also demonstrates severe folding and bosselation of the outer nuclear membrane in PTC [21, 22]. Conversely, invagination of the nuclear membrane is not present in artefactually vacuolated nuclei in follicular neoplasms [21]. We speculate that accumulation of intermediate filaments damaged by shearing forces on the nuclear membrane may contribute to ubiquitin accumulation in pseudoinclusions. It is uncertain whether this finding may be a result of absolute ubiquitin excess in the cell or abnormal localization of ubiquitinated-proteins. Nuclear pseudoinclusions have also been described in neoplasms outside of the thyroid including usual ductal hyperplasia of the breast, meningiomas, and melanocytic lesions [9, 23]. The nature of nuclear pseudoinclusions in other such neoplasms is beyond the scope of this study.
Nuclear features mimicking PTC can be found not only in NIFTPs but also in various benign or reactive thyroid conditions. Specifically, chromatin margination creating an optically clear nucleus may be present in the diffuse follicular hyperplasia of Graves disease as well as areas of nuclear injury in lymphocytic thyroiditis [8]. Fine needle aspiration (FNA) artifact can also result in chromatin clearing and other worrisome architectural features (such as seeding of the biopsy tract with tumor cells mimicking invasion). As most thyroid nodules are subject to preoperative FNA biopsy, such artifacts are encountered regularly in thyroid histopathology. Reviews have suggested that artifacts concerning for carcinoma are encountered in as many as 12% of thyroidectomies following FNA [24]. Reassuring signs of benignity in these situations include the lack of true pseudoinclusions and the focal nature of the nuclear changes [8]. Not only biologic processes but variability in tissue processing can also result in nuclear changes. Some artefacts simulating pseudoinclusions may result from overheating and water evaporation during routine tissue processing. When special precautions for drying tissues are performed experimentally, the presence of nuclear ‘bubbling’ is diminished [11].
Another common finding in PTC is the presence of psammoma bodies, defined as ovoid calcifications with concentric lamellations, present in cellular colloid-poor regions of PTC, and thought to be the result of infarcted papillary projections [8, 25, 26]. Coarse dystrophic calcifications distinct from psammoma bodies are common in FNA tracts and at the periphery of some nodules in an eggshell-like distribution [27]. Microcalcifications resembling psammoma bodies have also been reported in benign thyroid neoplasms and should be distinguished from true psammoma bodies of PTC. Microcalcifications (or so-called psammomatoid calcifications) can occasionally show concentric lamellations and are generally encountered within follicular lumina of oncocytic or Hurthle cell lesions [8, 25]. They are thought to represent inspissated colloid [26]. Two NIFTP cases in our study contained intrafollicular psammomatoid calcifications which were strongly positive for ubiquitin IHC, while true psammoma bodies in PTC cases were predominantly negative (67%). This finding has not previously been reported in the literature, though given the rarity of colloidal psammomatoid calcifications, the relevance may be limited. Although oncocytic NIFTPs remain controversial, they have not been definitively excluded, and were therefore included in this study in limited numbers [3].
In this study, true pseudoinclusions were only present in PTC and were absent in NIFTP. We also report an interesting and novel finding of strong ubiquitin expression in true intranuclear cytoplasmic pseudoinclusions in PTC, a pattern which was not present in papillary-like nuclei in NIFTP. This finding may aid in confirming the diagnosis of PTC in a thyroid mass by helping to separate true nuclear pseudoinclusions from pseudo-pseudoinclusions in non-malignant lesions, including chromatin clearing secondary to reactive changes or vacuoles created during tissue processing. We also uncovered two other unique phenomena, though in small number: a marginated pattern of ubiquitin expression in pseudo-pseudoinclusions and strong staining of colloidal psammomatoid calcifications in NIFTP. Though the precise mechanisms of these patterns of ubiquitin expression are yet to be determined, this study supports recent experimental evidence suggesting that ubiquitin and ubiquitin-related proteins may be increased in PTC [12–14]. Based on our data, true pseudoinclusions may be absent in NIFTP and ubiquitin immunostaining may help differentiate pseudoinclusions in PTC from nuclear artefacts.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
For this type of study formal consent is not required.
Research Involving Animal Rights
This article does not contain any studies with animals performed by any of the authors.
References
- 1.Al-Brahim N, Asa S. Papillary thyroid carcinoma: an overview. Arch Pathol Lab Med. 2006 doi: 10.1043/1543-2165(2006)130[1057:PTCAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seethala RR, Baloch ZW, Barletta JA, Khanafshar E, Mete O, Sadow PM, LiVolsi VA, Nikiforov YE, Thompson LD. Noninvasive follicular thyroid neoplasm with papillary-like nuclei: A review for pathologists. Mod pathol. 2017 doi: 10.1038/modpathol.2017.130. [DOI] [PubMed] [Google Scholar]
- 4.Hung YP, Barletta JA. A user’s guide to non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) Histopathol. 2018 doi: 10.1111/his.13363. [DOI] [PubMed] [Google Scholar]
- 5.Bizzarro T, Martini M, Capodimonti S, Straccia P, Lombardi CP, Pontecorvi A, Larocca LM, Rossi ED. Young investigator challenge: the morphologic analysis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on liquid-based cytology: some insights into their identification. Cancer Cytopathol. 2016 doi: 10.1002/cncy.2177. [DOI] [PubMed] [Google Scholar]
- 6.Howitt BE, Chang S, Eszlinger M, Paschke R, Drage MG, Krane JF, Barletta JA. Fine-needle aspiration diagnoses of noninvasive follicular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2015 doi: 10.1309/AJCPEIE12POICULI. [DOI] [PubMed] [Google Scholar]
- 7.Baloch Z, LiVolsi VA. Cytologic and architectural mimics of papillary thyroid carcinoma. Am J Clin Pathol. 2006;125(Suppl 1):S135-44. doi: 10.1309/YY72M308WPEKL1YY. [DOI] [PubMed] [Google Scholar]
- 8.Rosai J, Kuhn E, Carcangiu ML. Pitfalls in thyroid tumour pathology. Histopathol. 2006 doi: 10.1111/j.1365-2559.2006.02451.x. [DOI] [PubMed] [Google Scholar]
- 9.Ip Y, Filho M, Chan J. Nuclear inclusions and pseudoinclusions: friends or foes of the surgical pathologist? Int J Surg Pathol. 2010;18(6):465–481. doi: 10.1177/1066896910385342. [DOI] [PubMed] [Google Scholar]
- 10.Goellner JR, Caudill JL. Intranuclear holes (cytoplasmic pseudoinclusions) in parathyroid neoplasms, or “holes happen”. Cancer Cytopathol. 2000;90(1):41–46. doi: 10.1002/(SICI)1097-0142(20000225)90:1<41::AID-CNCR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Petrilli G, Fisogni S, Rosai J, Festa S, Torri L, Rodeschi A, Facchetti F. Nuclear bubbles (nuclear pseudo-pseudoinclusions): a pitfall in the interpretation of microscopic sections from the thyroid and other human organs. Am J Surg Pathol. 2017 doi: 10.1097/PAS.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 12.Tuccilli C, Baldini E, Sorrenti S, et al. Papillary thyroid cancer is characterized by altered expression of genes involved in the sumoylation process. J Biol Regul Homeost Agents. 2015;29(3):655–662. [PubMed] [Google Scholar]
- 13.Sipina LV, Bukurova YA, Nikitina IG, Krasnov GS, Sergeev SA, Lisitsyn NA, Karpov VL, Beresten SF. Identification of proteins overexpressed in papillary thyroid tumors. Biochem (Mosc) 2010;75(9):1148–1152. doi: 10.1134/S0006297910090087. [DOI] [PubMed] [Google Scholar]
- 14.Fluge Ø, Bruland O, Akslen LA, Lillehaug JR, Varhaug JE. Gene expression in poorly differentiated papillary thyroid carcinomas. Thyroid. 2006 doi: 10.1089/thy.2006.16.161. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson KD. The discovery of ubiquitin-dependent proteolysis. Proc Natl Acad Sci USA. 2005 doi: 10.1073/pnas.0504842102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002 doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DN, Furtado LV, Long BC, Zhen CJ, Wurst M, Mujacic I, Kadri S, Segal JP, Antic T, Cipriani NA. Noninvasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTPs) are genetically and biologically similar to adenomatous nodules and distinct from papillary thyroid carcinomas with extensive follicular growth. Arch Pathol Lab Med. 2016 doi: 10.5858/arpa.2017-0118-OA. [DOI] [PubMed] [Google Scholar]
- 18.Afkhami M, Karunamurthy A, Chiosea S, Nikiforova MN, Seethala RR, Nikiforov YE, Coyne C. Histopathologic and clinical characterization of thyroid tumors carrying the BRAFK601E mutation. Thyroid. 2016 doi: 10.1089/thy.2015.0227. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko C, Shamoto M, Niimi H, Osada A, Shimizu M, Shinzato M. Studies on intranuclear inclusions and nuclear grooves in papillary thyroid cancer by light, scanning electron and transmission electron microscopy. Acta Cytol. 1995;40:417–422. doi: 10.1159/000333892. [DOI] [PubMed] [Google Scholar]
- 20.Fischer AH, Taysavang P, Weber CJ, Wilson KL. Nuclear envelope organization in papillary thyroid carcinoma. Histol Histopathol. 2001;16:1–14. doi: 10.14670/HH-16.1. [DOI] [PubMed] [Google Scholar]
- 21.Asioli G, Bussolati G. Emerin immunohistochemistry reveals diagnostic features of nuclear membrane arrangement in thyroid lesions. Histopathol. 2009 doi: 10.1111/j.1365-2559.2009.03259.x. [DOI] [PubMed] [Google Scholar]
- 22.Bussolati G. Proper detection of the nuclear shape: ways and significance. Rom J Morphol Embryol. 2008;49(4):435–439. [PubMed] [Google Scholar]
- 23.Rose DS. Nuclear pseudoinclusions in melanocytic naevi and melanomas. J Clin Pathol. 1995;48:676–677. doi: 10.1136/jcp.48.7.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandit AA, Phulpagar MD. Worrisome histologic alterations following fine needle aspiration of the thyroid. Acta Cytol. 2001;45(2):173–179. doi: 10.1159/000327273. [DOI] [PubMed] [Google Scholar]
- 25.Pusztaszeri MP, Sadow PM, Faquin WC. Images in endocrine pathology: psammomatoid calcifications in on cocytic neoplasms of the thyroid, a potential pitfall for papillary carcinoma. Endo pathol. 2013 doi: 10.1007/s12022-013-9242-2. [DOI] [PubMed] [Google Scholar]
- 26.Johannessen JV, Sobrinho-Simoes M. The origin and significance of thyroid psammoma bodies. Lab Investig. 1980;43(3):287–96. [PubMed] [Google Scholar]
- 27.Kim BK, Choi YK, Kwon HJ, Lee JS, Heo JJ, Han YJ, Park YH, Kim JH. Relationship between patterns of calcification in thyroid nodules and histopathologic findings. Endo J. 2013;60(2):155–60. doi: 10.1507/endocrj.EJ12-0294. [DOI] [PubMed] [Google Scholar]